Gel Formulation of Nabumetone and a Newly Synthesized Analog: Microemulsion as a Photoprotective Topical Delivery System
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Instruments and Software
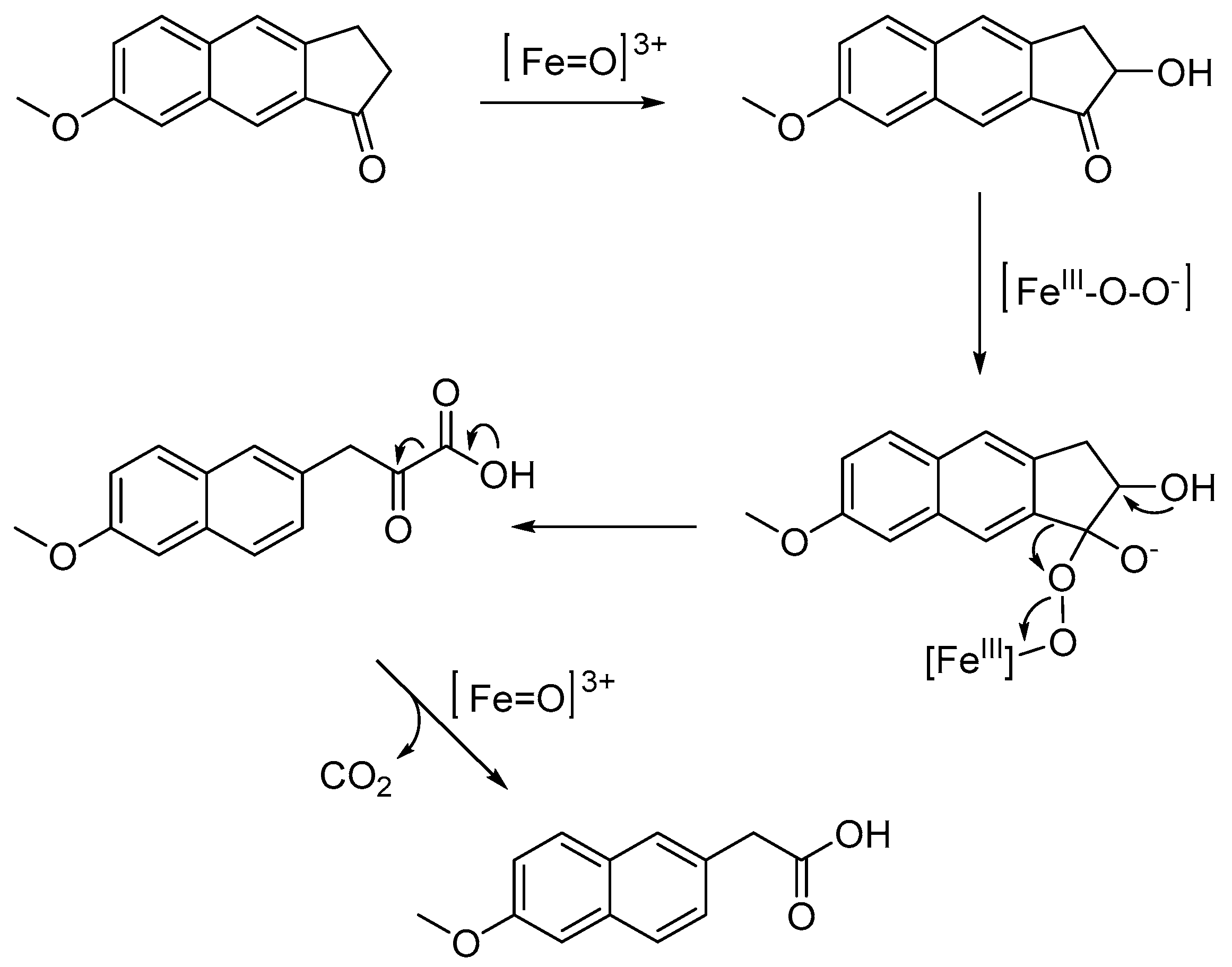
2.2. Chemistry
2.3. Sample Preparation
2.4. Size and Distribution Analysis
2.5. Photodegradation Test
2.6. Ex-Vivo Permeation Studies
3. Results
3.1. Micro-Emulsion Characterization
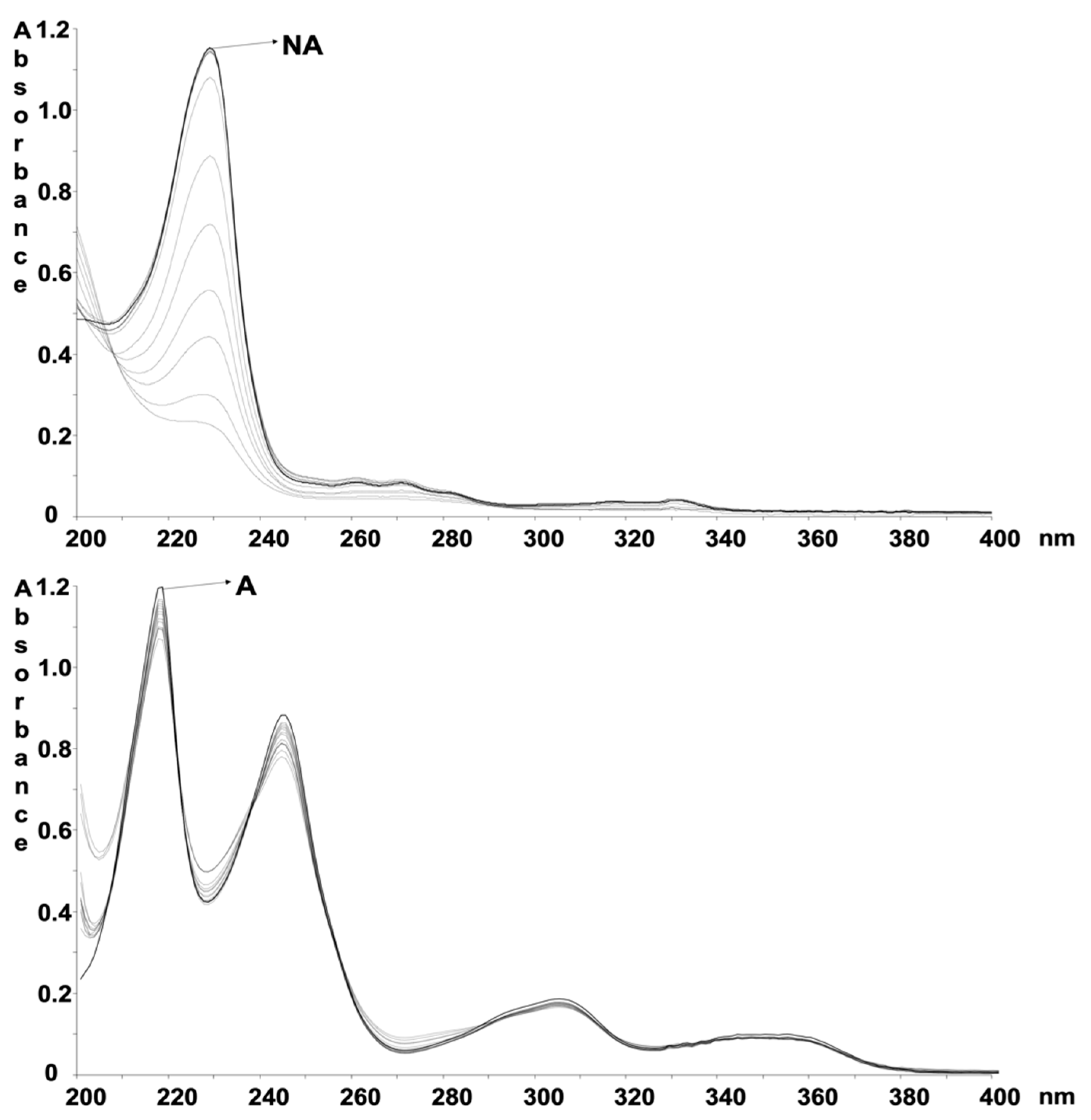
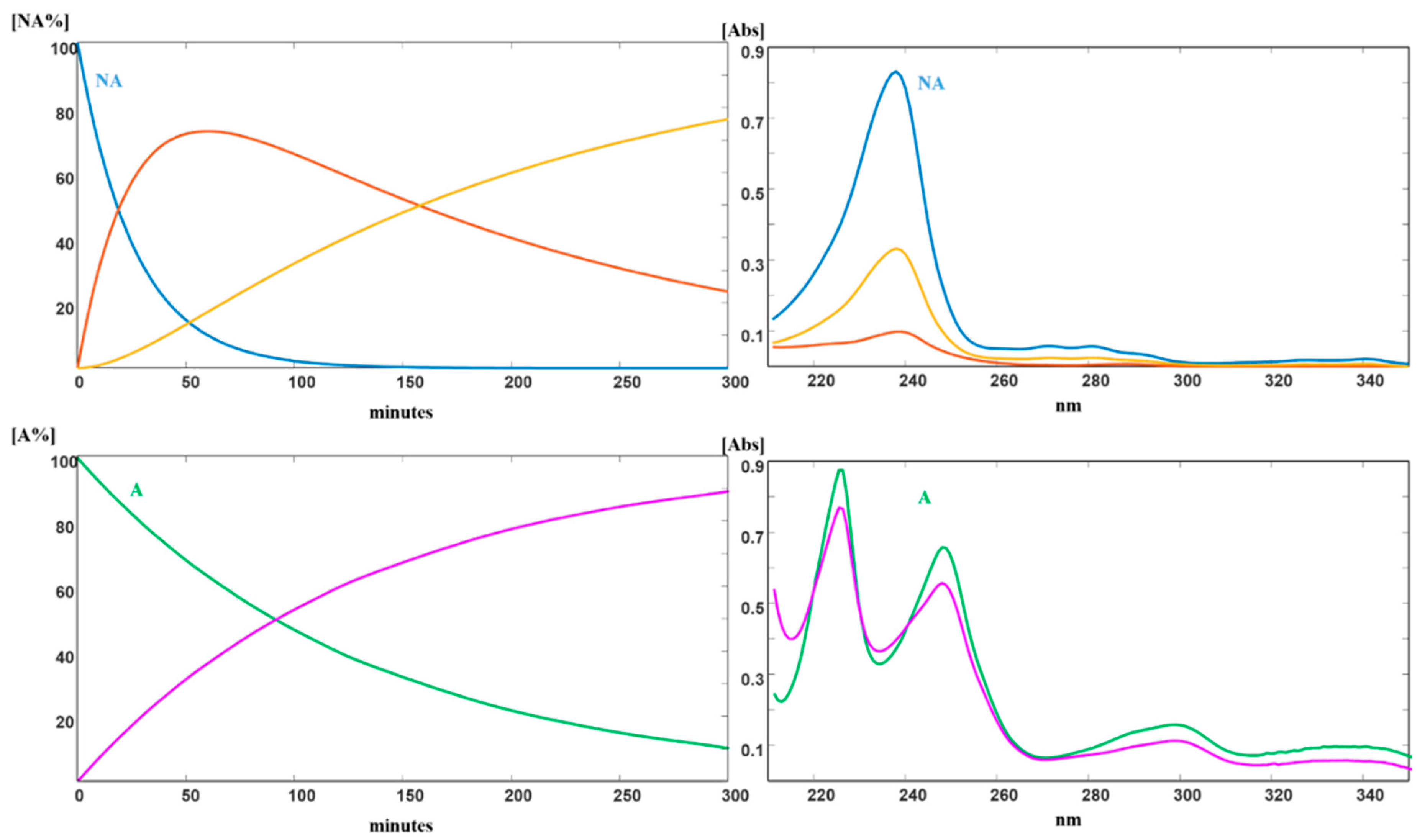
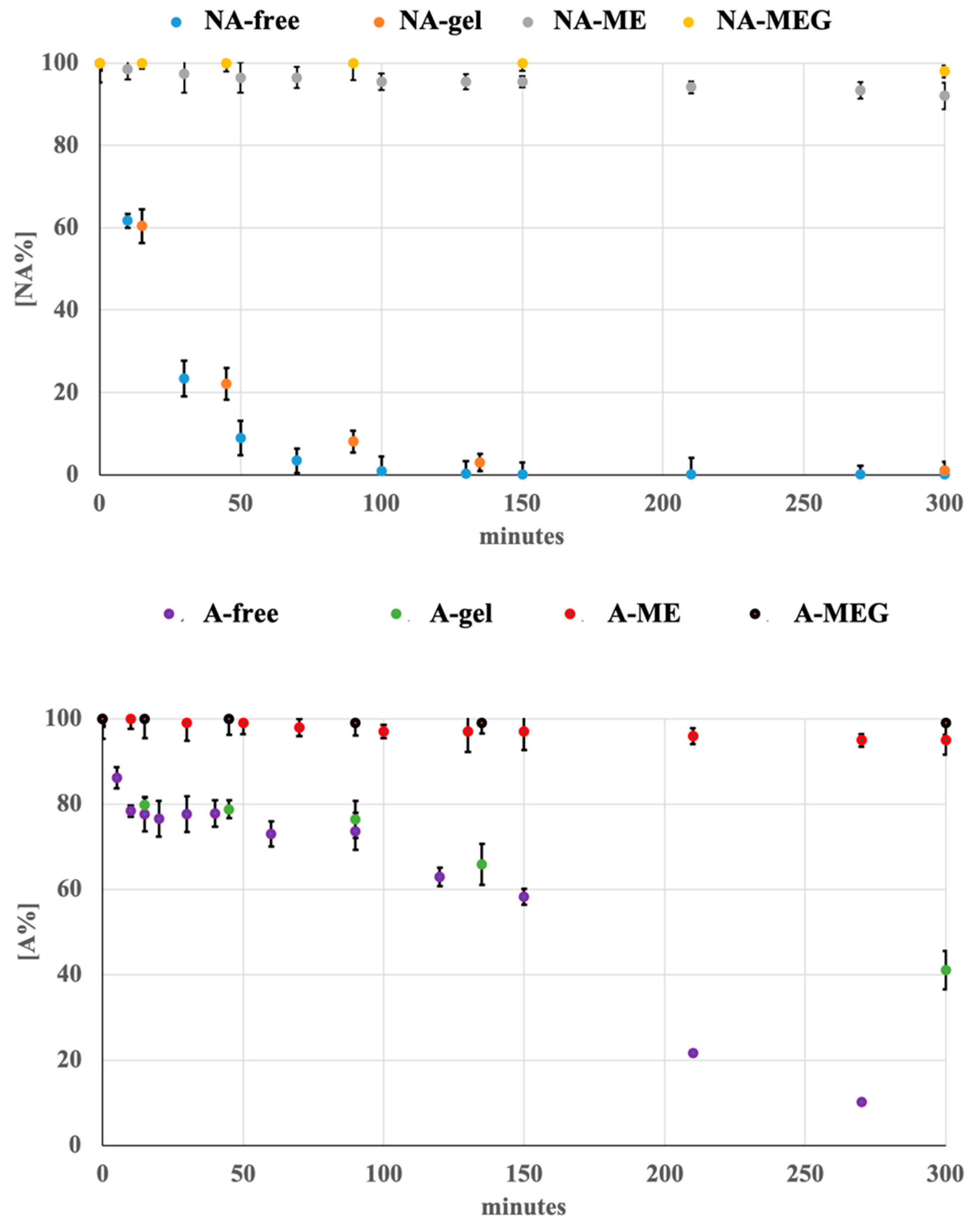
3.2. Photodegradation of Liquid Formulations
3.3. Photodegradation of Gel Formulations
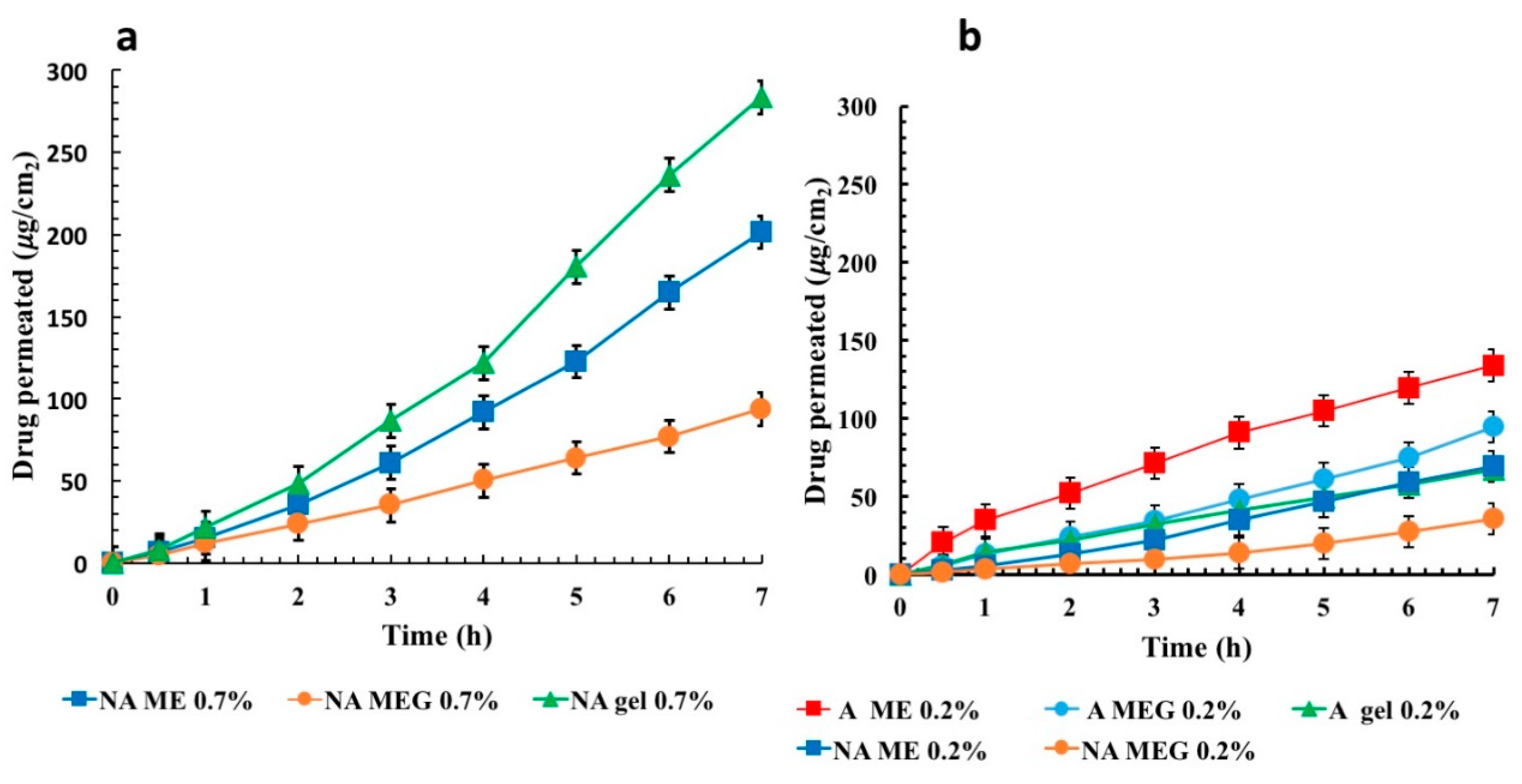
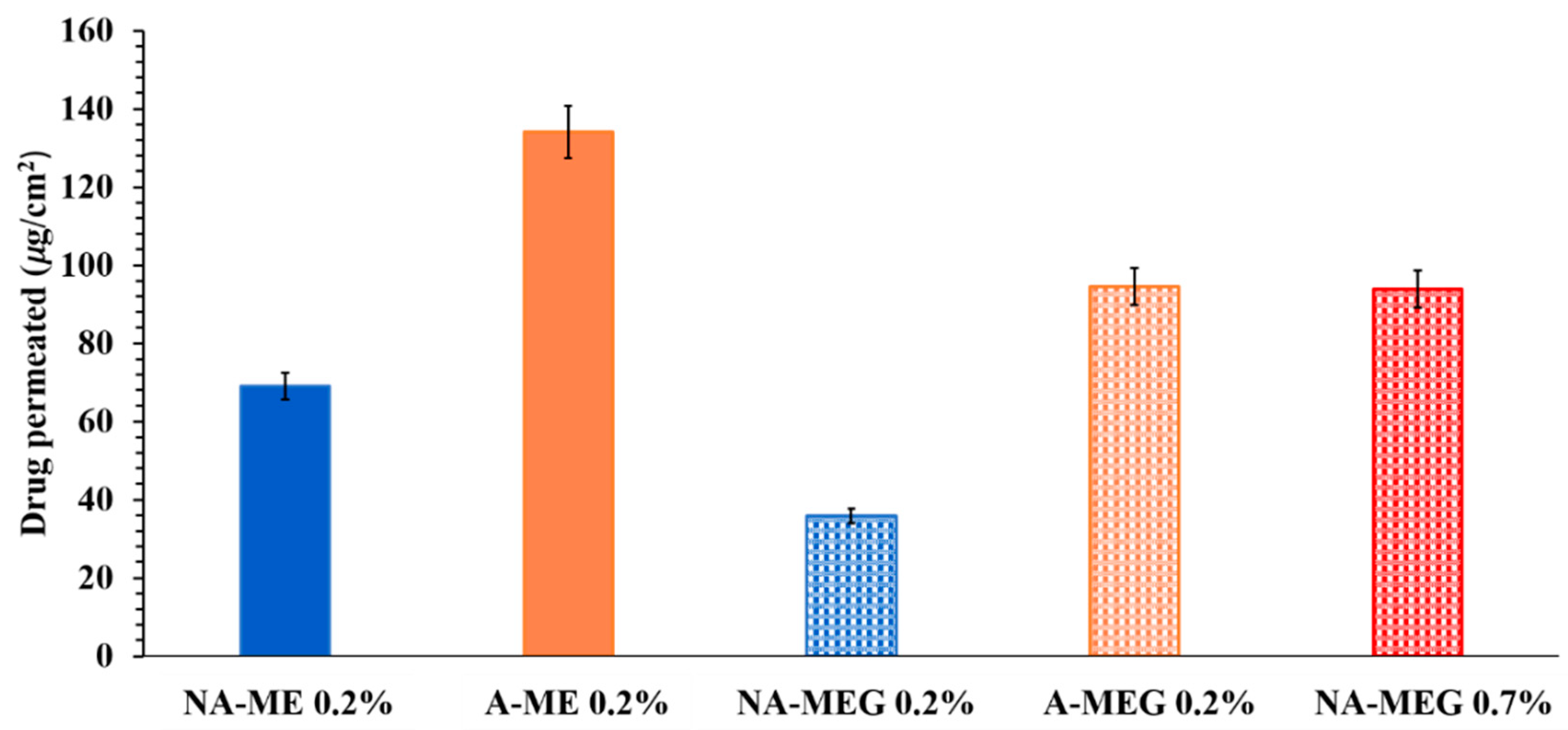
3.4. Ex-Vivo Permeation Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nobilis, M.; Mikusek, J.; Szotakova, B.; Jirasko, R.; Holcapek, M.; Chamseddin, C.; Jira, T.; Kucera, R.; Kunes, J.; Pour, M. Analytical power of LLE-HPLC-PDA-MS/MS in drug metabolism studies: Identification of new nabumetone metabolites. J. Pharmaceut. Biomed. 2013, 80, 164–172. [Google Scholar] [CrossRef]
- Blumenthal, K.G.; Lai, K.H.; Huang, M.; Wallace, Z.S.; Wickner, P.G.; Zhou, L. Adverse and Hypersensitivity Reactions to Prescription Nonsteroidal Anti-Inflammatory Agents in a Large Health Care System. J. Allergy Clin. Immunol. Pract. 2017, 5, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Kawisha, S.M.; Ahmeda, S.; Gull, A.; Aslam, M.; Pandit, J.; Aqil, M.; Sultana, Y. Development of nabumetone loaded lipid nano-scaffold for the effective oral delivery; optimization, characterization, drug release and pharmacodynamic study. J. Mol. Liq. 2017, 231, 514–522. [Google Scholar] [CrossRef]
- Valero, M. Photodegradation of Nabumetone in n-butanol solutions. J. Photoch. Photobio. A 2004, 163, 159–164. [Google Scholar] [CrossRef]
- Valero, M.; Costa Brito, S. Photodegradation of Nabumetone in aqueous solutions. J. Photoch. Photobio. A 2003, 157, 93–101. [Google Scholar] [CrossRef]
- Tonnesen, H.H. Formulation and stability testing of photolabile drugs. Inter. J. Pharm. 2001, 225, 1–14. [Google Scholar] [CrossRef]
- Coelho, L.; Almeida, I.F.; Sousa Lobo, J.M.; Sousa, E.S.J.P. Photostabilization strategies of photosensitive drugs. Inter. J. Pharm. 2018, 541, 19–25. [Google Scholar] [CrossRef]
- Ioele, G.; Tavano, L.; Luca, M.; Muzzalupo, R.; Mancuso, A.; Ragno, G. Light-sensitive drugs in topical formulations: Stability indicating methods and photostabilization strategies. Future Med. Chem. 2017, 9, 1795–1808. [Google Scholar] [CrossRef]
- De Luca, M.; Ioele, G.; Spatari, C.; Ragno, G. Photostabilization studies of antihypertensive 1,4-dihydropyridines using polymeric containers. Inter. J. Pharm. 2016, 505, 376–382. [Google Scholar] [CrossRef]
- Fiorucci, S.; Antonelli, E. Cyclo-oxygenase isoenzymes. Structural basis for selective inhibition of cyclo-oxygenases by anti-inflammatory agents. Dig. Liver Dis. 2001, 33 (Suppl. 2), S2–S7. [Google Scholar] [CrossRef]
- Organic Chemistry Portal. Available online: https://www.organic-chemistry.org/prog/peo/cLogP.html (accessed on 11 February 2019).
- Ragno, G.; Risoli, A.; Ioele, G.; Cione, E.; De Luca, M. Photostabilization of 1,4-dihydropyridine antihypertensives by incorporation into beta-cyclodextrin and liposomes. J. Nanosci. Nanotechnol. 2006, 6, 2979–2985. [Google Scholar] [CrossRef] [PubMed]
- Ioele, G.; De Luca, M.; Ragno, G. Photostability of barnidipine in combined cyclodextrin-in-liposome matrices. Future Med. Chem. 2014, 6, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Ioele, G.; De Luca, M.; Garofalo, A.; Ragno, G. Photosensitive drugs: A review on their photoprotection by liposomes and cyclodextrins. Drug Deliv. 2017, 24 (Suppl. 1), 33–44. [Google Scholar] [CrossRef] [PubMed]
- Ioele, G.; De Luca, M.; Tavano, L.; Ragno, G. The difficulties for a photolabile drug in topical formulations: The case of diclofenac. Inter. J. Pharm. 2014, 465, 284–290. [Google Scholar] [CrossRef]
- Ioele, G.; Tavano, L.; De Luca, M.; Ragno, G.; Picci, N.; Muzzalupo, R. Photostability and ex-vivo permeation studies on diclofenac in topical niosomal formulations. Inter. J. Pharm. 2015, 494, 490–497. [Google Scholar] [CrossRef]
- Ioele, G.; Gunduz, M.G.; Spatari, C.; De Luca, M.; Grande, F.; Ragno, G. A New Generation of Dihydropyridine Calcium Channel Blockers: Photostabilization of Liquid Formulations Using Nonionic Surfactants. Pharmaceutics 2019, 11, 28. [Google Scholar] [CrossRef]
- Vicentini, F.T.; Fonseca, Y.M.; Pitol, D.L.; Iyomasa, M.M.; Bentley, M.V.; Fonseca, M.J. Evaluation of protective effect of a water-in-oil microemulsion incorporating quercetin against UVB-induced damage in hairless mice skin. J. Pharm. Pharm. Sci. 2010, 13, 274–285. [Google Scholar] [CrossRef]
- Patel, M.R.; Patel, R.B.; Parikh, J.R.; Patel, B.G. Improving the isotretinoin photostability by incorporating in microemulsion matrix. ISRN Pharm. 2011, 2011, 838016. [Google Scholar] [CrossRef]
- Xia, L.; Zhongxiao, C.; Zhihao, L.; Xiaodong, M.; Ming, X.; Yan, T.; Xinyi, Z.; Bingqing, X.; Jianbin, Z.; Zeyao, T. Improvement of the solubility, photostability, antioxidant activity and UVB photoprotection of trans-resveratrol by essential oil based microemulsions for topical application. J. Drug Deliv. Sci. Technol. 2018, 48, 346–354. [Google Scholar]
- Nastiti, C.; Ponto, T.; Abd, E.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S. Topical Nano and Microemulsions for Skin Delivery. Pharmaceutics 2017, 9, 37. [Google Scholar] [CrossRef]
- Goswami, P.; Choudhury, A.; Kumar, D.B. Microemulsion -A Potential Carrier for Improved Bioavailability. Intern. J. Pharm. Biol. Sci. Arch. 2019, 10, 69–77. [Google Scholar]
- Jagdale, S.C.; Deore, G.K.; Chabukswar, A.R. Development of Microemulsion Based Nabumetone Transdermal Delivery for Treatment of Arthritis. Recent Pat. Drug Deliv. Formul. 2018, 12, 130–149. [Google Scholar] [CrossRef] [PubMed]
- ICH Harmonized Tripartite Guideline, Federal Register. Photostability Testing of New Drug Substance and Products; European Medicines Agency: London, UK, 1997; Volume 62.
- De Luca, M.; Mas, S.; Ioele, G.; Oliverio, F.; Ragno, G.; Tauler, R. Kinetic studies of nitrofurazone photodegradation by multivariate curve resolution applied to UV-spectral data. Inter. J. Pharm. 2010, 386, 99–107. [Google Scholar] [CrossRef]
- De Luca, M.; Tauler, R.; Ioele, G.; Ragno, G. Study of photodegradation kinetics of melatonin by multivariate curve resolution (MCR) with estimation of feasible band boundaries. Drug Test. Anal. 2013, 5, 96–102. [Google Scholar] [CrossRef]
- De Luca, M.; Ragno, G.; Ioele, G.; Tauler, R. Multivariate curve resolution of incomplete fused multiset data from chromatographic and spectrophotometric analyses for drug photostability studies. Anal. Chim. Acta. 2014, 837, 31–37. [Google Scholar] [CrossRef]
- De Luca, M.; Ioele, G.; Mas, S.; Tauler, R.; Ragno, G. A study of pH-dependent photodegradation of amiloride by a multivariate curve resolution approach to combined kinetic and acid-base titration UV data. Analyst 2012, 137, 5428–5435. [Google Scholar] [CrossRef]
- Dinç, E.; Ragno, G.; Baleanu, D.; De Luca, M.; Ioele, G. Fractional Wavelet Transform–Continous Wavelet Transform for the Quantification of Melatonin and Its Photodegradation Product. Spectrosc. Lett. 2012, 45, 337–343. [Google Scholar] [CrossRef]
- Ragno, G.; Vetuschi, C.; Risoli, A.; Ioele, G. Application of a classical least-squares regression method to the assay of 1,4-dihydropyridine antihypertensives and their photoproducts. Talanta 2003, 59, 375–382. [Google Scholar] [CrossRef]
- McPherson, M.L.; Cimino, N.M. Topical NSAID formulations. Pain Med. 2013, 14 (Suppl. 1), S35–S39. [Google Scholar] [CrossRef]
- Makris, U.E.; Kohler, M.J.; Fraenkel, L. Adverse effects of topical nonsteroidal antiinflammatory drugs in older adults with osteoarthritis: A systematic literature review. J. Rheumatol. 2010, 37, 1236–1243. [Google Scholar] [CrossRef]
- Mimori, S.; Koshikawa, Y.; Mashima, Y.; Mitsunaga, K.; Kawada, K.; Kaneko, M.; Okuma, Y.; Nomura, Y.; Murakami, Y.; Kanzaki, T.; et al. Evaluation of synthetic naphthalene derivatives as novel chemical chaperones that mimic 4-phenylbutyric acid. Bioorg. Med. Chem. Lett. 2015, 25, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Council of Europe. 2010 European Pharmacopoeia; Council of Europe: Strasbourg, Fance, 2010. [Google Scholar]
- Wang, Z.; Diao, Z.; Liu, F.; Li, G.; Zhang, G. Microstructure and rheological properties of liquid crystallines formed in Brij 97/water/IPM system. J. Colloid Interf. Sci. 2006, 297, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Makai, M.; Csanyi, E.; Nemeth, Z.; Palinkas, J.; Eros, I. Structure and drug release of lamellar liquid crystals containing glycerol. Inter. J. Pharm. 2003, 256, 95–107. [Google Scholar] [CrossRef]
- Cho, Y.H.; Kim, S.; Bae, E.K.; Mok, C.K.; Park, J. Formulation of a cosurfactant-free O/W microemulsion using nonionic surfactant mixtures. J. Food Sci. 2008, 73, E115–E121. [Google Scholar] [CrossRef] [PubMed]
- Provencher, S.W. CONTIN: A general purpose constrained regularization program for inverting noisy linear algebraic and integral equations. Comput. Phys. Commun. 1982, 27, 229–242. [Google Scholar] [CrossRef]
- Zhao, Z.; Lian, Y.; Zhu, Y.; Ye, H.; Liu, M.; Lif, J. Depot lidocaine-loaded microemulsion for prolonged local anesthesia: Different efficacy model studies. J. Drug Deliv. Sci. Technol. 2020, 55, 101404. [Google Scholar] [CrossRef]
- Jin, S.E.; Kim, C.K. Charge-mediated topical delivery of plasmid DNA with cationic lipid nanoparticles to the skin. Colloids Surf. B Biointerfaces 2014, 116, 582–590. [Google Scholar] [CrossRef]
- Danielsson, I.; Lindman, B. The definition of a microemulsion. Colloids Surf. B 1981, 3, 391–392. [Google Scholar] [CrossRef]
- Mazzotta, E.; Rossi, C.O.; Muzzalupo, R. Different BRIJ97 colloid systems as potential enhancers of acyclovir skin permeation and depot. Colloid Surf. 2019, 173, 623–631. [Google Scholar] [CrossRef]
- Hedner, T.; Samulesson, O.; Wahrborg, P.; Wadenvik, H.; Ung, K.A.; Ekbom, A. Nabumetone: Therapeutic use and safety profile in the management of osteoarthritis and rheumatoid arthritis. Drugs 2004, 64, 2315–2343. [Google Scholar] [CrossRef]
- Turpeinen, M.; Hofmann, U.; Klein, K.; Murdter, T.; Schwab, M.; Zanger, U.M. A predominate role of CYP1A2 for the metabolism of nabumetone to the active metabolite, 6-methoxy-2-naphthylacetic acid, in human liver microsomes. Drug Metab. Dispos. 2009, 37, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Varfaj, F.; Zulkifli, S.N.; Park, H.G.; Challinor, V.L.; De Voss, J.J.; Ortiz de Montellano, P.R. Carbon–carbon bond cleavage in activation of the prodrug nabumetone. Drug Metab. Dispos. 2014, 42, 828–838. [Google Scholar] [CrossRef]
- Murakami, M.; Takahashi, K.; Amii, H.; Ito, Y. Rhodium(I)-catalyzed successive double cleavage of carbon–carbon bonds of strained spiro cyclobutanones. J. Am. Chem. Soc. 1997, 119, 9307–9308. [Google Scholar] [CrossRef]
- De Rosa, F.S.; Tedesco, A.C.; Lopez, R.F.; Pierre, M.B.; Lange, N.; Marchetti, J.M.; Rotta, J.C.; Bentley, M.V. In vitro skin permeation and retention of 5-aminolevulinic acid ester derivatives for photodynamic therapy. J. Control. Release 2003, 89, 261–269. [Google Scholar] [CrossRef]
- Lopes, L.B. Overcoming the cutaneous barrier with microemulsions. Pharmaceutics 2014, 6, 52–77. [Google Scholar] [CrossRef] [PubMed]

| Samples | Diameter Droplet (nm) | PDI | Diameter Droplet (nm) | PDI |
|---|---|---|---|---|
| After 1 Day | After 6 Months | |||
| ME | 20.16 ± 1.70 | 0.214 | 20.87 ± 1.05 | 0.223 |
| NA-ME 0.2% | 13.17 ± 0.50 | 0.158 | 13.01 ± 0.65 | 0.163 |
| NA-ME 0.7% | 23.64 ± 3.21 | 0.169 | 24.52 ± 2.74 | 0.175 |
| A-ME 0.2% | 13.71 ± 0.32 | 0.153 | 13.54 ± 0.45 | 0.162 |
| Samples | K × 10−3 | t0.1 (min) | t0.5 (min) | R2 | |
|---|---|---|---|---|---|
| NA liquid formulations | NA-free | 48.2 | 2.08 | 14.31 | 0.999 |
| NA-ME | 0.20 | 500.95 | - | 0.910 | |
| NA semisolid formulations | NA-gel 0.2% | 23.4 | 4.27 | 29.49 | 0.977 |
| NA-gel 0.7% | 22.9 | 4.37 | 30.56 | 0.985 | |
| NA-MEG | 0.005 | - | - | 0.947 | |
| A liquid formulations | A-free | 6.9 | 14.49 | 100.00 | 0.903 |
| A-ME | 0.2 | 500.00 | - | 0.945 | |
| A semisolid formulations | A-gel | 2.6 | 38.46 | 265.40 | 0.951 |
| A-MEG | 0.07 | - | - | 0.924 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grande, F.; Ragno, G.; Muzzalupo, R.; Occhiuzzi, M.A.; Mazzotta, E.; De Luca, M.; Garofalo, A.; Ioele, G. Gel Formulation of Nabumetone and a Newly Synthesized Analog: Microemulsion as a Photoprotective Topical Delivery System. Pharmaceutics 2020, 12, 423. https://doi.org/10.3390/pharmaceutics12050423
Grande F, Ragno G, Muzzalupo R, Occhiuzzi MA, Mazzotta E, De Luca M, Garofalo A, Ioele G. Gel Formulation of Nabumetone and a Newly Synthesized Analog: Microemulsion as a Photoprotective Topical Delivery System. Pharmaceutics. 2020; 12(5):423. https://doi.org/10.3390/pharmaceutics12050423
Chicago/Turabian StyleGrande, Fedora, Gaetano Ragno, Rita Muzzalupo, Maria Antonietta Occhiuzzi, Elisabetta Mazzotta, Michele De Luca, Antonio Garofalo, and Giuseppina Ioele. 2020. "Gel Formulation of Nabumetone and a Newly Synthesized Analog: Microemulsion as a Photoprotective Topical Delivery System" Pharmaceutics 12, no. 5: 423. https://doi.org/10.3390/pharmaceutics12050423
APA StyleGrande, F., Ragno, G., Muzzalupo, R., Occhiuzzi, M. A., Mazzotta, E., De Luca, M., Garofalo, A., & Ioele, G. (2020). Gel Formulation of Nabumetone and a Newly Synthesized Analog: Microemulsion as a Photoprotective Topical Delivery System. Pharmaceutics, 12(5), 423. https://doi.org/10.3390/pharmaceutics12050423