Comparison between Colistin Sulfate Dry Powder and Solution for Pulmonary Delivery
Abstract
1. Introduction
2. Materials and Methods
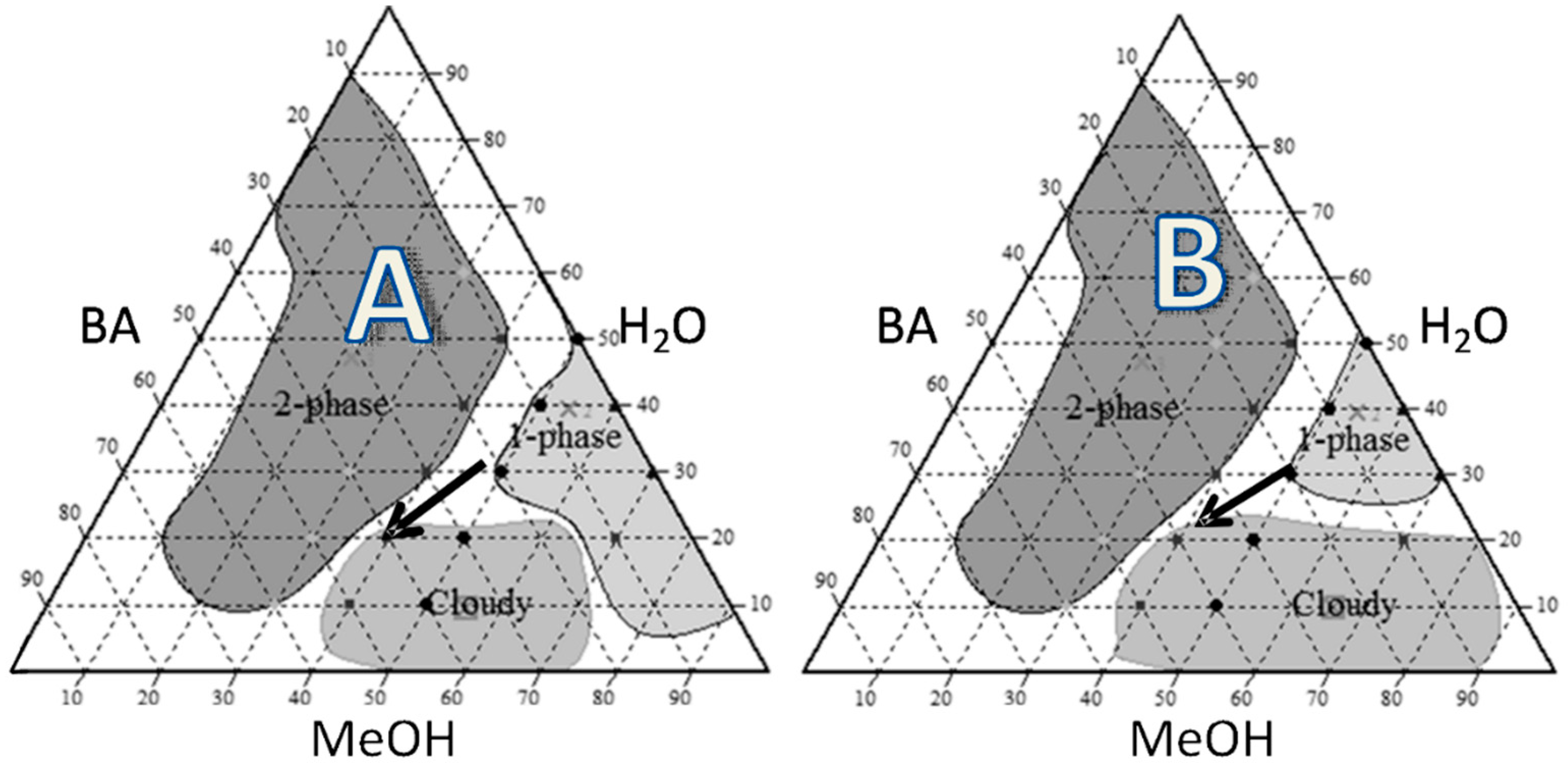
2.1. Pseudoternary Phase Diagram
2.2. Spray Drying
2.3. Characterization of the Particles
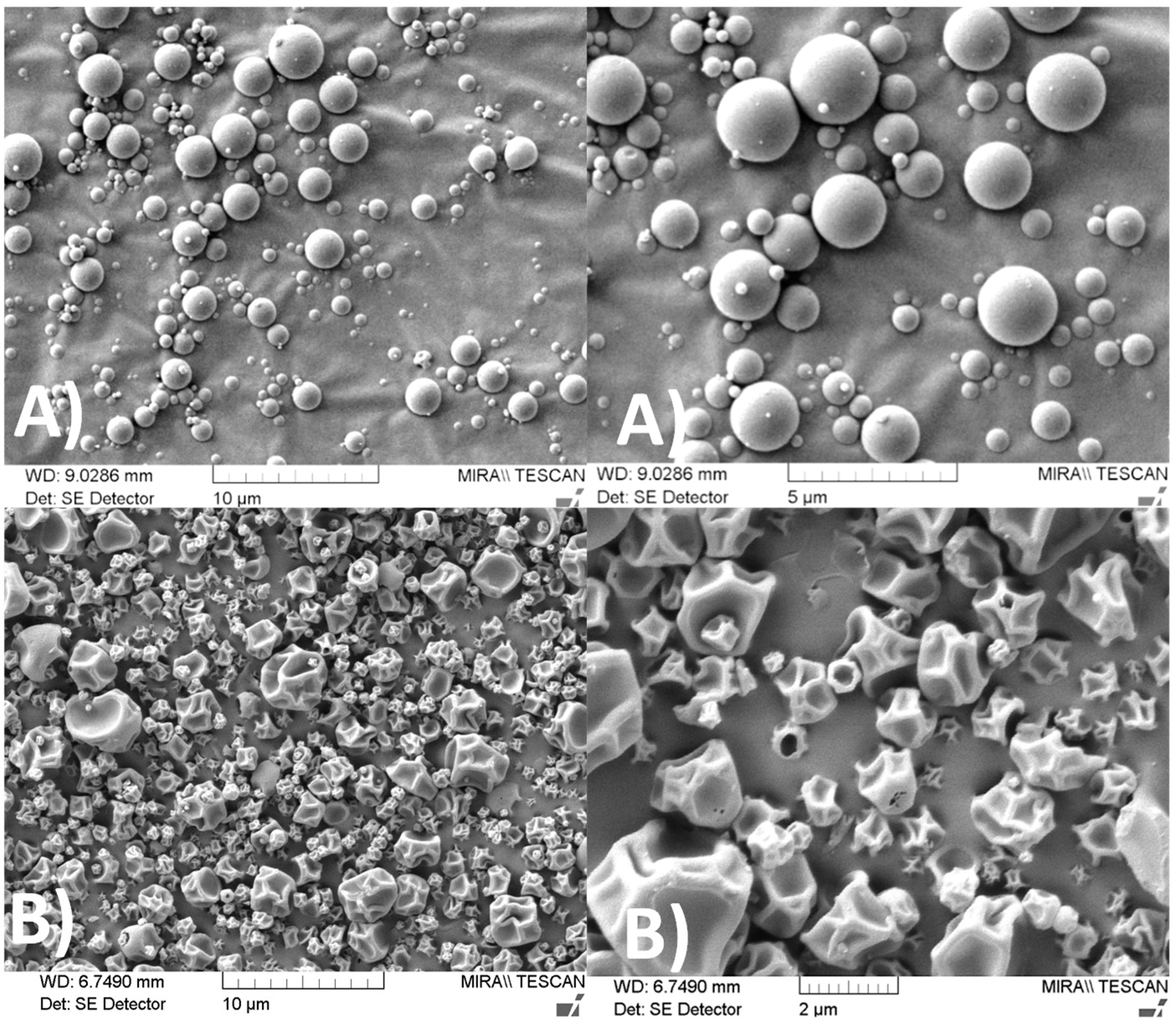
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. Particle Size Distribution Analysis
2.3.3. Aerodynamic Particle Diameter Analysis
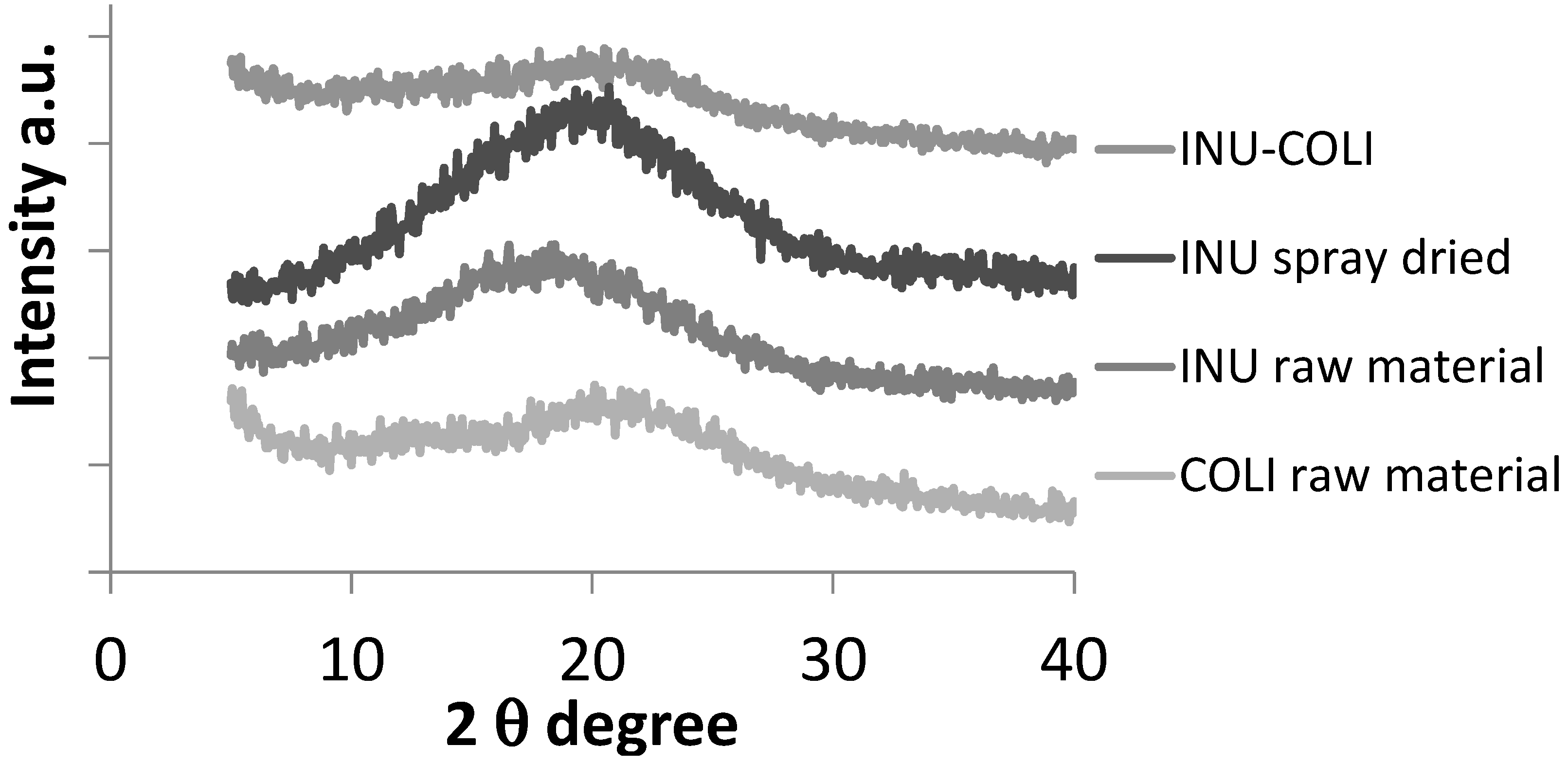
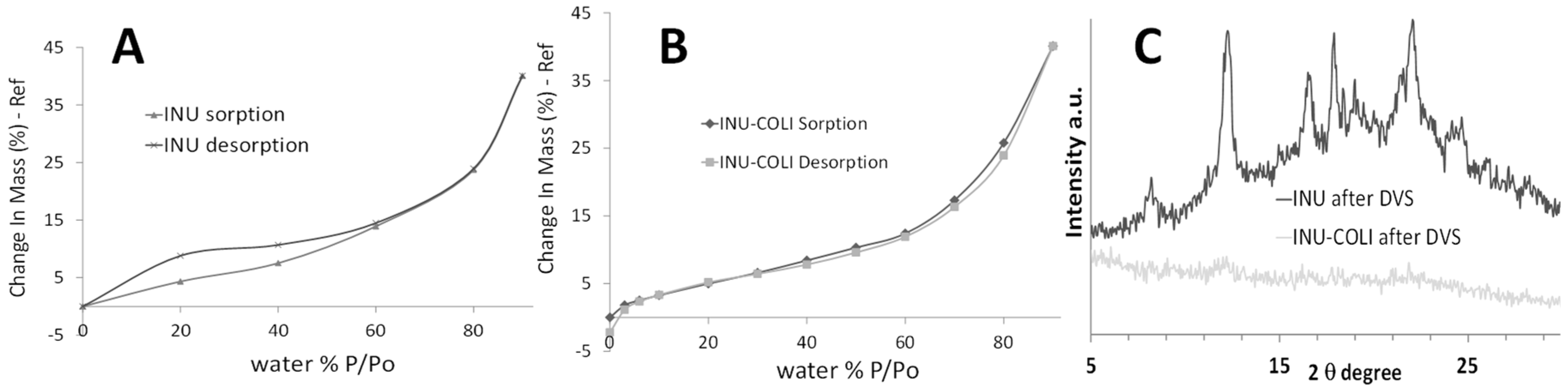
2.3.4. Powder X-Ray Diffraction (XRD)
2.4. Pharmacokinetics
2.4.1. Animals
2.4.2. Intratracheal Administration of COLI as Particles or Solution
2.4.3. Samples for the Plasma PK Study (n = 44)
2.4.4. Samples for Determination of Concentrations in ELF (n = 24)
2.4.5. Simultaneous PK Modeling of Plasma and ELF Concentrations of COLI
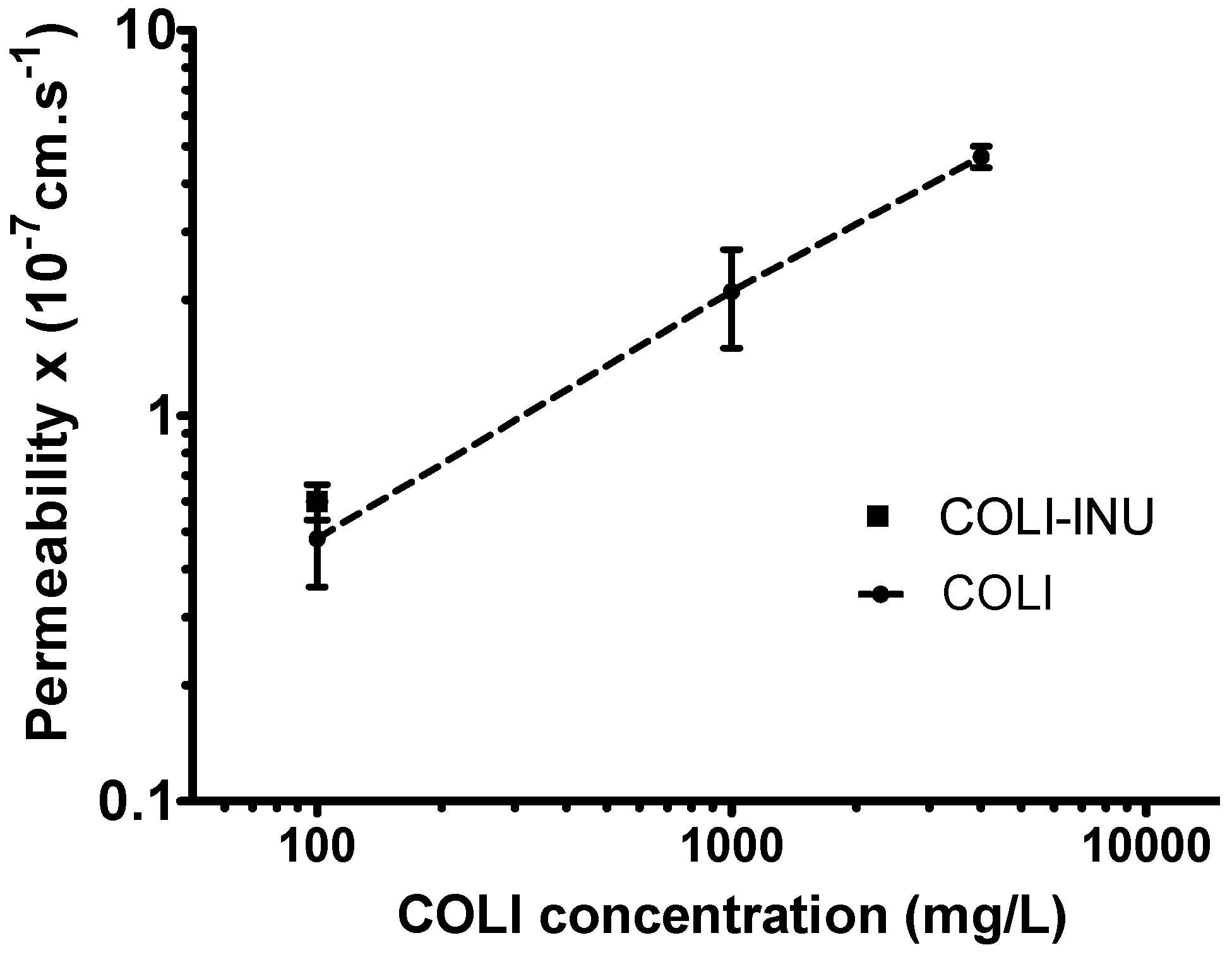
2.5. In-Vitro Transport Study
2.5.1. Calu-3 Cell Culture
2.5.2. Transport Study
2.6. Analytical Assays
2.6.1. HPLC Assay of Colistin
2.6.2. COLI Analysis in Plasma, BALF, and Calu-3 Transport Medium
2.6.3. Urea Analysis in BALF and Plasma
3. Results and Discussion
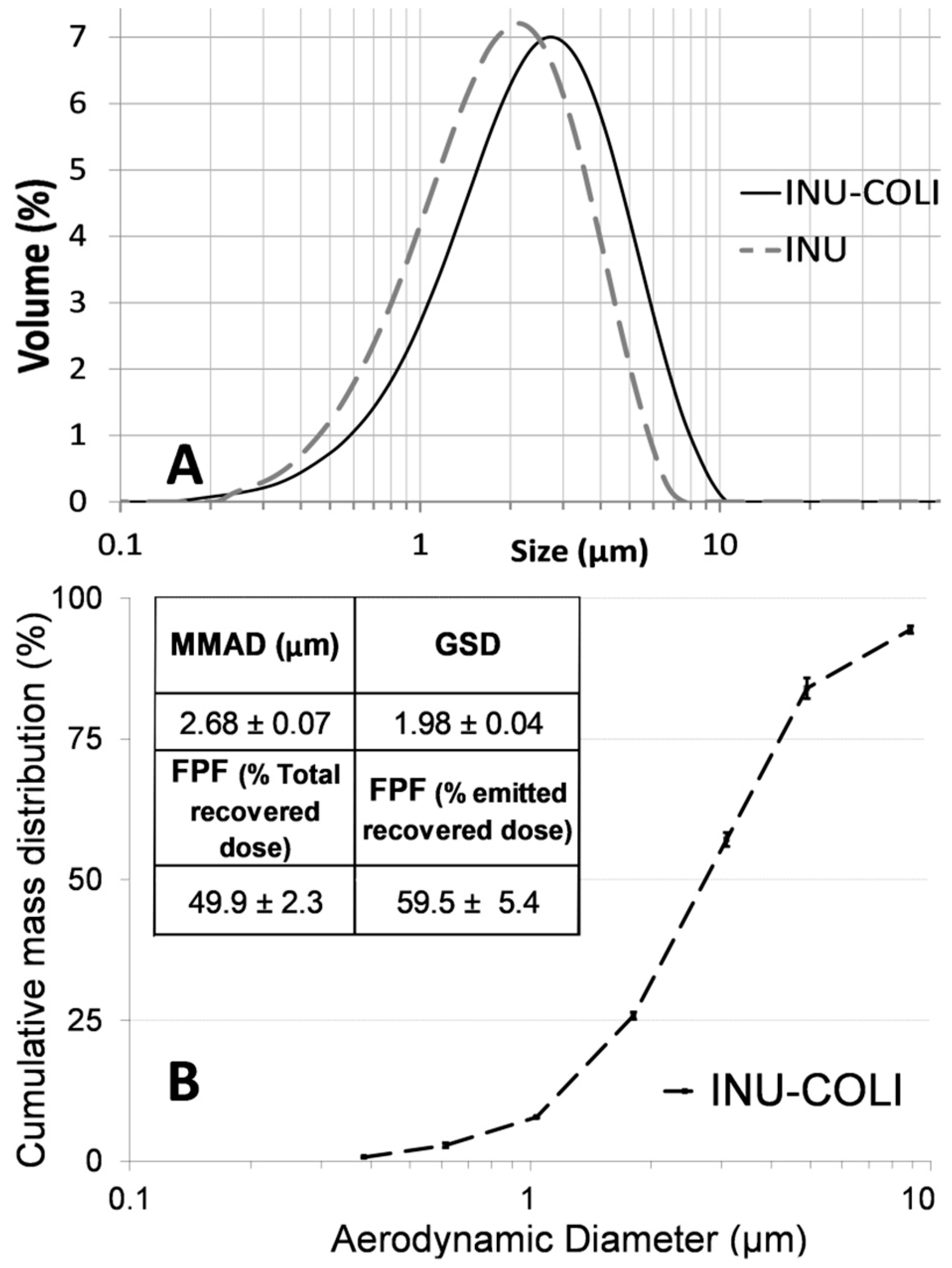
3.1. Microparticle Formulation and Characterization
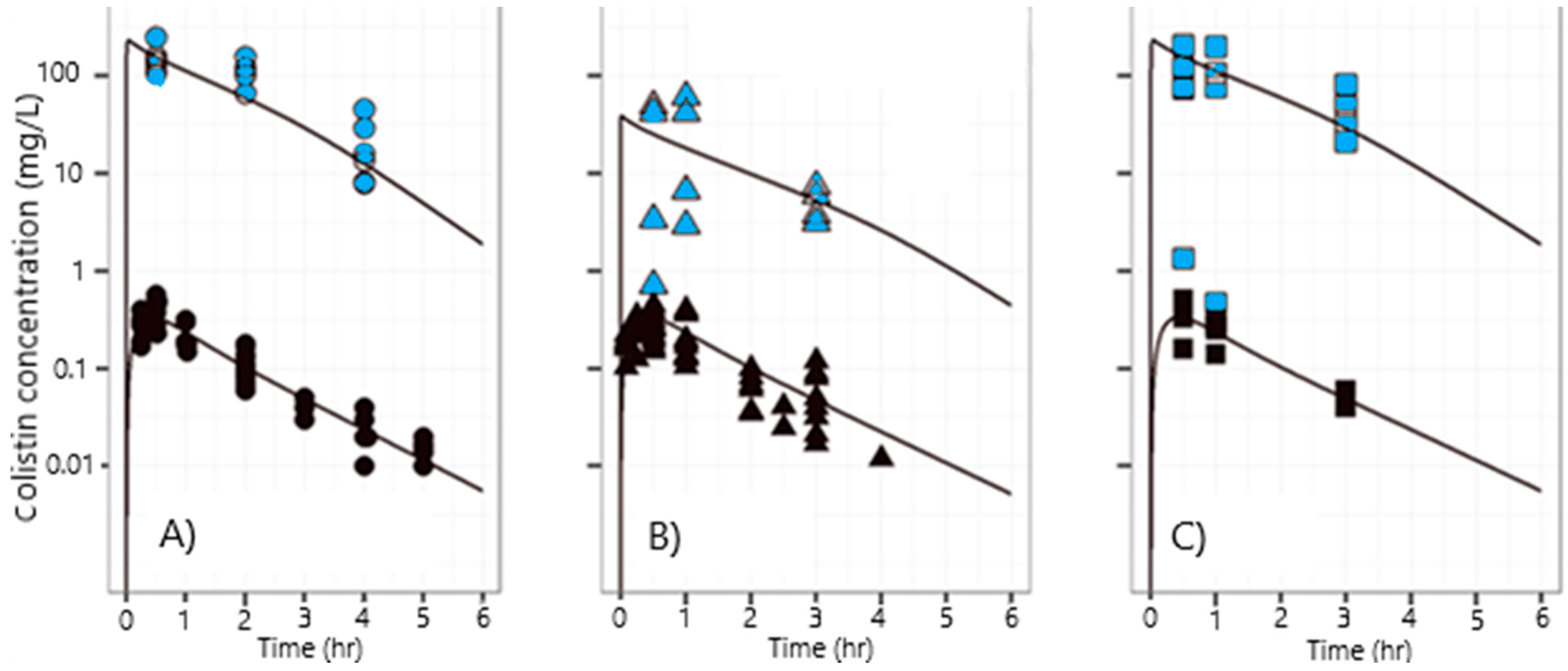
3.2. Pharmacokinetics
3.3. In-Vitro Transport Study on Calu-3 Broncho-Epithelial Cell Line
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
Appendix B
Appendix C
Appendix D
| Parameter Name | Description | Units | Typical Value (RSE) | Inter-Individual Variability (CV%) |
|---|---|---|---|---|
| Vc | Volume of distribution in the central compartment | (L·kg−1) | 0.22 (7%) | 25% (95%) |
| Vp | Volume of distribution of the peripheral compartment | (L·kg−1) | 0.18 (1%) | - |
| VELF | Volume of distribution in the ELF compartment | (L·kg−1) | 30 × 10−6 Fixed | - |
| Faero | Systemic bioavailability after nebulization/aerosol | (%) | 64 (6%) | 26% (29%) |
| CLT | Total systemic clearance | (L·h−1·kg−1) | 0.39 (5%) | 22% (40%) |
| Q | Clearance of distribution between central and peripheral compartments | (L·h−1·kg−1) | 0.32 (1%) | - |
| QELF | Diffusion clearance between central and ELF compartments | (L·h−1·kg−1) | 3.0 × 10−5 (1%) | - |
| ka | First-order transfer rate constant from depot compartment towards ELF compartment | (h−1) | 2.39 (1%) | - |
| Vmax,in | Maximal rate of transfer from ELF compartment towards central compartment | (mg·h−1·kg−1) | 0.98 (2%) | - |
| γ | The slope factor | 4.0 (1%) | - | |
| km for solutions made from raw COLI or INU-COLI powder | ELF concentration for which the rate of transfer from ELF compartment towards the central compartment is Vmax/2 | (µg·mL−1) | 236 (1%) | - |
| km for particles as dry powder | (µg·mL−1) | 38 (5%) | - | |
| Proportional error on plasma concentrations | (CV%) | 13 | ||
| Proportional error on ELF concentrations | (CV%) | 85 | ||
Appendix E
| Colistin µg/mL | Fluorescein Papp 10−7 cm/s |
|---|---|
| 100 | 0.62 ± 0.08 |
| 1000 | 0.63 ± 0.18 |
| 4000 | 1.73 ± 0.02 |
| Incubation Time in HBSS/HEPES (h) | Fluorescein Papp 10−7 cm/s |
|---|---|
| 1 | 0.54 ± 0.03 |
| 3 | 0.51 ± 0.04 |
| 6 | 0.64 ± 0.06 |
References
- Westerman, E.M.; Le Brun, P.P.H.; Touw, D.J.; Frijlink, H.W.; Heijerman, H.G.M. Effect of nebulized colistin sulphate and colistin sulphomethate on lung function in patients with cystic fibrosis: A pilot study. J. Cyst. Fibros. 2004, 3, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Bergen, P.J.; Li, J.; Rayner, C.R.; Nation, R.L. Colistin methanesulfonate is an inactive prodrug of colistin against pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2006, 50, 1953–1958. [Google Scholar] [CrossRef] [PubMed]
- Boisson, M.; Grégoire, N.; Cormier, M.; Gobin, P.; Marchand, S.; Couet, W.; Mimoz, O. Pharmacokinetics of nebulized colistin methanesulfonate in critically ill patients. J. Antimicrob. Chemother. 2017, 72, 2607–2612. [Google Scholar] [CrossRef]
- Boisson, M.; Jacobs, M.; Grégoire, N.; Gobin, P.; Marchand, S.; Couet, W.; Mimoz, O. Comparison of intrapulmonary and systemic pharmacokinetics of colistin methanesulfonate (cms) and colistin after aerosol delivery and intravenous administration of cms in critically ill patients. Antimicrob. Agents Chemother. 2014, 58, 7331–7339. [Google Scholar] [CrossRef]
- Couet, W.; Gregoire, N.; Marchand, S.; Mimoz, O. Colistin pharmacokinetics: The fog is lifting. Clin. Microbiol. Infect. 2012, 18, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Marchand, S.; Gobin, P.; Brillault, J.; Baptista, S.; Adier, C.; Olivier, J.-C.; Mimoz, O.; Couet, W. Aerosol therapy with colistin methanesulfonate: A biopharmaceutical issue illustrated in rats. Antimicrob. Agents Chemother. 2010, 54, 3702–3707. [Google Scholar] [CrossRef]
- Jong, T.; Li, J.; Morton, D.A.; Zhou, Q.T.; Larson, I. Investigation of the changes in aerosolization behavior between the jet-milled and spray-dried colistin powders through surface energy characterization. J. Pharm. Sci. 2016, 105, 1156–1163. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, Q.T.; Sun, S.-P.; Denman, J.A.; Gengenbach, T.R.; Barraud, N.; Rice, S.A.; Li, J.; Yang, M.; Chan, H.-K. Effects of surface composition on the aerosolisation and dissolution of inhaled antibiotic combination powders consisting of colistin and rifampicin. AAPS J. 2016, 18, 372–384. [Google Scholar] [CrossRef]
- Zhou, Q.T.; Morton, D.A.; Yu, H.H.; Jacob, J.; Wang, J.; Li, J.; Chan, H.K. Colistin powders with high aerosolisation efficiency for respiratory infection: Preparation and in vitro evaluation. J. Pharm. Sci. 2013, 102, 3736–3747. [Google Scholar] [CrossRef]
- Lin, Y.-W.; Zhou, Q.T.; Hu, Y.; Onufrak, N.J.; Sun, S.; Wang, J.; Forrest, A.; Chan, H.-K.; Li, J. Pulmonary pharmacokinetics of colistin following administration of dry powder aerosols in rats. Antimicrob. Agents Chemother. 2017, 61, 1–12. [Google Scholar] [CrossRef]
- Le Brun, P.P.H.; De Boer, A.H.; Mannes, G.P.M.; De Frature, D.M.I.; Brimicombe, R.W.; Touw, D.J.; Vinks, A.A.; Frijlink, H.W.; Heijerman, H.G.M. Dry powder inhalation of antibiotics in cystic fibrosis therapy: Part 2. Inhalation of a novel colistin dry powder formulation: A feasibility study in healthy volunteers and patients. Eur. J. Pharm. Biopharm. 2002, 54, 25–32. [Google Scholar] [CrossRef]
- Tiddens, H.A.; Bos, A.C.; Mouton, J.W.; Devadason, S.; Janssens, H.M. Inhaled antibiotics: Dry or wet? Eur. Respir. J. 2014, 44, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Tewes, F.; Gobbo, O.L.; Amaro, M.I.; Tajber, L.; Corrigan, O.I.; Ehrhardt, C.; Healy, A.M. Evaluation of HPΒCD–PEG microparticles for salmon calcitonin administration via pulmonary delivery. Mol. Pharm. 2011, 8, 1887–1898. [Google Scholar] [CrossRef] [PubMed]
- Tewes, F.; Ehrhardt, C.; Healy, A. Superparamagnetic iron oxide nanoparticles (SPIONS)-loaded trojan microparticles for targeted aerosol delivery to the lung. Eur. J. Pharm. Biopharm. 2013, 86, 98–104. [Google Scholar] [CrossRef]
- Amaro, M.I.; Tewes, F.; Gobbo, O.; Tajber, L.; Corrigan, O.I.; Ehrhardt, C.; Healy, A.M. Formulation, stability and pharmacokinetics of sugar-based salmon calcitonin-loaded nanoporous/nanoparticulate microparticles (NPMPS) for inhalation. Int. J. Pharm. 2015, 483, 6–18. [Google Scholar] [CrossRef]
- Tewes, F.; Tajber, L.; Corrigan, O.I.; Ehrhardt, C.; Healy, A.M. Development and characterisation of soluble polymeric particles for pulmonary peptide delivery. Eur. J. Pharm. Sci. 2010, 41, 337–352. [Google Scholar] [CrossRef]
- Gontijo, A.V.L.; Grégoire, N.; Lamarche, I.; Gobin, P.; Couet, W.; Marchand, S. Biopharmaceutical characterization of nebulized antimicrobial agents in rats: 2. Colistin. Antimicrob. Agents Chemother. 2014, 58, 3950–3956. [Google Scholar] [CrossRef]
- Marchand, S.; Lamarche, I.; Gobin, P.; Couet, W. Dose-ranging pharmacokinetics of colistin methanesulphonate (CMS) and colistin in rats following single intravenous cms doses. J. Antimicrob. Chemother. 2010, 65, 1753–1758. [Google Scholar] [CrossRef]
- Bulitta, J.B.; Bingölbali, A.; Shin, B.S.; Landersdorfer, C.B. Development of a new pre-and post-processing tool (sadapt-tran) for nonlinear mixed-effects modeling in s-adapt. AAPS J. 2011, 13, 201–211. [Google Scholar] [CrossRef]
- Li, J.; Milne, R.W.; Nation, R.L.; Turnidge, J.D.; Smeaton, T.C.; Coulthard, K. Use of high-performance liquid chromatography to study the pharmacokinetics of colistin sulfate in rats following intravenous administration. Antimicrob. Agents Chemother. 2003, 47, 1766–1770. [Google Scholar] [CrossRef]
- Beal, S.L. Ways to fit a pk model with some data below the quantification limit. J. Pharm. Pharm. 2001, 28, 481–504. [Google Scholar]
- Brillault, J.; De Castro, W.V.; Harnois, T.; Kitzis, A.; Olivier, J.-C.; Couet, W. P-glycoprotein-mediated transport of moxifloxacin in a calu-3 lung epithelial cell model. Antimicrob. Agents Chemother. 2009, 53, 1457–1462. [Google Scholar] [CrossRef] [PubMed]
- Tewes, F.; Brillault, J.; Couet, W.; Olivier, J.-C. Formulation of rifampicin–cyclodextrin complexes for lung nebulization. J. Control. Release 2008, 129, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Gobin, P.; Lemaître, F.; Marchand, S.; Couet, W.; Olivier, J.C. Assay of colistin and colistin methanesulfonate in plasma and urine by liquid chromatography-tandem mass spectrometry. Antimicrob. Agents Chemother. 2010, 54, 1941–1948. [Google Scholar] [CrossRef]
- Van Drooge, D.; Hinrichs, W.; Wegman, K.; Visser, M.; Eissens, A.; Frijlink, H. Solid dispersions based on inulin for the stabilisation and formulation of δ9-tetrahydrocannabinol. Eur. J. Pharm. Sci. 2004, 21, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, G.S.; Ponsioen, B.J.; Hummel, S.A.; Sanders, N.; Hinrichs, W.L.; de Boer, A.H.; Frijlink, H.W. Formulation and process development of (recombinant human) deoxyribonuclease i as a powder for inhalation. Pharm. Dev. Technol. 2009, 14, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Van Drooge, D.; Hinrichs, W.; Frijlink, H. Incorporation of lipophilic drugs in sugar glasses by lyophilization using a mixture of water and tertiary butyl alcohol as solvent. J. Pharm. Sci. 2004, 93, 713–725. [Google Scholar] [CrossRef]
- Tewes, F.; Gobbo, O.L.; Ehrhardt, C.; Healy, A.M. Amorphous calcium carbonate based-microparticles for peptide pulmonary delivery. ACS Appl. Mater. Interfaces 2016, 8, 1164–1175. [Google Scholar] [CrossRef]
- Healy, A.M.; Amaro, M.I.; Paluch, K.J.; Tajber, L. Dry powders for oral inhalation free of lactose carrier particles. Adv. Drug Deliv. Rev. 2014, 75, 32–52. [Google Scholar] [CrossRef]
- Carvalho, T.C.; Peters, J.I.; Williams, R.O., III. Influence of particle size on regional lung deposition–what evidence is there? Int. J. Pharm. 2011, 406, 1–10. [Google Scholar] [CrossRef]
- Stockmann, C.; Roberts, J.K.; Yellepeddi, V.K.; Sherwin, C.M. Clinical pharmacokinetics of inhaled antimicrobials. Clin. Pharmacokinet. 2015, 54, 473–492. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.R.; Lewis, S.A. Colistin interactions with the mammalian urothelium. Am. J. Physiol. Cell Physiol. 2004, 286, C913–C922. [Google Scholar] [CrossRef] [PubMed]
- Wallace, S.J.; Nation, R.L.; Li, J.; Boyd, B.J. Physicochemical aspects of the coformulation of colistin and azithromycin using liposomes for combination antibiotic therapies. J. Pharm. Sci. 2013, 102, 1578–1587. [Google Scholar] [CrossRef]
- Mestres, C.; Alsina, M.; Busquets, M.; Muranyi, I.; Reig, F. Interaction of colistin with lipids in liposomes and monolayers. Int. J. Pharm. 1998, 160, 99–107. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kondo, S.; Juni, K. Pulmonary delivery of salmon calcitonin dry powders containing absorption enhancers in rats. Pharm. Res. 1996, 13, 80–83. [Google Scholar] [CrossRef]
- Hussain, A.; Majumder, Q.H.; Ahsan, F. Inhaled insulin is better absorbed when administered as a dry powder compared to solution in the presence or absence of alkylglycosides. Pharm. Res. 2006, 23, 138–147. [Google Scholar] [CrossRef]
- Patton, J.S.; Brain, J.D.; Davies, L.A.; Fiegel, J.; Gumbleton, M.; Kim, K.-J.; Sakagami, M.; Vanbever, R.; Ehrhardt, C. The particle has landed—characterizing the fate of inhaled pharmaceuticals. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, S-71–S-87. [Google Scholar] [CrossRef]
- Maillet, A.; Guilleminault, L.; Lemarié, E.; Lerondel, S.; Azzopardi, N.; Montharu, J.; Congy-Jolivet, N.; Reverdiau, P.; Legrain, B.; Parent, C. The airways, a novel route for delivering monoclonal antibodies to treat lung tumors. Pharm. Res. 2011, 28, 2147–2156. [Google Scholar] [CrossRef]
- Gagnadoux, F.; Le Pape, A.; Urban, T.; Montharu, J.; Vecellio, L.; Dubus, J.-C.; Leblond, V.; Diot, P.; Grimbert, D.; Racineux, J.-L. Safety of pulmonary administration of gemcitabine in rats. J. Aerosol Med. 2005, 18, 198–206. [Google Scholar] [CrossRef]
- Cheah, S.-E.; Wang, J.; Nguyen, V.T.T.; Turnidge, J.D.; Li, J.; Nation, R.L. New pharmacokinetic/pharmacodynamic studies of systemically administered colistin against pseudomonas aeruginosa and acinetobacter baumannii in mouse thigh and lung infection models: Smaller response in lung infection. J. Antimicrob. Chemother. 2015, 70, 3291–3297. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tewes, F.; Brillault, J.; Gregoire, N.; Olivier, J.-C.; Lamarche, I.; Adier, C.; Healy, A.-M.; Marchand, S. Comparison between Colistin Sulfate Dry Powder and Solution for Pulmonary Delivery. Pharmaceutics 2020, 12, 557. https://doi.org/10.3390/pharmaceutics12060557
Tewes F, Brillault J, Gregoire N, Olivier J-C, Lamarche I, Adier C, Healy A-M, Marchand S. Comparison between Colistin Sulfate Dry Powder and Solution for Pulmonary Delivery. Pharmaceutics. 2020; 12(6):557. https://doi.org/10.3390/pharmaceutics12060557
Chicago/Turabian StyleTewes, Frédéric, Julien Brillault, Nicolas Gregoire, Jean-Christophe Olivier, Isabelle Lamarche, Christophe Adier, Anne-Marie Healy, and Sandrine Marchand. 2020. "Comparison between Colistin Sulfate Dry Powder and Solution for Pulmonary Delivery" Pharmaceutics 12, no. 6: 557. https://doi.org/10.3390/pharmaceutics12060557
APA StyleTewes, F., Brillault, J., Gregoire, N., Olivier, J.-C., Lamarche, I., Adier, C., Healy, A.-M., & Marchand, S. (2020). Comparison between Colistin Sulfate Dry Powder and Solution for Pulmonary Delivery. Pharmaceutics, 12(6), 557. https://doi.org/10.3390/pharmaceutics12060557






