Microneedle-Based Delivery: An Overview of Current Applications and Trends
Abstract
1. Introduction
2. Microneedle-Based Transdermal Delivery Approaches
2.1. Solid Microneedles for “Poke and Patch”
2.2. Coated Microneedles for “Coat and Poke”
2.3. Dissolving and Hydrogel-Forming Microneedles for “Poke and Release”.
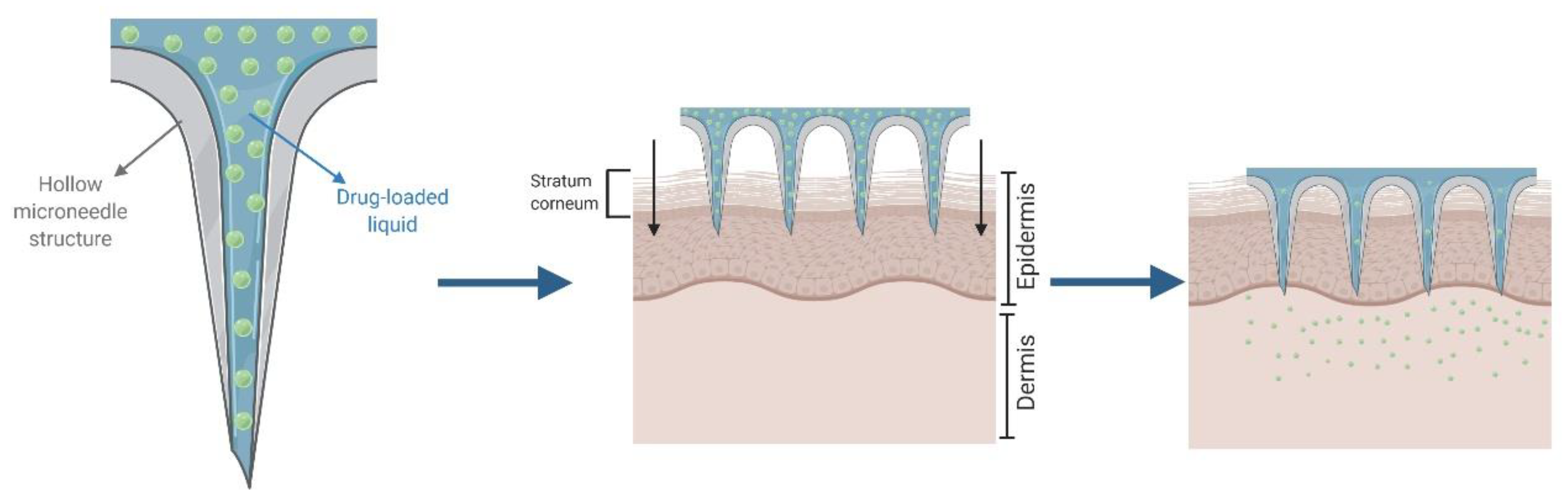
2.4. Hollow Microneedles for “Poke and Flow”
3. MNA Fabrication
3.1. Materials
3.2. Manufacturing Processes
4. Applications in Drug and Vaccine Delivery
4.1. Immunization
4.2. Therapy
4.2.1. Therapeutic Proteins
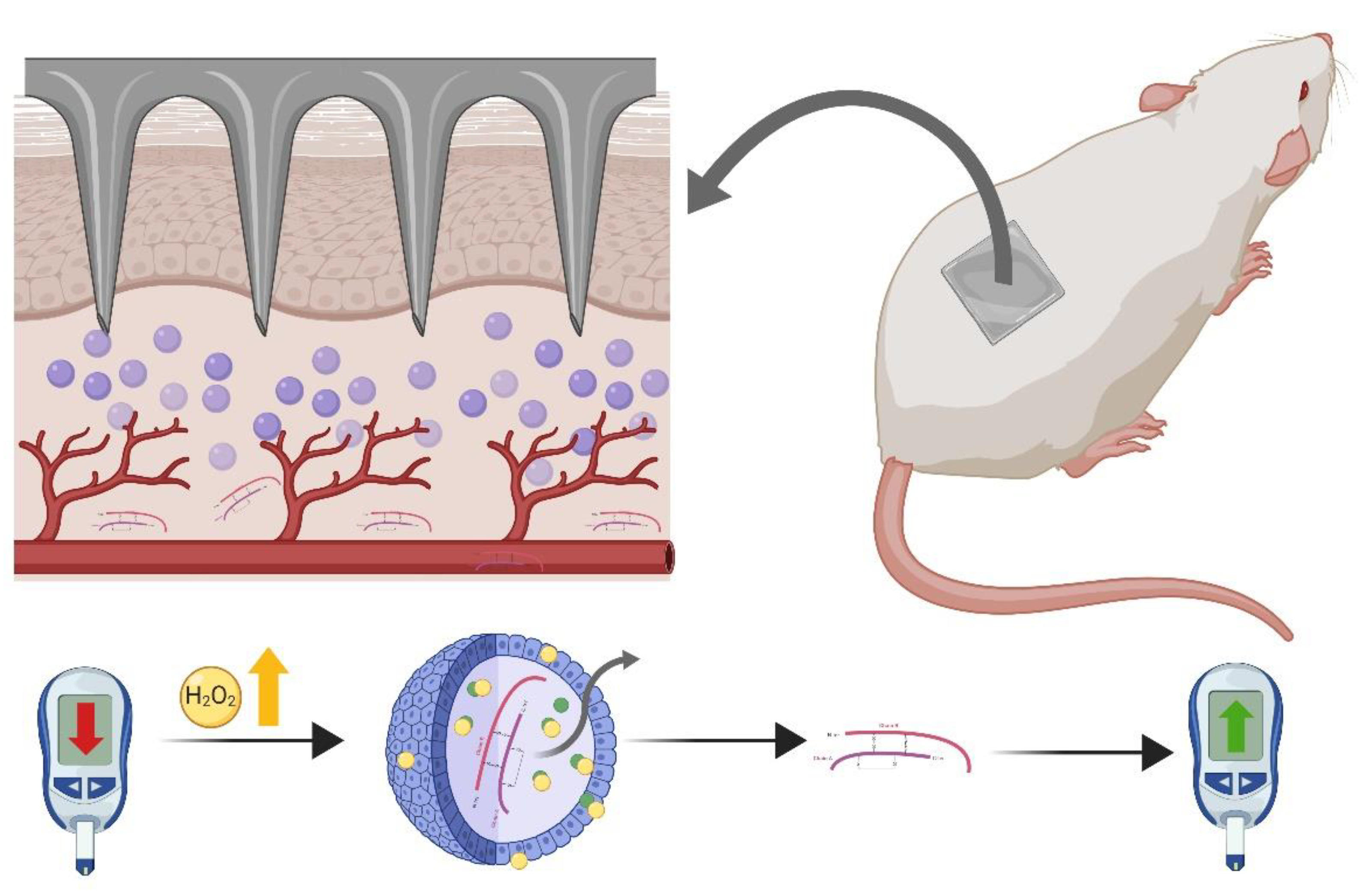
4.2.2. Insulin
4.2.3. Vitamins
4.2.4. Antibiotics
4.2.5. Natural Compounds
4.3. Cosmeceuticals
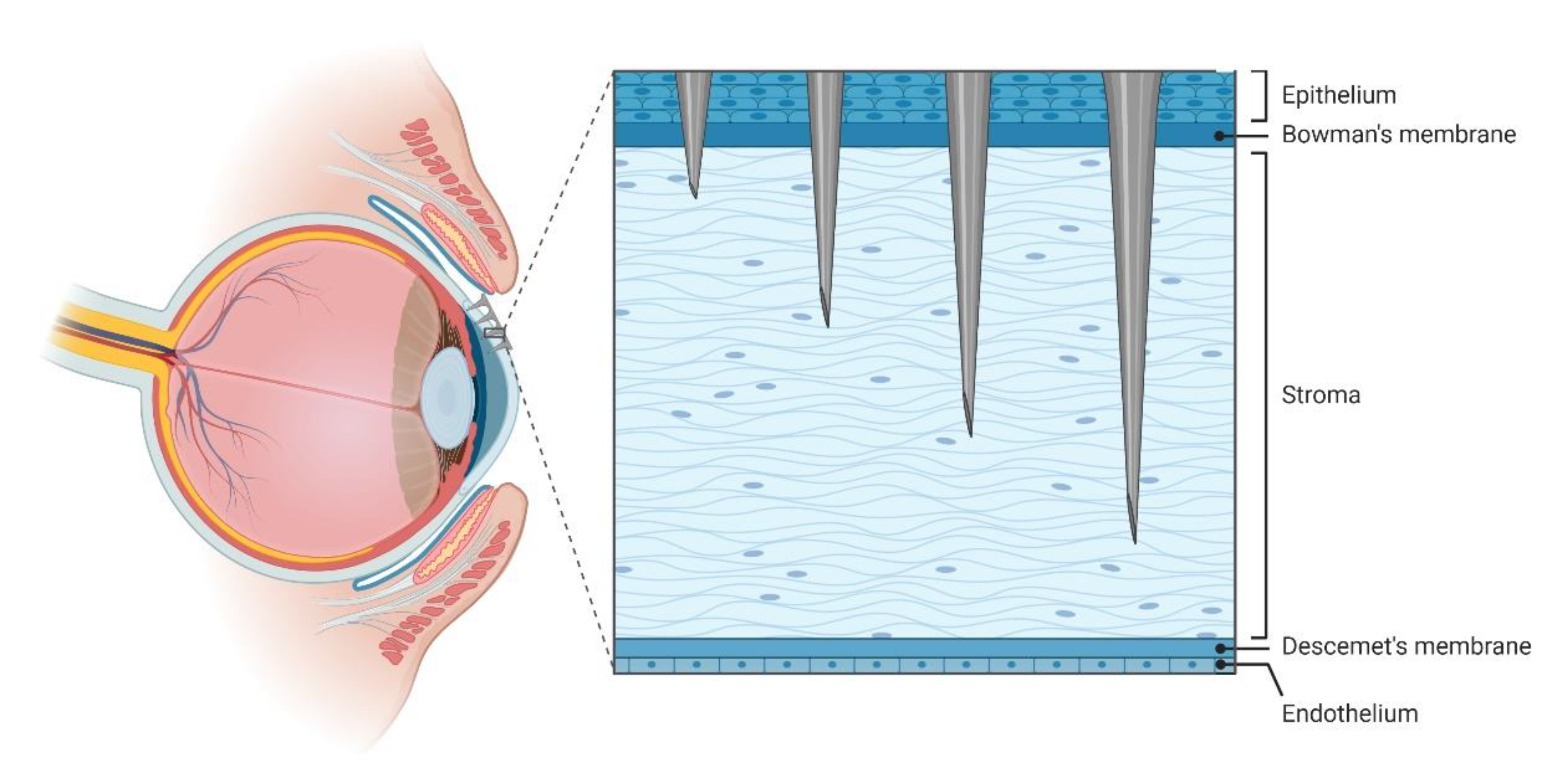
5. Use of MNA in Other Organs, Stimuli Responsive MNA, and Delivery of Cells
6. Safety Considerations
6.1. Pain
6.2. Infections
6.3. Biocompatibility, Immunogenicity, and Local Skin Reactions
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- MacGregor, R.R.; Graziani, A.L. Oral Administration of Antibiotics: A Rational Alternative to the Parenteral Route. Clin. Infect. Dis. 1997, 24, 457–467. [Google Scholar] [CrossRef]
- Darji, M.A.; Lalge, R.M.; Marathe, S.P.; Mulay, T.D.; Fatima, T.; Alshammari, A.; Lee, H.K.; Repka, M.A.; Narasimha Murthy, S. Excipient Stability in Oral Solid Dosage Forms: A Review. AAPS PharmSciTech 2018, 19, 12–26. [Google Scholar] [CrossRef]
- Davis, S.S.; Hardy, J.G.; Fara, J.W. Transit of Pharmaceutical Dosage Forms through the Small Intestine. Gut 1986, 27, 886–892. [Google Scholar] [CrossRef]
- Maderuelo, C.; Lanao, J.M.; Zarzuelo, A. Enteric Coating of Oral Solid Dosage Forms as a Tool to Improve Drug Bioavailability. Eur. J. Pharm. Sci. 2019, 138, 105019. [Google Scholar] [CrossRef]
- Parsons, R.L. Drug Absorption in Gastrointestinal Disease with Particular Reference to Malabsorption Syndromes. Clin. Pharmacokinet. 1977, 2, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Pond, S.M.; Tozer, T.N. First-Pass Elimination. Basic Concepts and Clinical Consequences. Clin. Pharmacokinet. 1984, 9, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Jimmy, B.; Jose, J. Patient Medication Adherence: Measures in Daily Practice. Oman. Med. J. 2011, 26, 155–159. [Google Scholar] [CrossRef]
- Ruiz, F.; Vallet, T.; Dufaÿ Wojcicki, A.; Belissa, É.; Fontan, J.-E.; de Pontual, L.; Nathanson, S.; Chevallier, A.; Laribe-Caget, S.; Boudy, V. Dosage Form Suitability in Vulnerable Populations: A Focus on Paracetamol Acceptability from Infants to Centenarians. PLoS ONE 2019, 14, e0221261. [Google Scholar] [CrossRef] [PubMed]
- Bruce, J.; Wong, I. Parenteral Drug Administration Errors by Nursing Staff on an Acute Medical Admissions Ward during Day Duty. Drug Saf. 2001, 24, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Hua, S. Physiological and Pharmaceutical Considerations for Rectal Drug Formulations. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Secret, E.; Smith, K.; Dubljevic, V.; Moore, E.; Macardle, P.; Delalat, B.; Rogers, M.-L.; Johns, T.G.; Durand, J.-O.; Cunin, F.; et al. Antibody-Functionalized Porous Silicon Nanoparticles for Vectorization of Hydrophobic Drugs. Adv. Healthc Mater. 2013, 2, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, G.; Baeza, A. Targeting Strategies for Improving the Efficacy of Nanomedicine in Oncology. Beilstein. J. Nanotechnol. 2019, 10, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Macri, L.K.; Kaplan, H.M.; Kohn, J. Nanoparticles and Nanofibers for Topical Drug Delivery. J. Control. Release 2016, 240, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Smola, M.; Vandamme, T.; Sokolowski, A. Nanocarriers as Pulmonary Drug Delivery Systems to Treat and to Diagnose Respiratory and Non Respiratory Diseases. Int. J. Nanomed. 2008, 3, 1–19. [Google Scholar] [CrossRef]
- Arroyo, C.M.; Quinteros, D.; Cózar-Bernal, M.J.; Palma, S.D.; Rabasco, A.M.; González-Rodríguez, M.L. Ophthalmic Administration of a 10-Fold-Lower Dose of Conventional Nanoliposome Formulations Caused Levels of Intraocular Pressure Similar to Those Induced by Marketed Eye Drops. Eur. J. Pharm. Sci. 2018, 111, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Koulich, E.; Roland, P.S.; Pawlowski, K.S. Comparison of Systemic and Otic Administration of Ofloxacin. Laryngoscope 2010, 120, 2083–2088. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.; Harry, R. Harry’s Cosmeticology, 7th ed.; Chemical Publishing: New York, NY, USA, 1982; pp. 1–41. [Google Scholar]
- Beck, R.; Guterres, S.; Pohlmann, A. Nanocosmetics and Nanomedicines: New Approaches for Skin Care; Springer: Berlin, Germany, 2011. [Google Scholar] [CrossRef]
- Fox, L.T.; Gerber, M.; Plessis, J.D.; Hamman, J.H. Transdermal Drug Delivery Enhancement by Compounds of Natural Origin. Molecules 2011, 16, 10507–10540. [Google Scholar] [CrossRef]
- Mitragotri, S.; Anissimov, Y.G.; Bunge, A.L.; Frasch, H.F.; Guy, R.H.; Hadgraft, J.; Kasting, G.B.; Lane, M.E.; Roberts, M.S. Mathematical Models of Skin Permeability: An Overview. Int. J. Pharm. 2011, 418, 115–129. [Google Scholar] [CrossRef]
- Singh, S.; Singh, J. Transdermal Drug Delivery by Passive Diffusion and Iontophoresis: A Review. Med. Res. Rev. 1993, 13, 569–621. [Google Scholar] [CrossRef]
- Bos, J.D.; Meinardi, M.M. The 500 Dalton Rule for the Skin Penetration of Chemical Compounds and Drugs. Exp. Dermatol. 2000, 9, 165–169. [Google Scholar] [CrossRef]
- Paudel, K.S.; Milewski, M.; Swadley, C.L.; Brogden, N.K.; Ghosh, P.; Stinchcomb, A.L. Challenges and Opportunities in Dermal/Transdermal Delivery. Ther. Deliv. 2010, 1, 109–131. [Google Scholar] [CrossRef]
- Hern, S.; Mortimer, P.S. Visualization of Dermal Blood Vessels—Capillaroscopy. Clin. Exp. Dermatol. 1999, 24, 473–478. [Google Scholar] [CrossRef]
- Asbill, C.S.; El-Kattan, A.F.; Michniak, B. Enhancement of Transdermal Drug Delivery: Chemical and Physical Approaches. Crit. Rev. Ther. Drug Carrier Syst. 2000, 17, 621–658. [Google Scholar] [CrossRef] [PubMed]
- Prasanthi, D.; Lakshmi, P.K. Effect of Chemical Enhancers in Transdermal Permeation of Alfuzosin Hydrochloride. ISRN Pharm. 2012, 2012, 965280. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, Y.B.; Tsai, Y.H.; Chang, J.S.; Liu, J.C.; Tsai, M.J.; Wu, P.C. Effect of Antioxidants and Anti-Irritants on the Stability, Skin Irritation and Penetration Capacity of Captopril Gel. Int. J. Pharm. 2002, 241, 345–351. [Google Scholar] [CrossRef]
- Karande, P.; Jain, A.; Ergun, K.; Kispersky, V.; Mitragotri, S. Design Principles of Chemical Penetration Enhancers for Transdermal Drug Delivery. Proc. Natl. Acad. Sci. USA 2005, 102, 4688–4693. [Google Scholar] [CrossRef]
- Singh, R.; Vyas, S.P. Topical Liposomal System for Localized and Controlled Drug Delivery. J. Dermatol. Sci. 1996, 13, 107–111. [Google Scholar] [CrossRef]
- N’Da, D.D. Prodrug Strategies for Enhancing the Percutaneous Absorption of Drugs. Molecules 2014, 19, 20780–20807. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, T.H.; Lee, S.H.; Lee, A.Y. Treatment of Palmar Hyperhidrosis with Tap Water Iontophoresis: A Randomized, Sham-Controlled, Single-Blind, and Parallel-Designed Clinical Trial. Ann. Dermatol. 2017, 29, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Dhote, V.; Bhatnagar, P.; Mishra, P.K.; Mahajan, S.C.; Mishra, D.K. Iontophoresis: A Potential Emergence of a Transdermal Drug Delivery System. Sci. Pharm. 2012, 80, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Nagar, R.; Sengar, S.S. A Simple User-Made Iontophoresis Device for Palmoplantar Hyperhidrosis. J. Cutan. Aesthet. Surg. 2016, 9, 32–33. [Google Scholar] [CrossRef] [PubMed]
- Kotzki, S.; Roustit, M.; Arnaud, C.; Godin-Ribuot, D.; Cracowski, J.-L. Effect of Continuous vs Pulsed Iontophoresis of Treprostinil on Skin Blood Flow. Eur. J. Pharm. Sci. 2015, 72, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Roustit, M.; Blaise, S.; Cracowski, J.-L. Trials and Tribulations of Skin Iontophoresis in Therapeutics. Br. J. Clin. Pharmacol. 2014, 77, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Bose, V.G.; Langer, R.; Weaver, J.C. Electroporation of Mammalian Skin: A Mechanism to Enhance Transdermal Drug Delivery. Proc. Natl. Acad. Sci. USA 1993, 90, 10504–10508. [Google Scholar] [CrossRef]
- Babiuk, S.; Baca-Estrada, M.E.; Foldvari, M.; Baizer, L.; Stout, R.; Storms, M.; Rabussay, D.; Widera, G.; Babiuk, L. Needle-Free Topical Electroporation Improves Gene Expression from Plasmids Administered in Porcine Skin. Mol. Ther. 2003, 8, 992–998. [Google Scholar] [CrossRef]
- Vranić, E. Sonophoresis-Mechanisms and Application. Bosn J. Basic Med. Sci. 2004, 4, 25–32. [Google Scholar] [CrossRef]
- Rich, K.T.; Hoerig, C.L.; Rao, M.B.; Mast, T.D. Relations between Acoustic Cavitation and Skin Resistance during Intermediate- and High-Frequency Sonophoresis. J. Control. Release 2014, 194, 266–277. [Google Scholar] [CrossRef]
- Rao, R.; Nanda, S. Sonophoresis: Recent Advancements and Future Trends. J. Pharm. Pharmacol. 2009, 61, 689–705. [Google Scholar] [CrossRef]
- Escobar-Chávez, J.J.; Bonilla-Martínez, D.; Villegas-González, M.A.; Rodríguez-Cruz, I.M.; Domínguez-Delgado, C.L. The Use of Sonophoresis in the Administration of Drugs throughout the Skin. J. Pharm. Pharm. Sci. 2009, 12, 88–115. [Google Scholar] [CrossRef]
- Zhu, Q.; Shen, Y.; Zhang, A.; Xu, L.X. Numerical Study of the Influence of Water Evaporation on Radiofrequency Ablation. Biomed. Eng. Online 2013, 12, 127. [Google Scholar] [CrossRef]
- Lin, C.H.; Aljuffali, I.A.; Fang, J.Y. Lasers as an Approach for Promoting Drug Delivery via Skin. Expert. Opin. Drug Deliv. 2014, 11, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, H.; Atobe, S.; Suzuki, T.; Iga, H.; Terai, K. Development of Pyro-Drive Jet Injector with Controllable Jet Pressure. J. Pharm. Sci. 2019, 108, 2415–2420. [Google Scholar] [CrossRef] [PubMed]
- Barolet, D.; Benohanian, A. Current Trends in Needle-Free Jet Injection: An Update. Clin. Cosmet. Investig. Dermatol. 2018, 11, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.R.; Hodgins, B.; Hendin, H.E.; Patel, A.; Menassa, K.; Menassa, C.; Menassa, M.; Pereira, J.A.; Ward, B.J. Needle-Free Delivery of Influenza Vaccine Using the Med-Jet® H4 Is Efficient and Elicits the Same Humoral and Cellular Responses as Standard IM Injection: A Randomized Trial. Vaccine 2019, 37, 1332–1339. [Google Scholar] [CrossRef]
- Hoffman, P.N.; Abuknesha, R.A.; Andrews, N.J.; Samuel, D.; Lloyd, J.S. A Model to Assess the Infection Potential of Jet Injectors Used in Mass Immunisation. Vaccine 2001, 19, 4020–4027. [Google Scholar] [CrossRef]
- Rzhevskiy, A.S.; Singh, T.R.R.; Donnelly, R.F.; Anissimov, Y.G. Microneedles as the Technique of Drug Delivery Enhancement in Diverse Organs and Tissues. J. Control. Release 2018, 270, 184–202. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Dave, K.; Venuganti, V.V.K. Microneedles in the Clinic. J. Control. Release 2017, 260, 164–182. [Google Scholar] [CrossRef]
- Cheung, K.; Das, D.B. Microneedles for Drug Delivery: Trends and Progress. Drug Deliv. 2016, 23, 2338–2354. [Google Scholar] [CrossRef]
- Singh, T.R.R.; Dunne, N.J.; Cunningham, E.; Donnelly, R.F. Review of Patents on Microneedle Applicators. Recent Pat. Drug Deliv. Formul. 2011, 5, 11–23. [Google Scholar] [CrossRef]
- Lhernould, M.S.; Tailler, S.; Deleers, M.; Delchambre, A. Review of Patents for Microneedle Application Devices Allowing Fluid Injections through the Skin. Recent Pat. Drug Deliv. Formul. 2015, 9, 146–157. [Google Scholar] [CrossRef]
- Bariya, S.H.; Gohel, M.C.; Mehta, T.A.; Sharma, O.P. Microneedles: An Emerging Transdermal Drug Delivery System. J. Pharm. Pharmacol. 2012, 64, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Chávez, J.J.; Bonilla-Martínez, D.; Villegas-González, M.A.; Molina-Trinidad, E.; Casas-Alancaster, N.; Revilla-Vázquez, A.L. Microneedles: A Valuable Physical Enhancer to Increase Transdermal Drug Delivery. J. Clin. Pharmacol. 2011, 51, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R. Engineering Microneedle Patches for Vaccination and Drug Delivery to Skin. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 177–200. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-Z.; Huo, M.-R.; Zhou, J.-P.; Zhou, Y.-Q.; Hao, B.-H.; Liu, T.; Zhang, Y. Super-Short Solid Silicon Microneedles for Transdermal Drug Delivery Applications. Int. J. Pharm. 2010, 389, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Gill, H.S.; Andrews, S.N.; Prausnitz, M.R. Kinetics of Skin Resealing after Insertion of Microneedles in Human Subjects. J. Control. Release 2011, 154, 148–155. [Google Scholar] [CrossRef]
- Larrañeta, E.; Lutton, R.E.M.; Woolfson, A.D.; Donnelly, R.F. Microneedle Arrays as Transdermal and Intradermal Drug Delivery Systems: Materials Science, Manufacture and Commercial Development. Mater. Sci. Eng. R Rep. 2016, 104, 1–32. [Google Scholar] [CrossRef]
- Kalluri, H.; Banga, A.K. Formation and Closure of Microchannels in Skin Following Microporation. Pharm. Res. 2011, 28, 82–94. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Singh, T.R.R.; Alkilani, A.Z.; McCrudden, M.T.C.; O’Neill, S.; O’Mahony, C.; Armstrong, K.; McLoone, N.; Kole, P.; Woolfson, A.D. Hydrogel-Forming Microneedle Arrays Exhibit Antimicrobial Properties: Potential for Enhanced Patient Safety. Int. J. Pharm. 2013, 45, 76–91. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, B.Z.; Wang, Q.L.; Jin, X.; Guo, X.D. Fabrication of Coated Polymer Microneedles for Transdermal Drug Delivery. J. Control. Release 2017, 265, 14–21. [Google Scholar] [CrossRef]
- Li, S.; Li, W.; Prausnitz, M. Individually Coated Microneedles for Co-Delivery of Multiple Compounds with Different Properties. Drug Deliv. Transl. Res. 2018, 8, 1043–1052. [Google Scholar] [CrossRef]
- Iliescu, F.; Dumitrescu-Ionescu, D.; Petrescu, M.; Iliescu, C. A Review on Transdermal Drug Delivery Using Microneedles: Current Research and Perspective. Ann. Acad. Rom. Sci. Series Sci. Technol. Inf. 2014, 7, 7–34. [Google Scholar]
- Koutsonanos, D.G.; del Pilar Martin, M.; Zarnitsyn, V.G.; Sullivan, S.P.; Compans, R.W.; Prausnitz, M.R.; Skountzou, I. Transdermal Influenza Immunization with Vaccine-Coated Microneedle Arrays. PLoS ONE 2009, 4, e4773. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, A.M.; McCrudden, M.T.C.; Vincente-Perez, E.M.; Dubois, A.V.; Ingram, R.J.; Larrañeta, E.; Kissenpfennig, A.; Donnelly, R.F. Design and Characterisation of a Dissolving Microneedle Patch for Intradermal Vaccination with Heat-Inactivated Bacteria: A Proof of Concept Study. Int. J. Pharm. 2018, 549, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-J.; Han, M.-R.; Kim, J.-S.; Park, J.-H. A Tearable Dissolving Microneedle System for Shortening Application Time. Expert Opin. Drug Deliv. 2019, 16, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Yoshimitsu, J.-I.; Shiroyama, K.; Sugioka, N.; Takada, K. Self-Dissolving Microneedles for the Percutaneous Absorption of EPO in Mice. J. Drug Target 2006, 14, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Wei, L.; Wu, F.; Wu, Z.; Chen, L.; Liu, Z.; Yuan, W. Dissolving and Biodegradable Microneedle Technologies for Transdermal Sustained Delivery of Drug and Vaccine. Drug Des. Devel. Ther. 2013, 7, 945–952. [Google Scholar] [CrossRef]
- Zhu, D.D.; Wang, Q.L.; Liu, X.B.; Guo, X.D. Rapidly Separating Microneedles for Transdermal Drug Delivery. Acta Biomater. 2016, 41, 312–319. [Google Scholar] [CrossRef]
- Chen, M.-C.; Huang, S.-F.; Lai, K.-Y.; Ling, M.-H. Fully Embeddable Chitosan Microneedles as a Sustained Release Depot for Intradermal Vaccination. Biomaterials 2013, 34, 3077–3086. [Google Scholar] [CrossRef]
- Zhu, D.D.; Zhang, X.P.; Shen, C.B.; Cui, Y.; Guo, X.D. The Maximum Possible Amount of Drug in Rapidly Separating Microneedles. Drug Deliv. Transl. Res. 2019, 9, 1133–1142. [Google Scholar] [CrossRef]
- Li, W.; Terry, R.N.; Tang, J.; Feng, M.R.; Schwendeman, S.P.; Prausnitz, M.R. Rapidly Separable Microneedle Patch for the Sustained Release of a Contraceptive. Nat. Biomed. Eng. 2019, 3, 220–229. [Google Scholar] [CrossRef]
- Singh, T.R.R.; Garland, M.J.; Migalska, K.; Salvador, E.C.; Shaikh, R.; McCarthy, H.O.; Woolfson, A.D.; Donnelly, R.F. Influence of a Pore-Forming Agent on Swelling, Network Parameters, and Permeability of Poly(Ethylene Glycol)-Crosslinked Poly(Methyl Vinyl Ether-Co-Maleic Acid) Hydrogels: Application in Transdermal Delivery Systems. J. Appl. Polym. Sci. 2012, 125, 2680–2694. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Singh, T.R.R.; Garland, M.J.; Migalska, K.; Majithiya, R.; McCrudden, C.M.; Kole, P.L.; Mahmood, T.M.T.; McCarthy, H.O.; Woolfson, A.D. Hydrogel-Forming Microneedle Arrays for Enhanced Transdermal Drug Delivery. Adv. Funct. Mater. 2012, 22, 4879–4890. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.J.; Choi, S.-O.; Tong, N.T.; Aiyar, A.R.; Patel, S.R.; Prausnitz, M.R.; Allen, M.G. Hollow Microneedles for Intradermal Injection Fabricated by Sacrificial Micromolding and Selective Electrodeposition. Biomed. Microdevices 2013, 15, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Martanto, W.; Moore, J.S.; Kashlan, O.; Kamath, R.; Wang, P.M.; O’Neal, J.M.; Prausnitz, M.R. Microinfusion Using Hollow Microneedles. Pharm. Res. 2006, 23, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Dharadhar, S.; Majumdar, A.; Dhoble, S.; Patravale, V. Microneedles for Transdermal Drug Delivery: A Systematic Review. Drug Dev. Ind. Pharm. 2019, 45, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; Roxhed, N.; Shafagh, R.Z.; Haraldson, T.; Fischer, A.C.; van der Wijngaart, W.; Stemme, G.; Niklaus, F. Flexible and Stretchable Microneedle Patches with Integrated Rigid Stainless Steel Microneedles for Transdermal Biointerfacing. PLoS ONE 2016, 11, e0166330. [Google Scholar] [CrossRef]
- Verbaan, F.J.; Bal, S.M.; van den Berg, D.J.; Groenink, W.H.H.; Verpoorten, H.; Lüttge, R.; Bouwstra, J.A. Assembled Microneedle Arrays Enhance the Transport of Compounds Varying over a Large Range of Molecular Weight across Human Dermatomed Skin. J. Control. Release 2007, 117, 238–245. [Google Scholar] [CrossRef]
- Jiang, J.; Gill, H.S.; Ghate, D.; McCarey, B.E.; Patel, S.R.; Edelhauser, H.F.; Prausnitz, M.R. Coated Microneedles for Drug Delivery to the Eye. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4038–4043. [Google Scholar] [CrossRef]
- Li, J.; Liu, B.; Zhou, Y.; Chen, Z.; Jiang, L.; Yuan, W.; Liang, L. Fabrication of a Ti Porous Microneedle Array by Metal Injection Molding for Transdermal Drug Delivery. PLoS ONE 2017, 12, e0172043. [Google Scholar] [CrossRef]
- Parker, E.R.; Rao, M.; Foster, K.; Meinhart, C.; MacDonald, N.C. Bulk Micromachined Titanium Microneedles. Microelectromechanical Syst. J. 2007, 16, 289–295. [Google Scholar] [CrossRef]
- Senel, M.; Dervisevic, M.; Voelcker, N.H. Gold Microneedles Fabricated by Casting of Gold Ink Used for Urea Sensing. Mater. Lett. 2019, 243, 50–53. [Google Scholar] [CrossRef]
- Davis, S.P.; Martanto, W.; Allen, M.G.; Prausnitz, M.R. Hollow Metal Microneedles for Insulin Delivery to Diabetic Rats. IEEE Trans. Biomed. Eng. 2005, 52, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Indermun, S.; Luttge, R.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Modi, G.; Pillay, V. Current Advances in the Fabrication of Microneedles for Transdermal Delivery. J. Control. Release 2014, 185, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, M.; Bystrova, S.; Winnubst, L.; Qureshi, H.; de Gruijl, T.D.; Scheper, R.J.; Luttge, R. Applying Ceramic Nanoporous Microneedle Arrays as a Transport Interface in Egg Plants and an Ex-Vivo Human Skin Model. Microelectron. Eng. 2012, 98, 659–662. [Google Scholar] [CrossRef]
- Van der Maaden, K.; Luttge, R.; Vos, P.J.; Bouwstra, J.; Kersten, G.; Ploemen, I. Microneedle-Based Drug and Vaccine Delivery via Nanoporous Microneedle Arrays. Drug Deliv. Transl. Res. 2015, 5, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Ita, K. Ceramic Microneedles and Hollow Microneedles for Transdermal Drug Delivery: Two Decades of Research. J. Drug Deliv. Sci. Technol. 2018, 44, 314–322. [Google Scholar] [CrossRef]
- Wilke, N.; Mulcahy, A.; Ye, S.-R.; Morrissey, A. Process Optimization and Characterization of Silicon Microneedles Fabricated by Wet Etch Technology. Microelectron. J. 2005, 36, 650–656. [Google Scholar] [CrossRef]
- Das, A.; Singha, C.; Bhattacharyya, A. Development of Silicon Microneedle Arrays with Spontaneously Generated Micro-Cavity Ring for Transdermal Drug Delivery. Microelectron. Eng. 2019, 210, 14–18. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Yang, R.; Laffitte, Y.; Schmill, U.; Hu, W.; Kaddoura, M.; Blondeel, E.J.M.; Cui, B. Fabrication of Sharp Silicon Hollow Microneedles by Deep-Reactive Ion Etching towards Minimally Invasive Diagnostics. Microsyst. Nanoeng. 2019, 5, 1–11. [Google Scholar] [CrossRef]
- Kim, H.; Theogarajan, L.; Pennathur, S. A Repeatable and Scalable Fabrication Method for Sharp, Hollow Silicon Microneedles. J. Micromech. Microeng. 2018, 28. [Google Scholar] [CrossRef]
- McAllister, D.V.; Wang, P.M.; Davis, S.P.; Park, J.-H.; Canatella, P.J.; Allen, M.G.; Prausnitz, M.R. Microfabricated Needles for Transdermal Delivery of Macromolecules and Nanoparticles: Fabrication Methods and Transport Studies. Proc. Natl. Acad. Sci. USA 2003, 100, 13755–13760. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Park, S.S.; Bondy, B.; Felner, E.I.; Prausnitz, M.R. Infusion Pressure and Pain during Microneedle Injection into Skin of Human Subjects. Biomaterials 2011, 32, 6823–6831. [Google Scholar] [CrossRef] [PubMed]
- Sivamani, R.K.; Liepmann, D.; Maibach, H.I. Microneedles and Transdermal Applications. Expert Opin. Drug Deliv. 2007, 4, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Miyano, T.; Tobinaga, Y.; Kanno, T.; Matsuzaki, Y.; Takeda, H.; Wakui, M.; Hanada, K. Sugar Micro Needles as Transdermic Drug Delivery System. Biomed. Microdevices 2005, 7, 185–188. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, S.-O.; Felner, E.I.; Prausnitz, M.R. Dissolving Microneedle Patch for Transdermal Delivery of Human Growth Hormone. Small 2011, 7, 531–539. [Google Scholar] [CrossRef]
- Raphael, A.P.; Crichton, M.L.; Falconer, R.J.; Meliga, S.; Chen, X.; Fernando, G.J.P.; Huang, H.; Kendall, M.A.F. Formulations for Microprojection/Microneedle Vaccine Delivery: Structure, Strength and Release Profiles. J. Control. Release 2016, 225, 40–52. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Morrow, D.I.J.; Singh, T.R.R.; Migalska, K.; McCarron, P.A.; O’Mahony, C.; Woolfson, A.D. Processing Difficulties and Instability of Carbohydrate Microneedle Arrays. Drug Dev. Ind. Pharm. 2009, 35, 1242–1254. [Google Scholar] [CrossRef]
- Sun, W.; Inayathullah, M.; Manoukian, M.A.C.; Malkovskiy, A.V.; Manickam, S.; Marinkovich, M.P.; Lane, A.T.; Tayebi, L.; Seifalian, A.M.; Rajadas, J. Transdermal Delivery of Functional Collagen Via Polyvinylpyrrolidone Microneedles. Ann. Biomed. Eng. 2015, 43, 2978–2990. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, P.S.; Domínguez Delgado, C.L.; Rodríguez Cruz, I.M.; Melgoza Contreras, L.M.; Molina Trinidad, E.M.; Cervantes, M.L.; Escobar-Chávez, J.J. Development of Poly (Methyl Vinyl Ether-Alt-Maleic Acid) Microneedles for Transdermal Delivery of Atorvastatin Calcium. Curr. Pharm. Biotechnol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Gittard, S.D.; Ovsianikov, A.; Akar, H.; Chichkov, B.; Monteiro-Riviere, N.A.; Stafslien, S.; Chisholm, B.; Shin, C.-C.; Shih, C.-M.; Lin, S.-J.; et al. Two Photon Polymerization-Micromolding of Polyethylene Glycol-Gentamicin Sulfate Microneedles. Adv. Eng. Mater. 2010, 12, B77–B82. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-H.; Park, H.-H.; Do, K.-Y.; Han, M.; Hyun, D.-H.; Kim, C.-G.; Kim, C.-H.; Lee, S.S.; Hwang, S.-J.; Shin, S.-C.; et al. Influence of the Delivery Systems Using a Microneedle Array on the Permeation of a Hydrophilic Molecule, Calcein. Eur. J. Pharm. Biopharm. 2008, 69, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.X.; Bozorg, B.D.; Kim, Y.; Wieber, A.; Birk, G.; Lubda, D.; Banga, A.K. Poly (Vinyl Alcohol) Microneedles: Fabrication, Characterization, and Application for Transdermal Drug Delivery of Doxorubicin. Eur. J. Pharm. Biopharm. 2018, 129, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Wang, H.; Pant, A.; Pastorin, G.; Lee, C. Development of Vertical SU-8 Microneedles for Transdermal Drug Delivery by Double Drawing Lithography Technology. Biomicrofluidics 2013, 7, 66501. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Han, M.-R.; Kim, Y.-H.; Shin, S.-W.; Nam, S.-Y.; Park, J.-H. Tip-Loaded Dissolving Microneedles for Transdermal Delivery of Donepezil Hydrochloride for Treatment of Alzheimer’s Disease. Eur. J. Pharm. Biopharm. 2016, 105, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-T.; Park, S.-J.; Park, J.-H. Microneedles Containing Cross-Linked Hyaluronic Acid Particulates for Control of Degradation and Swelling Behaviour after Administration into Skin. J. Drug Target 2018, 26, 884–894. [Google Scholar] [CrossRef]
- Park, Y.-H.; Ha, S.K.; Choi, I.; Kim, K.S.; Park, J.; Choi, N.; Kim, B.; Sung, J.H. Fabrication of Degradable Carboxymethyl Cellulose (CMC) Microneedle with Laser Writing and Replica Molding Process for Enhancement of Transdermal Drug Delivery. Biotechnol. Bioproc. E 2016, 21, 110–118. [Google Scholar] [CrossRef]
- Nguyen, H.X.; Banga, A.K. Delivery of Methotrexate and Characterization of Skin Treated by Fabricated PLGA Microneedles and Fractional Ablative Laser. Pharm. Res. 2018, 35, 68. [Google Scholar] [CrossRef]
- Patel, A.; Cholkar, K.; Mitra, A.K. Recent Developments in Protein and Peptide Parenteral Delivery Approaches. Ther. Deliv. 2014, 5, 337–365. [Google Scholar] [CrossRef]
- Bull, S.P.; Hong, Y.; Khutoryanskiy, V.V.; Parker, J.K.; Faka, M.; Methven, L. Whey Protein Mouth Drying Influenced by Thermal Denaturation. Food Qual. Prefer. 2017, 56 (Pt B), 233–240. [Google Scholar] [CrossRef]
- Muheem, A.; Shakeel, F.; Jahangir, M.A.; Anwar, M.; Mallick, N.; Jain, G.K.; Warsi, M.H.; Ahmad, F.J. A Review on the Strategies for Oral Delivery of Proteins and Peptides and Their Clinical Perspectives. Saudi Pharm. J. 2016, 24, 413–428. [Google Scholar] [CrossRef]
- Collin, M.; Milne, P. Langerhans Cell Origin and Regulation. Curr. Opin. Hematol. 2016, 23, 28–35. [Google Scholar] [CrossRef]
- Van der Maaden, K.; Jiskoot, W.; Bouwstra, J. Microneedle Technologies for (Trans)Dermal Drug and Vaccine Delivery. J. Control. Release 2012, 161, 645–655. [Google Scholar] [CrossRef]
- Du, G.; Hathout, R.M.; Nasr, M.; Nejadnik, M.R.; Tu, J.; Koning, R.I.; Koster, A.J.; Slütter, B.; Kros, A.; Jiskoot, W.; et al. Intradermal Vaccination with Hollow Microneedles: A Comparative Study of Various Protein Antigen and Adjuvant Encapsulated Nanoparticles. J. Control. Release 2017, 266, 109–118. [Google Scholar] [CrossRef] [PubMed]
- McCrudden, M.T.C.; Torrisi, B.M.; Al-Zahrani, S.; McCrudden, C.M.; Zaric, M.; Scott, C.J.; Kissenpfennig, A.; McCarthy, H.O.; Donnelly, R.F. Laser-Engineered Dissolving Microneedle Arrays for Protein Delivery: Potential for Enhanced Intradermal Vaccination. J. Pharm. Pharmacol. 2015, 67, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Erdos, G.; Huang, S.; Kenniston, T.W.; Balmert, S.C.; Carey, C.D.; Raj, V.S.; Epperly, M.W.; Klimstra, W.B.; Haagmans, B.L.; et al. Microneedle Array Delivered Recombinant Coronavirus Vaccines: Immunogenicity and Rapid Translational Development. EBioMedicine 2020. [Google Scholar] [CrossRef] [PubMed]
- Van der Maaden, K.; Trietsch, S.J.; Kraan, H.; Varypataki, E.M.; Romeijn, S.; Zwier, R.; van der Linden, H.J.; Kersten, G.; Hankemeier, T.; Jiskoot, W.; et al. Novel Hollow Microneedle Technology for Depth-Controlled Microinjection-Mediated Dermal Vaccination: A Study with Polio Vaccine in Rats. Pharm. Res. 2014, 31, 1846–1854. [Google Scholar] [CrossRef] [PubMed]
- De Groot, A.M.; Du, G.; Mönkäre, J.; Platteel, A.C.M.; Broere, F.; Bouwstra, J.A.; Sijts, A.J.A.M. Hollow Microneedle-Mediated Intradermal Delivery of Model Vaccine Antigen-Loaded PLGA Nanoparticles Elicits Protective T Cell-Mediated Immunity to an Intracellular Bacterium. J. Control. Release 2017, 266, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, S.; Duan, Y.; Niu, Y.; Gu, H.; Zhao, Z.; Zhang, S.; Yang, Y.; Wang, X.; Gao, Y.; et al. Transcutaneous Immunization of Recombinant Staphylococcal Enterotoxin B Protein Using a Dissolving Microneedle Provides Potent Protection against Lethal Enterotoxin Challenge. Vaccine 2019, 37, 3810–3819. [Google Scholar] [CrossRef]
- Lee, J.; Arun Kumar, S.; Jhan, Y.Y.; Bishop, C.J. Engineering DNA Vaccines against Infectious Diseases. Acta Biomater. 2018, 80, 31–47. [Google Scholar] [CrossRef]
- Song, J.-M.; Kim, Y.-C.; Eunju, O.; Compans, R.W.; Prausnitz, M.R.; Kang, S.-M. DNA Vaccination in the Skin Using Microneedles Improves Protection against Influenza. Mol. Ther. 2012, 20, 1472–1480. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Mikszta, J.A.; Cormier, M.; Andrianov, A.K. Microneedle-Based Vaccines. Curr. Top. Microbiol. Immunol. 2009, 333, 369–393. [Google Scholar] [CrossRef] [PubMed]
- Pamornpathomkul, B.; Niyomtham, N.; Yingyongnarongkul, B.-E.; Prasitpuriprecha, C.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P. Cationic Niosomes for Enhanced Skin Immunization of Plasmid DNA-Encoding Ovalbumin via Hollow Microneedles. AAPS PharmSciTech 2018, 19, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Kines, R.C.; Zarnitsyn, V.; Johnson, T.R.; Pang, Y.-Y.S.; Corbett, K.S.; Nicewonger, J.D.; Gangopadhyay, A.; Chen, M.; Liu, J.; Prausnitz, M.R.; et al. Vaccination with Human Papillomavirus Pseudovirus-Encapsidated Plasmids Targeted to Skin Using Microneedles. PLoS ONE 2015, 10, e0120797. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Cheng, Z.; Liu, H.; Shan, W.; Cheng, Z.; Dai, X.; Xue, Y.; Chen, F. Enhancement of Ag85B DNA Vaccine Immunogenicity against Tuberculosis by Dissolving Microneedles in Mice. Vaccine 2018, 36, 4471–4476. [Google Scholar] [CrossRef] [PubMed]
- Bellofatto, V.; Wilusz, J. Transcription and MRNA Stability: Parental Guidance Suggested. Cell 2011, 147, 1438–1439. [Google Scholar] [CrossRef]
- Golombek, S.; Pilz, M.; Steinle, H.; Kochba, E.; Levin, Y.; Lunter, D.; Schlensak, C.; Wendel, H.; Avci-Adali, M. Intradermal Delivery of Synthetic MRNA Using Hollow Microneedles for Efficient and Rapid Production of Exogenous Proteins in Skin. Mol. Ther. Nucleic Acids 2018, 11. [Google Scholar] [CrossRef]
- Cheung, K.; West, G.; Das, D.B. Delivery of Large Molecular Protein Using Flat and Short Microneedles Prepared Using Focused Ion Beam (FIB) as a Skin Ablation Tool. Drug Deliv. Transl. Res. 2015, 5, 462–467. [Google Scholar] [CrossRef]
- Mönkäre, J.; Reza Nejadnik, M.; Baccouche, K.; Romeijn, S.; Jiskoot, W.; Bouwstra, J.A. IgG-Loaded Hyaluronan-Based Dissolving Microneedles for Intradermal Protein Delivery. J. Control. Release 2015, 218, 53–62. [Google Scholar] [CrossRef]
- Courtenay, A.J.; McCrudden, M.T.C.; McAvoy, K.J.; McCarthy, H.O.; Donnelly, R.F. Microneedle-Mediated Transdermal Delivery of Bevacizumab. Mol. Pharm. 2018, 15, 3545–3556. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, Y.; Zhang, S.; Gao, Y. Dissolving Microneedle-Based Intradermal Delivery of Interferon-α-2b. Drug Dev. Ind. Pharm. 2016, 42, 890–896. [Google Scholar] [CrossRef]
- Xie, X.; Pascual, C.; Lieu, C.; Oh, S.; Wang, J.; Zou, B.; Xie, J.; Li, Z.; Xie, J.; Yeomans, D.C.; et al. Analgesic Microneedle Patch for Neuropathic Pain Therapy. ACS Nano 2017, 11, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Resnik, D.; Možek, M.; Pečar, B.; Janež, A.; Urbančič, V.; Iliescu, C.; Vrtačnik, D. In Vivo Experimental Study of Noninvasive Insulin Microinjection through Hollow Si Microneedle Array. Micromachines (Basel) 2018, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-C.; Lin, W.-M.; Shu, J.-C.; Tsai, S.-W.; Chen, C.-H.; Tsai, M.-T. Formulation of Two-Layer Dissolving Polymeric Microneedle Patches for Insulin Transdermal Delivery in Diabetic Mice. J. Biomed. Mater. Res. A 2017, 105, 84–93. [Google Scholar] [CrossRef]
- Chen, M.-C.; Ling, M.-H.; Kusuma, S.J. Poly-γ-Glutamic Acid Microneedles with a Supporting Structure Design as a Potential Tool for Transdermal Delivery of Insulin. Acta Biomater. 2015, 24, 106–116. [Google Scholar] [CrossRef]
- Tong, Z.; Zhou, J.; Zhong, J.; Tang, Q.; Lei, Z.; Luo, H.; Ma, P.; Liu, X. Glucose- and H2O2-Responsive Polymeric Vesicles Integrated with Microneedle Patches for Glucose-Sensitive Transcutaneous Delivery of Insulin in Diabetic Rats. ACS Appl. Mater. Interfaces 2018, 10, 20014–20024. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, Y.; Ye, Y.; DiSanto, R.; Sun, W.; Ranson, D.; Ligler, F.S.; Buse, J.B.; Gu, Z. Microneedle-Array Patches Loaded with Hypoxia-Sensitive Vesicles Provide Fast Glucose-Responsive Insulin Delivery. PNAS 2015, 112, 8260–8265. [Google Scholar] [CrossRef]
- Dong, R.; Sun, S.; Liu, X.-Z.; Shen, Z.; Chen, G.; Zheng, S. Fat-Soluble Vitamin Deficiency in Pediatric Patients with Biliary Atresia. Gastroenterol. Res. Pract. 2017, 2017, 7496860. [Google Scholar] [CrossRef]
- Godala, M.; Materek-Kuśmierkiewicz, I.; Moczulski, D.; Rutkowski, M.; Szatko, F.; Gaszyńska, E.; Tokarski, S.; Kowalski, J. The Risk of Plasma Vitamin A, C, E and D Deficiency in Patients with Metabolic Syndrome: A Case-Control Study. Adv. Clin. Exp. Med. 2017, 26, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Okebukola, P.O.; Kansra, S.; Barrett, J. Vitamin E Supplementation in People with Cystic Fibrosis. Cochrane Database Syst. Rev. 2014, CD009422. [Google Scholar] [CrossRef]
- Kim, H.-G.; Gater, D.L.; Kim, Y.-C. Development of Transdermal Vitamin D3 (VD3) Delivery System Using Combinations of PLGA Nanoparticles and Microneedles. Drug Deliv. Transl. Res. 2018, 8, 281–290. [Google Scholar] [CrossRef]
- Vora, L.K.; Donnelly, R.F.; Larrañeta, E.; González-Vázquez, P.; Thakur, R.R.S.; Vavia, P.R. Novel Bilayer Dissolving Microneedle Arrays with Concentrated PLGA Nano-Microparticles for Targeted Intradermal Delivery: Proof of Concept. J. Control. Release 2017, 265, 93–101. [Google Scholar] [CrossRef]
- Hutton, A.R.J.; Quinn, H.L.; McCague, P.J.; Jarrahian, C.; Rein-Weston, A.; Coffey, P.S.; Gerth-Guyette, E.; Zehrung, D.; Larrañeta, E.; Donnelly, R.F. Transdermal Delivery of Vitamin K Using Dissolving Microneedles for the Prevention of Vitamin K Deficiency Bleeding. Int. J. Pharm. 2018, 541, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Ramöller, I.K.; Tekko, I.A.; McCarthy, H.O.; Donnelly, R.F. Rapidly Dissolving Bilayer Microneedle Arrays—A Minimally Invasive Transdermal Drug Delivery System for Vitamin B12. Int. J. Pharm. 2019, 566, 299–306. [Google Scholar] [CrossRef]
- Risbud, M.V.; Bhonde, R.R. Polyacrylamide-Chitosan Hydrogels: In Vitro Biocompatibility and Sustained Antibiotic Release Studies. Drug Deliv. 2000, 7, 69–75. [Google Scholar] [CrossRef]
- González-Vázquez, P.; Larrañeta, E.; McCrudden, M.T.C.; Jarrahian, C.; Rein-Weston, A.; Quintanar-Solares, M.; Zehrung, D.; McCarthy, H.; Courtenay, A.J.; Donnelly, R.F. Transdermal Delivery of Gentamicin Using Dissolving Microneedle Arrays for Potential Treatment of Neonatal Sepsis. J. Control. Release 2017, 265, 30–40. [Google Scholar] [CrossRef]
- Lee, H.S.; Ryu, H.R.; Roh, J.Y.; Park, J.-H. Bleomycin-Coated Microneedles for Treatment of Warts. Pharm. Res. 2017, 34, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.; Permana, A.D.; Ahmed, N.; Khan, G.M.; Rehman, A.; ur Donnelly, R.F. Enhancement in Site-Specific Delivery of Carvacrol for Potential Treatment of Infected Wounds Using Infection Responsive Nanoparticles Loaded into Dissolving Microneedles: A Proof of Concept Study. Eur. J. Pharm. Biopharm. 2020, 147, 57–68. [Google Scholar] [CrossRef]
- Xu, J.; Danehy, R.; Cai, H.; Ao, Z.; Pu, M.; Nusawardhana, A.; Rowe-Magnus, D.; Guo, F. Microneedle Patch-Mediated Treatment of Bacterial Biofilms. ACS Appl. Mater. Interfaces 2019, 11, 14640–14646. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Kaur, A.; Sharma, S. A review on advances of sustained release drug delivery system. Int. Res. J. Pharm. 2013, 4, 1–5. [Google Scholar] [CrossRef]
- Paleco, R.; Vučen, S.R.; Crean, A.M.; Moore, A.; Scalia, S. Enhancement of the in Vitro Penetration of Quercetin through Pig Skin by Combined Microneedles and Lipid Microparticles. Int. J. Pharm. 2014, 472, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Patel, M.G.; Bakshi, P.; Sharma, D.; Banga, A.K. Enhancement in the Transdermal and Localized Delivery of Honokiol Through Breast Tissue. AAPS PharmSciTech 2018, 19, 3501–3511. [Google Scholar] [CrossRef] [PubMed]
- Puri, A.; Nguyen, H.X.; Banga, A.K. Microneedle-Mediated Intradermal Delivery of Epigallocatechin-3-Gallate. Int. J. Cosmet. Sci. 2016, 38, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Kochhar, J.S.; Tan, J.J.Y.; Kwang, Y.C.; Kang, L. Recent Trends in Microneedle Development & Applications in Medicine and Cosmetics (2013–2018). In Microneedles for Transdermal Drug Delivery; Kochhar, J.S., Tan, J.J.Y., Kwang, Y.C., Kang, L., Eds.; Springer International Publishing: Cham, Germany, 2019; pp. 95–144. [Google Scholar] [CrossRef]
- Kim, M.; Yang, H.; Kim, H.; Jung, H.; Jung, H. Novel Cosmetic Patches for Wrinkle Improvement: Retinyl Retinoate- and Ascorbic Acid-Loaded Dissolving Microneedles. Int. J. Cosmet. Sci. 2014, 36, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Eom, Y.A.; Yang, H.; Jang, M.; Jung, S.U.; Park, Y.O.; Lee, S.E.; Jung, H. Skin Barrier Restoration and Moisturization Using Horse Oil-Loaded Dissolving Microneedle Patches. Skin Pharmacol. Physiol. 2018, 31, 163–171. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, H.U.; Lee, Y.-C.; Kim, G.H.; Park, E.C.; Han, S.H.; Lee, J.G.; Choi, S.; Heo, N.S.; Kim, D.L.; et al. Wound Healing Potential of Antibacterial Microneedles Loaded with Green Tea Extracts. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 42, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.; Zhang, X.; Chen, C.; Shao, C.; Zhao, Y.; Wang, Y. Antibacterial and Angiogenic Chitosan Microneedle Array Patch for Promoting Wound Healing. Bioact. Mater. 2020, 5, 253–259. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Saju, A.; Cheerla, K.D.; Gade, S.K.; Garg, P.; Venuganti, V.V.K. Corneal Delivery of Besifloxacin Using Rapidly Dissolving Polymeric Microneedles. Drug Deliv. Transl. Res. 2018, 8, 473–483. [Google Scholar] [CrossRef]
- Mahadevan, G.; Sheardown, H.; Selvaganapathy, P. PDMS Embedded Microneedles as a Controlled Release System for the Eye. J. Biomater. Appl. 2013, 28, 20–27. [Google Scholar] [CrossRef]
- Patel, S.R.; Berezovsky, D.E.; McCarey, B.E.; Zarnitsyn, V.; Edelhauser, H.F.; Prausnitz, M.R. Targeted Administration into the Suprachoroidal Space Using a Microneedle for Drug Delivery to the Posterior Segment of the Eye. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4433–4441. [Google Scholar] [CrossRef]
- Miquel-Clopés, A.; Bentley, E.G.; Stewart, J.P.; Carding, S.R. Mucosal Vaccines and Technology. Clin. Exp. Immunol. 2019, 196, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Jang, Y.-S. The Development of Mucosal Vaccines for Both Mucosal and Systemic Immune Induction and the Roles Played by Adjuvants. Clin. Exp. Vaccine Res. 2017, 6, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Hasséus, B.; Jontell, M.; Bergenholtz, G.; Dahlgren, U.I. T-Cell Costimulatory Capacity of Oral and Skin Epithelial Cells in Vitro: Presence of Suppressive Activity in Supernatants from Skin Epithelial Cell Cultures. Eur. J. Oral Sci. 2004, 112, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Creighton, R.L.; Woodrow, K.A. Microneedle-Mediated Vaccine Delivery to the Oral Mucosa. Adv. Healthc. Mater. 2019, 8, e1801180. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Tao, W.; Krebs, S.J.; Sutton, W.F.; Haigwood, N.L.; Gill, H.S. Vaccine Delivery to the Oral Cavity Using Coated Microneedles Induces Systemic and Mucosal Immunity. Pharm. Res. 2014, 31, 2393–2403. [Google Scholar] [CrossRef]
- Seon-Woo, H.-S.; Kim, H.J.; Roh, J.Y.; Park, J.-H. Dissolving Microneedle Systems for the Oral Mucosal Delivery of Triamcinolone Acetonide to Treat Aphthous Stomatitis. Macromol. Res. 2019, 27, 282–289. [Google Scholar] [CrossRef]
- Duong, H.T.T.; Kim, N.W.; Thambi, T.; Giang Phan, V.H.; Lee, M.S.; Yin, Y.; Jeong, J.H.; Lee, D.S. Microneedle Arrays Coated with Charge Reversal PH-Sensitive Copolymers Improve Antigen Presenting Cells-Homing DNA Vaccine Delivery and Immune Responses. J. Control. Release 2018, 269, 225–234. [Google Scholar] [CrossRef]
- Duong, H.T.T.; Yin, Y.; Thambi, T.; Nguyen, T.L.; Giang Phan, V.H.; Lee, M.S.; Lee, J.E.; Kim, J.; Jeong, J.H.; Lee, D.S. Smart Vaccine Delivery Based on Microneedle Arrays Decorated with Ultra-PH-Responsive Copolymers for Cancer Immunotherapy. Biomaterials 2018, 185, 13–24. [Google Scholar] [CrossRef]
- Heathman, T.R.J.; Nienow, A.W.; McCall, M.J.; Coopman, K.; Kara, B.; Hewitt, C.J. The Translation of Cell-Based Therapies: Clinical Landscape and Manufacturing Challenges. Regen. Med. 2015, 10, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Gualeni, B.; Coulman, S.A.; Shah, D.; Eng, P.F.; Ashraf, H.; Vescovo, P.; Blayney, G.J.; Piveteau, L.-D.; Guy, O.J.; Birchall, J.C. Minimally Invasive and Targeted Therapeutic Cell Delivery to the Skin Using Microneedle Devices. Br. J. Dermatol. 2018, 178, 731–739. [Google Scholar] [CrossRef]
- Gill, H.S.; Denson, D.D.; Burris, B.A.; Prausnitz, M.R. Effect of Microneedle Design on Pain in Human Subjects. Clin. J. Pain 2008, 24, 585–594. [Google Scholar] [CrossRef]
- Gill, H.S.; Prausnitz, M.R. Does Needle Size Matter? J. Diabetes Sci. Technol. 2007, 1, 725–729. [Google Scholar] [CrossRef]
- Bal, S.M.; Caussin, J.; Pavel, S.; Bouwstra, J.A. In Vivo Assessment of Safety of Microneedle Arrays in Human Skin. Eur. J. Pharm. Sci. 2008, 35, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, X.H.M.; van der Linde, P.; Homburg, E.F.G.A.; van Breemen, L.C.A.; de Jong, A.M.; Luttge, R. Insertion Process of Ceramic Nanoporous Microneedles by Means of a Novel Mechanical Applicator Design. Pharmaceutics 2015, 7, 503–522. [Google Scholar] [CrossRef] [PubMed]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A Smart Approach and Increasing Potential for Transdermal Drug Delivery System. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Singh, T.R.R.; Tunney, M.M.; Morrow, D.I.J.; McCarron, P.A.; O’Mahony, C.; Woolfson, A.D. Microneedle Arrays Allow Lower Microbial Penetration Than Hypodermic Needles In Vitro. Pharm. Res. 2009, 26, 2513–2522. [Google Scholar] [CrossRef]
- Vicente-Perez, E.M.; Larrañeta, E.; McCrudden, M.T.C.; Kissenpfennig, A.; Hegarty, S.; McCarthy, H.O.; Donnelly, R.F. Repeat Application of Microneedles Does Not Alter Skin Appearance or Barrier Function and Causes No Measurable Disturbance of Serum Biomarkers of Infection, Inflammation or Immunity in Mice in Vivo. Eur. J. Pharm. Biopharm. 2017, 117, 400–407. [Google Scholar] [CrossRef]
- Serhan, H.; Slivka, M.; Albert, T.; Kwak, S.D. Is Galvanic Corrosion between Titanium Alloy and Stainless Steel Spinal Implants a Clinical Concern? Spine J 2004, 4, 379–387. [Google Scholar] [CrossRef]
- Jung, P.; Lee, T.; Oh, D.; Hwang, S.; Jung, I.; Lee, S.; Ko, J. Nickel Microneedles Fabricated by Sequential Copper and Nickel Electroless Plating and Copper Chemical Wet Etching. Sens. Mater. 2008, 20, 45–53. [Google Scholar]
- Ermolli, M.; Menné, C.; Pozzi, G.; Serra, M.; Clerici, L. Nickel, Cobalt and Chromium-Induced Cytotoxicity and Intracellular Accumulation in Human Hacat Keratinocytes. Toxicology 2001, 159, 23–31. [Google Scholar] [CrossRef]
- Jeong, H.-R.; Lee, H.-S.; Choi, I.-J.; Park, J.-H. Considerations in the Use of Microneedles: Pain, Convenience, Anxiety and Safety. J. Drug Target 2017, 25, 29–40. [Google Scholar] [CrossRef]
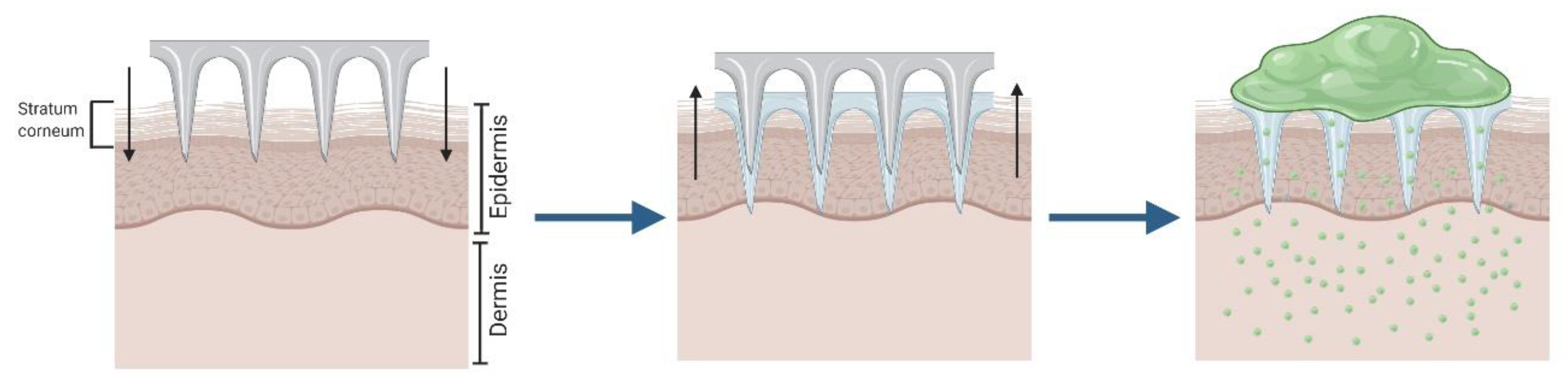
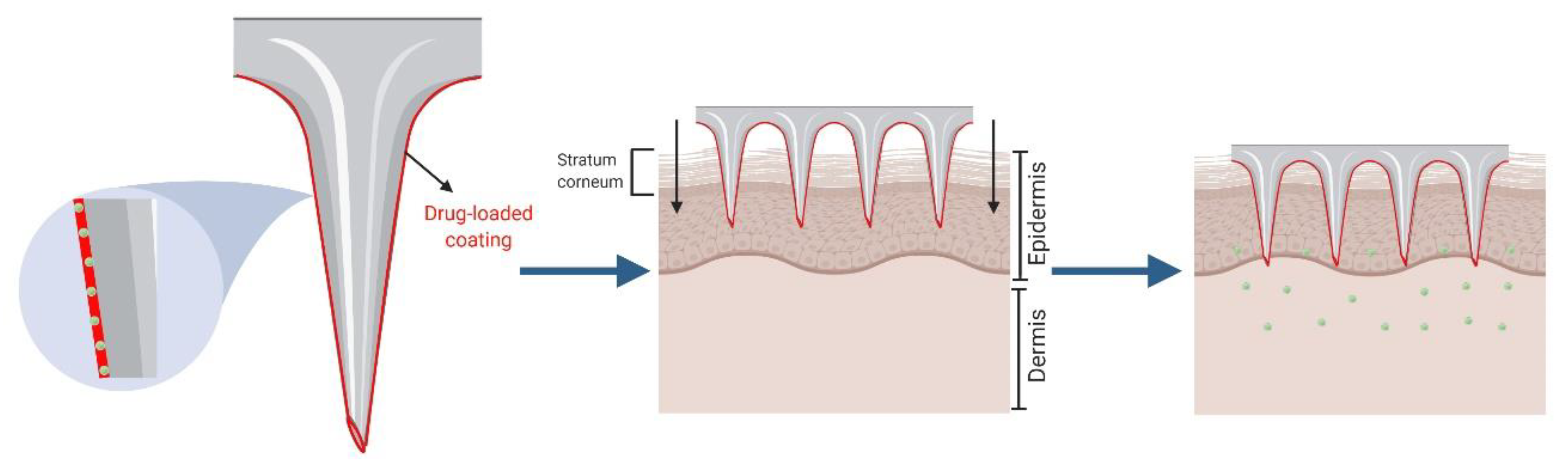
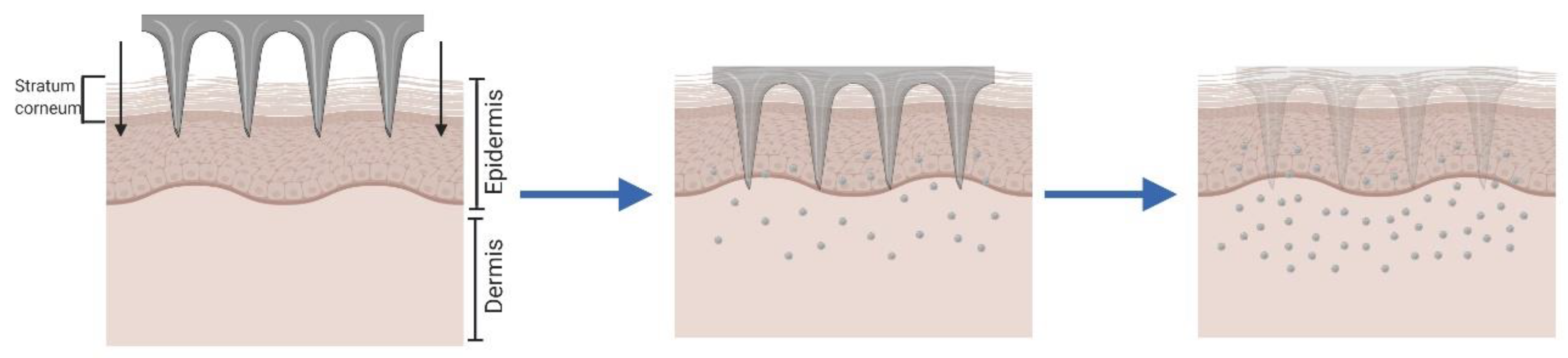
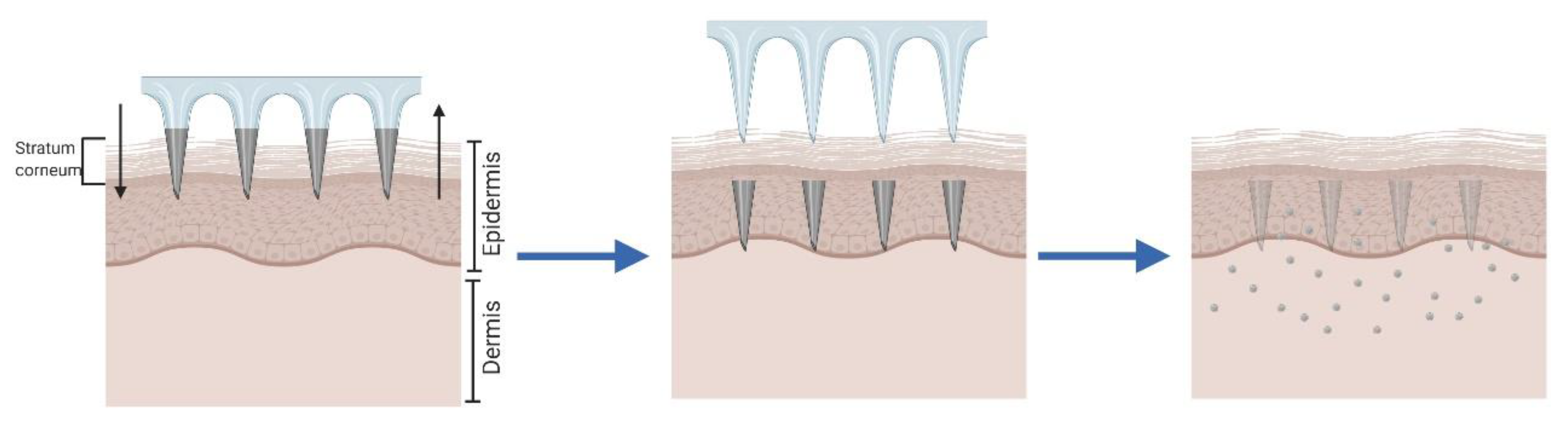

| Method | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Chemical enhancers | High effectiveness in combination with small molecules | Poor effectiveness in combination with macromolecules and hydrophilic molecules Inability to locate the effects on the stratum corneum (SC) Skin reactions (irritation, inflammation, erythema) Anti-inflammatory, anti-irritation pre-treatments are recommended | [26,27,28] |
| Microsystems and nanosystems | Possibility to localize the effects and drug release in the first layers of the skin | Large size can hinder the penetration of the system into the skin | [29] |
| Prodrugs | Improve chemical stability Avoiding skin reactions | Large size can hinder the diffusion through the skin | [30] |
| Iontophoresis | Rapidly responsive molecular transport Control of transport magnitude | Devices are expensive Not applicable for long periods of time due to the polarization of the skin Inability to locate the effects on the SC Skin reactions (irritation, inflammation, erythema) | [31,32,33,34,35] |
| Electroporation | Rapidly responsive molecular transport Control of transport magnitude | Devices are expensive Inability to locate the effects on the SC Skin reactions (irritation, inflammation, erythema) | [36,37] |
| Sonophoresis | Rapidly responsive molecular transport Control of transport magnitude Good effectiveness in combination with hydrophilic drugs and medium-large molecular weight | Devices are expensive Poor range of molecules administered safely Inability to locate the effects on the SC Skin reactions (irritation, inflammation, erythema) | [38,39,40,41] |
| Thermal methods | Possibility to diffuse large-size molecules | Inability to locate the effects on the SC Intense skin reactions (irritation, inflammation, erythema) | [42,43] |
| Jet injectors | Delivery of solid particles or liquids Possibility to control de depth where the drug is deposited Useful for vaccination | Not applicable for long periods of time Possibility of contamination of the devices with interstitial fluids | [44,45,46,47] |
| Material | MNA Type | Fabrication Process | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|
| Stainless steel, Titanium, Nickel, Gold | Solid, Hollow, Coated (array) | Laser cutting, laser ablation, etching, electropolishing, lithography, and microstereolithography | Desirable mechanical properties and high tensile strength | Fractures, corrosion, and poor biocompatibility of some metals | [78,79,80,81,82,83,84] |
| Alumina, Zirconia, Calcium phosphate/sulphate | Ceramic | Lithography and ceramic sintering | Good biocompatibility | Fractures | [85,86,87,88] |
| Silicon | Solid, Hollow, Coated (array) | Etching, Lithography | Desirable mechanical properties | High material cost, long fabrication, and fractures | [89,90,91,92] |
| Borosilicates (glass) | Hollow | Pulling pipettes | Good biocompatibility | Fractures | [75,93,94,95] |
| Sugars | Solid, Dissolving | Solvent casting or micromolding | Good biocompatibility | Mechanical properties are more difficult to achieve, stability problems, storage issues. | [96,97,98,99] |
| Polymers | Dissolving, Hydrogel-forming, Coated (coating) | Solvent casting or micromolding | Optimal biocompatibility, biodegradation, and absence of waste after use | Mechanical properties are more difficult to achieve | [69,100,101,102,103,104,105,106,107,108,109] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillot, A.J.; Cordeiro, A.S.; Donnelly, R.F.; Montesinos, M.C.; Garrigues, T.M.; Melero, A. Microneedle-Based Delivery: An Overview of Current Applications and Trends. Pharmaceutics 2020, 12, 569. https://doi.org/10.3390/pharmaceutics12060569
Guillot AJ, Cordeiro AS, Donnelly RF, Montesinos MC, Garrigues TM, Melero A. Microneedle-Based Delivery: An Overview of Current Applications and Trends. Pharmaceutics. 2020; 12(6):569. https://doi.org/10.3390/pharmaceutics12060569
Chicago/Turabian StyleGuillot, Antonio José, Ana Sara Cordeiro, Ryan F. Donnelly, M. Carmen Montesinos, Teresa M. Garrigues, and Ana Melero. 2020. "Microneedle-Based Delivery: An Overview of Current Applications and Trends" Pharmaceutics 12, no. 6: 569. https://doi.org/10.3390/pharmaceutics12060569
APA StyleGuillot, A. J., Cordeiro, A. S., Donnelly, R. F., Montesinos, M. C., Garrigues, T. M., & Melero, A. (2020). Microneedle-Based Delivery: An Overview of Current Applications and Trends. Pharmaceutics, 12(6), 569. https://doi.org/10.3390/pharmaceutics12060569









