Real Life Population Pharmacokinetics Modelling of Eight Factors VIII in Patients with Severe Haemophilia A: Is It Always Relevant to Switch to an Extended Half-Life?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Subjects, Sample Collection and Assay Method
2.2. PK Analysis
2.2.1. First Step—Basic Model Building
2.2.2. Second Step—Covariate Analysis
2.3. Internal Evaluation of the Model
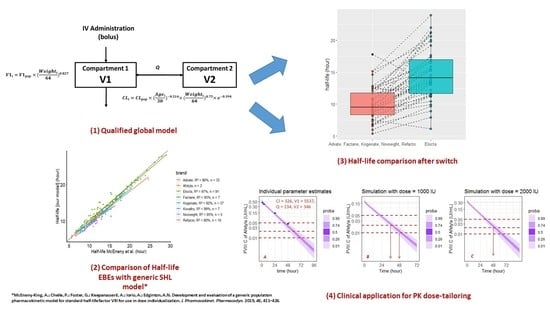
2.4. Model Qualification Process
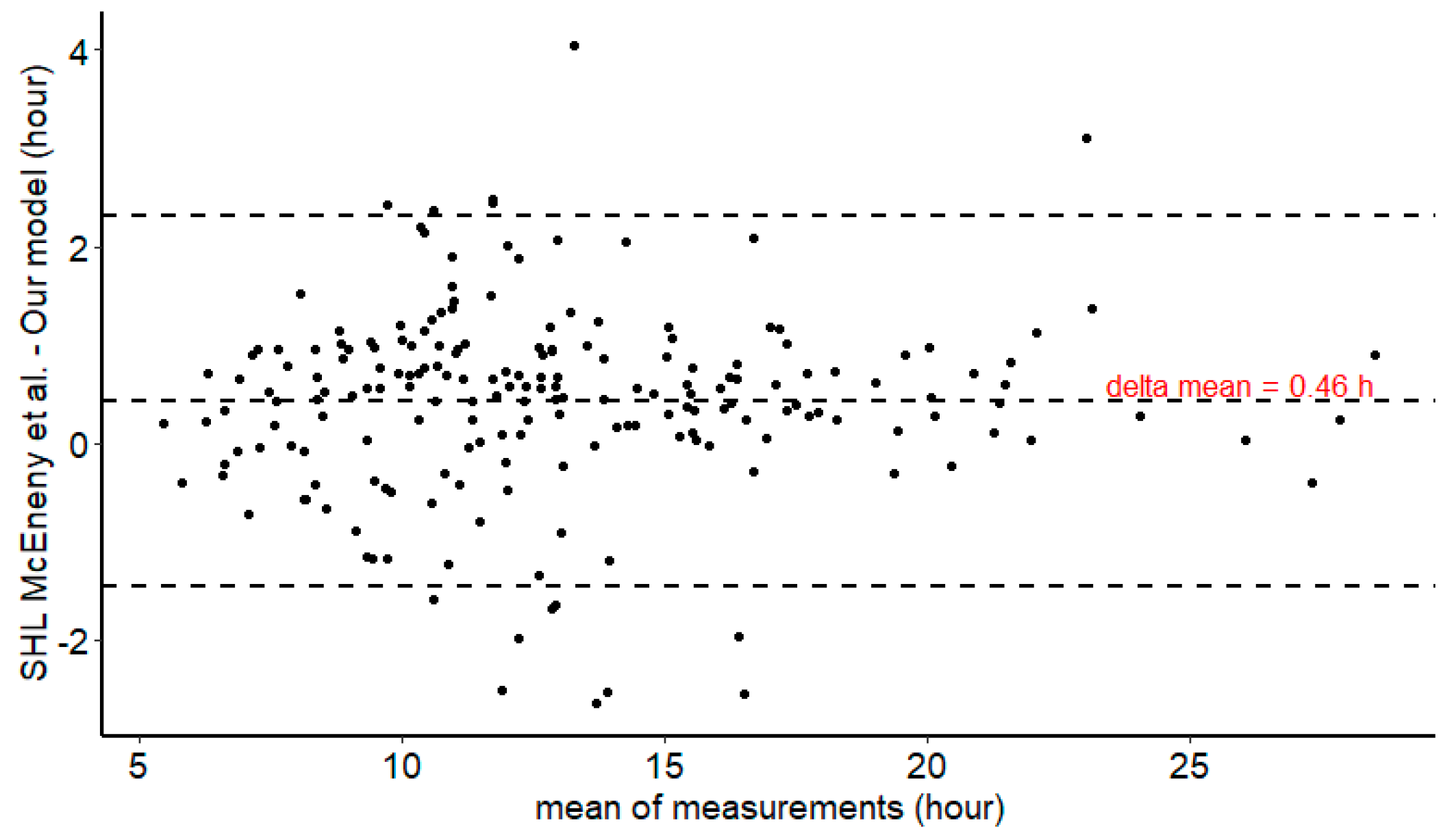
2.5. External Comparison of Half-Life Estimates Between Our Model and Mc Eneny-King SHL Model [21]
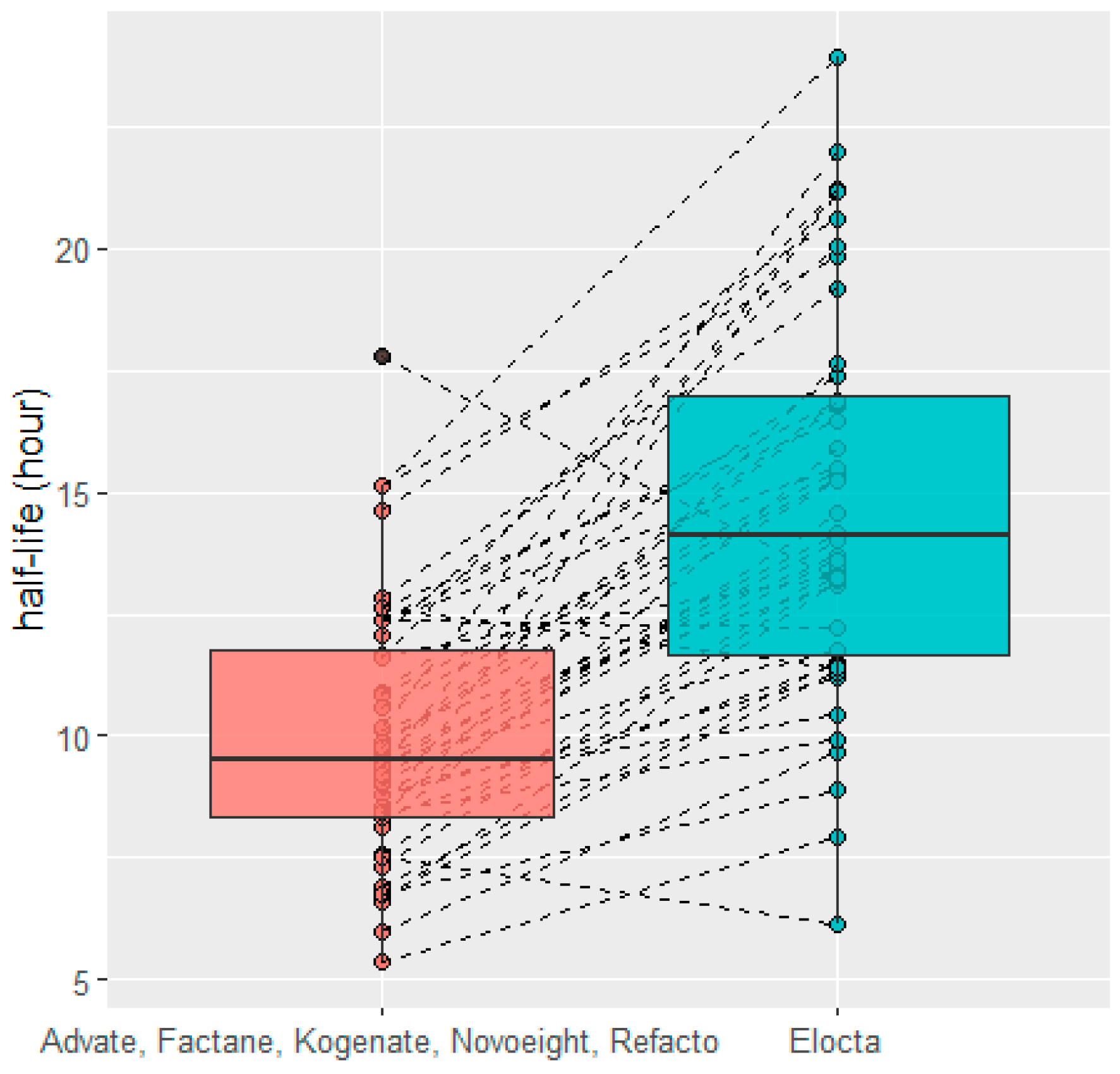
2.6. HL Comparison after Switch
3. Results
3.1. Subject Characteristics
3.2. Basic Model Building
3.3. Covariate Analysis
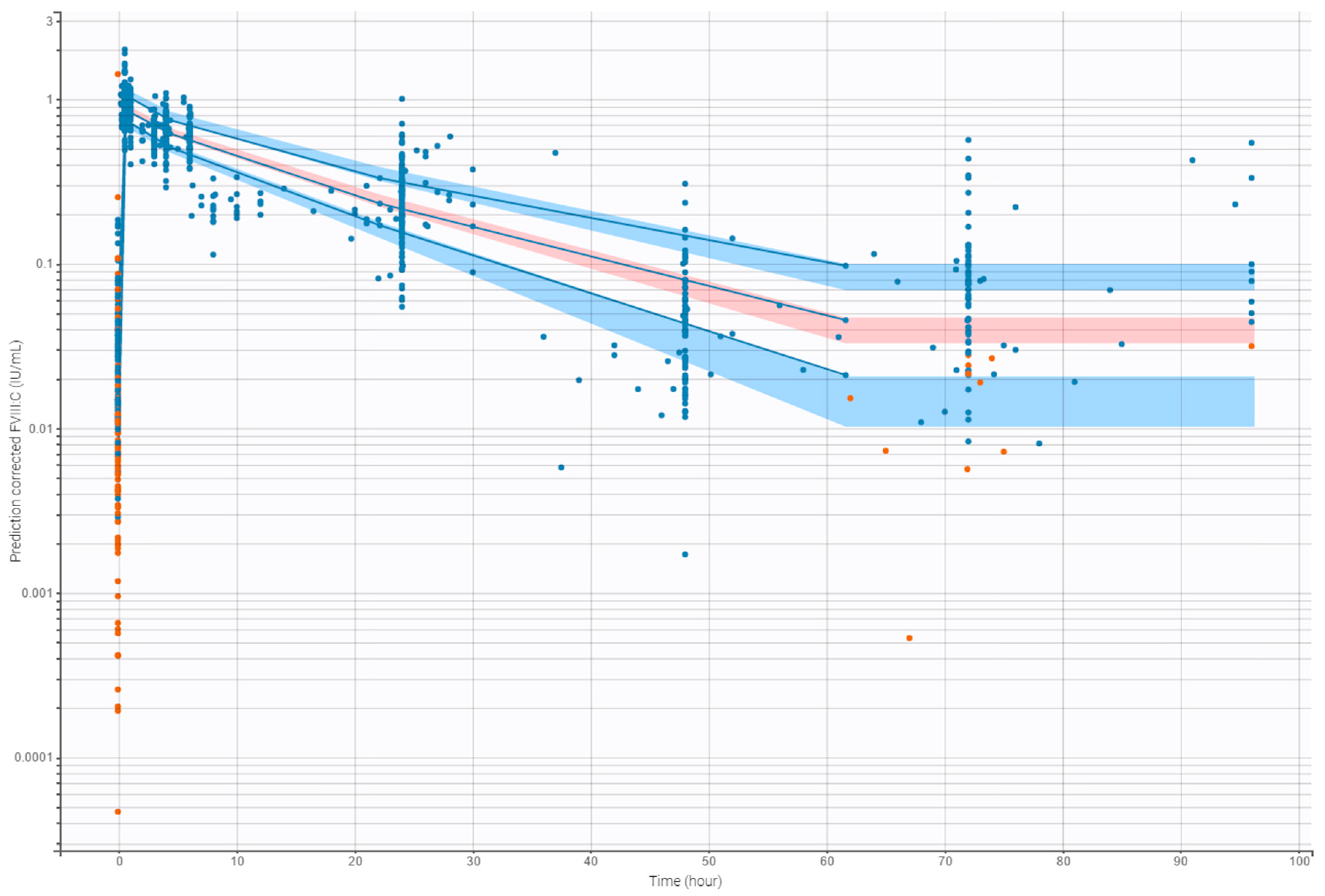
3.4. Internal Evaluation of the Final Model
3.5. HL Comparison between Our Global Model and SHL Generic Model of McEneny-King et al. [21]
3.6. HL Comparison after Switch
4. Discussion
5. Clinical Application (Example)
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lieuw, K. Many factor VIII products available in the treatment of hemophilia A: An embarrassment of riches? J. Blood Med. 2017, 8, 67–73. [Google Scholar] [CrossRef]
- Iorio, A.; Iserman, E.; Blanchette, V.; Dolan, G.; Escuriola Ettingshausen, C.; Hermans, C.; Negrier, C.; Oldenburg, J.; Reininger, A.; Rodriguez-Merchan, C.; et al. Target plasma factor levels for personalized treatment in haemophilia: A Delphi consensus statement. Haemophilia 2017, 23, e170–e179. [Google Scholar] [CrossRef] [PubMed]
- Delavenne, X.; Dargaud, Y.; Ollier, E.; Négrier, C. Dose tailoring of human cell line-derived recombinant factor VIII simoctocog alfa: Using a limited sampling strategy in patients with severe haemophilia A. Br. J. Clin. Pharmacol. 2019, 85, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Croteau, S.E.; Callaghan, M.U.; Davis, J.; Dunn, A.L.; Guerrera, M.; Khan, O.; Neufeld, E.J.; Raffini, L.J.; Recht, M.; Wang, M.; et al. Focusing in on use of pharmacokinetic profiles in routine hemophilia care. Res. Pract. Thromb. Haemost. 2018, 2, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Ostrenga, A.; Latzman, R.D.; Payne, C.; Hunt, Q.; Morris, A.; Iyer, R. Pharmacoeconomic impact of obesity in severe haemophilia children on clotting factor prophylaxis in a single institution. Haemophilia 2011, 17, 717–718. [Google Scholar] [CrossRef] [PubMed]
- Megías-Vericat, J.E.; Bonanad, S.; Haya, S.; Cid, A.R.; Marqués, M.R.; Monte, E.; Pérez-Alenda, S.; Bosch, P.; Querol, F.; Poveda, J.L. Bayesian pharmacokinetic-guided prophylaxis with recombinant factor VIII in severe or moderate haemophilia A. Thromb. Res. 2019, 174, 151–162. [Google Scholar] [CrossRef]
- Carlsson, M.; Berntorp, E.; Björkman, S.; Lethagen, S.; Ljung, R. Improved cost-effectiveness by pharmacokinetic dosing of factor VIII in prophylactic treatment of haemophilia A. Haemophilia 1997, 3, 96–101. [Google Scholar] [CrossRef]
- Iorio, A.; Keepanasseril, A.; Foster, G.; Navarro-Ruan, T.; McEneny-King, A.; Edginton, A.N.; Thabane, L. WAPPS-Hemo co-investigator network Development of a Web-Accessible Population Pharmacokinetic Service-Hemophilia (WAPPS-Hemo): Study Protocol. JMIR Res. Protoc. 2016, 5, e239. [Google Scholar] [CrossRef]
- McEneny-King, A.; Foster, G.; Iorio, A.; Edginton, A.N. Data Analysis Protocol for the Development and Evaluation of Population Pharmacokinetic Models for Incorporation Into the Web-Accessible Population Pharmacokinetic Service—Hemophilia (WAPPS-Hemo). JMIR Res. Protoc. 2016, 5, e232. [Google Scholar] [CrossRef]
- Iorio, A.; Blanchette, V.; Blatny, J.; Collins, P.; Fischer, K.; Neufeld, E. Estimating and interpreting the pharmacokinetic profiles of individual patients with hemophilia A or B using a population pharmacokinetic approach: Communication from the SSC of the ISTH. J. Thromb. Haemost. JTH 2017, 15, 2461–2465. [Google Scholar] [CrossRef]
- Lavielle, M.; Mentré, F. Estimation of population pharmacokinetic parameters of saquinavir in HIV patients with the MONOLIX software. J. Pharmacokinet. Pharmacodyn. 2007, 34, 229–249. [Google Scholar] [CrossRef] [PubMed]
- Cazaubon, Y.; Talineau, Y.; Feliu, C.; Konecki, C.; Russello, J.; Mathieu, O.; Djerada, Z. Population Pharmacokinetics Modelling and Simulation of Mitotane in Patients with Adrenocortical Carcinoma: An Individualized Dose Regimen to Target All Patients at Three Months? Pharmaceutics 2019, 11, 566. [Google Scholar] [CrossRef] [PubMed]
- Samson, A.; Lavielle, M.; Mentré, F. Extension of the SAEM algorithm to left-censored data in nonlinear mixed-effects model: Application to HIV dynamics model. Comput. Stat. Data Anal. 2006, 51, 1562–1574. [Google Scholar] [CrossRef]
- Oualha, M.; Urien, S.; Spreux-Varoquaux, O.; Bordessoule, A.; D’Agostino, I.; Pouard, P.; Tréluyer, J.-M. Pharmacokinetics, hemodynamic and metabolic effects of epinephrine to prevent post-operative low cardiac output syndrome in children. Crit. Care Lond. Engl. 2014, 18, R23. [Google Scholar] [CrossRef] [PubMed]
- Al-Sallami, H.S.; Goulding, A.; Grant, A.; Taylor, R.; Holford, N.; Duffull, S.B. Prediction of Fat-Free Mass in Children. Clin. Pharmacokinet. 2015, 54, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Cazaubon, Y.; Mauprivez, C.; Feliu, C.; Binet, L.; Oget, O.; Gozalo, C.; Djerada, Z. Population pharmacokinetics of articaine with 1:200,000 epinephrine during third molar surgery and simulation of high-dose regimens. Eur. J. Pharm. Sci. 2018, 114, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Bergstrand, M.; Hooker, A.C.; Wallin, J.E.; Karlsson, M.O. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011, 13, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Thai, H.-T.; Mentré, F.; Holford, N.H.G.; Veyrat-Follet, C.; Comets, E. Evaluation of bootstrap methods for estimating uncertainty of parameters in nonlinear mixed-effects models: A simulation study in population pharmacokinetics. J. Pharmacokinet. Pharmacodyn. 2014, 41, 15–33. [Google Scholar] [CrossRef]
- Djerada, Z.; Feliu, C.; Cazaubon, Y.; Smati, F.; Gomis, P.; Guerrot, D.; Charbit, B.; Fernandes, O.; Malinovsky, J.-M. Population Pharmacokinetic-Pharmacodynamic Modeling of Ropivacaine in Spinal Anesthesia. Clin. Pharmacokinet. 2017, 59, 1135–1147. [Google Scholar] [CrossRef]
- Lavielle, M.; Chauvin, J. Rsmlx: R Speaks “Monolix”; Lixoft: Antony, France, 2019. [Google Scholar]
- McEneny-King, A.; Chelle, P.; Foster, G.; Keepanasseril, A.; Iorio, A.; Edginton, A.N. Development and evaluation of a generic population pharmacokinetic model for standard half-life factor VIII for use in dose individualization. J. Pharmacokinet. Pharmacodyn. 2019, 46, 411–426. [Google Scholar] [CrossRef]
- Back, H.-M.; Lee, J.B.; Han, N.; Goo, S.; Jung, E.; Kim, J.; Song, B.; An, S.H.; Kim, J.T.; Rhie, S.J.; et al. Application of Size and Maturation Functions to Population Pharmacokinetic Modeling of Pediatric Patients. Pharmaceutics 2019, 11, 259. [Google Scholar] [CrossRef] [PubMed]
- Nestorov, I.; Neelakantan, S.; Ludden, T.M.; Li, S.; Jiang, H.; Rogge, M. Population pharmacokinetics of recombinant factor VIII Fc fusion protein. Clin. Pharmacol. Drug Dev. 2015, 4, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Carcao, M.D.; Chelle, P.; Clarke, E.; Kim, L.; Tiseo, L.; Morfini, M.; Hossain, T.; Rand, M.L.; Brown, C.; Edginton, A.; et al. Comparative pharmacokinetics of two extended half-life FVIII concentrates (Eloctate and Adynovate) in adolescents with hemophilia A: Is there a difference? J. Thromb. Haemost. JTH 2019, 17, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Hazendonk, H.; Fijnvandraat, K.; Lock, J.; Driessens, M.; van der Meer, F.; Meijer, K.; Kruip, M.; Gorkom, B.L.; Peters, M.; de Wildt, S.; et al. A population pharmacokinetic model for perioperative dosing of factor VIII in hemophilia A patients. Haematologica 2016, 101, 1159–1169. [Google Scholar] [CrossRef]
- Zhang, Y.; Roberts, J.; Tortorici, M.; Veldman, A.; St Ledger, K.; Feussner, A.; Sidhu, J. Population pharmacokinetics of recombinant coagulation factor VIII-SingleChain in patients with severe hemophilia A. J. Thromb. Haemost. JTH 2017, 15, 1106–1114. [Google Scholar] [CrossRef]
- Klarmann, D.; Eggert, C.; Geisen, C.; Becker, S.; Seifried, E.; Klingebiel, T.; Kreuz, W. Association of ABO(H) and I blood group system development with von Willebrand factor and Factor VIII plasma levels in children and adolescents. Transfusion (Paris) 2010, 50, 1571–1580. [Google Scholar] [CrossRef]
- Mahlangu, J.; Powell, J.S.; Ragni, M.V.; Chowdary, P.; Josephson, N.C.; Pabinger, I.; Hanabusa, H.; Gupta, N.; Kulkarni, R.; Fogarty, P.; et al. Phase 3 study of recombinant factor VIII Fc fusion protein in severe hemophilia A. Blood 2014, 123, 317–325. [Google Scholar] [CrossRef]
- Karafoulidou, A.; Suarez, E.; Anastasopoulou, I.; Katsarou, O.; Kouramba, A.; Kotsi, P.; Zografidis, A.; Lukas, J.C. Population pharmacokinetics of recombinant factor VIII:C (ReFacto) in adult HIV-negative and HIV-positive haemophilia patients. Eur. J. Clin. Pharmacol. 2009, 65, 1121–1130. [Google Scholar] [CrossRef]




| Patients Characteristics | Median (Min-Max) |
|---|---|
| Age (year) | 30 (3–77) |
| Weight (kg) | 64 (15.1–130) |
| Haemophilia status | Severe, n = 244; moderate, n = 11; minor, n = 3 |
| Treatment | Factane, n = 8; Advate, n = 44; Kogenate, n = 34; Kovaltry, n = 7; Afstyla, n = 5; Refacto, n = 18; Novoeight, n = 6; Elocta, n = 136 |
| Sampling Information | |
| Number of BLQ samples (%) | 99 (10.6%) |
| Number of samples per patient | 4 (1–11) |
| Number of patients with one sample | 63, no residual and FVIII:C ≥ LLOQ Factane, n = 6; Advate, n = 18; Kogenate, n = 7; Kovaltry, n = 4; Afstyla, n = 2; Refacto, n = 8; Novoeight, n = 4; Elocta, n = 14 |
| Dosing (IU) per patient | 2750 (500–5000) |
| Parameter | Value (RSE %) | Median of Bootstrap † (95% CI) |
|---|---|---|
| Fixed Effects | ||
| Cl (mL·h−1) | 204 (3.25) | 203 (188–218) |
| βEHL * | −0.394 (10.1) | −0.393 (–0.473–−0.313) |
| βAge ** | −0.214 (21.1) | −0.211 (–0.306–−0.115) |
| βWeight | 0.75 FIX | - |
| V1 (mL) | 2640 (2.04) | 2630 (2510–2749) |
| βWeight *** | 0.827 (4.75) | 0.833 (0.752–0.917) |
| Q (mL·h−1) | 135 (20.5) | 136 (54–241) |
| V2 (mL) | 339 (10.7) | 327 (221–433) |
| Between-Subject Variability | ||
| ω Cl (%) | 34.9 (5.13) | 34.6 (30.9–38.6) |
| ω V1 (%) | 23.2 (6.4) | 23.0 (18.9–27.2) |
| ω V2 (%) | 46.8 (17.4) | 50.0 (19.4–84.4) |
| Residual Variability | ||
| a (constant, IU·mL−1) | 0.00868 (8.88) | 0.00868 (0.00633–0.0112) |
| b (proportional, %) | 10.8 (6.6) | 10.6 (7.4–13.8) |
| Correlations | ||
| Corr (V1, Cl) | 0.599 (9.75) | 0.601 (0.457–0.743) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allard, Q.; Djerada, Z.; Pouplard, C.; Repessé, Y.; Desprez, D.; Galinat, H.; Frotscher, B.; Berger, C.; Harroche, A.; Ryman, A.; et al. Real Life Population Pharmacokinetics Modelling of Eight Factors VIII in Patients with Severe Haemophilia A: Is It Always Relevant to Switch to an Extended Half-Life? Pharmaceutics 2020, 12, 380. https://doi.org/10.3390/pharmaceutics12040380
Allard Q, Djerada Z, Pouplard C, Repessé Y, Desprez D, Galinat H, Frotscher B, Berger C, Harroche A, Ryman A, et al. Real Life Population Pharmacokinetics Modelling of Eight Factors VIII in Patients with Severe Haemophilia A: Is It Always Relevant to Switch to an Extended Half-Life? Pharmaceutics. 2020; 12(4):380. https://doi.org/10.3390/pharmaceutics12040380
Chicago/Turabian StyleAllard, Quentin, Zoubir Djerada, Claire Pouplard, Yohann Repessé, Dominique Desprez, Hubert Galinat, Birgit Frotscher, Claire Berger, Annie Harroche, Anne Ryman, and et al. 2020. "Real Life Population Pharmacokinetics Modelling of Eight Factors VIII in Patients with Severe Haemophilia A: Is It Always Relevant to Switch to an Extended Half-Life?" Pharmaceutics 12, no. 4: 380. https://doi.org/10.3390/pharmaceutics12040380
APA StyleAllard, Q., Djerada, Z., Pouplard, C., Repessé, Y., Desprez, D., Galinat, H., Frotscher, B., Berger, C., Harroche, A., Ryman, A., Flaujac, C., Chamouni, P., Guillet, B., Volot, F., Szymezak, J., Nguyen, P., & Cazaubon, Y. (2020). Real Life Population Pharmacokinetics Modelling of Eight Factors VIII in Patients with Severe Haemophilia A: Is It Always Relevant to Switch to an Extended Half-Life? Pharmaceutics, 12(4), 380. https://doi.org/10.3390/pharmaceutics12040380