Fabrication of Transgelosomes for Enhancing the Ocular Delivery of Acetazolamide: Statistical Optimization, In Vitro Characterization, and In Vivo Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preliminary Screening Studies
2.2.2. Preparation of ACZ-Loaded TNVs
2.2.3. Experimental Design of ACZ-Loaded TNVs Using 32 Factorial Design
2.2.4. Determination of EE% of ACZ-Loaded TNVs
2.2.5. In Vitro Release Study of ACZ-Loaded TNVs
2.2.6. Statistical Optimization of ACZ-Loaded TNVs
2.2.7. Characterization of the Optimized ACZ-Loaded TNVs
Scanning Electron Microscopy (SEM)
Zeta Potential Determination
Measurement of Vesicle Elasticity
Fourier Transform Infrared Spectroscopy (FT-IR)
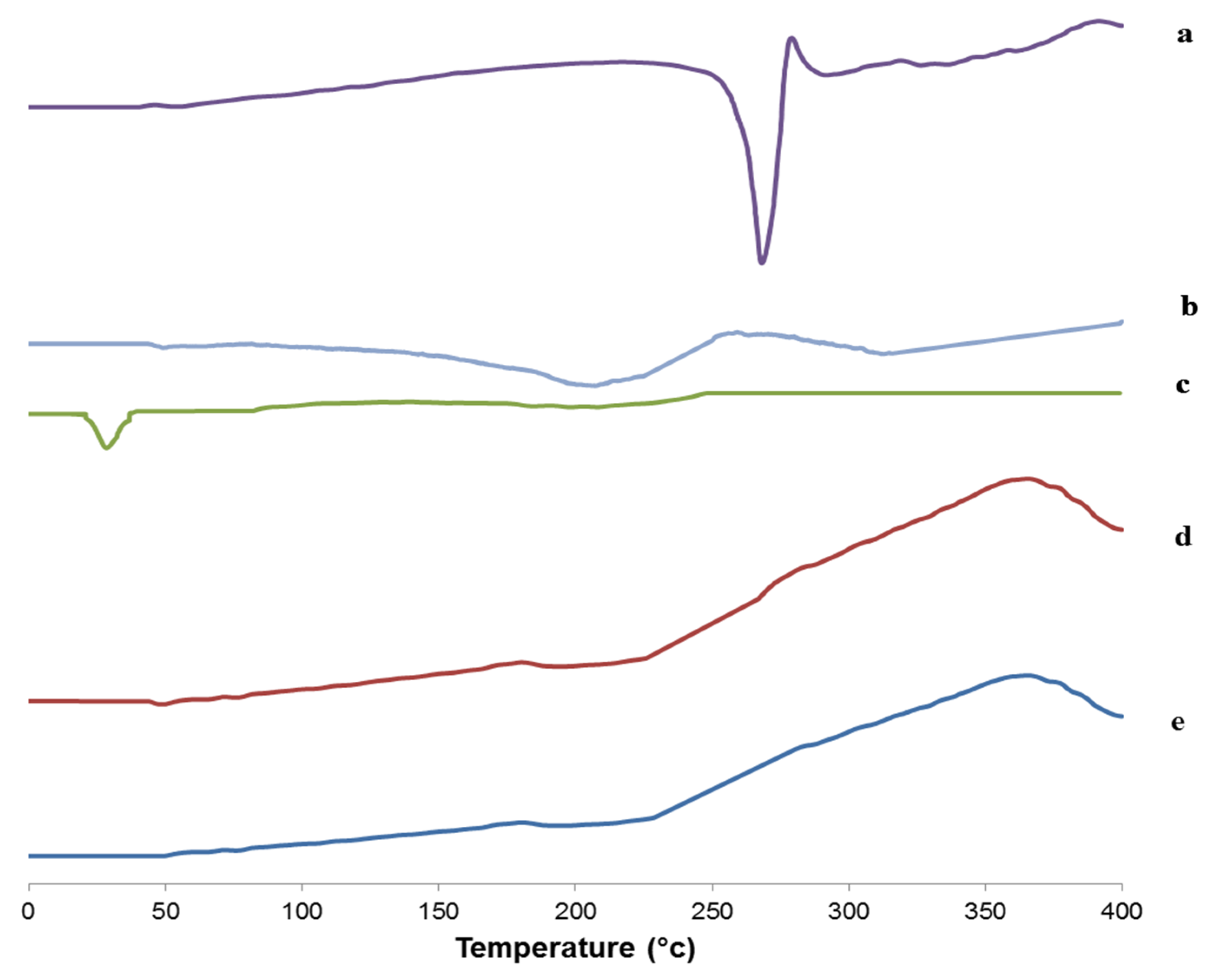
Differential Scanning Calorimetry (DSC)
Effect of Storage on the Stability of the Optimized ACZ-Loaded TNVs
2.2.8. Formulation of the ACZ-Loaded TGS
2.2.9. Evaluation of the ACZ-Loaded TGS
Homogeneity
Spreadability
pH
Drug Content
Gel Strength
Measurement of Gelation Temperature (GT)
Determination of Gelation Time and Gel Residence Time
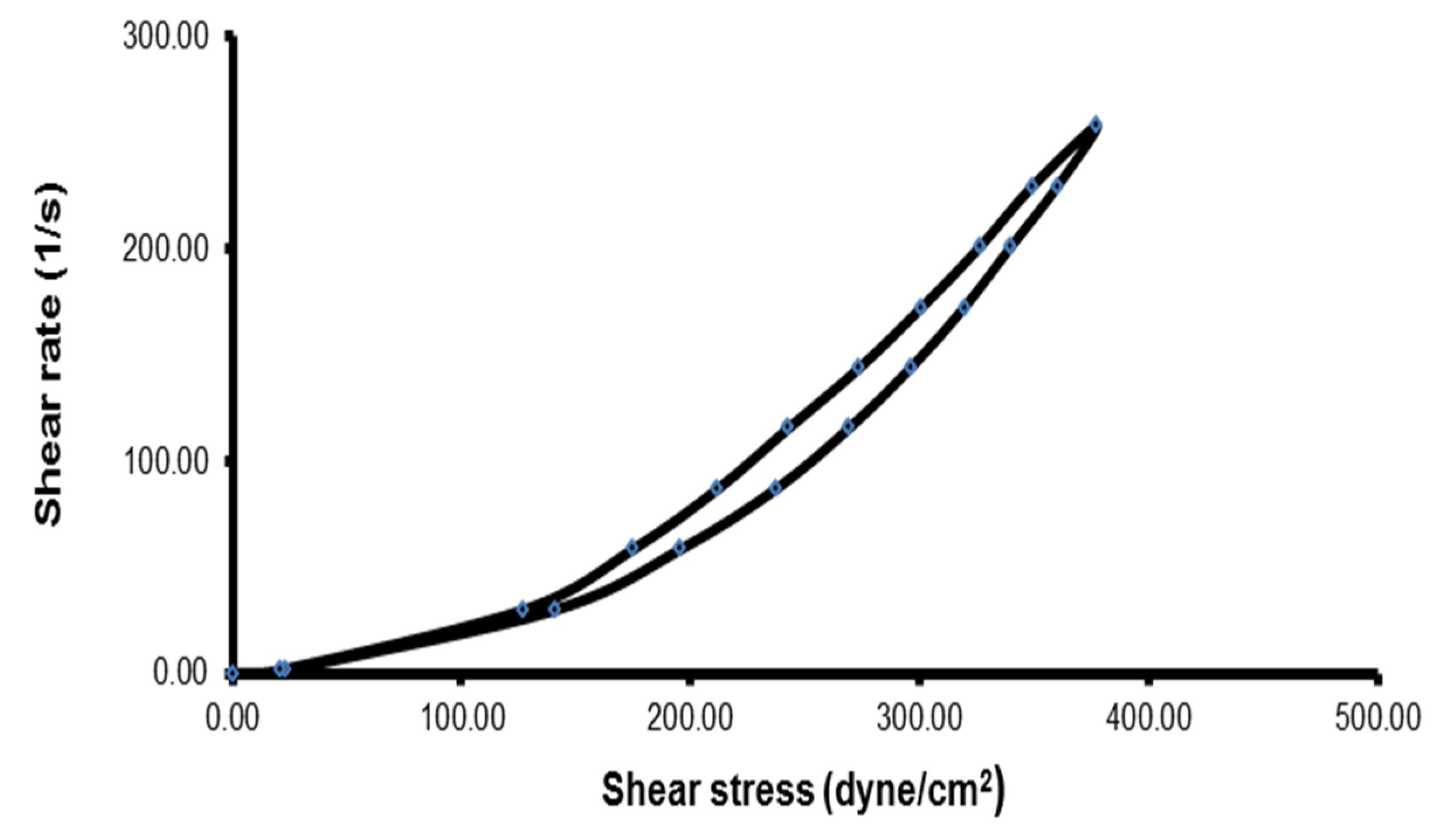
Determination of the Rheological Properties
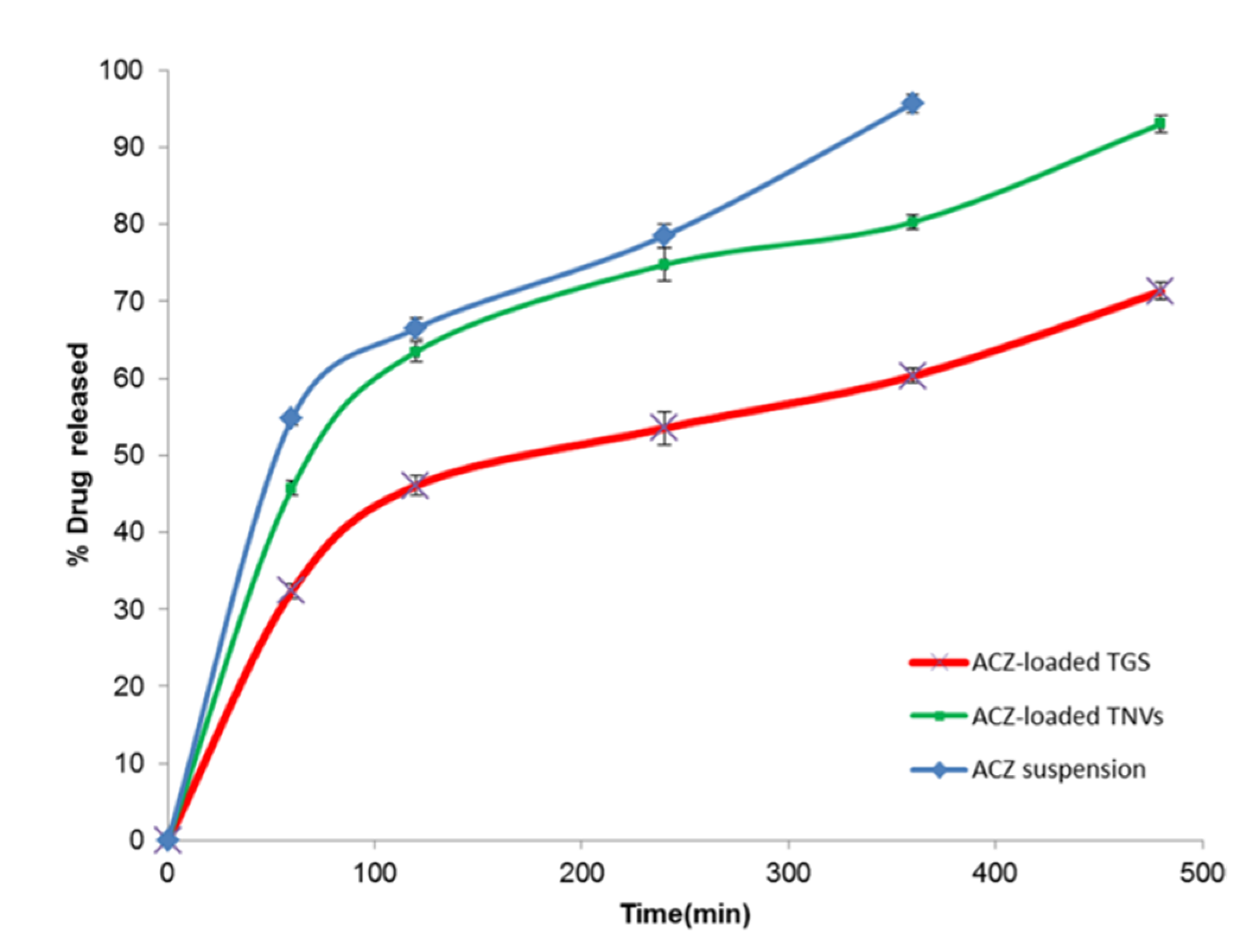
2.2.10. In Vitro Release Study of ACZ-Loaded TGS
2.2.11. Ex Vivo Corneal Permeation Study of ACZ-Loaded TGS
2.2.12. In Vivo Study of the ACZ-Loaded TGS
Assessment of Ocular Irritancy
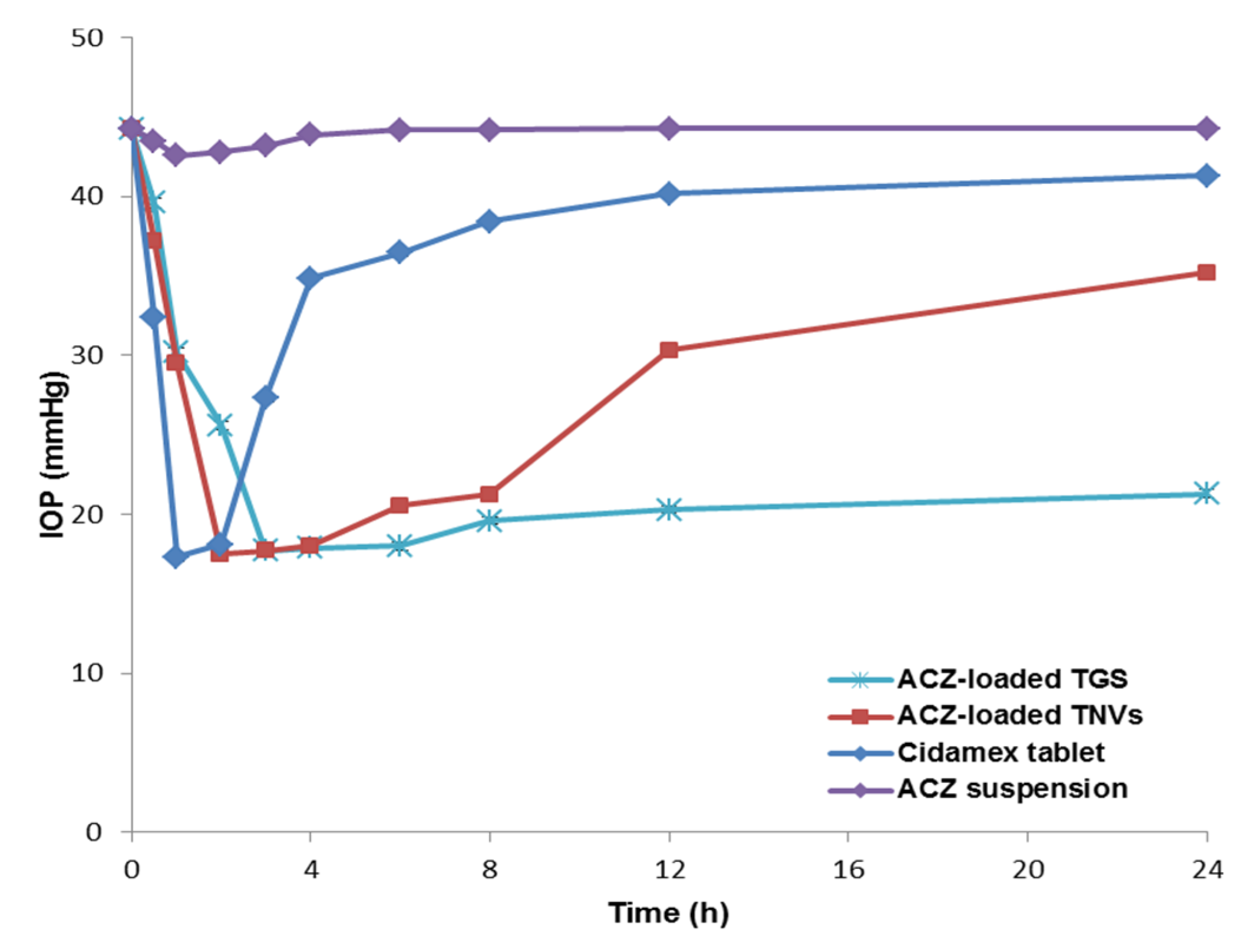
Pharmacodynamic Study
Pharmacokinetic Studies in Aqueous Humor
2.2.13. Statistical Analysis
3. Results
3.1. Preliminary Screening Studies
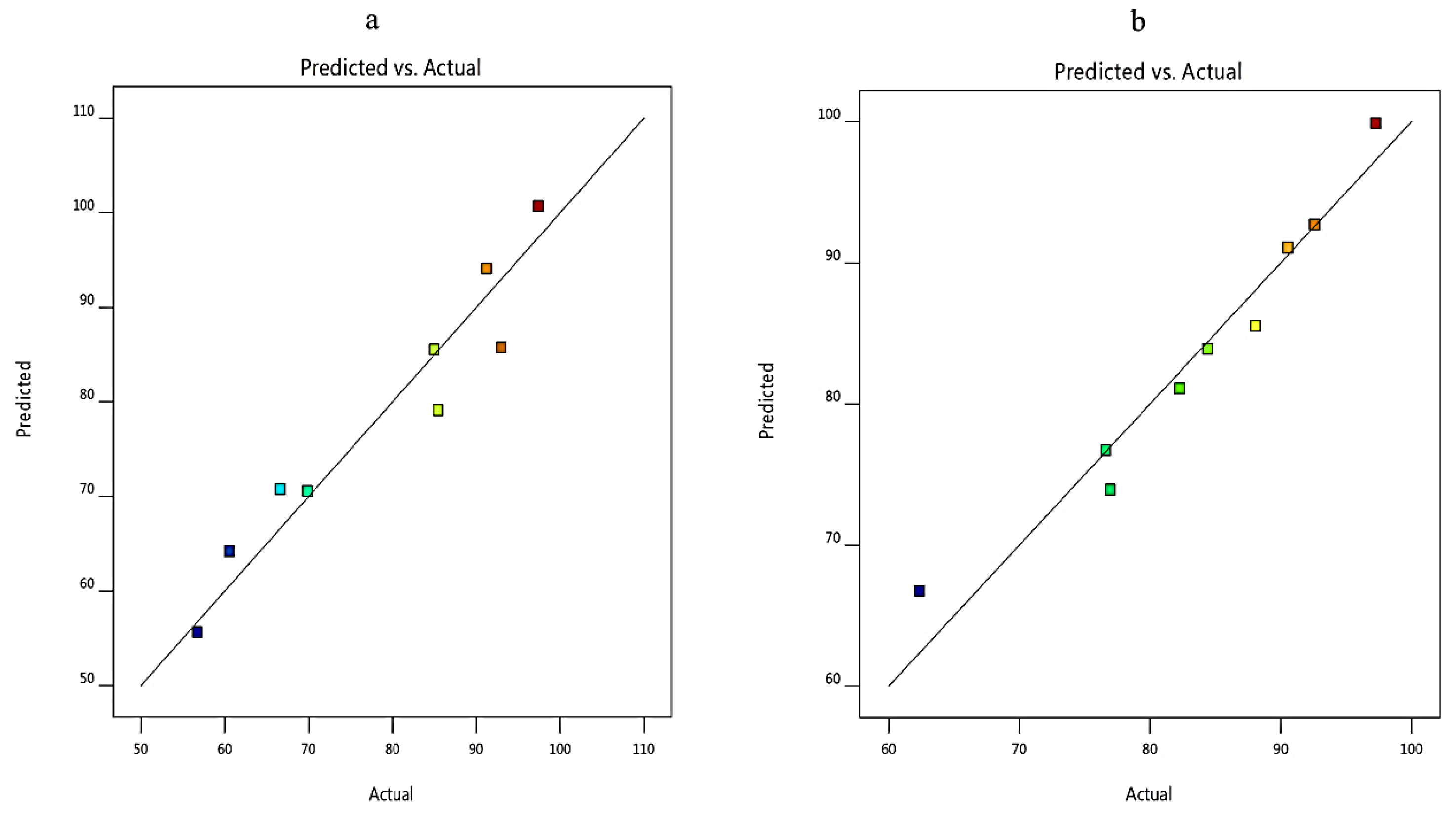
3.2. Analysis of Factorial Design
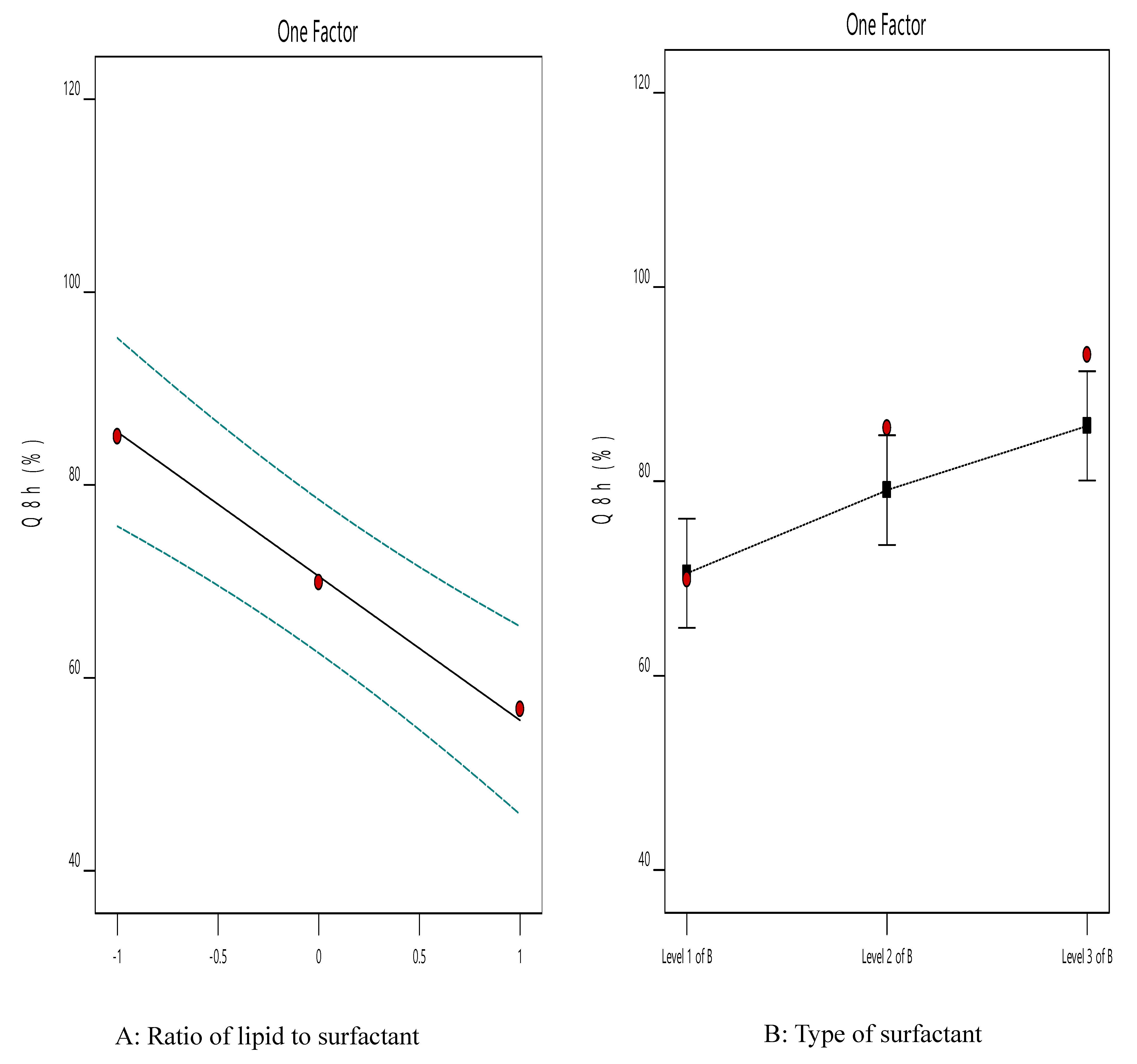
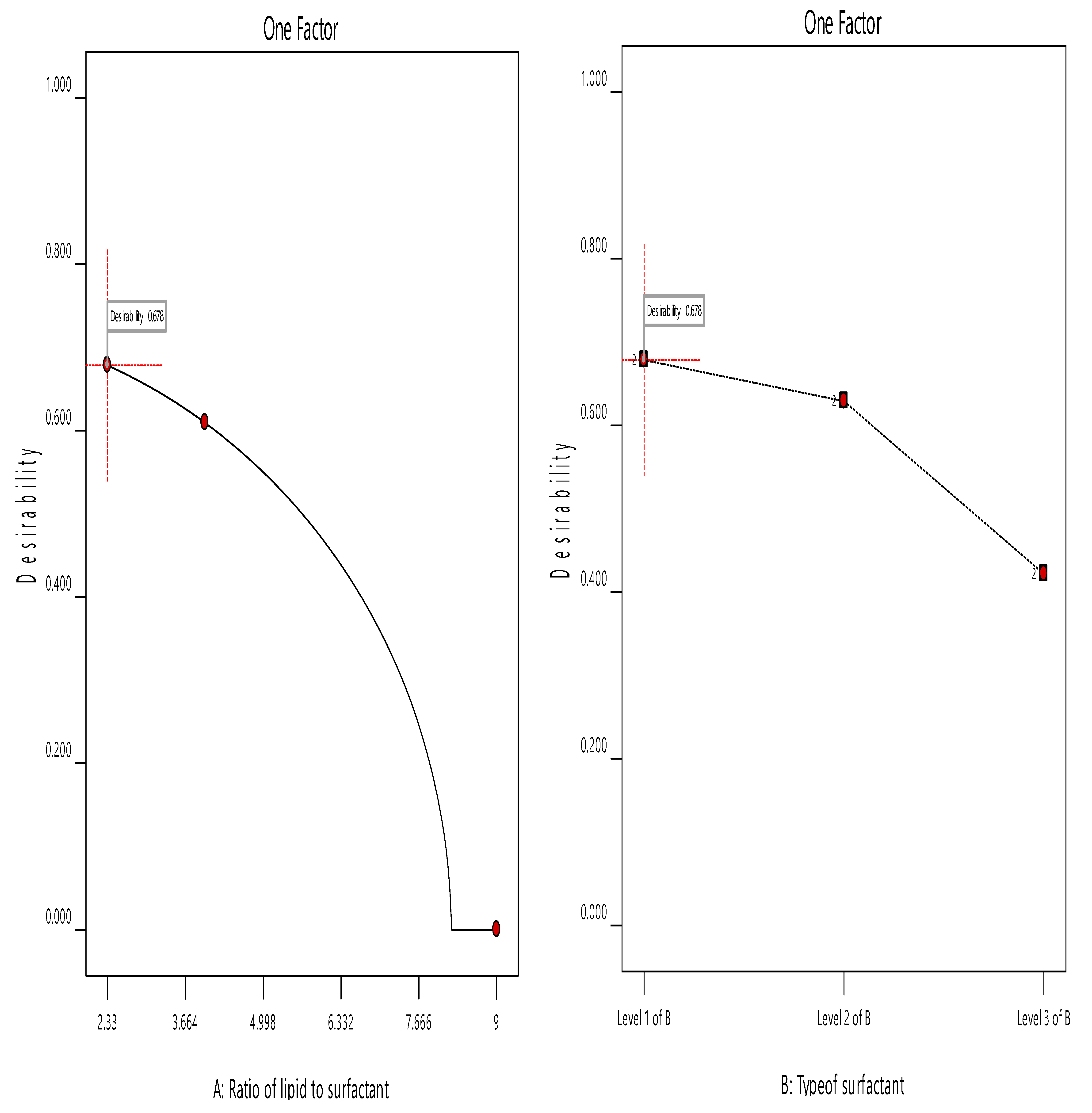
3.3. The Effect of Formulation Variables on Q8h of ACZ-Loaded TNVs
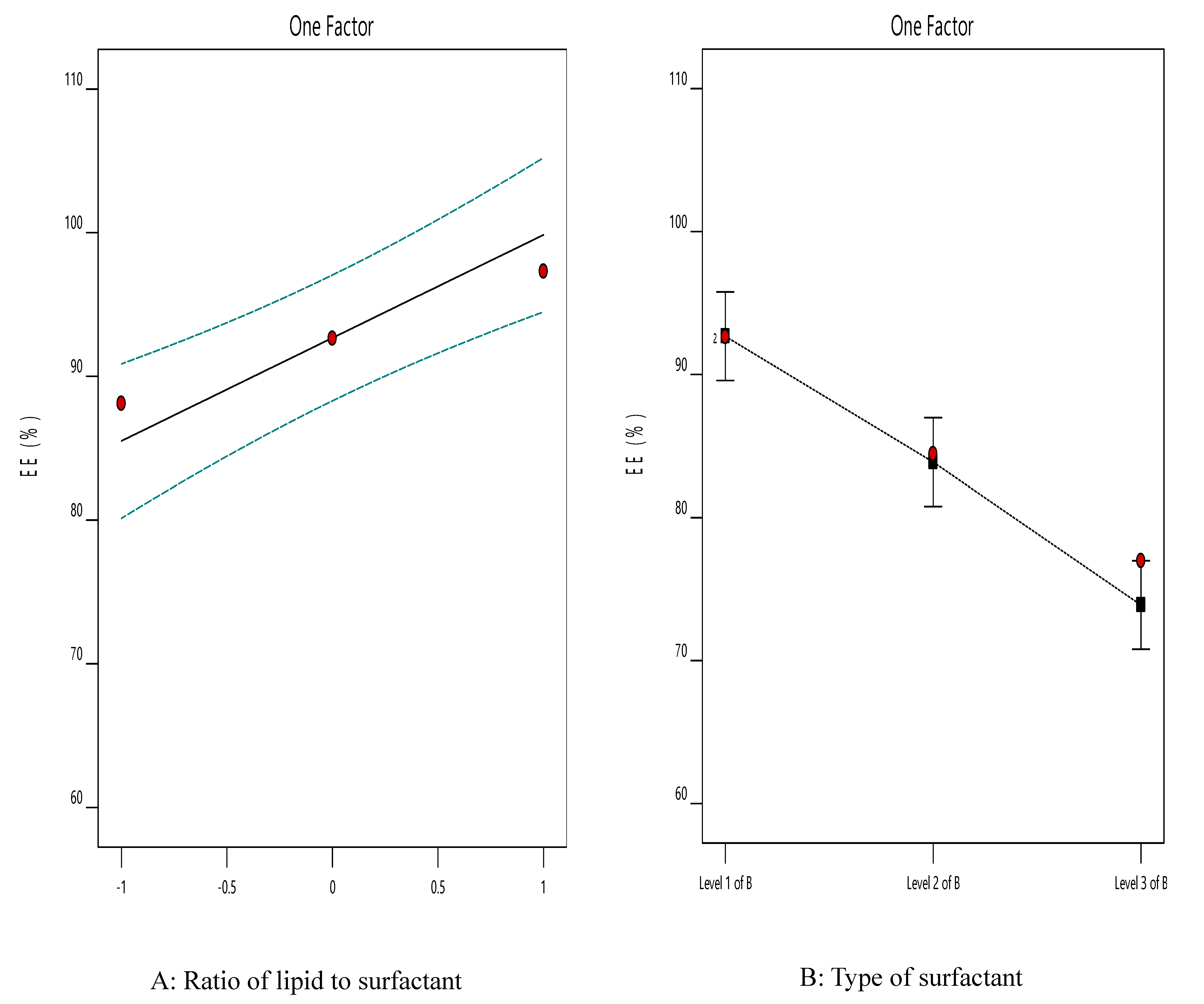
3.4. The Effect of Formulation Variables on EE% of ACZ-Loaded TNVs
3.5. Optimization of the ACZ-Loaded TNVs
3.6. Characterization of the Optimized ACZ-Loaded TNVs
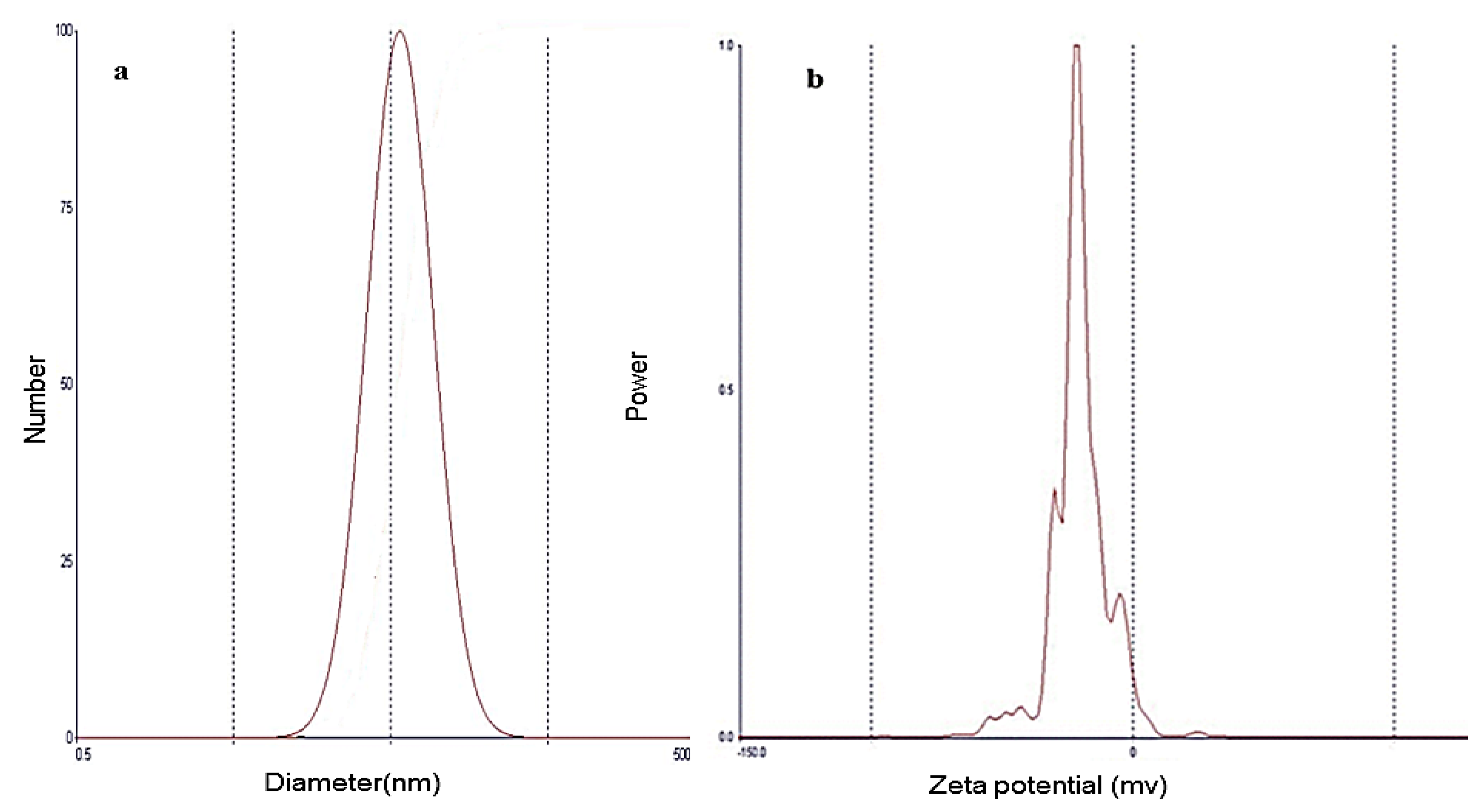
3.6.1. Vesicle Size and Zeta Potential Determination
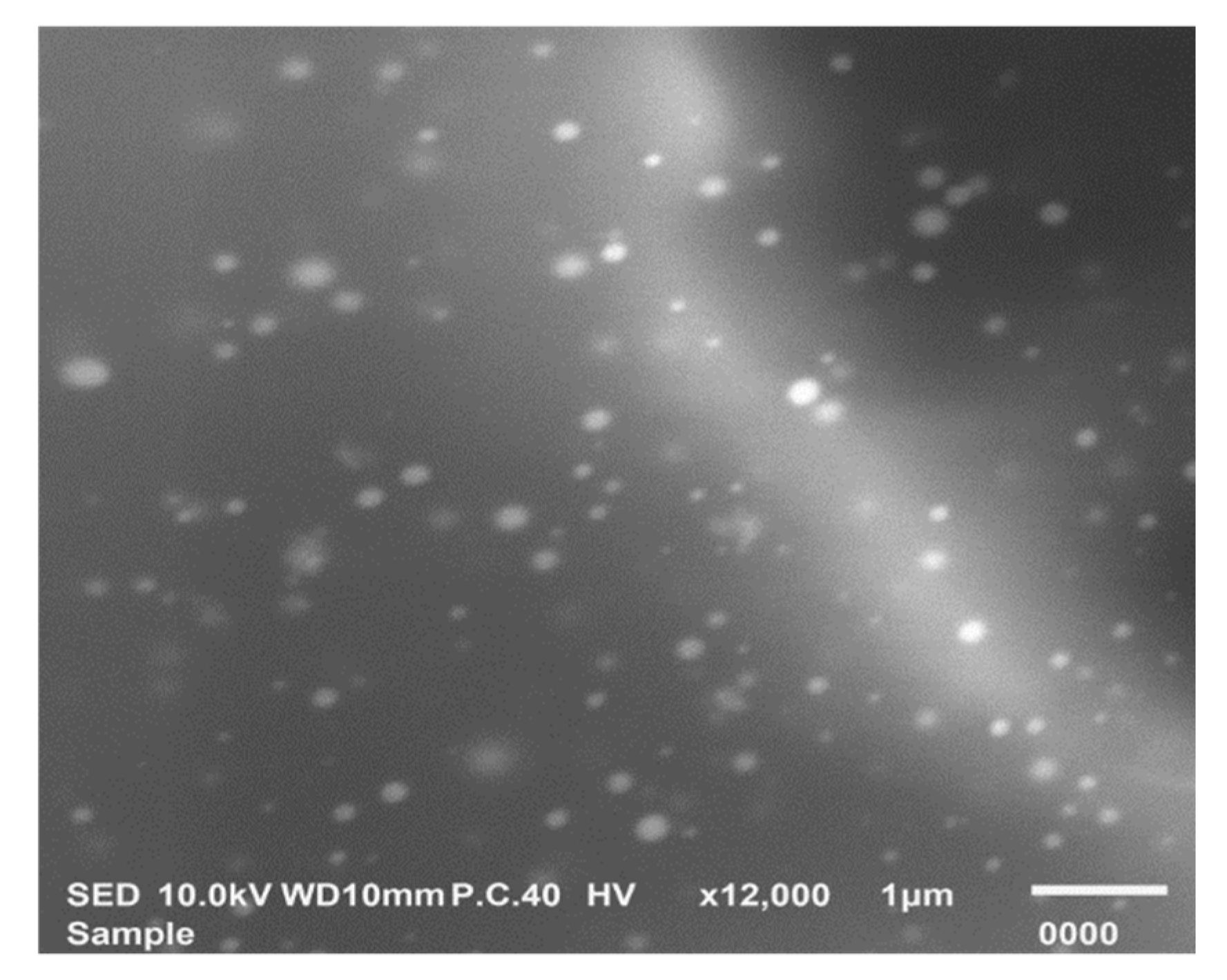
3.6.2. Morphological Characterization by SEM
3.6.3. Measurement of Vesicle Elasticity
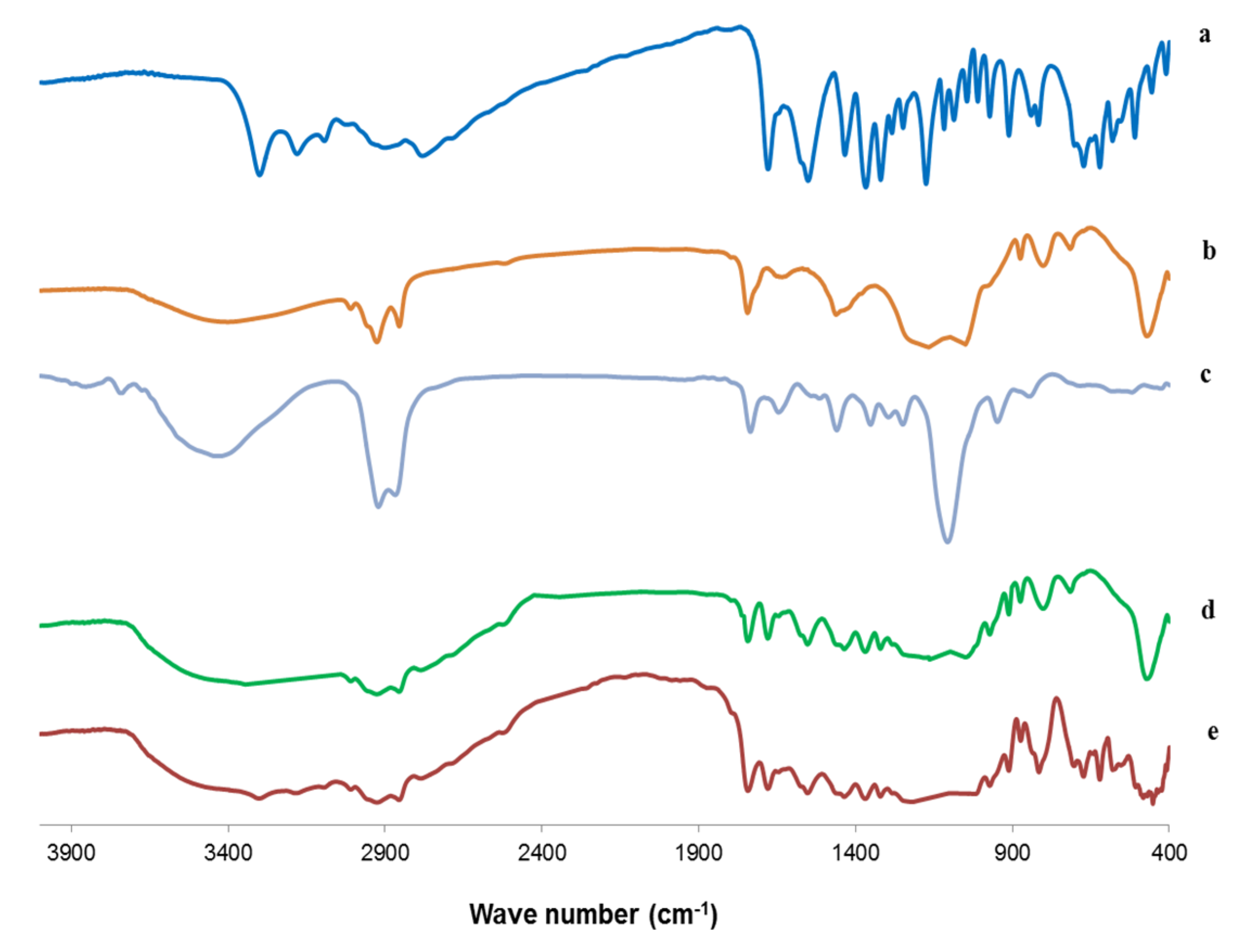
3.6.4. Fourier Transform Infrared (FTIR) Spectroscopy
3.6.5. Differential Scanning Calorimetry (DSC) Study
3.6.6. Effect of Storage on the Stability of the Optimized ACZ-Loaded TNVs
3.7. Formulation of ACZ-Loaded TGS
3.8. Evaluation of ACZ-Loaded TGS
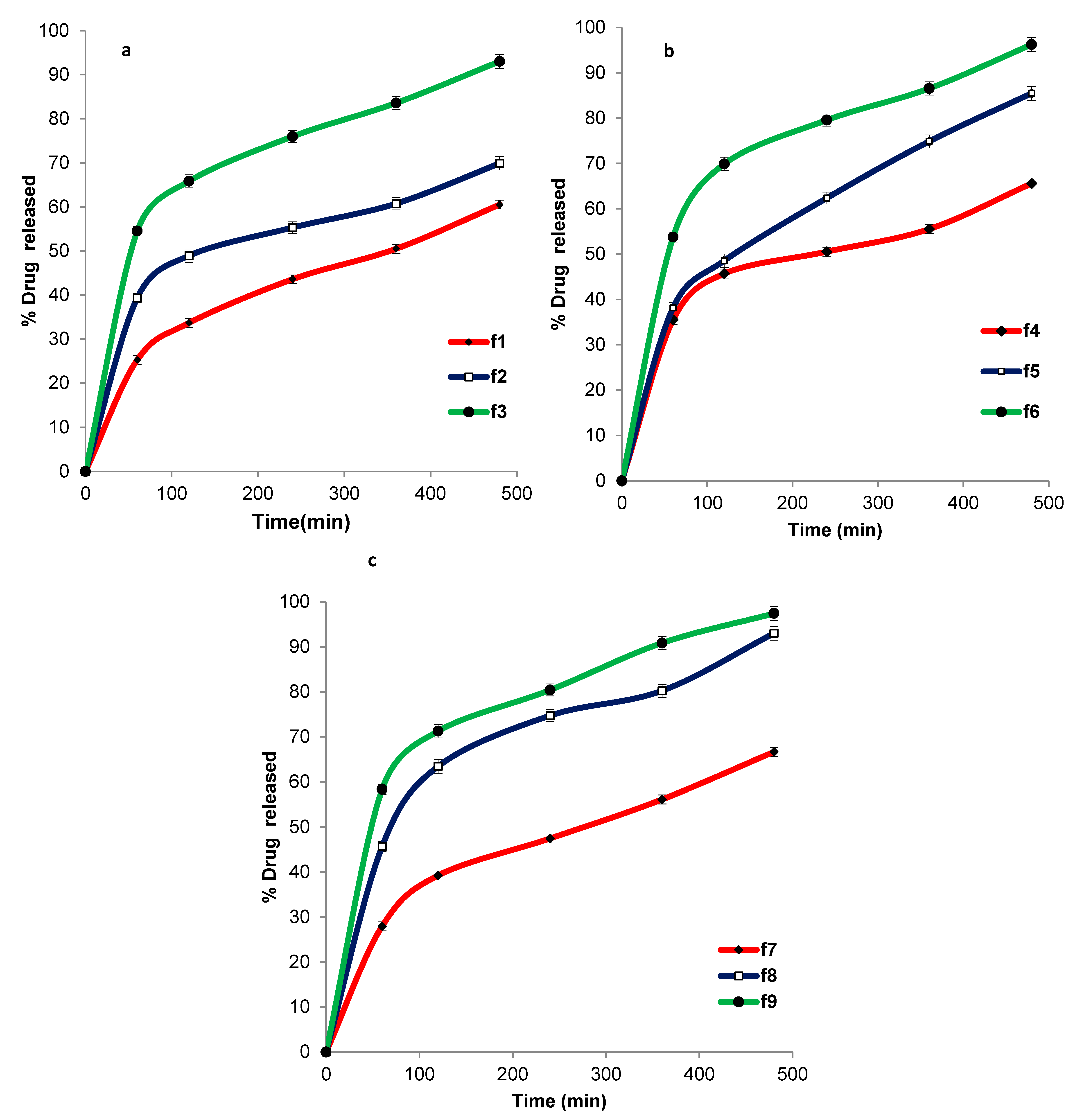
3.9. In Vitro Release Study of ACZ-Loaded TGS
3.10. Ex Vivo Corneal Permeation Study of ACZ-Loaded TGS
3.11. In Vivo Study for ACZ ACZ-Loaded TGS
3.11.1. Ocular Irritancy Test
3.11.2. Pharmacodynamic Study
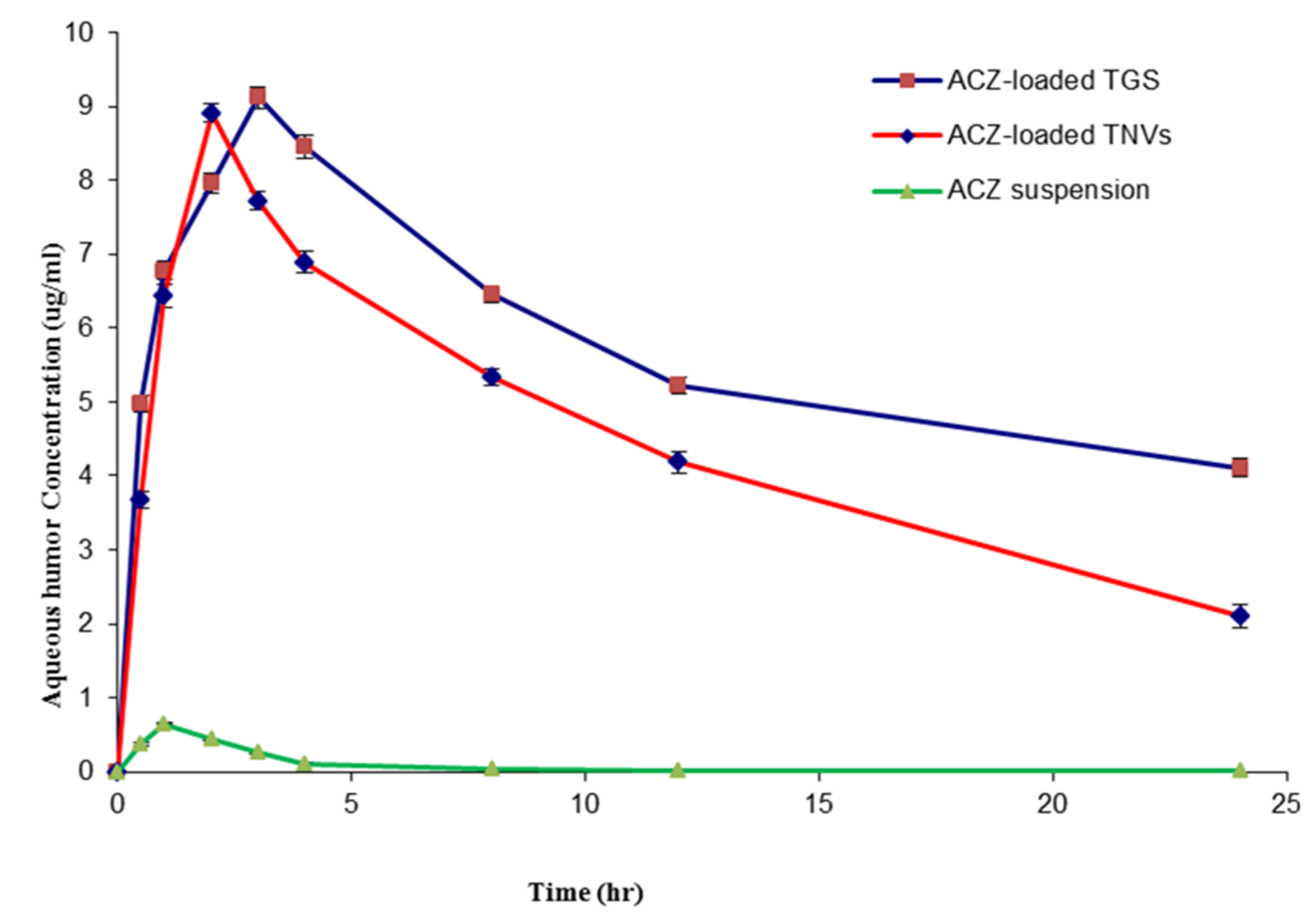
3.11.3. Pharmacokinetics Study in Aqueous Humor
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Weinreb, R.N.; Khaw, P.T. Primary open-angle glaucoma. Lancet 2004, 363, 1711–1720. [Google Scholar] [CrossRef]
- Juliana, F.R.; Kesse, S.; Boakye-Yiadom, K.O.; Veroniaina, H.; Wang, H.; Sun, M. Promising Approach in the Treatment of Glaucoma Using Nanotechnology and Nanomedicine-Based Systems. Molecules 2019, 24, 3805. [Google Scholar] [CrossRef] [PubMed]
- Nagasubramanian, S.; Bloom, J.; Poinoosawmy, D.; Hitchings, R. The effects of a topical acetazolamide preparation on intraocular pressure in patients with ocular hypertension. In Glaucoma Update III; Springer: Heidelberg, Germany, 1987; pp. 255–259. [Google Scholar]
- Kaur, I.P.; Smitha, R.; Aggarwal, D.; Kapil, M. Acetazolamide: Future perspective in topical glaucoma therapeutics. Int. J. Pharm. 2002, 248, 1–14. [Google Scholar] [CrossRef]
- Hathout, R.M.; Mansour, S.; Mortada, N.D.; Guinedi, A.S. Liposomes as an ocular delivery system for acetazolamide: In vitro and in vivo studies. AAPS PharmSciTech 2007, 8, E1–E12. [Google Scholar] [CrossRef] [PubMed]
- Friedman, Z.; Allen, R.C.; Raph, S.M. Topical acetazolamide and methazolamide delivered by contact lenses. Arch. Ophthalmol. 1985, 103, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Shawky Tous, S.; Abd-El Nasser, K. Acetazolamide topical formulation and ocular effect. STP Pharma Sci. 1992, 2, 125–131. [Google Scholar]
- Loftsson, T.; Friðriksdóttir, H.; Stefansson, E.; Thorisdottir, S.; Guðmundsson, Ö.; Sigthorsson, T. Topically effective ocular hypotensive acetazolamide and ethoxyzolamide formulations in rabbits. J. Pharm. Pharmacol. 1994, 46, 503–504. [Google Scholar] [CrossRef]
- Kaur, I.P.; Singh, M.; Kanwar, M. Formulation and evaluation of ophthalmic preparations of acetazolamide. Int. J. Pharm. 2000, 199, 119–127. [Google Scholar] [CrossRef]
- Kaur, I.P.; Smitha, R. Penetration enhancers and ocular bioadhesives: Two new avenues for ophthalmic drug delivery. Drug Dev. Ind. Pharm. 2002, 28, 353–369. [Google Scholar] [CrossRef]
- Morsi, N.; Mohamed, M.; Refai, H.; El Sorogy, H. Nanoemulsion as a novel ophthalmic delivery system for acetazolamide. Int. J. Pharm. Pharm. Sci. 2014, 6, 227–236. [Google Scholar]
- Pitta, S.K.; Dudhipala, N.; Narala, A.; Veerabrahma, K. Development of zolmitriptan transfersomes by Box–Behnken design for nasal delivery: In vitro and in vivo evaluation. Drug Dev. Ind. Pharm. 2018, 44, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.-S.; Fang, X.-Q.; Wang, L.-L.; Zhang, Y.-J. Preparation and quality assessment of itraconazole transfersomes. Int. J. Pharm. 2012, 436, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Rother, M.; Lavins, B.J.; Kneer, W.; Lehnhardt, K.; Seidel, E.J.; Mazgareanu, S. Efficacy and safety of epicutaneous ketoprofen in Transfersome (IDEA-033) versus oral celecoxib and placebo in osteoarthritis of the knee: Multicentre randomised controlled trial. Ann. Rheum. Dis. 2007, 66, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Yeole, P.; Iyer, D. Thermosensitive in situ gel of Timolol Maleate for the treatment of open angle glaucoma. RJPBCS 2011, 2, 1048–1064. [Google Scholar]
- Gaudana, R.; Jwala, J.; Boddu, S.H.; Mitra, A.K. Recent perspectives in ocular drug delivery. Pharm. Res. 2009, 26, 1197. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Alexandridis, P. Formulation of poloxamers for drug delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef]
- Duman, G.; Aslan, İ.; Yekta Özer, A.; İnanç, İ.; Taralp, A. Liposome, gel and lipogelosome formulations containing sodium hyaluronate. J. Liposome Res. 2014, 24, 259–269. [Google Scholar] [CrossRef]
- Garg, V.; Singh, H.; Bimbrawh, S.; Kumar Singh, S.; Gulati, M.; Vaidya, Y.; Kaur, P. Ethosomes and transfersomes: Principles, perspectives and practices. Curr. Drug Deliv. 2017, 14, 613–633. [Google Scholar] [CrossRef]
- Rai, S.; Pandey, V.; Rai, G. Transfersomes as versatile and flexible nano-vesicular carriers in skin cancer therapy: The state of the art. Nano Rev. Exp. 2017, 8, 1325708. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons: New York, NY, USA, 2017. [Google Scholar]
- El Gamal, S.S.; Naggar, V.F.; Allam, A.N. Optimization of acyclovir oral tablets based on gastroretention technology: Factorial design analysis and physicochemical characterization studies. Drug Dev. Ind. Pharm. 2011, 37, 855–867. [Google Scholar] [CrossRef]
- Duangjit, S.; Opanasopit, P.; Rojanarata, T.; Ngawhirunpat, T. Evaluation of meloxicam-loaded cationic transfersomes as transdermal drug delivery carriers. AAPS PharmSciTech 2013, 14, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Larsson, J. Methods for Measurement of Solubility and Dissolution Rate of Sparingly Soluble Drugs. Master’s Thesis, Lund University, Lund, Sweden, 2009. [Google Scholar]
- Zhang, C.; Xu, T.; Zhang, D.; He, W.; Wang, S.; Jiang, T. Disulfiram thermosensitive in-situ gel based on solid dispersion for cataract. AJPS 2018, 13, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Abdelbary, G.A.; Amin, M.M.; Zakaria, M.Y. Ocular ketoconazole-loaded proniosomal gels: Formulation, ex vivo corneal permeation and in vivo studies. Drug Deliv. 2017, 24, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Younes, N.F.; Abdel-Halim, S.A.; Elassasy, A.I. Solutol HS15 based binary mixed micelles with penetration enhancers for augmented corneal delivery of sertaconazole nitrate: Optimization, in vitro, ex vivo and in vivo characterization. Drug Deliv. 2018, 25, 1706–1717. [Google Scholar] [CrossRef]
- Fotaki, N.; Klein, S. In Vitro Drug Release Testing of Special Dosage Forms; John Wiley & Sons: New York, NY, USA, 2019. [Google Scholar]
- Liu, R. Water-Insoluble Drug Formulation; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Mazyed, E.A.; Zakaria, S. Enhancement of dissolution characteristics of clopidogrel bisulphate by proniosomes. Int. J. Appl. Pharm. 2019, 11, 77–85. [Google Scholar] [CrossRef]
- Betha, S.; Reddy, B.P.; Swamy, P.; Varma, M.M.; Raju, D.B.; Kolapalli, V.R.M. Dose calculation, design and development of nateglinide matrix tablets using quality by design approach and its pharmacokinetic evaluation in animal model. J. Pharm. Investig. 2015, 45, 515–528. [Google Scholar] [CrossRef]
- Bansal, S.; Aggarwal, G.; Chandel, P.; Harikumar, S. Design and development of cefdinir niosomes for oral delivery. J. Pharm. Bioall. Sci. 2013, 5, 318. [Google Scholar]
- Das, B.; Sen, S.O.; Maji, R.; Nayak, A.K.; Sen, K.K. Transferosomal gel for transdermal delivery of risperidone: Formulation optimization and ex vivo permeation. J. Drug Deliv. Sci. Technol. 2017, 38, 59–71. [Google Scholar] [CrossRef]
- Al-mahallawi, A.M.; Khowessah, O.M.; Shoukri, R.A. Nano-transfersomal ciprofloxacin loaded vesicles for non-invasive trans-tympanic ototopical delivery: In-vitro optimization, ex-vivo permeation studies, and in-vivo assessment. Int. J. Pharm. 2014, 472, 304–314. [Google Scholar] [CrossRef]
- Maheswari, U.; Mullaicharam, A. Development and In-Vitro evaluation of nanosuspension formulation containing acyclovir for the treatment of ocular infections. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 463–480. [Google Scholar]
- Sezgin-Bayindir, Z.; Yuksel, N. Investigation of formulation variables and excipient interaction on the production of niosomes. AAPS PharmSciTech 2012, 13, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-P.; Wei, Y.-H.; Zhou, Y.; Li, Y.-Q.; Wu, X.-A. Ethosomes, binary ethosomes and transfersomes of terbinafine hydrochloride: A comparative study. Arch. Pharm. Res. 2012, 35, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Schmolka, I.R. Artificial skin I. Preparation and properties of pluronic F-127 gels for treatment of burns. J. Biomed. Mater. Res. 1972, 6, 571–582. [Google Scholar] [CrossRef]
- Zhang, K.; Shi, X.; Lin, X.; Yao, C.; Shen, L.; Feng, Y. Poloxamer-based in situ hydrogels for controlled delivery of hydrophilic macromolecules after intramuscular injection in rats. Drug Deliv. 2015, 22, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Gadad, A.P.; Wadklar, P.D.; Dandghi, P.; Patil, A. Thermosensitive in situ gel for ocular delivery of lomefloxacin. Indian J. Pharm. Educ. Res. 2016, 50, S96–S105. [Google Scholar] [CrossRef]
- Soliman, S.M.; Malak, N.A.; El-Gazayerly, O.N.; Rehim, A.A. Formulation of microemulsion gel systems for transdermal delivery of celecoxib: In vitro permeation, anti-inflammatory activity and skin irritation tests. Drug Discov. Ther. 2010, 4, 459–471. [Google Scholar]
- Firoz, S.; Padmini, K.; Padmasree, K.; Sravani, N.; Hemalatha, A.; Vennelarani, Y. Formulation, development and evaluation of carbopol-incorporated thermoreversible gels of pseudoephedrine for rectal drug delivery. Asian J. Pharm. Clin. Res. 2019, 12, 231–235. [Google Scholar] [CrossRef]
- Rajan, R.; Vasudevan, D.T. Effect of permeation enhancers on the penetration mechanism of transfersomal gel of ketoconazole. J. Adv. Pharm. Technol. Res. 2012, 3, 112. [Google Scholar]
- Slettedal, J.K.; Ringvold, A. Correlation between corneal and ambient temperature with particular focus on polar conditions. Acta Ophthalmol. 2015, 93, 422–426. [Google Scholar] [CrossRef]
- Baranowski, P.; Karolewicz, B.; Gajda, M.; Pluta, J. Ophthalmic drug dosage forms: Characterisation and research methods. Sci. World J. 2014, 2014, 861904. [Google Scholar] [CrossRef]
- Dol, H.; Gandhi, S.; Pardhi, D.; Vyawahare, N. Formulation and evaluation of in situ ophthalmic gel of moxifloxacin hydrochloride. Pharma Innov. 2014, 3, 60. [Google Scholar]
- Viram, P.; Lumbhani, A. Development and evaluation of ion-dependent in situ nasal gelling systems of metoclopramide hydrochloride as an antimigraine model drug. IJLRST 2012, 1, 80–89. [Google Scholar]
- Qi, H.; Li, L.; Huang, C.; Li, W.; Wu, C. Optimization and physicochemical characterization of thermosensitive poloxamer gel containing puerarin for ophthalmic use. Chem. Pharm. Bull. 2006, 54, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Thakkar, V.; Metalia, V.; Baldaniya, L.; Gandhi, T.; Gohel, M. Formulation and development of ophthalmic in situ gel for the treatment ocular inflammation and infection using application of quality by design concept. Drug Dev. Ind. Pharm. 2016, 42, 1406–1423. [Google Scholar] [CrossRef]
- Dorraj, G.; Moghimi, H.R. Preparation of SLN-containing thermoresponsive in-situ forming gel as a controlled nanoparticle delivery system and investigating its rheological, thermal and erosion behavior. IJPR 2015, 14, 347. [Google Scholar]
- Cao, F.; Zhang, X.; Ping, Q. New method for ophthalmic delivery of azithromycin by poloxamer/carbopol-based in situ gelling system. Drug Deliv. 2010, 17, 500–507. [Google Scholar] [CrossRef]
- Augusthy, A.R.; Chandran, S.C.; Vipin, K. Design and Evaluation of an in Situ Forming Implant System of an Anti-Inflammatory Drug. Indo Am. J. Pharm. Sci. 2017, 4, 983–994. [Google Scholar]
- Gad, S.; Ahmed, A.; Ghourab, M.; Queshawy, M. Design, formulation, and evaluation of Piroxicam niosomal gel. Int. J. PharmTech. Res 2014, 6, 185–195. [Google Scholar]
- Farghaly, D.A.; Aboelwafa, A.A.; Hamza, M.Y.; Mohamed, M.I. Topical delivery of fenoprofen calcium via elastic nano-vesicular spanlastics: Optimization using experimental design and in vivo evaluation. AAPS PharmSciTech 2017, 18, 2898–2909. [Google Scholar] [CrossRef]
- Hollands, C. The Animals (scientific procedures) Act 1986. Lancet 1986, 2, 32. [Google Scholar] [CrossRef]
- Basha, M.; Abd El-Alim, S.H.; Shamma, R.N.; Awad, G.E. Design and optimization of surfactant-based nanovesicles for ocular delivery of Clotrimazole. J. Liposome Res. 2013, 23, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Manthey, A.L.; Chiu, K.; Do, C.-W. Methods to induce chronic ocular hypertension: Reliable rodent models as a platform for cell transplantation and other therapies. Cell Transplant. 2018, 27, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Hassan, D.H.; Abdelmonem, R.; Abdellatif, M.M. Formulation and characterization of carvedilol leciplex for glaucoma treatment: In-vitro, ex-vivo and in-vivo study. Pharmaceutics 2018, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- El-Gazayerly, O.N.; Hikal, A.H. Preparation and evaluation of acetazolamide liposomes as an ocular delivery system. Int. J. Pharm. 1997, 158, 121–127. [Google Scholar] [CrossRef]
- Xingqi, W.; Yong, Z.; Xing, L.; Yang, W.; Jie, H.; Rongfeng, H.; Shuangying, G.; Xiaoqin, C. Cubic and hexagonal liquid crystal gels for ocular delivery with enhanced effect of pilocarpine nitrate on anti-glaucoma treatment. Drug Deliv. 2019, 26, 952–964. [Google Scholar] [CrossRef]
- Mishra, V.; Jain, N. Acetazolamide encapsulated dendritic nano-architectures for effective glaucoma management in rabbits. Int. J. Pharm. 2014, 461, 380–390. [Google Scholar] [CrossRef]
- Ibrahim, S.S. The Role of Surface Active Agents in Ophthalmic Drug Delivery: A Comprehensive Review. J. Pharm. Sci. 2019, 108, 1923–1933. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Inactive Ingredient Search for Approved Drug Products; FDA Database: Silver Spring, MD, USA, 2017. [Google Scholar]
- US Food and Drug Administration. GRAS Substances (SCOGS) Database; 2012. Available online: https://www.fda.gov/food/generally-recognized-safe-gras/gras-substances-scogs-database (accessed on 20 April 2020).
- Sahoo, R.K.; Biswas, N.; Guha, A.; Sahoo, N.; Kuotsu, K. Nonionic surfactant vesicles in ocular delivery: Innovative approaches and perspectives. BioMed Res. Int. 2014, 2014, 263604. [Google Scholar] [CrossRef]
- Janga, K.Y.; Tatke, A.; Dudhipala, N.; Balguri, S.P.; Ibrahim, M.M.; Maria, D.N.; Jablonski, M.M.; Majumdar, S. Gellan gum based sol-to-gel transforming system of natamycin transfersomes improves topical ocular delivery. J. Pharmacol. Exp. Ther. 2019, 370, 814–822. [Google Scholar] [CrossRef]
- Eldeeb, A.E.; Salah, S.; Ghorab, M. Proniosomal gel-derived niosomes: An approach to sustain and improve the ocular delivery of brimonidine tartrate; formulation, in-vitro characterization, and in-vivo pharmacodynamic study. Drug Deliv. 2019, 26, 509–521. [Google Scholar] [CrossRef]
- Tiwari, R.; Dubey, V.; Kesavan, K. Ocular self-microemulsifying drug delivery system of prednisolone improves therapeutic effectiveness in the treatment of experimental uveitis. Ocul. Immunol. Inflamm. 2019, 27, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Guldiken, B.; Gibis, M.; Boyacioglu, D.; Capanoglu, E.; Weiss, J. Impact of liposomal encapsulation on degradation of anthocyanins of black carrot extract by adding ascorbic acid. Food Funct. 2017, 8, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Duangjit, S.; Opanasopit, P.; Rojanarata, T.; Ngawhirunpat, T. Characterization and in vitro skin permeation of meloxicam-loaded liposomes versus transfersomes. J. Drug Deliv. 2011, 2011, 418316. [Google Scholar] [CrossRef] [PubMed]
- Elsheikh, M.A.; Elnaggar, Y.S.; Hamdy, D.A.; Abdallah, O.Y. Novel cremochylomicrons for improved oral bioavailability of the antineoplastic phytomedicine berberine chloride: Optimization and pharmacokinetics. Int. J. Pharm. 2018, 535, 316–324. [Google Scholar] [CrossRef]
- Abdelbary, G.; El-gendy, N. Niosome-encapsulated gentamicin for ophthalmic controlled delivery. AAPS PharmSciTech 2008, 9, 740–747. [Google Scholar] [CrossRef]
- Anderson, M.J.; Whitcomb, P.J. Design of experiments. Kirk Othmer Encycl. Chem. Technol. 2000, 1–22. [Google Scholar] [CrossRef]
- Turk, C.T.S.; Oz, U.C.; Serim, T.M.; Hascicek, C. Formulation and optimization of nonionic surfactants emulsified nimesulide-loaded PLGA-based nanoparticles by design of experiments. AAPS PharmSciTech 2014, 15, 161–176. [Google Scholar] [CrossRef]
- Al-Mahallawi, A.M.; Khowessah, O.M.; Shoukri, R.A. Enhanced non invasive trans-tympanic delivery of ciprofloxacin through encapsulation into nano-spanlastic vesicles: Fabrication, in-vitro characterization, and comparative ex-vivo permeation studies. Int. J. Pharm. 2017, 522, 157–164. [Google Scholar] [CrossRef]
- Bou-Chacra, N.; Melo, K.J.C.; Morales, I.A.C.; Stippler, E.S.; Kesisoglou, F.; Yazdanian, M.; Löbenberg, R. Evolution of choice of solubility and dissolution media after two decades of biopharmaceutical classification system. AAPS J. 2017, 19, 989–1001. [Google Scholar]
- El Maghraby, G.M.; Williams, A.C.; Barry, B.W. Skin delivery of oestradiol from lipid vesicles: Importance of liposome structure. Int. J. Pharm. 2000, 204, 159–169. [Google Scholar] [CrossRef]
- Oh, Y.K.; Kim, M.Y.; Shin, J.Y.; Kim, T.W.; Yun, M.O.; Yang, S.J.; Choi, S.S.; Jung, W.W.; Kim, J.A.; Choi, H.G. Skin permeation of retinol in Tween 20-based deformable liposomes: In-vitro evaluation in human skin and keratinocyte models. J. Pharm. Pharmacol. 2006, 58, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Petchsomrit, A.; Sermkaew, N.; Wiwattanapatapee, R. Effect of alginate and surfactant on physical properties of oil entrapped alginate bead formulation of curcumin. Int. J. Med. Health Biomed. Bioeng. Pharm. Eng. 2013, 7, 864–868. [Google Scholar]
- Singh, C.H.; Jain, C.; Kumar, B.N. Formulation, characterization, stability and invitro evaluation of nimesulide niosomes. Pharmacophore 2011, 3, e185. [Google Scholar]
- Setthacheewakul, S.; Mahattanadul, S.; Phadoongsombut, N.; Pichayakorn, W.; Wiwattanapatapee, R. Development and evaluation of self-microemulsifying liquid and pellet formulations of curcumin, and absorption studies in rats. Eur. J. Pharm. Biopharm. 2010, 76, 475–485. [Google Scholar] [CrossRef]
- Modi, A.; Tayade, P. A comparative solubility enhancement profile of valdecoxib with different solubilization approaches. Indian J. Pharm. Sci. 2007, 69, 274. [Google Scholar]
- Fang, J.-Y.; Yu, S.-Y.; Wu, P.-C.; Huang, Y.-B.; Tsai, Y.-H. In vitro skin permeation of estradiol from various proniosome formulations. Int. J. Pharm. 2001, 215, 91–99. [Google Scholar] [CrossRef]
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes—Novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J. Control. Release 2000, 65, 403–418. [Google Scholar] [CrossRef]
- Pandit, J.; Garg, M.; Jain, N.K. Miconazole nitrate bearing ultraflexible liposomes for the treatment of fungal infection. J. Liposome Res. 2014, 24, 163–169. [Google Scholar] [CrossRef]
- Khalil, R.M.; El-Bary, A.A.; Kassem, M.A.; Ghorab, M.M.; Basha, M. Influence of formulation parameters on the physicochemical properties of meloxicam-loaded solid lipid nanoparticles. Egypt Pharm. J. 2013, 12, 63. [Google Scholar]
- Lv, Q.; Yu, A.; Xi, Y.; Li, H.; Song, Z.; Cui, J.; Cao, F.; Zhai, G. Development and evaluation of penciclovir-loaded solid lipid nanoparticles for topical delivery. Int. J. Pharm. 2009, 372, 191–198. [Google Scholar] [CrossRef]
- Jeong, I.-J.; Kim, K.-J. An interactive desirability function method to multiresponse optimization. Eur. J. Oper. Res. 2009, 195, 412–426. [Google Scholar] [CrossRef]
- Thomas, L.; Viswanad, V. Formulation and optimization of clotrimazole-loaded proniosomal gel using 32 factorial design. Sci. Pharm. 2012, 80, 731–748. [Google Scholar] [CrossRef] [PubMed]
- Studart, A.R.; Amstad, E.; Gauckler, L.J. Colloidal stabilization of nanoparticles in concentrated suspensions. Langmuir 2007, 23, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Leonyza, A.; Surini, S. Optimization of sodium deoxycholate-based transfersomes for percutaneous delivery of peptides and proteins. Int. J. Appl. Pharm. 2019, 11, 329–332. [Google Scholar] [CrossRef]
- Nzai, J.; Proctor, A. Determination of phospholipids in vegetable oil by Fourier transform infrared spectroscopy. J. Am. Oil Chem. Soc. 1998, 75, 1281–1289. [Google Scholar] [CrossRef]
- Meng, X.; Pan, Q.; Ding, Y.; Jiang, L. Rapid determination of phospholipid content of vegetable oils by FTIR spectroscopy combined with partial least-square regression. Food Chem. 2014, 147, 272–278. [Google Scholar] [CrossRef]
- El Maghraby, G.M.; Ghanem, S.F. Preparation and evaluation of rapidly dissolving tablets of raloxifene hydrochloride by ternary system formation. Int. J. Pharm. Pharm. Sci. 2016, 8, 127–136. [Google Scholar]
- Manchanda, S.; Sahoo, P.K. Topical delivery of acetazolamide by encapsulating in mucoadhesive nanoparticles. AJPS 2017, 12, 550–557. [Google Scholar] [CrossRef]
- Yusuf, M.; Sharma, V.; Pathak, K. Nanovesicles for transdermal delivery of felodipine: Development, characterization, and pharmacokinetics. Int. J. Pharm. Investig. 2014, 4, 119. [Google Scholar]
- Yeo, L.; Olusanya, T.; Chaw, C.; Elkordy, A. Brief effect of a small hydrophobic drug (cinnarizine) on the physicochemical characterisation of Niosomes produced by thin-film hydration and microfluidic methods. Pharmaceutics 2018, 10, 185. [Google Scholar] [CrossRef]
- El-Sayed, M.M.; Hussein, A.K.; Sarhan, H.A.; Mansour, H.F. Flurbiprofen-loaded niosomes-in-gel system improves the ocular bioavailability of flurbiprofen in the aqueous humor. Drug Dev. Ind. Pharm. 2017, 43, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Villa, C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef] [PubMed]
- Al Khateb, K.; Ozhmukhametova, E.K.; Mussin, M.N.; Seilkhanov, S.K.; Rakhypbekov, T.K.; Lau, W.M.; Khutoryanskiy, V.V. In situ gelling systems based on Pluronic F127/Pluronic F68 formulations for ocular drug delivery. Int. J. Pharm. 2016, 502, 70–79. [Google Scholar] [CrossRef]
- Soliman, K.A.; Ullah, K.; Shah, A.; Jones, D.S.; Thakur, R.R. Poloxamer-based in situ gelling thermoresponsive systems for ocular drug delivery applications. Drug Discov. Today 2019, 24, 1575–1586. [Google Scholar] [CrossRef] [PubMed]
- Galgatte, U.C.; Chaudhari, P.D. Preformulation study of poloxamer 407 gels: Effect of additives. Int. J. Pharm. Pharm. Sci. 2014, 6, 130–133. [Google Scholar]
- Fathalla, Z.M.; Vangala, A.; Longman, M.; Khaled, K.A.; Hussein, A.K.; El-Garhy, O.H.; Alany, R.G. Poloxamer-based thermoresponsive ketorolac tromethamine in situ gel preparations: Design, characterisation, toxicity and transcorneal permeation studies. Eur. J. Pharm. Biopharm. 2017, 114, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Preetha, J.P.; Karthika, K.; Rekha, N.; Elshafie, K. Formulation and evaluation of in situ ophthalmic gels of Diclofenac sodium. J. Chem. Pharm. Res. 2010, 2, 528–535. [Google Scholar]
- Jothi, M.; Harikumar, S.; Aggarwal, G. In-situ ophthalmic gels for the treatment of eye diseases. Int. J. Pharm. Sci. Res. 2012, 3, 1891. [Google Scholar]
- Uddin, M.S.; Al Mamun, A.; Kabir, M.T.; Setu, J.R.; Zaman, S.; Begum, Y.; Amran, M.S. Quality control tests for ophthalmic pharmaceuticals: Pharmacopoeial standards and specifications. J. Adv. Med. Pharm. Sci. 2017, 14, 1–17. [Google Scholar] [CrossRef]
- Tatke, A.; Dudhipala, N.; Janga, K.Y.; Balguri, S.P.; Avula, B.; Jablonski, M.M.; Majumdar, S. In situ gel of triamcinolone acetonide-loaded solid lipid nanoparticles for improved topical ocular delivery: Tear kinetics and ocular disposition studies. Nanomaterials 2019, 9, 33. [Google Scholar] [CrossRef]
- Fathalla, D.; Abdel-Mageed, A.; Abdel-Hamid, F.; Ahmed, M. In-vitro and in-vivo evaluation of niosomal gel containing aceclofenac for sustained drug delivery. IJPSR 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Moawad, F.A.; Ali, A.A.; Salem, H.F. Nanotransfersomes-loaded thermosensitive in situ gel as a rectal delivery system of tizanidine HCl: Preparation, in vitro and in vivo performance. Drug Deliv. 2017, 24, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Mukherjee, B. Design, development, physicochemical, and in vitro and in vivo evaluation of transdermal patches containing diclofenac diethylammonium salt. J. Pharm. Sci. 2002, 91, 2076–2089. [Google Scholar] [CrossRef] [PubMed]
- Salama, H.A.; Mahmoud, A.A.; Kamel, A.O.; Abdel Hady, M.; Awad, G.A. Brain delivery of olanzapine by intranasal administration of transfersomal vesicles. J. Liposome Res. 2012, 22, 336–345. [Google Scholar] [CrossRef]
- Almeida, H.; Amaral, M.H.; Lobão, P.; Lobo, J.M.S. Pluronic® F-127 and Pluronic Lecithin Organogel (PLO): Main features and their applications in topical and transdermal administration of drugs. J. Pharm. Pharm. Sci. 2012, 15, 592–605. [Google Scholar] [CrossRef]
- Patel, K.K.; Kumar, P.; Thakkar, H.P. Formulation of niosomal gel for enhanced transdermal lopinavir delivery and its comparative evaluation with ethosomal gel. AAPS PharmSciTech 2012, 13, 1502–1510. [Google Scholar] [CrossRef]
- Ban, J.; Zhang, Y.; Huang, X.; Deng, G.; Hou, D.; Chen, Y.; Lu, Z. Corneal permeation properties of a charged lipid nanoparticle carrier containing dexamethasone. Int. J. Nanomed. 2017, 12, 1329. [Google Scholar] [CrossRef]

| Formula | Lipid-to-Surfactant Ratio | Type of Surfactant | EE% |
|---|---|---|---|
| E1 | 90:10 | Span 60 | 94.60 ± 1.74 |
| E2 | 80:20 | Span 60 | 90.63 ± 2.23 |
| E3 | 70:30 | Span 60 | 84.60 ± 1.25 |
| E4 | 60:40 | Span 60 | 68.50 ± 1.27 |
| E5 | 90:10 | Tween 80 | 80.04 ± 1.32 |
| E6 | 80:20 | Tween 80 | 74.32 ± 1.53 |
| E7 | 70:30 | Tween 80 | 59.12 ± 1.35 |
| E8 | 60:40 | Tween 80 | 38.33 ± 1.35 |
| E9 | 90:10 | Cremophor RH | 88.62 ± 1.25 |
| E10 | 80:20 | Cremophor RH | 82.17 ± 1.21 |
| E11 | 70:30 | Cremophor RH | 73.04 ± 1.38 |
| E12 | 60:40 | Cremophor RH | 53.18 ± 1.65 |
| E13 | 90:10 | Brij 35 | 60.32 ± 1.71 |
| E14 | 80:20 | Brij 35 | 52.24 ± 1.09 |
| E15 | 70:30 | Brij 35 | 39.11 ± 2.12 |
| E16 | 60:40 | Brij 35 | 28.61 ± 1.33 |
| Formula | Lipid-to- Surfactant Ratio | Type of Surfactant | EE% |
|---|---|---|---|
| S1 | 90:10 | Span 60 | 97.30 ± 1.68 |
| S2 | 80:20 | Span 60 | 92.63 ± 2.74 |
| S3 | 70:30 | Span 60 | 88.10 ± 1.62 |
| S4 | 60:40 | Span 60 | 70.40 ± 1.16 |
| S5 | 90:10 | Tween 80 | 82.3 ± 1.64 |
| S6 | 80:20 | Tween 80 | 76.98 ± 2.72 |
| S7 | 70:30 | Tween 80 | 62.39 ± 1.55 |
| S8 | 60:40 | Tween 80 | 40.14 ± 1.14 |
| S9 | 90:10 | Cremophor RH | 90.57 ± 2.67 |
| S10 | 80:20 | Cremophor RH | 84.44 ± 2.82 |
| S11 | 70:30 | Cremophor RH | 76.63 ± 0.49 |
| S12 | 60:40 | Cremophor RH | 56.17 ± 2.13 |
| S13 | 90:10 | Brij 35 | 63.44 ± 1.52 |
| S14 | 80:20 | Brij 35 | 56.28 ± 2.11 |
| S15 | 70:30 | Brij 35 | 42.51 ± 1.45 |
| S16 | 60:40 | Brij 35 | 31.51 ± 1.45 |
| Independent Variables | High (+1) | Medium (0) | Low (−1) |
| X1 | 90:10 | 80:20 | 70:30 |
| X2 | Cremophor RH | Tween 80 | Span 60 |
| Responses | Desirability | ||
| Y1 | Maximize | ||
| Y2 | Maximize | ||
| Formula | Independent Variables | Dependent Variables | ||
|---|---|---|---|---|
| X1 | X2 | Y1 | Y2 | |
| F1 | 1 | −1 | 56.75 ± 0.82 | 97.30 ± 1.68 |
| F2 | 0 | −1 | 69.89 ± 1.57 | 92.63 ± 2.74 |
| F3 | −1 | −1 | 85.03 ± 2.11 | 88.10 ± 1.62 |
| F4 | 1 | 0 | 60.59 ± 1.24 | 82.3 ± 1.64 |
| F5 | 0 | 0 | 85.48 ± 1.58 | 76.98 ± 2.72 |
| F6 | −1 | 0 | 91.23 ± 2.22 | 62.39 ± 1.55 |
| F7 | 1 | 1 | 66.66 ± 0.58 | 90.57 ± 2.67 |
| F8 # | 0 | 1 | 93.01 ± 3.76 | 84.44 ± 2.82 |
| F9 | −1 | 1 | 97.43 ± 1.89 | 76.63 ± 0.49 |
| Responses | R2 | Adjusted R2 | Predicted R2 | Adequate Precision |
|---|---|---|---|---|
| Q8h (Y1) | 0.9669 | 0.9470 | 0.8973 | 19.284 |
| EE% (Y2) | 0.8973 | 0.8357 | 0.6914 | 11.0919 |
| Dependent Variable | Source | SS | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|---|
| Y1 | Model | 1769.45 | 3 | 589.82 | 48.68 | 0.0004 |
| X1 | 1423.11 | 1 | 1423.11 | 117.45 | 0.0001 | |
| X2 | 346.34 | 2 | 173.17 | 14.29 | 0.0086 | |
| Y2 | Model | 791.97 | 3 | 263.99 | 14.56 | 0.0066 |
| X1 | 261.85 | 1 | 261.85 | 14.45 | 0.0126 | |
| X2 | 530.12 | 2 | 265.06 | 14.62 | 0.0081 |
| Formula | Zero Order | First Order | Higuchi Model | Hixson Crowell | Korsmeyer– Pappas |
|---|---|---|---|---|---|
| F1 | 0.9928 | −0.9957 | 0.9967 | 0.9958 | 0.9911 |
| F2 | 0.9822 | −0.9874 | 0.9891 | 0.9873 | 0.9868 |
| F3 | 0.9851 | −0.9820 | 0.9964 | 0.9925 | 0.9904 |
| F4 | 0.9746 | −0.9778 | 0.9790 | 0.9781 | 0.9757 |
| F5 | 0.9950 | −0.9936 | 0.9993 | 0.9988 | 0.9921 |
| F6 | 0.9649 | −0.9679 | 0.9857 | 0.9849 | 0.9853 |
| F7 | 0.9863 | −0.9948 | 0.9949 | 0.9933 | 0.9944 |
| F8 | 0.9620 | −0.9675 | 0.9818 | 0.9789 | 0.9768 |
| F9 | 0.9785 | −0.9764 | 0.9945 | 0.9940 | 0.9941 |
| Parameter | Fresh Formula | Stored Formula |
|---|---|---|
| Q8h (%) | 93.01 ± 3.76 | 88.40 ± 0.89 |
| EE (%) | 84.44 ± 2.82 | 78.09 ± 0.96 |
| Dilution with SLF | Maximum Viscosity (CP) | Minimum Viscosity (CP) | Thixotropic Behavior (cm2) | Farrow’s Constant |
|---|---|---|---|---|
| Before dilution with SLF | 1788.57 | 146.34 | 4.2 | 2.21 |
| After dilution with SLF | 1784.32 | 143.09 | 4.0 | 2.20 |
| Formula | Zero Order | First Order | Higuchi Model | Hixson Crowell | Korsmeyer–Pappas |
|---|---|---|---|---|---|
| ACZ dispersion | 0.9948 | −0.9544 | 0.9924 | 0.9770 | 0.9703 |
| ACZ-loaded TGS | 0.9746 | −0.9846 | 0.9858 | 0.9838 | 0.9841 |
| Formula | Jss (µg cm−2 h−1) | KP (cm h−1) | ER |
|---|---|---|---|
| ACZ dispersion | 3.39 ± 0.52 | 6.78E-05 ± 0.14 | - |
| ACZ-loaded TNVs | 15.50 ± 1.25 | 0.00030 ± 0.11 | 4.57 |
| ACZ-loaded TGS | 26.63 ± 1.75 | 0.00053 ± 0.17 | 7.86 |
| Formula | Zero Order | First Order | Higuchi Model | Hixson Crowell | Korsmeyer–Pappas |
|---|---|---|---|---|---|
| ACZ dispersion | 0.9848 | −0.9863 | 0.9915 | 0.9858 | 0.9901 |
| ACZ-loaded TNVs | 0.9661 | −0.9827 | 0.9962 | 0.9777 | 0.9910 |
| ACZ-loaded TGS | 0.9941 | −0.9990 | 0.9993 | 0.9992 | 0.9900 |
| Parameter | ACZ Aqueous Dispersion | ACZ-Loaded TNVs | ACZ-Loaded TGS |
|---|---|---|---|
| Cmax (µg mL−1) | 0.651 ± 0.04 | 8.91 ± 0.16 | 9.12 ± 0.13 |
| AUC0–24 (µg h mL−1) | 1.95 ± 0.08 | 107.91 ± 1.32 | 138.08 ± 2.13 |
| MRT (h) | 3.92 ± 0.11 | 12.77 ± 0.45 | 16.76 ± 0.63 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazyed, E.A.; Abdelaziz, A.E. Fabrication of Transgelosomes for Enhancing the Ocular Delivery of Acetazolamide: Statistical Optimization, In Vitro Characterization, and In Vivo Study. Pharmaceutics 2020, 12, 465. https://doi.org/10.3390/pharmaceutics12050465
Mazyed EA, Abdelaziz AE. Fabrication of Transgelosomes for Enhancing the Ocular Delivery of Acetazolamide: Statistical Optimization, In Vitro Characterization, and In Vivo Study. Pharmaceutics. 2020; 12(5):465. https://doi.org/10.3390/pharmaceutics12050465
Chicago/Turabian StyleMazyed, Eman A., and Abdelaziz E. Abdelaziz. 2020. "Fabrication of Transgelosomes for Enhancing the Ocular Delivery of Acetazolamide: Statistical Optimization, In Vitro Characterization, and In Vivo Study" Pharmaceutics 12, no. 5: 465. https://doi.org/10.3390/pharmaceutics12050465
APA StyleMazyed, E. A., & Abdelaziz, A. E. (2020). Fabrication of Transgelosomes for Enhancing the Ocular Delivery of Acetazolamide: Statistical Optimization, In Vitro Characterization, and In Vivo Study. Pharmaceutics, 12(5), 465. https://doi.org/10.3390/pharmaceutics12050465




