Topical Administration of SLN-Based Gene Therapy for the Treatment of Corneal Inflammation by De Novo IL-10 Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SLNs and Vectors
2.3. Size and Zeta Potential of SLNs and Vectors
2.4. Adhesion Test
2.5. Rheology Studies
2.6. pH Measurement
2.7. In Vitro Studies
2.7.1. Transfection Efficacy of the Vectors Containing the Plasmid pUNO1-hIL10
2.7.2. In Vitro Cell Viability
2.8. In Vivo Studies
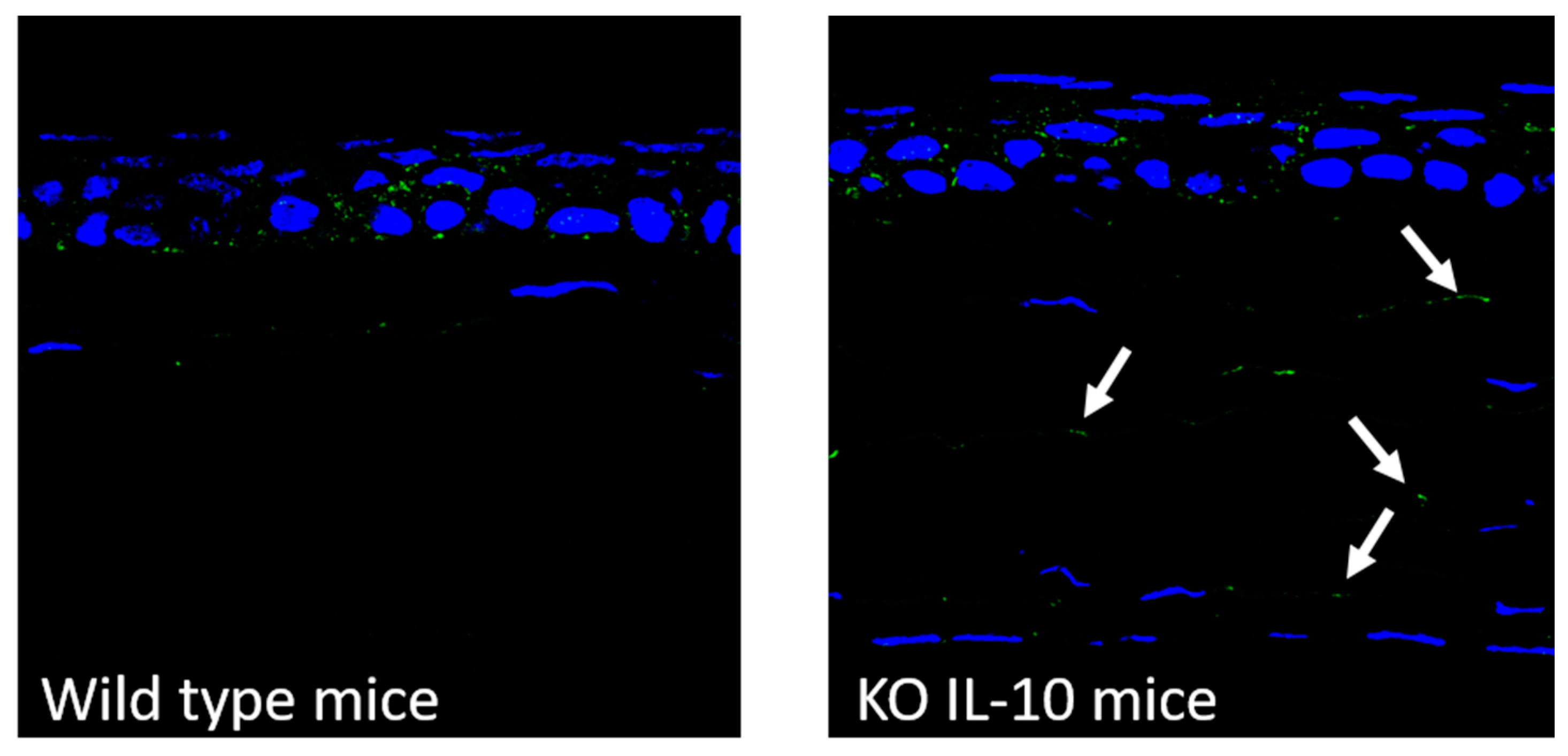
2.8.1. Detection of CD44 Receptor in Cornea from Wild Type and IL-10 KO Mice
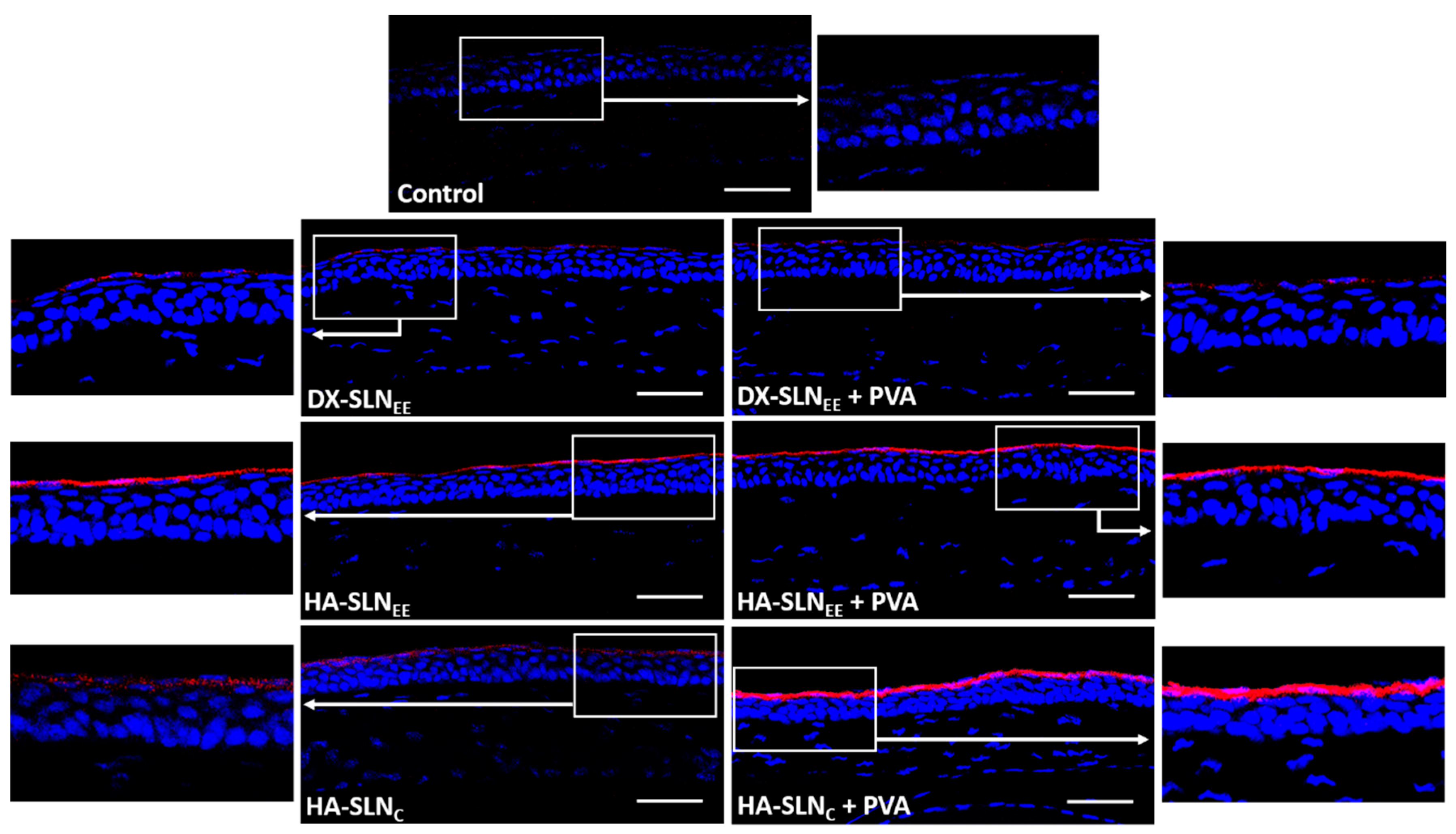
2.8.2. Corneal Localization of the Vectors
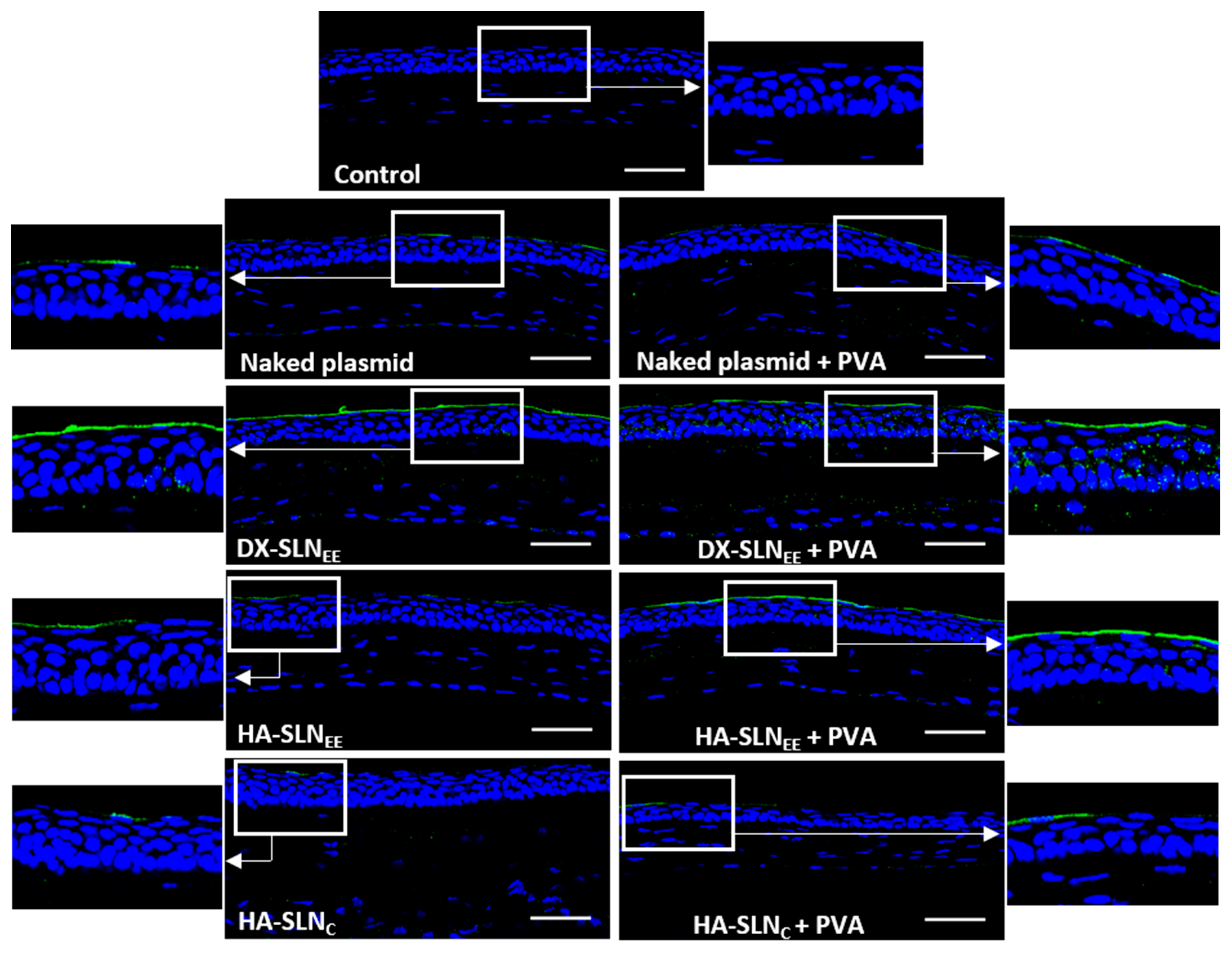
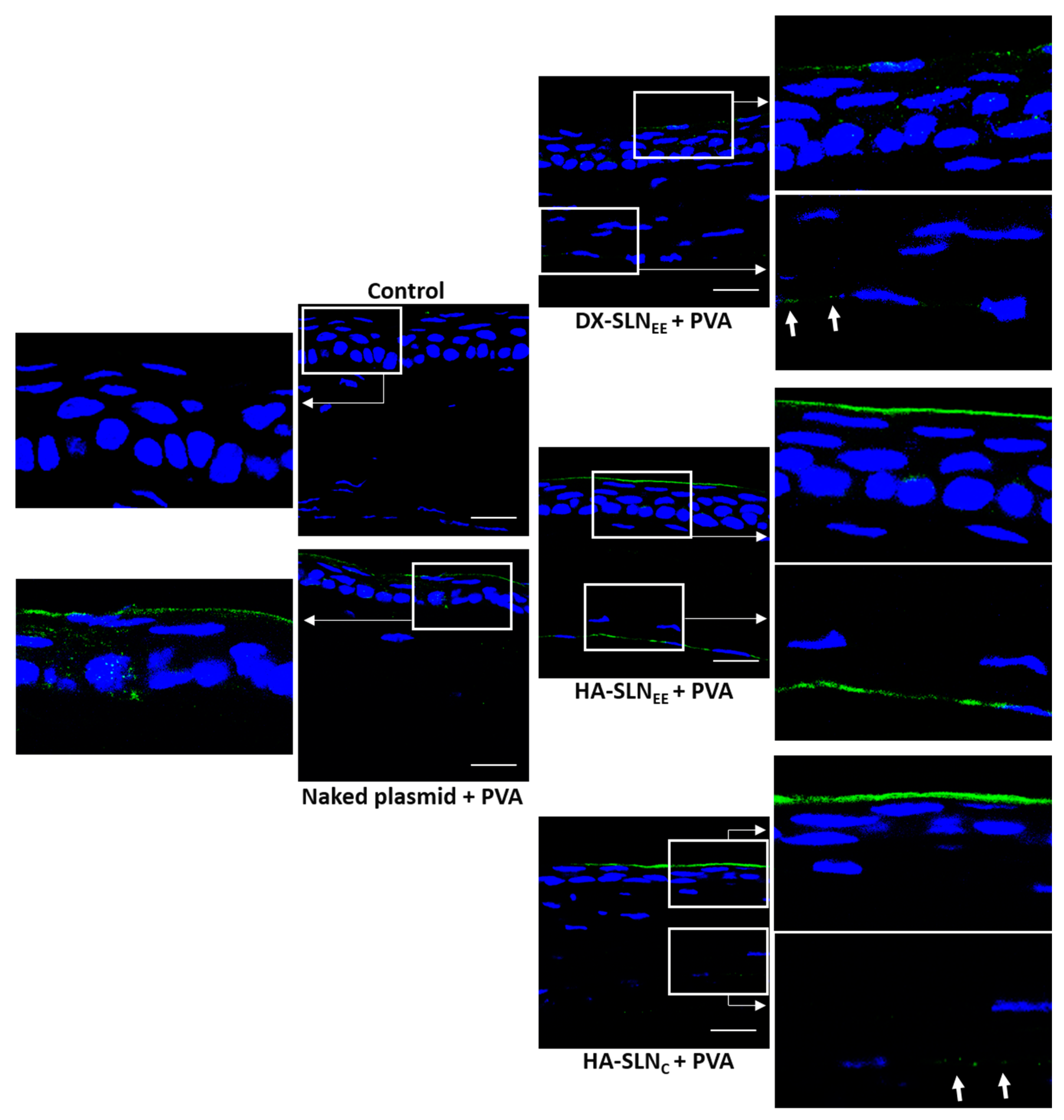
2.8.3. In Vivo Transfection Studies
Topical Administration
Evaluation of Gene Expression
Structural Analysis of the Cornea
2.9. Statistical Analysis
3. Results
3.1. Size and Zeta Potential of SLNs and Vectors
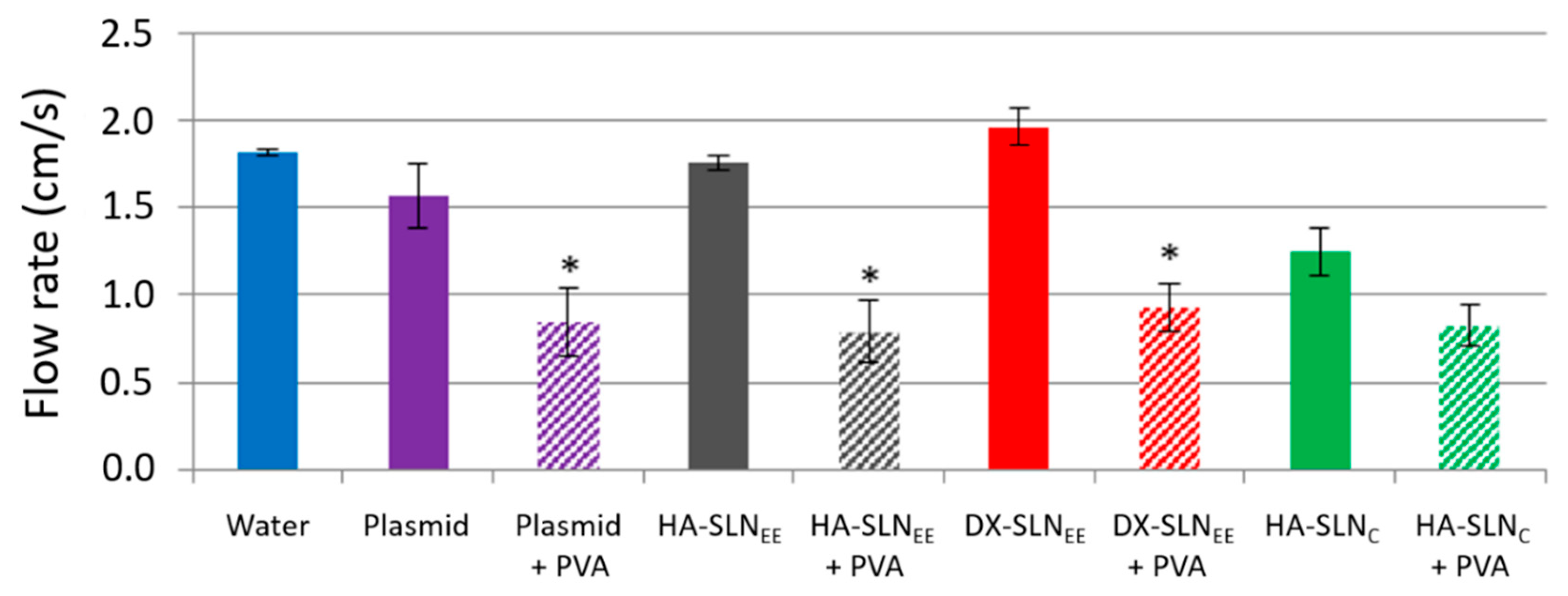
3.2. Adhesion Test: Flow Rates
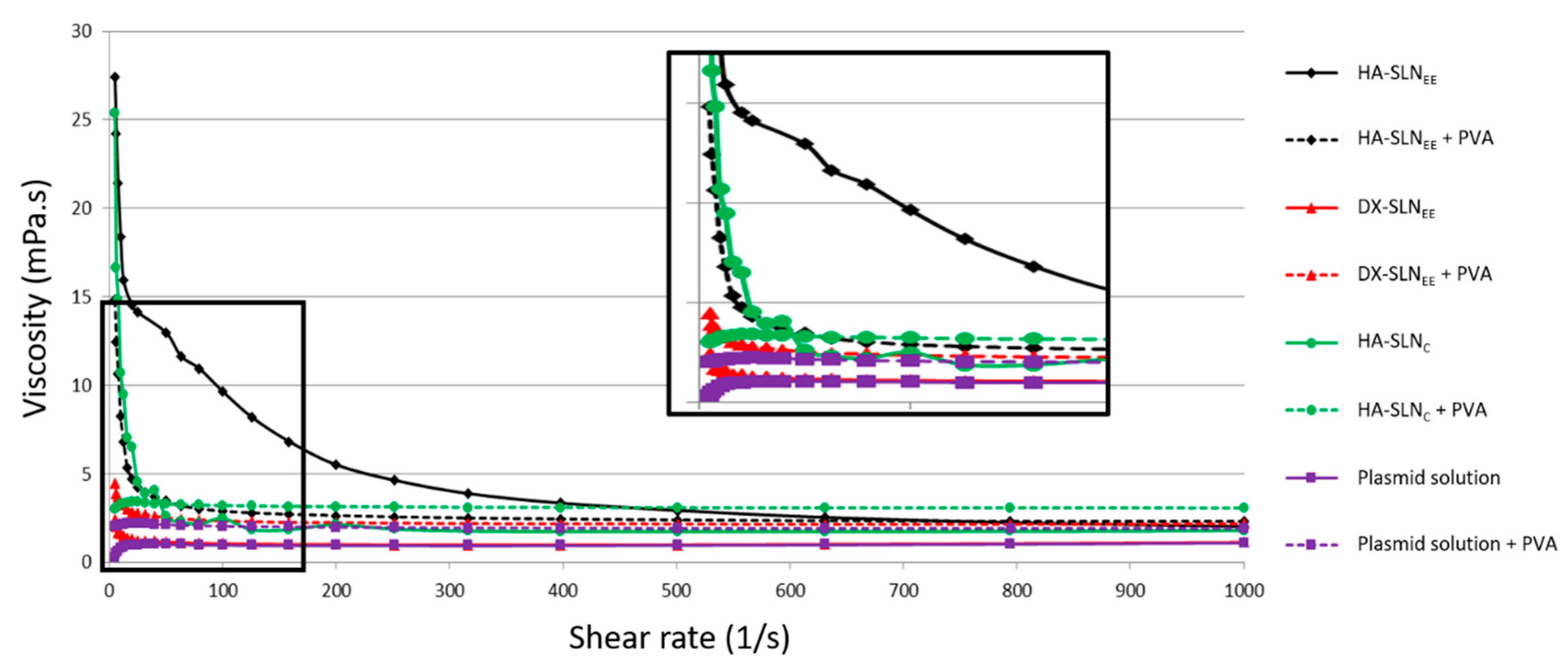
3.3. Rheology Studies
3.4. pH Values
3.5. In Vitro Studies
3.5.1. Transfection Efficacy of the Vectors Containing the Plasmid pUNO1-hIL10
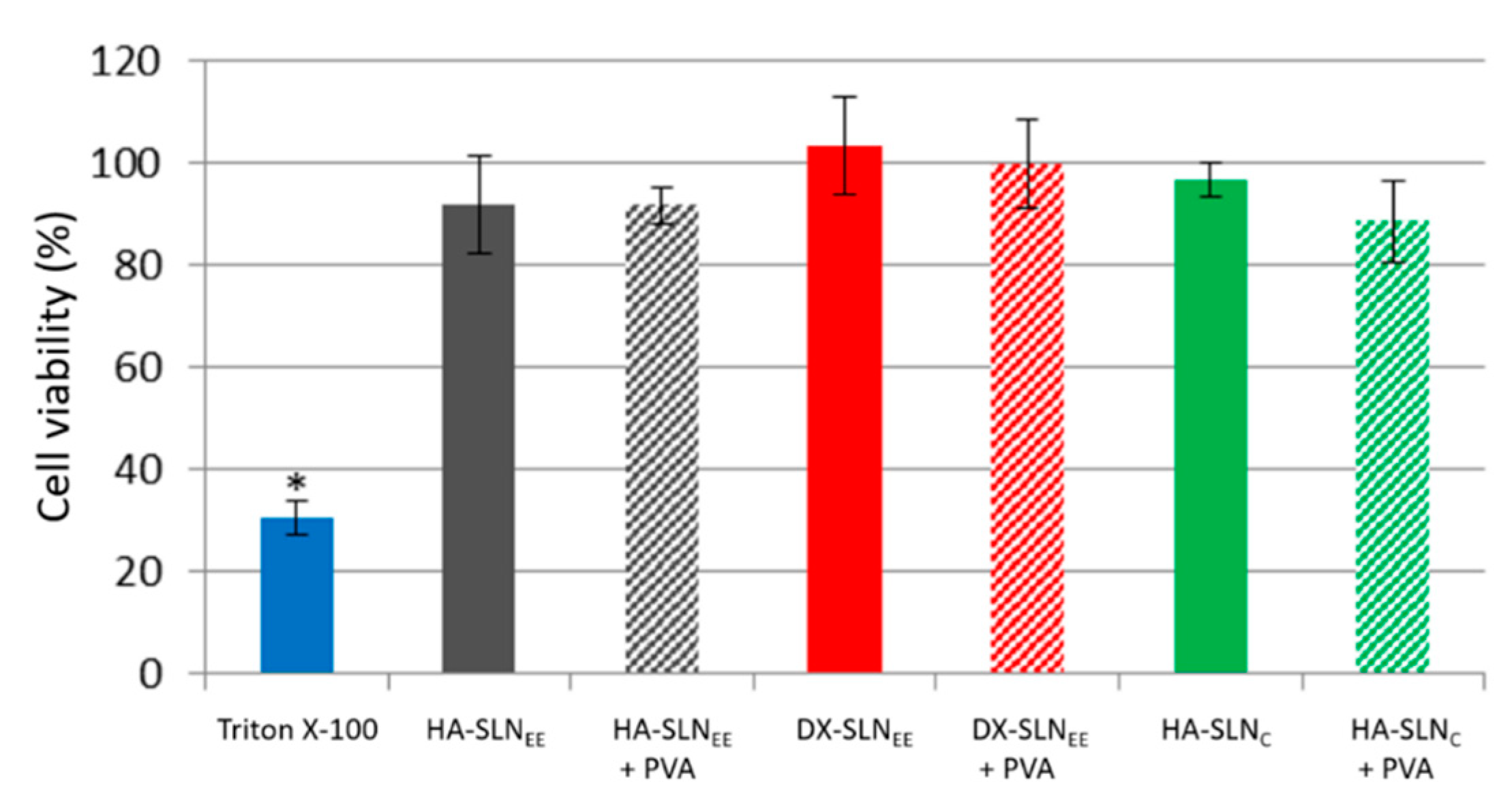
3.5.2. In Vitro Cell Viability
3.6. In Vivo Studies
3.6.1. Detection of CD44
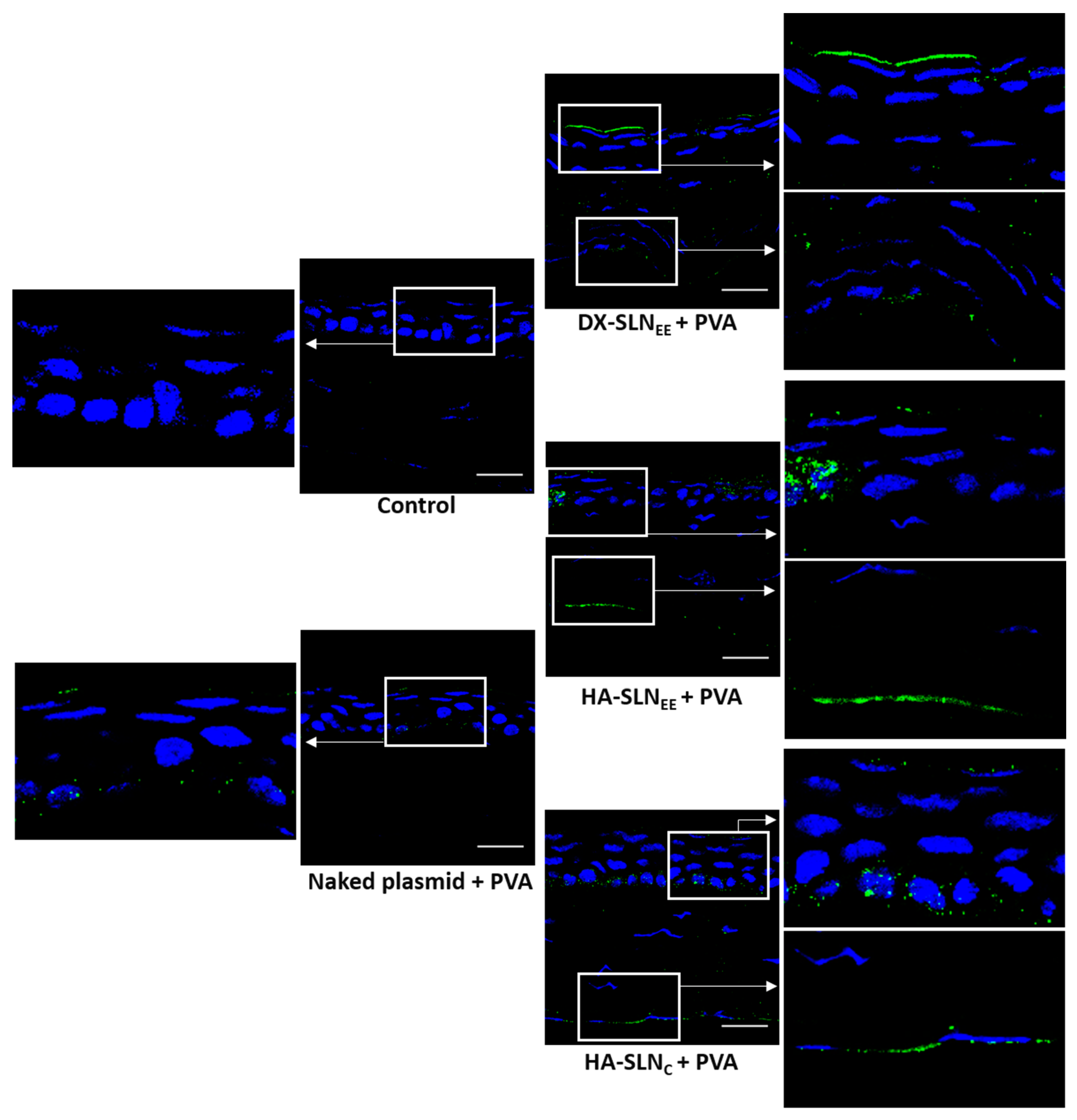
3.6.2. Corneal Localization of the Vectors
3.6.3. In Vivo Transfection with the Vectors Containing the Plasmid pcDNA3-EGFP
3.6.4. In Vivo Transfection with the Vectors Containing the pUNO1-hIL10 Plasmid
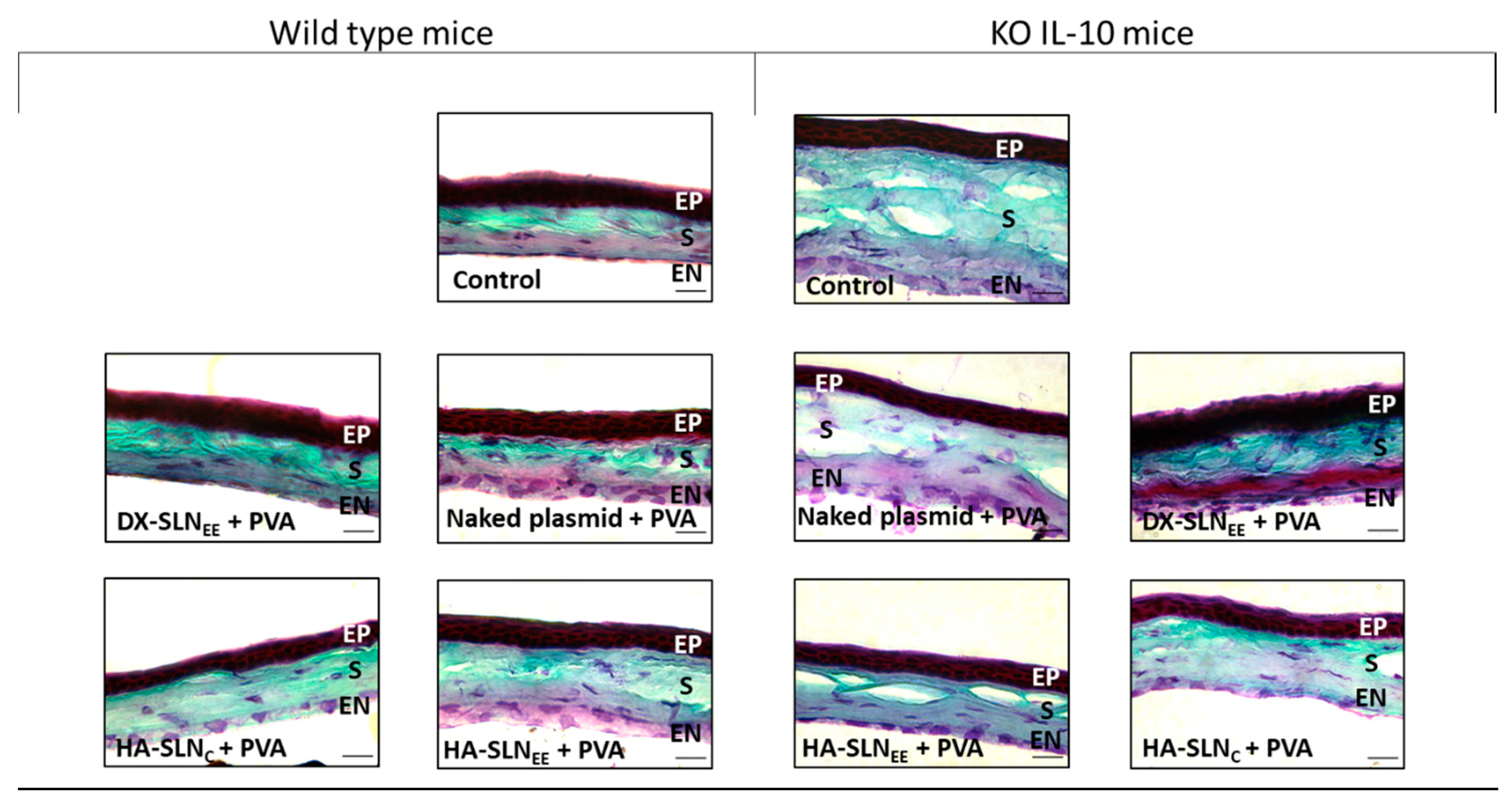
3.6.5. Structural Analysis of the Cornea
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torrecilla, J.; del Pozo-Rodríguez, A.; Vicente-Pascual, M.; Solinís, M.Á.; Rodríguez-Gascón, A. Targeting corneal inflammation by gene therapy: Emerging strategies for keratitis. Exp. Eye Res. J. 2018, 176, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, K.W.; Joo, K.; Kim, J.C. Angiogenin ameliorates corneal opacity and neovascularization via regulating immune response in corneal fibroblasts. BMC Ophthalmol. 2016, 17, 16–57. [Google Scholar] [CrossRef] [PubMed]
- Calles, J.A.; López-García, A.; Vallés, E.M.; Palma, S.D.; Diebold, Y. Preliminary characterization of dexamethasone-loaded cross-linked hyaluronic acid films for topical ocular therapy. Int. J. Pharm. 2016, 509, 237–243. [Google Scholar] [CrossRef] [PubMed]
- EMA (European Medicine Agency). Guideline on The Quality, Non-Clinical and Clinical Aspects of Gene Therapy Medicinal Product, 2018. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-quality-non-clinical-clinical-aspects-gene-therapy-medicinal-products_en.pdf (accessed on 15 May 2020).
- del Pozo-Rodríguez, A.; Rodríguez-Gascón, A.; Rodríguez-Castejón, J.; Vicente-Pascual, M.; Gómez-Aguado, I.; Battaglia, L.S.; Solinís, M.Á. Gene Therapy. In Current Applications of Pharmaceutical Biotechnology, Advances in Biochemical Engineering/Biotechnology, 1st ed.; Silva, A., Moreira, J., Lobo, J., Almeida, H., Eds.; Springer: Basel, Switzerland, 2020; Volume 171, pp. 321–368. [Google Scholar] [CrossRef]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the Interleukin-10 Receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef]
- De Waal Malefyt, R.; Abrams, J.; Figdor, C.G.; Bennett, B.; De Vries, J.E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: An autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 1991, 174, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- De Waal Malefyt, R.; Haanen, J.; Spits, H.; Roncarolo, M.G.; Te Velde, A.; Figdor, C.; Johnson, K.; Kastelein, R.; Yssel, H.; De Vries, J.E. Interleukin 10 (IL-10) and Viral IL-10 Strongly Reduce Antigen-specific Human T Cell Proliferation by Diminishing the Antigen-presenting Capacity of Monocytes via Downregulation of Class H Major Histocompatibility Complex Expression. J. Exp. Med. 1991, 174, 915–924. [Google Scholar] [CrossRef]
- Ralph, P.; Nakoinz, I.; Sampson-Johannes, A.; Fong, S.; Lowe, D.; Min, H.Y.; Lin, L. IL-10, T lymphocyte inhibitor of human blood cell production of IL-1 and tumor necrosis factor. J. Immunol. 1992, 148, 808–814. [Google Scholar]
- Cassatella, B.M.A.; Meda, L.; Bonora, S.; Ceska, M.; Constantin, G. Interleukin 10 (II.-10) Inhibits the Release of Proinflammatory Cytokines from Human Polymorphonuclear Leukocytes. Evidence for an Autocrine Role of Tumor Necrosis Factor and IL-10 in Mediating the Production of IL-8 Triggered by Lipopolysaccharide. J. Exp. Med. 1993, 178, 2207–2211. [Google Scholar] [CrossRef]
- Vicente-Pascual, M.; Albano, A.; Solinís, M.Á.; Serpe, L.; Rodríguez-Gascón, A.; Foglietta, F.; Muntoni, E.; Torrecilla, J.; del Pozo-Rodríguez, A.D.; Battaglia, L. Gene delivery in the cornea: In vitro & ex vivo evaluation of solid lipid nanoparticle-based vectors. Nanomed. J. 2018, 13, 1847–1864. [Google Scholar] [CrossRef]
- Luo, L.J.; Nguyen, D.D.; Lai, J.Y. Dually functional hollow ceria nanoparticle platform for intraocular drug delivery: A push beyond the limits of static and dynamic ocular barriers toward glaucoma therapy. Biomaterials 2020, 243, 119961. [Google Scholar] [CrossRef]
- Irimia, T.; Ghica, M.V.; Popa, L.; Anuţa, V.; Arsene, A.L.; Dinu-Pîrvu, C.E. Strategies for improving ocular drug bioavailability and corneal wound healing with chitosan-based delivery systems. Polymers-Basel 2018, 10, 1221. [Google Scholar] [CrossRef] [PubMed]
- Dubashynskaya, N.V.; Poshina, D.N.; Raik, S.V.; Urtti, A. Polysaccharides in Ocular Drug Delivery. Pharmaceutics 2019, 12, 22. [Google Scholar] [CrossRef]
- Pathak, Y.V.; Sutariya, V.; Hirani, A.A. Nano-Biomaterials for Ophthalmic Drug Delivery, 1st ed.; Springer: Basel, Switzerland, 2016; p. 627. [Google Scholar]
- Seyfoddin, A.; Al-Kassas, R. Development of solid lipid nanoparticles and nanostructured lipid carriers for improving ocular delivery of acyclovir. Drug Dev. Ind. Pharm. 2013, 39, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, L.; Serpe, L.; Foglietta, F.; Muntoni, E.; Gallarate, M.; del Pozo-Rodríguez, A.; Solinís, M.Á. Application of lipid nanoparticles to ocular drug delivery. Expert Opin. Drug Deliv. 2016, 13, 1743–1757. [Google Scholar] [CrossRef]
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.F.; Karla, P.K.; Boddu, S.H.S. Ocular drug delivery barriers—Role of nanocarriers in the treatment of anterior segment ocular diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef]
- Maiti, S.; Jana, S. Biocomposites in ocular drug delivery. In Biopolymer-Based Composites, 1st ed.; Jana, S., Maiti, S., Jana, S., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 139–168. [Google Scholar] [CrossRef]
- Hao, J.; Wang, X.; Bi, Y.; Teng, Y.; Wang, J.; Li, F.; Li, Q.; Zhang, J.; Guo, F.; Liu, J. Fabrication of a composite system combining solid lipid nanoparticles and thermosensitive hydrogel for challenging ophthalmic drug delivery. Colloids Surf. B 2014, 114, 111–120. [Google Scholar] [CrossRef]
- Battaglia, L.; Gallarate, M.; Serpe, L.; Foglietta, F.; Muntoni, E.; del Pozo-Rodríguez, A.; Solinís, M.Á. Ocular delivery of solid lipid nanoparticles. In Lipid Nanocarriers for Drug Targeting, 1st ed.; Grumezescu, A.M., Ed.; William Andrew: Norwich, NY, USA, 2018; pp. 269–312. [Google Scholar] [CrossRef]
- Apaolaza, P.S.; del Pozo-Rodríguez, A.; Solinís, M.Á.; Rodríguez, J.M.; Friedrich, U.; Torrecilla, J.; Weber, B.H.; Rodríguez-Gascón, A. Structural recovery of the retina in a retinoschisin-deficient mouse after gene replacement therapy by solid lipid nanoparticles. Biomaterials 2016, 90, 40–49. [Google Scholar] [CrossRef]
- Rodríguez-Gascón, A.; Solinís, M.A.; del Pozo-Rodríguez, A.; Delgado, D.; Pedraz, J.L. Lipid Nanoparticles for Gene Therapy. EP2460516A2, 20 September 2017. [Google Scholar]
- Rodríguez-Gascón, A.; Solinís, M.A.; del Pozo-Rodríguez, A.; Delgado, D.; Jover, E.F. Lipid Nanoparticles for Treating Ocular Diseases. EP2656837B1, 15 May 2019. [Google Scholar]
- Gallarate, M.; Chirio, D.; Bussano, R.; Peira, E.; Battaglia, L.; Baratta, F.; Trotta, M. Development of O/W nanoemulsions for ophthalmic administration of timolol. Int. J. Pharm. 2013, 440, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Coffey, M.J.; Decory, H.H.; Lane, S.S. Development of a non-settling gel formulation of 0.5% loteprednol etabonate for anti-inflammatory use as an ophthalmic drop. Clin. Ophthalmol. 2013, 7, 299–312. [Google Scholar] [CrossRef][Green Version]
- Nwosu, O.U.; Ewulon, C.M. Rheological Behaviour of Eco-friendly Drilling Fluids from Biopolymers. J. Polym. Biopolym. Phys. Chem. 2014, 2, 50–54. [Google Scholar] [CrossRef]
- Maftoonazad, N.; Shahamirian, M.; John, D.; Ramaswamy, H. Development and evaluation of antibacterial electrospun pea protein isolate-polyvinyl alcohol nanocomposite mats incorporated with cinnamaldehyde. Mater. Sci. Eng. C 2019, 94, 393–402. [Google Scholar] [CrossRef]
- Kühn, R.; Löhler, J.; Rennick, D.; Rajewsky, K.; Müller, W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 1993, 75, 263–274. [Google Scholar] [CrossRef]
- Rodríguez-Gascón, A.; del Pozo-Rodríguez, A.; Isla, A.; Solinís, M.A. Gene Therapy in the Cornea; eLS John Wiley & Sons, Ltd.: Chichester, UK, 2016. [Google Scholar] [CrossRef]
- Solinís, M.Á.; del Pozo-Rodríguez, A.; Apaolaza, P.S.; Rodríguez-Gascón, A. Treatment of ocular disorders by gene therapy. Eur. J. Pharm. Biopharm. 2015, 95, 331–342. [Google Scholar] [CrossRef]
- Alvarez-Rivera, F.; Rey-Rico, A.; Venkatesan, J.K.; Diaz-Gomez, L.; Cucchiarini, M.; Concheiro, A.; Alvarez-Lorenzo, C. Controlled release of rAAV vectors from APMA-functionalized contact lenses for corneal gene therapy. Pharmaceutics 2020, 12, 335. [Google Scholar] [CrossRef]
- de la Fuente, M.; Seijo, B.; Alonso, M.J. Bioadhesive hyaluronan-chitosan nanoparticles can transport genes across the ocular mucosa and transfect ocular tissue. Gene Ther. 2008, 15, 668–676. [Google Scholar] [CrossRef]
- Delgado, D.; del Pozo-Rodríguez, A.; Solinís, M.Á.; Avilés-Triqueros, M.; Weber, B.H.F.; Fernández, E.; Rodríguez-Gascón, A. Dextran and Protamine-Based Solid Lipid Nanoparticles as Potential Vectors for the Treatment of X-Linked Juvenile Retinoschisis. Hum. Gene Ther. 2012, 23, 345–355. [Google Scholar] [CrossRef]
- Bauer, D.; Lu, M.; Wasmuth, S.; Li, H.; Yang, Y.; Roggendorf, M.; Steuhl, K.P.; Heiligenhaus, A. Immunomodulation by topical particle-mediated administration of cytokine plasmid DNA suppresses herpetic stromal keratitis without impairment of antiviral defense. Graefe’s Arch. Clin. Exp. Ophthalmol. 2006, 244, 216–225. [Google Scholar] [CrossRef]
- Gupta, S.; Fink, M.K.; Ghosh, A.; Tripathi, R.; Sinha, P.R.; Sharma, A.; Hesemann, N.P.; Chaurasia, S.S.; Giuliano, E.A.; Mohan, R.R. Novel combination BMP7 and HGF gene therapy instigates selective myofibroblast apoptosis and reduces corneal haze in vivo. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1045–1057. [Google Scholar] [CrossRef]
- Siene, N.W.; Binley, K.; Song, B.; Morgan, J.E. Use of magnetic nanoparticles and oscillating magnetic field for non-viral gene transfer into mouse cornea. Lancet 2015, 385, S75. [Google Scholar] [CrossRef]
- Contreras-Ruiz, L.; Zorzi, G.K.; Hileeto, D.; López-García, A.; Calonge, M.; Seijo, B.; Sánchez, A.; Diebold, Y. A nanomedicine to treat ocular surface inflammation: Performance on an experimental dry eye murine model. Gene Ther. 2013, 20, 467–477. [Google Scholar] [CrossRef]
- Gupta, R.; Tandon, A.; Hansen, E.T.; Cebulko, T.C.; Hemmat, Y.J.; Fortune, J.A.; Klibanov, A.M.; Mohan, R.R. Rapid and Substantial Gene Delivery into Cornea In Vivo and In Vitro with Linearized Polyethyleneimine Nanoparticles. Investig. Ophthalmol. Vis. Sci. 2011, 52, 494. [Google Scholar]
- Sharma, A.; Tandon, A.; Tovey, J.C.; Gupta, R.; Robertson, J.D.; Fortune, J.A.; Klibanov, A.M.; Cowden, J.W.; Rieger, F.G.; Mohan, R.R. Polyethylenimine-conjugated gold nanoparticles: Gene transfer potential and low toxicity in the cornea. Nanomedicine 2011, 7, 505–513. [Google Scholar] [CrossRef]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular drug delivery systems: An overview. World J. Pharmacol. 2015, 2, 47–64. [Google Scholar] [CrossRef]
- Apaolaza, P.S.; Delgado, D.; del Pozo-Rodríguez, A.; Rodríguez-Gascón, A.; Solinís, M.Á. A novel gene therapy vector based on hyaluronic acid and solid lipid nanoparticles for ocular diseases. Int. J. Pharm. 2014, 65, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Delgado, D.; del Pozo-Rodríguez, A.; Solinís, M.Á.; Rodríguez-Gascón, A. Understanding the mechanism of protamine in solid lipid nanoparticle-based lipofection: The importance of the entry pathway. Eur. J. Pharm. Biopharm. 2011, 79, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Alonso, M.J.; Vila-Jato, J.L.; Robinson, J.R. Improved Ocular Bioavailability of Indomethacin by Novel Ocular Drug Carriers. J. Pharm. Pharmacol. 1996, 48, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Qaddoumi, M.G.; Ueda, H.; Yang, J.; Davda, J.; Labhasetwar, V.; Lee, V.H.L. The characteristics and mechanisms of uptake of PLGA nanoparticles in rabbit conjunctival epithelial cell layers. Pharm. Res. 2004, 21, 641–648. [Google Scholar] [CrossRef]
- Huang, H.Y.; Wang, M.C.; Chen, Z.Y.; Chiu, W.Y.; Chen, K.H.; Lin, I.C.; Yang, W.V.; Wu, C.C.; Tseng, C.L. Gelatin–epigallocatechin gallate nanoparticles with hyaluronic acid decoration as eye drops can treat rabbit dry-eye syndrome effectively via inflammatory relief. Int. J. Nanomed. 2018, 13, 7251–7273. [Google Scholar] [CrossRef] [PubMed]
- Tatke, A.; Dudhipala, N.; Janga, K.Y.; Balguri, S.P.; Avula, B.; Jablonski, M.M.; Majumdar, S. In situ gel of triamcinolone acetonide-loaded solid lipid nanoparticles for improved topical ocular delivery: Tear kinetics and ocular disposition studies. J. Nanomater. 2019, 9, 33. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Espina, M.; Doktorovova, S.; Souto, E.B.; García, M.L. Lipid nanoparticles (SLN, NLC): Overcoming the anatomical and physiological barriers of the eye—Part I—Barriers and determining factors in ocular delivery. Eur. J. Pharm. Biopharm. 2017, 110, 70–75. [Google Scholar] [CrossRef]
- Gan, L.; Wang, J.; Jiang, M.; Bartlett, H.; Ouyang, D.; Eperjesi, F.; Liu, J.; Gan, Y. Recent advances in topical ophthalmic drug delivery with lipid-based nanocarriers. Drug Discov. Today 2013, 18, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Gasco, M.R.; Saettone, M.F.; Zara, G.P. Pharmaceutical Compositions Suitable for the Treatment of Ophthalmic Diseases. U.S. Patent 10/533,512, 2 February 2006. [Google Scholar]
- Almeida, H.; Amaral, M.H.; Lobão, P.; Silva, A.C.; Lobo, J.M.S. Applications of polymeric and lipid nanoparticles in ophthalmic pharmaceutical formulations: Present and future considerations. J. Pharm. Pharm. Sci. 2014, 17, 278–293. [Google Scholar] [CrossRef]
- Misra, A.; Shahiwala, A. Applications of Polymers in Drug Delivery, 1st ed.; Smithers Rapra: Shawbury, UK, 2014; p. 546. [Google Scholar]
- Mundada, A.S. Update on Polymers for Ocular Drug Delivery, 1st ed.; Smithers Rapra: Shawbury, UK, 2011; p. 198. [Google Scholar]
- Tummala, L.; Mihranyan, F. Biocompatibility of Nanocellulose-Reinforced PVA Hydrogel with Human Corneal Epithelial Cells for Ophthalmic Applications. J. Funct. Biomater. 2019, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Chen, C.; Liu, K.; Tu, Y.; Zhang, L.; Li, Y. Preparation of PVA hydrogel with high-transparence and investigations on its transparent mechanism. RSC Adv. 2015, 5, 24023–24030. [Google Scholar] [CrossRef]
- Salzillo, R.; Schiraldi, C.; Corsuto, L.; D’Agostino, A.; Filosa, R.; De Rosa, M.; La Gatta, A. Optimization of hyaluronan-based eye drop formulations. Carbohydr. Polym. 2016, 153, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Oechsner, M.; Keipert, S. Polyacrylic acid/polyvinylpyrrolidone bipolymeric systems I Rheological and mucoadhesive properties of formulations potentially useful for the treatment of dry-eye-syndrome. Eur. J. Pharm. Biopharm. 1999, 47, 113–118. [Google Scholar] [CrossRef]
- Mucha, M. Rheological properties of chitosan blends with poly(ethylene oxide) and poly(vinyl alcohol) in solution. React. Funct. Polym. 1998, 38, 19–25. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Huang, J.; Xia, M.; Liu, L.; Tian, C.; Hu, R.; Gui, S.; Chu, X. Self-assembled hexagonal liquid crystalline gels as novel ocular formulation with enhanced topical delivery of pilocarpine nitrate. Int. J. Pharm. 2019, 562, 31–41. [Google Scholar] [CrossRef]
- Achouri, D.; Alhanout, K.; Piccerelle, P.; Andrieu, V. Recent advances in ocular drug delivery. Drug Dev. Ind. Pharm. 2013, 39, 1599–1617. [Google Scholar] [CrossRef]
- Abelson, M.B.; Udell, I.J.; Weston, J.H. Normal human tear pH by direct measurement. Arch. Ophthalmol 1981, 99, 301. [Google Scholar] [CrossRef]
- Yamada, M.; Mochizuki, H.; Kawai, M.; Yoshino, M.; Mashima, Y. Fluorophotometric measurement of pH of human tears in vivo. Curr. Eye Res. 1997, 16, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Stein, H.A.; Stein, R.M.; Freeman, M.I. The Ophthalmic Assistant: A Text for Allied and Associated Ophthalmic Personnel, 10th ed.; Elsevier: Amsterdam, The Netherlands, 2012; p. 894. [Google Scholar]
- Battaglia, L.; Gallarate, M.; Cavalli, R.; Trotta, M. Solid lipid nanoparticles produced through a coacervation method. J. Microencapsul. 2010, 27, 78–85. [Google Scholar] [CrossRef]
- del Pozo-Rodríguez, A.; Delgado, D.; Solinís, M.Á.; Gascón, A.R.; Pedraz, J.L. Solid lipid nanoparticles for retinal gene therapy: Transfection and intracellular trafficking in RPE cells. Int. J. Pharm. 2008, 360, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Ruponen, M.; Rönkkö, S.; Honkakoski, P.; Pelkonen, J.; Tammi, M.; Urtti, A. Extracellular Glycosaminoglycans Modify Cellular Trafficking of Lipoplexes and Polyplexes. J. Biol. Chem. 2001, 276, 33875–33880. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Cunha, G.M.; Na, K.S.; Putra, I.; Lee, H.J.; Hull, S.; Cheng, Y.C.; Blanco, I.J.; Eslani, M.; Djalilian, A.R.; Myung, D. Corneal Wound Healing Effects of Mesenchymal Stem Cell Secretome Delivered Within a Viscoelastic Gel Carrier. Stem Cells Transl. Med. 2019, 8, 478–489. [Google Scholar] [CrossRef]
- Tahvildari, M.; Emami-Naeini, P.; Omoto, M.; Mashaghi, A.; Chauhan, S.K.; Dana, R. Treatment of donor corneal tissue with immunomodulatory cytokines: A novel strategy to promote graft survival in high-risk corneal transplantation. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Azher, T.N.; Yin, X.T.; Stuart, P.M. Understanding the role of chemokines and cytokines in experimental models of herpes simplex keratitis. J. Immunol. Res. 2017, 2017, 2–6. [Google Scholar] [CrossRef]
- Keadle, T.L.; Stuart, P.M. Interleukin-10 (IL-10) ameliorates corneal disease in a mouse model of recurrent herpetic keratitis. Microb. Pathog. 2005, 38, 13–21. [Google Scholar] [CrossRef]
- Apaolaza, P.S.; del Pozo-Rodríguez, A.; Torrecilla, J.; Rodríguez-Gascón, A.; Rodríguez, J.M.; Friedrich, U.; Weber, B.H.F.; Solinís, M.A. Solid lipid nanoparticle-based vectors intended for the treatment of X-linked juvenile retinoschisis by gene therapy: In vivo approaches in Rs1h-deficient mouse model. J. Control. Release 2015, 217, 273–283. [Google Scholar] [CrossRef]
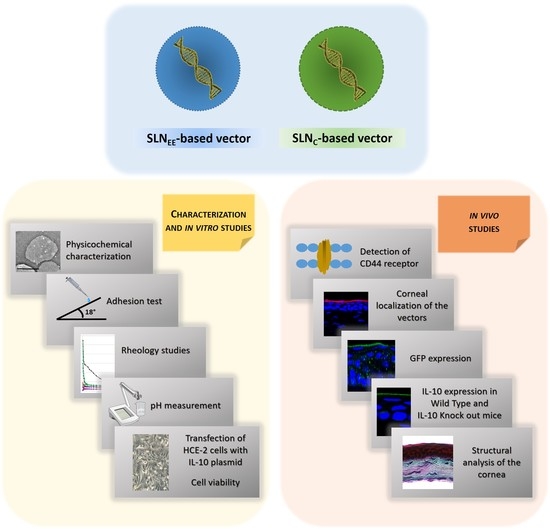
| Name of the Complex | Weight Ratio |
|---|---|
| DX-SLNEE | DX:P:DNA:SLNEE 1:2:1:5 |
| HA-SLNEE | HA:P:DNA:SLNEE 0.5:2:1:2 |
| DNA-SLNC | DNA:SLNC 1:10 |
| HA-SLNC | HA:P:DNA:SLNC 0.5:1:1:10 |
| Size (nm) | PDI | Zeta Potential (mV) | |
|---|---|---|---|
| SLNEE | 202.2 ± 28.2 * | 0.25 ± 0.01 | +51.3 ± 2.2 |
| HA-SLNEE | |||
| pcDNA3-EGFP | 204.9 ± 15.0 | 0.18 ± 0.07 | +29.2 ± 3.1 |
| pUNO1-hIL10 | 266.1 ± 11.4 | 0.34 ± 0.03 | +29.7 ± 1.2 |
| DX-SLNEE | |||
| pcDNA3-EGFP | 177.3 ± 23.2 # | 0.33 ± 0.06 | +39.3 ± 1.5 # |
| pUNO1-hIL10 | 159.4 ± 4.9 ‡ | 0.27 ± 0.02 | +34.1 ± 0.6 ‡ |
| SLNC | 453.9 ± 13.6 | 0.26 ± 0.03 | +33.8 ± 2.5 |
| DNA-SLNC | |||
| pcDNA3-EGFP | 404.1 ± 7.2 # | 0.27 ± 0.02 | +20.8 ± 1.5 |
| pUNO1-hIL10 | 447.9 ± 17.0 ‡ | 0.29 ± 0.04 | +14.7 ± 0.7 & |
| HA-SLNC | |||
| pcDNA3-EGFP | 368.5 ± 7.4 | 0.24 ± 0.02 | +21.9 ± 0.9 |
| pUNO1-hIL10 | 374.7 ± 14.5 | 0.29 ± 0.02 | +15.8 ± 2.4 & |
| Sample | R2 | Viscosity 10 s−1 (mPa·s) | Viscosity 500 s−1 (mPa·s) | K (Pa·sn) | n |
|---|---|---|---|---|---|
| Water | 0.9947 | 0.73 | 0.87 | 0.001 | 1.065 |
| Plasmid solution | 0.9847 | 0.78 | 0.98 | 0.001 | 1.11 |
| Plasmid solution + PVA | 0.9995 | 2.14 | 1.91 | 0.002 | 0.976 |
| HA-SLNEE | 0.9588 | 18.40 | 2.92 | 0.067 | 0.519 |
| HA-SLNEE + PVA | 0.9592 | 8.26 | 2.38 | 0.015 | 0.681 |
| DX-SLNEE | 0.9921 | 1.60 | 1.00 | 0.002 | 0.876 |
| DX-SLNEE + PVA | 0.9969 | 3.31 | 2.16 | 0.004 | 0.879 |
| HA-SLNC | 0.8642 | 10.70 | 1.75 | 0.028 | 0.525 |
| HA-SLNC + PVA | 0.9995 | 3.29 | 3.08 | 0.003 | 0.989 |
| Sample | pH |
|---|---|
| Plasmid solution | 7.4 ± 0.13 |
| Plasmid solution + PVA | 7.3 ± 0.13 |
| HA-SLNEE | 7.3 ± 0.03 |
| HA-SLNEE + PVA | 7.2 ± 0.01 |
| DX-SLNEE | 7.5 ± 0.10 |
| DX-SLNEE + PVA | 7.0 ± 0.18 |
| HA-SLNC | 4.0 ± 0.07 |
| HA-SLNC + PVA | 4.3 ± 0.11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicente-Pascual, M.; Gómez-Aguado, I.; Rodríguez-Castejón, J.; Rodríguez-Gascón, A.; Muntoni, E.; Battaglia, L.; del Pozo-Rodríguez, A.; Solinís Aspiazu, M.Á. Topical Administration of SLN-Based Gene Therapy for the Treatment of Corneal Inflammation by De Novo IL-10 Production. Pharmaceutics 2020, 12, 584. https://doi.org/10.3390/pharmaceutics12060584
Vicente-Pascual M, Gómez-Aguado I, Rodríguez-Castejón J, Rodríguez-Gascón A, Muntoni E, Battaglia L, del Pozo-Rodríguez A, Solinís Aspiazu MÁ. Topical Administration of SLN-Based Gene Therapy for the Treatment of Corneal Inflammation by De Novo IL-10 Production. Pharmaceutics. 2020; 12(6):584. https://doi.org/10.3390/pharmaceutics12060584
Chicago/Turabian StyleVicente-Pascual, Mónica, Itziar Gómez-Aguado, Julen Rodríguez-Castejón, Alicia Rodríguez-Gascón, Elisabetta Muntoni, Luigi Battaglia, Ana del Pozo-Rodríguez, and María Ángeles Solinís Aspiazu. 2020. "Topical Administration of SLN-Based Gene Therapy for the Treatment of Corneal Inflammation by De Novo IL-10 Production" Pharmaceutics 12, no. 6: 584. https://doi.org/10.3390/pharmaceutics12060584
APA StyleVicente-Pascual, M., Gómez-Aguado, I., Rodríguez-Castejón, J., Rodríguez-Gascón, A., Muntoni, E., Battaglia, L., del Pozo-Rodríguez, A., & Solinís Aspiazu, M. Á. (2020). Topical Administration of SLN-Based Gene Therapy for the Treatment of Corneal Inflammation by De Novo IL-10 Production. Pharmaceutics, 12(6), 584. https://doi.org/10.3390/pharmaceutics12060584