Pyrrolylquinoxaline-2-One Derivative as a Potent Therapeutic Factor for Brain Trauma Rehabilitation
Abstract
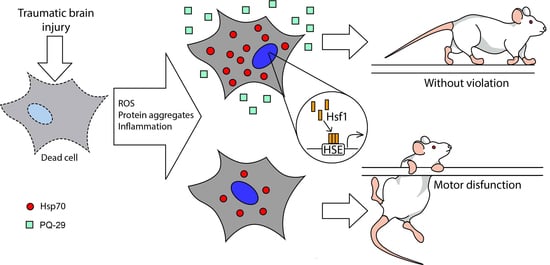
1. Introduction
2. Materials and Methods
2.1. Reporter System and Screening
2.2. Cells
2.3. Electrophoresis and Immunoblotting
2.4. RNA Isolation and Real-Time PCR
2.5. Animals
2.6. Three Methods Were Utilized to Determine the Physiological Characteristics of C6 Cells Responding to PQ-29
2.6.1. Analysis of Proliferation
2.6.2. Viability Test
2.6.3. Apoptosis Analysis
2.7. Immunohistochemistry
2.8. Caspase-3 Assay
2.9. Statistics
3. Results
3.1. Identification of Hsp70 Inducers
3.2. In Vitro Model of Secondary Damage Caused by TBI
3.3. PQ-29 Rescues C6 Cells from the Cytotoxicity of CSF from Traumatized Rats
3.4. Therapeutic Effect of PQ-29 in a Rat Model of TBI
3.5. PQ-29 Reduces the Cytotoxicity of CSF from Traumatized Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beez, T.; Steiger, H.J.; Etminan, N. Pharmacological targeting of secondary brain damage following ischemic or hemorrhagic stroke, traumatic brain injury, and bacterial meningitis—A systematic review and meta-analysis. BMC Neurol. 2017, 17, 209. [Google Scholar] [CrossRef] [PubMed]
- Smrcka, M.; Vidlák, M.; Máca, K.; Smrcka, V.; Gál, R. The influence of mild hypothermia on ICP, CPP and outcome in patients with primary and secondary brain injury. Acta Neurochir. Suppl. 2005, 95, 273–275. [Google Scholar] [PubMed]
- Hart, T.; Sander, A. Memory and Traumatic Brain Injury. Arch Phys. Med. Rehabil. 2017, 98, 407–408. [Google Scholar] [CrossRef] [PubMed]
- Falchook, A.D.; Porges, E.C.; Nadeau, S.E.; Leon, S.A.; Williamson, J.B.; Heilman, K.M. Cognitive-motor dysfunction after severe traumatic brain injury: A cerebral interhemispheric disconnection syndrome. J. Clin. Exp. Neuropsychol. 2015, 37, 1062–1073. [Google Scholar] [CrossRef]
- Broussard, J.I.; Acion, L.; De Jesús-Cortés, H.; Yin, T.; Britt, J.K.; Salas, R.; Costa-Mattioli, M.; Robertson, C.; Pieper, A.A.; Arciniegas, D.B.; et al. Repeated mild traumatic brain injury produces neuroinflammation, anxiety-like behaviour and impaired spatial memory in mice. Brain Inj. 2018, 32, 113–122. [Google Scholar] [CrossRef]
- Hiebert, J.B.; Shen, Q.; Thimmesch, A.R.; Pierce, J.D. Traumatic Brain Injury and Mitochondrial Dysfunction. Am. J. Med. Sci. 2015, 350, 132–138. [Google Scholar] [CrossRef]
- Quillinan, N.; Herson, P.S.; Traystman, R.J. Neuropathophysiology of Brain Injury. Anesthesiol. Clin. 2016, 34, 453–464. [Google Scholar] [CrossRef]
- Curvello, V.; Hekierski, H.; Pastor, P.; Vavilala, M.S.; Armstead, W.M. Dopamine protects cerebral autoregulation and prevents hippocampal necrosis after traumatic brain injury via block of ERK MAPK in juvenile pigs. Brain Res. 2017, 1670, 118–124. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Mestre, H.; Nedergaard, M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018, 17, 1016–1024. [Google Scholar] [CrossRef]
- Agoston, D.V.; Shutes-David, A.; Peskind, E.R. Biofluid biomarkers of traumatic brain injury. Brain Inj. 2017, 31, 1195–1203. [Google Scholar] [CrossRef]
- Lazarev, V.F.; Dutysheva, E.A.; Komarova, E.Y.; Mikhaylova, E.R.; Guzhova, I.V.; Margulis, B.A. GAPDH-targeted therapy – A new approach for secondary damage after traumatic brain injury on rats. Biochem. Biophys. Res. Commun. 2018, 501, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.E.; Hipp, M.S.; Bracher, A.; Hayer-Hartl, M.; Ulrich Hartl, F. Molecular Chaperone Functions in Protein Folding and Proteostasis. Annu. Rev. Biochem. 2013, 82, 323–355. [Google Scholar] [CrossRef] [PubMed]
- Lazarev, V.F.; Mikhaylova, E.R.; Guzhova, I.V.; Margulis, B.A. Possible function of molecular chaperones in diseases caused by propagating amyloid aggregates. Front. Neurosci. 2017, 11, 277. [Google Scholar] [CrossRef]
- Kroemer, G. Heat Shock Protein 70 Neutralizes Apoptosis-Inducing Factor. Sci. World J. 2001, 1, 590. [Google Scholar] [CrossRef] [PubMed]
- Komarova, E.Y.; Afanasyeva, E.A.; Bulatova, M.M.; Cheetham, M.E.; Margulis, B.A.; Guzhova, I.V. Downstream caspases are novel targets for the antiapoptotic activity of the molecular chaperone Hsp70. Cell Stress Chaperones 2004, 9, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Duncan, E.J.; Cheetham, M.E.; Chapple, J.P.; van der Spuy, J. The Role of HSP70 and Its Co-chaperones in Protein Misfolding, Aggregation and Disease. Subcell. Biochem. 2015, 78, 243–273. [Google Scholar]
- Guzhova, I.V.; Lazarev, V.F.; Kaznacheeva, A.V.; Ippolitova, M.V.; Muronetz, V.I.; Kinev, A.V.; Margulis, B.A. Novel mechanism of Hsp70 chaperone-mediated prevention of polyglutamine aggregates in a cellular model of huntington disease. Hum. Mol. Genet. 2011, 20, 3953–3963. [Google Scholar] [CrossRef]
- Ekimova, I.V.; Plaksina, D.V.; Pastukhov, Y.F.; Lapshina, K.V.; Lazarev, V.F.; Mikhaylova, E.R.; Polonik, S.G.; Pani, B.; Margulis, B.A.; Guzhova, I.V.; et al. New HSF1 inducer as a therapeutic agent in a rodent model of Parkinson’s disease. Exp. Neurol. 2018, 306, 199–208. [Google Scholar] [CrossRef]
- Chow, A.M.; Tang, D.W.F.; Hanif, A.; Brown, I.R. Induction of heat shock proteins in cerebral cortical cultures by celastrol. Cell Stress Chaperones 2013, 18, 155–160. [Google Scholar] [CrossRef]
- Nakazono, A.; Adachi, N.; Takahashi, H.; Seki, T.; Hamada, D.; Ueyama, T.; Sakai, N.; Saito, N. Pharmacological induction of heat shock proteins ameliorates toxicity of mutant PKCγ in spinocerebellar ataxia type 14. J. Biol. Chem. 2018, 293, 14758–14774. [Google Scholar] [CrossRef]
- Katsuno, M.; Sang, C.; Adachi, H.; Minamiyama, M.; Waza, M.; Tanaka, F.; Doyu, M.; Sobue, G. Pharmacological induction of heat-shock proteins alleviates polyglutamine-mediated motor neuron disease. Proc. Natl. Acad. Sci. USA 2005, 102, 16801–16806. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, N.; Zheng, Z.; Lee, J.E.; Yenari, M.A. The 70 kDa heat shock protein protects against experimental traumatic brain injury. Neurobiol. Dis. 2013, 58, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Kim, J.Y.; Yenari, M.A. Pharmacological induction of the 70-kDa heat shock protein protects against brain injury. Neuroscience 2015, 284, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Tandean, S.; Japardi, I.; Loe, M.L.; Riawan, W.; July, J. Protective effects of propolis extract in a rat model of traumatic brain injury via hsp70 induction. Open Access Maced. J. Med. Sci. 2019, 7, 2763–2766. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, X.; Gu, C.; Zhang, H.; Zhang, R.; Dong, X.; Liu, C.; Hu, X.; Ji, X.; Huang, S.; et al. Celastrol ameliorates Cd-induced neuronal apoptosis by targeting NOX2-derived ROS-dependent PP5-JNK signaling pathway. J. Neurochem. 2017, 141, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Westerheide, S.D.; Bosman, J.D.; Mbadugha, B.N.A.; Kawahara, T.L.A.; Matsumoto, G.; Kim, S.; Gu, W.; Devlin, J.P.; Silverman, R.B.; Morimoto, R.I. Celastrols as inducers of the heat shock response and cytoprotection. J. Biol. Chem. 2004, 279, 56053–56060. [Google Scholar] [CrossRef]
- Utepova, I.A.; Trestsova, M.A.; Chupakhin, O.N.; Charushin, V.N.; Rempel, A.A. Aerobic oxidative C–H/C–H coupling of azaaromatics with indoles and pyrroles in the presence of TiO2 as a photocatalyst. Green Chem. 2015, 17, 4401–4410. [Google Scholar] [CrossRef]
- Lazarev, V.F.; Nikotina, A.D.; Mikhaylova, E.R.; Nudler, E.; Polonik, S.G.; Guzhova, I.V.; Margulis, B.A. Hsp70 chaperone rescues C6 rat glioblastoma cells from oxidative stress by sequestration of aggregating GAPDH. Biochem. Biophys. Res. Commun. 2016, 470, 766–771. [Google Scholar] [CrossRef]
- Lasunskaia, E.B.; Fridlianskaia, I.; Arnoldt, A.V.; Kanashiro, M.; Guzhova, I.; Margulis, B. Sub-lethal heat shock induces plasma membrane translocation of 70-kDa heat shock protein in viable, but not in apoptotic, U-937 leukaemia cells. APMIS 2010, 118, 179–187. [Google Scholar] [CrossRef]
- Mychasiuk, R.; Farran, A.; Angoa-Perez, M.; Briggs, D.; Kuhn, D.; Esser, M.J. A Novel Model of Mild Traumatic Brain Injury for Juvenile Rats. J. Vis. Exp. 2014. [Google Scholar] [CrossRef]
- Lazarev, V.F.; Nikotina, A.D.; Semenyuk, P.I.; Evstafyeva, D.B.; Mikhaylova, E.R.; Muronetz, V.I.; Shevtsov, M.A.; Tolkacheva, A.V.; Dobrodumov, A.V.; Shavarda, A.L.; et al. Small molecules preventing GAPDH aggregation are therapeutically applicable in cell and rat models of oxidative stress. Free Radic. Biol. Med. 2016, 92, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Lazarev, V.F.; Sverchinskyi, D.V.; Ippolitova, M.V.; Stepanova, A.V.; Guzhova, I.V.; Margulis, B.A. Factors affecting aggregate formation in cell models of huntington’s disease and amyotrophic lateral sclerosis. Acta Nat. 2013, 5, 81–89. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Academic Press: Sydney, Australia, 1982; ISBN 978-0-12-547620-1. [Google Scholar]
- Denault, J.B.; Drag, M.; Salvesen, G.S.; Alves, J.; Heidt, A.B.; Deveraux, Q.; Harris, J.L. Small molecules not direct activators of caspases. Nat. Chem. Biol. 2007, 3, 519. [Google Scholar] [CrossRef]
- Lin, C.H.; Wu, Y.R.; Kung, P.J.; Chen, W.L.; Lee, L.C.; Lin, T.H.; Chao, C.Y.; Chen, C.M.; Chang, K.H.; Janreddy, D.; et al. The potential of indole and a synthetic derivative for polyQ aggregation reduction by enhancement of the chaperone and autophagy systems. ACS Chem. Neurosci. 2014, 5, 1063–1074. [Google Scholar] [CrossRef]
- Jokar, A.; Ahmadi, K.; Salehi, T.; Sharif-Alhoseini, M.; Rahimi-Movaghar, V. The effect of tranexamic acid in traumatic brain injury: A randomized controlled trial. Chin. J. Traumatol. Engl. Ed. 2017, 20, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.Y.; Lee, A.Y.W. Traumatic Brain Injuries: Pathophysiology and Potential Therapeutic Targets. Front. Cell. Neurosci. 2019, 13, 528. [Google Scholar] [CrossRef]
- Akamatsu, Y.; Hanafy, K.A. Cell Death and Recovery in Traumatic Brain Injury. Neurotherapeutics 2020. [Google Scholar] [CrossRef]
- Glushakova, O.Y.; Jeromin, A.; Martinez, J.; Johnson, D.; Denslow, N.; Streeter, J.; Hayes, R.L.; Mondello, S. Cerebrospinal fluid protein biomarker panel for assessment of neurotoxicity induced by kainic acid in rats. Toxicol. Sci. 2012, 130, 158–167. [Google Scholar] [CrossRef]
- Mishra, P.S.; Dhull, D.K.; Nalini, A.; Vijayalakshmi, K.; Sathyaprabha, T.N.; Alladi, P.A.; Raju, T.R. Astroglia acquires a toxic neuroinflammatory role in response to the cerebrospinal fluid from amyotrophic lateral sclerosis patients. J. Neuroinflammation 2016, 13, 212. [Google Scholar] [CrossRef]
- De, S.; Whiten, D.R.; Ruggeri, F.S.; Hughes, C.; Rodrigues, M.; Sideris, D.I.; Taylor, C.G.; Aprile, F.A.; Muyldermans, S.; Knowles, T.P.J.; et al. Soluble aggregates present in cerebrospinal fluid change in size and mechanism of toxicity during Alzheimer’s disease progression. Acta Neuropathol. Commun. 2019, 7, 120. [Google Scholar] [CrossRef]
- Kong, P.; Zhang, B.S.; Lei, P.; Kong, X.D.; Zhang, S.S.; Li, D.; Zhang, Y. Neurotoxicity of cerebro-spinal fluid from patients with Parkinson’s disease on mesencephalic primary cultures as an in vitro model of dopaminergic neurons. Mol. Med. Rep. 2015, 12, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- Lazarev, V.F.; Onokhin, K.V.; Antimonova, O.I.; Polonik, S.G.; Guzhova, I.V.; Margulis, B.A. Kinetics of chaperone activity of proteins Hsp70 and Hdj1 in human leukemia u-937 cells after preconditioning with thermal shock or compound u-133. Biochemistry (Mosc.) 2011, 76, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Kalmar, B.; Greensmith, L. Activation of the heat shock response in a primary cellular model of motoneuron neurodegeneration—Evidence for neuroprotective and neurotoxic effects. Cell. Mol. Biol. Lett. 2009, 14, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Teshima, S.; Kusumoto, K.; Kawahara, T.; Kondo, K.; Kishi, K.; Rokutan, K. A non-toxic heat-shock protein 70 inducer, geranyl-geranyl-acetone, restores the heat shock response in gastric mucosa of protein-malnourished rats. J. Lab. Clin. Med. 2000, 136, 138–148. [Google Scholar] [CrossRef]
- Zhao, Z.; Faden, A.I.; Loane, D.J.; Lipinski, M.M.; Sabirzhanov, B.; Stoica, B.A. Neuroprotective effects of geranylgeranylacetone in experimental traumatic brain injury. J. Cereb. Blood Flow Metab. 2013, 33, 1897–1908. [Google Scholar] [CrossRef]
- Rosen, H.J.; Boeve, B.F.; Boxer, A.L. Tracking disease progression in familial and sporadic frontotemporal lobar degeneration: Recent findings from ARTFL and LEFFTDS. Alzheimers. Dement. 2020, 16, 71–78. [Google Scholar] [CrossRef]
- Mantri, S.; Morley, J.F.; Siderowf, A.D. The importance of preclinical diagnostics in Parkinson disease. Park. Relat. Disord. 2019, 64, 20–28. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutysheva, E.A.; Mikeladze, M.A.; Trestsova, M.A.; Aksenov, N.D.; Utepova, I.A.; Mikhaylova, E.R.; Suezov, R.V.; Charushin, V.N.; Chupakhin, O.N.; Guzhova, I.V.; et al. Pyrrolylquinoxaline-2-One Derivative as a Potent Therapeutic Factor for Brain Trauma Rehabilitation. Pharmaceutics 2020, 12, 414. https://doi.org/10.3390/pharmaceutics12050414
Dutysheva EA, Mikeladze MA, Trestsova MA, Aksenov ND, Utepova IA, Mikhaylova ER, Suezov RV, Charushin VN, Chupakhin ON, Guzhova IV, et al. Pyrrolylquinoxaline-2-One Derivative as a Potent Therapeutic Factor for Brain Trauma Rehabilitation. Pharmaceutics. 2020; 12(5):414. https://doi.org/10.3390/pharmaceutics12050414
Chicago/Turabian StyleDutysheva, Elizaveta A., Marina A. Mikeladze, Maria A. Trestsova, Nikolay D. Aksenov, Irina A. Utepova, Elena R. Mikhaylova, Roman V. Suezov, Valery N. Charushin, Oleg N. Chupakhin, Irina V. Guzhova, and et al. 2020. "Pyrrolylquinoxaline-2-One Derivative as a Potent Therapeutic Factor for Brain Trauma Rehabilitation" Pharmaceutics 12, no. 5: 414. https://doi.org/10.3390/pharmaceutics12050414
APA StyleDutysheva, E. A., Mikeladze, M. A., Trestsova, M. A., Aksenov, N. D., Utepova, I. A., Mikhaylova, E. R., Suezov, R. V., Charushin, V. N., Chupakhin, O. N., Guzhova, I. V., Margulis, B. A., & Lazarev, V. F. (2020). Pyrrolylquinoxaline-2-One Derivative as a Potent Therapeutic Factor for Brain Trauma Rehabilitation. Pharmaceutics, 12(5), 414. https://doi.org/10.3390/pharmaceutics12050414