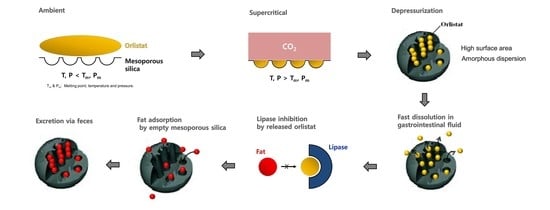
Pharmaceutical Characterization and In Vivo Evaluation of Orlistat Formulations Prepared by the Supercritical Melt-Adsorption Method Using Carbon Dioxide: Effects of Mesoporous Silica Type
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Mesoporous Silica Santa Barbara Amorphous (SBA-15)
2.3. Supercritical Melt-Adsorption Process Using CO2
2.4. Solid-State Characterization
2.4.1. Measurements of Specific Surface Area, Total Pore Volume and Pore Diameter
2.4.2. Differential Scanning Calorimetry
2.4.3. Powder X-ray Diffraction
2.4.4. Scanning Electron Microscopy
2.5. In-Vitro Dissolution Test
2.6. HPLC-UV Method for Orlistat Quantification
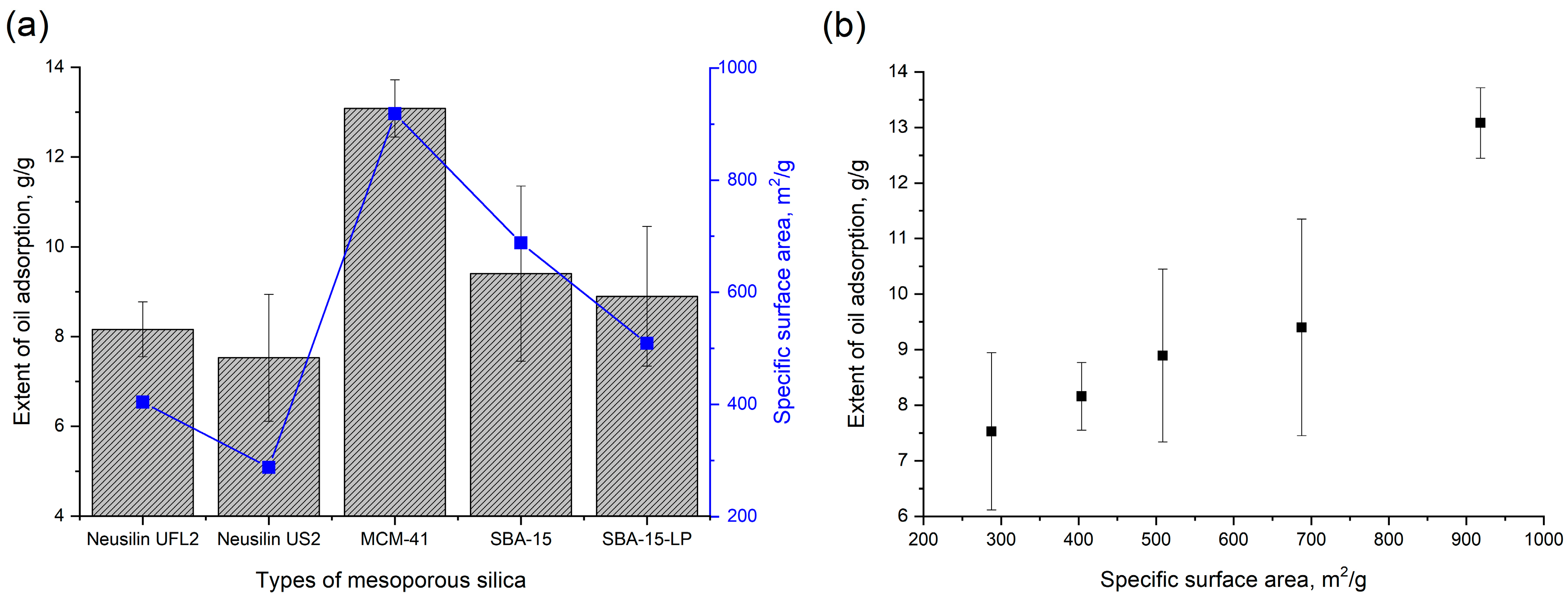
2.7. In Vitro Oil Adsorption Test
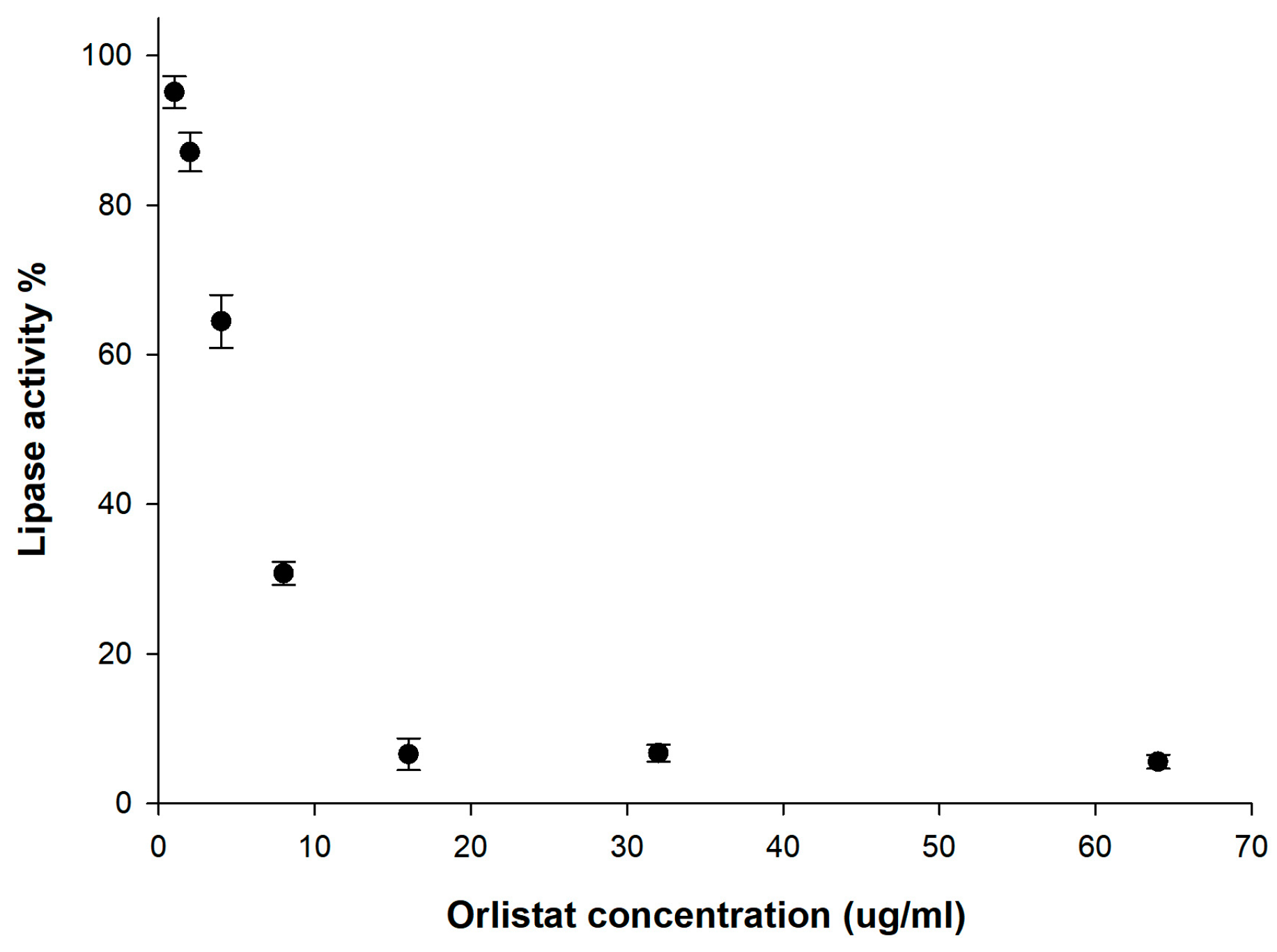
2.8. In Vitro Lipase Inhibition Test
2.9. In Vivo Animal Studies
2.9.1. Serum Triglyceride Levels in Sprague Dawley (SD) Rats after Administration of Orlistat Formulation
2.9.2. Data Analysis
2.9.3. Fat Excretion via Feces in ICR Mice
2.9.4. Oily Spotting Test
2.10. Statistical Analysis
3. Results and Discussions
3.1. Physicochemical Characterization of Orlistat-Loaded Mesoporous Silica
3.1.1. Morphology of Mesoporous Silica before and after Orlistat Adsorption by SCMA
3.1.2. Specific Surface Area, Total Pore Volume, and Pore Diameter of Various Mesoporous Silica before and after Orlistat Adsorption by SCMA
3.1.3. DSC and PXRD Analyses
3.2. In Vitro Dissolution
3.3. In Vitro Lipase Inhibition
3.4. In Vitro Oil Adsorption Capacity of Mesoporous Silica
3.5. In Vivo Study of Orlistat-Loaded Mesoporous Silica
3.5.1. Serum TG Level
3.5.2. Fat Excretion via Feces
3.5.3. Oily Spotting Number
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hadvary, P.; Lengsfeld, H.; Wolfer, H. Inhibition of pancreatic lipase in vitro by the covalent inhibitor tetrahydrolipstatin. Biochem. J. 1988, 256, 357–361. [Google Scholar]
- Borgström, B. Mode of action of tetrahydrolipstatin: A derivative of the naturally occurring lipase inhibitor lipstatin. BBA Lipids Lipid Metab. 1988, 962, 308–316. [Google Scholar]
- Zhi, J.; Melia, A.T.; Eggers, H.; Joly, R.; Patel, I.H. Review of limited systemic absorption of orlistat, a lipase inhibitor, in healthy human volunteers. J. Clin. Pharmacol. 1995, 35, 1103–1108. [Google Scholar]
- Schwartz, S.M.; Bansal, V.P.; Hale, C.; Rossi, M.; Engle, J.P. Compliance, behavior change, and weight loss with orlistat in an over-the-counter setting. Obesity 2008, 16, 623–629. [Google Scholar]
- Sahebkar, A.; Simental-Mendia, L.E.; Reiner, Ž.; Kovanen, P.T.; Simental-Mendía, M.; Bianconi, V.; Pirro, M. Effect of orlistat on plasma lipids and body weight: A systematic review and meta-analysis of 33 randomized controlled trials. Pharmacol. Res. 2017, 122, 53–65. [Google Scholar]
- Tonstad, S.; Pometta, D.; Erkelens, D.; Ose, L.; Moccetti, T.; Schouten, J.; Golay, A.; Reitsma, J.; Del Bufalo, A.; Pasotti, E. The effect of the gastrointestinal lipase inhibitor, orlistat, on serum lipids and lipoproteins in patients with primary hyperlipidaemia. Eur. J. Clin. Pharmacol. 1994, 46, 405–410. [Google Scholar]
- Heck, A.M.; Yanovski, J.A.; Calis, K.A. Orlistat, a new lipase inhibitor for the management of obesity. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2000, 20, 270–279. [Google Scholar]
- Clarysse, S.; Psachoulias, D.; Brouwers, J.; Tack, J.; Annaert, P.; Duchateau, G.; Reppas, C.; Augustijns, P. Postprandial changes in solubilizing capacity of human intestinal fluids for BCS class II drugs. Pharm. Res. 2009, 26, 1456–1466. [Google Scholar]
- Choi, Y.H.; Choi, Y.H.; Han, H.-K.; Han, H.-K. Nanomedicines: Current status and future perspectives in aspect of drug delivery and pharmacokinetics. J. Pharm. Investig. 2018, 48, 43–60. [Google Scholar]
- Ahsan, M.N.; Verma, P.R.P. Enhancement of in vitro dissolution and pharmacodynamic potential of olanzapine using solid SNEDDS. J. Pharm. Investig. 2018, 48, 269–278. [Google Scholar]
- Singh, D.; Bedi, N.; Tiwary, A.K. Enhancing solubility of poorly aqueous soluble drugs: Critical appraisal of techniques. J. Pharm. Investig. 2018, 48, 509–526. [Google Scholar]
- Davidson, M.H.; Hauptman, J.; DiGirolamo, M.; Foreyt, J.P.; Halsted, C.H.; Heber, D.; Heimburger, D.C.; Lucas, C.P.; Robbins, D.C.; Chung, J. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: A randomized controlled trial. JAMA 1999, 281, 235–242. [Google Scholar]
- Filippatos, T.D.; Derdemezis, C.S.; Gazi, I.F.; Nakou, E.S.; Mikhailidis, D.P.; Elisaf, M.S. Orlistat-associated adverse effects and drug interactions. Drug Saf. 2008, 31, 53–65. [Google Scholar]
- Park, J.W.; Bin, S.A.; Lee, J.A.; Kim, J.J. Pharmaceutical Composition Comprising Lipase Inhibitor and Lipophilic Oil Absorbent and Oral Formulation Prepared Therefrom. U.S. Patent 8,246,985, 21 August 2012. [Google Scholar]
- Bailly, J.; Fleury, A.; Hadvary, P.; Lengsfeld, H.; Steffen, H. Pharmaceutical Composition Containing Chitosan. U.S. Patent 6,030,953, 29 February 2000. [Google Scholar]
- Niazi, S.K. Pharmaceutical Composition Containing Psyllium Fiber and a Lipase Inhibitor. U.S. Patent 625,1421, 26 June 2001. [Google Scholar]
- Thompson, R.J. Method and Composition of a Carminative Herb or Natural Supplement to Decrease the Adverse Effects of Orlistat, and Oral Lipase Inhibitor. US Patent 11,585,633, 20 March 2008. [Google Scholar]
- Barbier, P.; Hadvary, P.; Lengsfeld, H. Method of Reducing Gastrointestinal Side Effects Associated with Orlistat Treatment. U.S. Patent 6,756,364, 29 June 2004. [Google Scholar]
- Li, W.; Liu, J.; Zhao, D. Mesoporous materials for energy conversion and storage devices. Nat. Rev. Mater. 2016, 1, 16023. [Google Scholar]
- Prokopowicz, M.; Przyjazny, A. Synthesis of sol-gel mesoporous silica materials providing a slow release of doxorubicin. J. Microencapsul. 2007, 24, 694–713. [Google Scholar]
- Tourné-Péteilh, C.; Lerner, D.A.; Charnay, C.; Nicole, L.; Bégu, S.; Devoisselle, J.M. The Potential of Ordered Mesoporous Silica for the Storage of Drugs: The Example of a Pentapeptide Encapsulated in a MSU-Tween 80. Chem. Phys. Chem. 2003, 4, 281–286. [Google Scholar]
- Tozuka, Y.; Sugiyama, E.; Takeuchi, H. Release profile of insulin entrapped on mesoporous materials by freeze–thaw method. Int. J. Pharm. 2010, 386, 172–177. [Google Scholar]
- Vallet-Regí, M.; Ruiz-González, L.; Izquierdo-Barba, I.; González-Calbet, J.M. Revisiting silica based ordered mesoporous materials: Medical applications. J. Mater. Chem. 2006, 16, 26–31. [Google Scholar]
- Hong, S.h.; Hong, S.h.; Choi, Y.; Choi, Y. Mesoporous silica-based nanoplatforms for the delivery of photodynamic therapy agents. J. Pharm. Investig. 2018, 48, 3–17. [Google Scholar] [CrossRef]
- Aerts, C.; Verraedt, E.; Depla, A.; Follens, L.; Froyen, L.; Van Humbeeck, J.; Augustijns, P.; Van den Mooter, G.; Mellaerts, R.; Martens, J. Potential of amorphous microporous silica for ibuprofen controlled release. Int. J. Pharm. 2010, 397, 84–91. [Google Scholar]
- Munoz, B.; Ramila, A.; Perez-Pariente, J.; Diaz, I.; Vallet-Regi, M. MCM-41 organic modification as drug delivery rate regulator. Chem. Mater. 2003, 15, 500–503. [Google Scholar]
- Shen, S.C.; Ng, W.K.; Chia, L.; Dong, Y.C.; Tan, R.B. Stabilized amorphous state of ibuprofen by co-spray drying with mesoporous SBA-15 to enhance dissolution properties. J. Pharm. Sci. 2010, 99, 1997–2007. [Google Scholar]
- Salonen, J.; Laitinen, L.; Kaukonen, A.M.; Tuura, J.; Björkqvist, M.; Heikkilä, T.; Vähä-Heikkilä, K.; Hirvonen, J.; Lehto, V.-P. Mesoporous silicon microparticles for oral drug delivery: Loading and release of five model drugs. J. Control. Release 2005, 108, 362–374. [Google Scholar]
- Mellaerts, R.; Mols, R.; Jammaer, J.A.; Aerts, C.A.; Annaert, P.; Van Humbeeck, J.; Van den Mooter, G.; Augustijns, P.; Martens, J.A. Increasing the oral bioavailability of the poorly water soluble drug itraconazole with ordered mesoporous silica. Eur. J. Pharm. Biopharm. 2008, 69, 223–230. [Google Scholar]
- Zhang, Y.; Zhi, Z.; Jiang, T.; Zhang, J.; Wang, Z.; Wang, S. Spherical mesoporous silica nanoparticles for loading and release of the poorly water-soluble drug telmisartan. J. Control. Release 2010, 145, 257–263. [Google Scholar]
- Charnay, C.; Bégu, S.; Tourné-Péteilh, C.; Nicole, L.; Lerner, D.; Devoisselle, J.-M. Inclusion of ibuprofen in mesoporous templated silica: Drug loading and release property. Eur. J. Pharm. Biopharm. 2004, 57, 533–540. [Google Scholar]
- Liu, X.; Feng, X.; Williams, R.O.; Zhang, F. Characterization of amorphous solid dispersions. J. Pharm. Investig. 2018, 48, 19–41. [Google Scholar]
- Sanganwar, G.P.; Gupta, R.B. Dissolution-rate enhancement of fenofibrate by adsorption onto silica using supercritical carbon dioxide. Int. J. Pharm. 2008, 360, 213–218. [Google Scholar]
- Cha, K.-H.; Cho, K.-J.; Kim, M.-S.; Kim, J.-S.; Park, H.J.; Park, J.; Cho, W.; Park, J.-S.; Hwang, S.-J. Enhancement of the dissolution rate and bioavailability of fenofibrate by a melt-adsorption method using supercritical carbon dioxide. Int. J. Nanomed. 2012, 7, 5565. [Google Scholar]
- Miura, H.; Kanebako, M.; Shirai, H.; Nakao, H.; Inagi, T.; Terada, K. Enhancement of dissolution rate and oral absorption of a poorly water-soluble drug, K-832, by adsorption onto porous silica using supercritical carbon dioxide. Eur. J. Pharm. Biopharm. 2010, 76, 215–221. [Google Scholar]
- Franco, P.; De Marco, I. Supercritical CO2 adsorption of non-steroidal anti-inflammatory drugs into biopolymer aerogels. J. CO2 Util. 2020, 36, 40–53. [Google Scholar]
- Reiser, S.; Shaban, M.; Weber, A.; Türk, M. CO2 assisted deposition of R/S-ibuprofen on different porous carrier materials: Influence of carrier properties on loading and dissolution behavior. J. CO2 Util. 2018, 25, 216–225. [Google Scholar]
- Park, H.J.; Kim, M.-S.; Kim, J.-S.; Cho, W.; Park, J.; Cha, K.-H.; Kang, Y.-S.; Hwang, S.-J. Solid-state carbon NMR characterization and investigation of intrinsic dissolution behavior of fluconazole polymorphs, anhydrate forms I and II. Int. J. Pharm. 2010, 58, 1243–1247. [Google Scholar]
- Abuzar, S.M.; Hyun, S.-M.; Kim, J.-H.; Park, H.J.; Kim, M.-S.; Park, J.-S.; Hwang, S.-J. Enhancing the solubility and bioavailability of poorly water-soluble drugs using supercritical antisolvent (SAS) process. Int. J. Pharm. 2018, 538, 1–13. [Google Scholar]
- Pasquali, I.; Bettini, R.; Giordano, F. Supercritical fluid technologies: An innovative approach for manipulating the solid-state of pharmaceuticals. Adv. Drug Deliv. Rev. 2008, 60, 399–410. [Google Scholar]
- Kim, M.-S.; Jin, S.-J.; Kim, J.-S.; Park, H.J.; Song, H.-S.; Neubert, R.H.; Hwang, S.-J. Preparation, characterization and in vivo evaluation of amorphous atorvastatin calcium nanoparticles using supercritical antisolvent (SAS) process. Eur. J. Pharm. Biopharm. 2008, 69, 454–465. [Google Scholar]
- Li, S.; Qiao, C.; Li, Z.; Hui, Y. The effect of permeability on supercritical CO2 diffusion coefficient and determination of diffusive tortuosity of porous media under reservoir conditions. J. CO2 Util. 2018, 28, 1–14. [Google Scholar]
- Han, Y.; Zheng, H.; Jing, X.; Zheng, L. Swelling behavior of polyester in supercritical carbon dioxide. J. CO2 Util. 2018, 26, 45–51. [Google Scholar]
- Wang, B.-C.; Su, C.-S. Solid solubility measurement of ipriflavone in supercritical carbon dioxide and microparticle production through the rapid expansion of supercritical solutions process. J. CO2 Util. 2020, 37, 285–294. [Google Scholar]
- Knez, Ž.; Škerget, M.; Hrnčič, M.K.; Čuček, D. Particle formation using sub-and supercritical fluids. In Supercritical Fluid Technology for Energy and Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 31–67. [Google Scholar]
- Weidner, E. High pressure micronization for food applications. J. Supercrit. Fluids 2009, 47, 556–565. [Google Scholar]
- Fischer, K.; Wilken, M.; Gmehling, J. The effect of gas pressure on the melting behavior of compounds. Fluid Phase Equilib. 2003, 210, 199–214. [Google Scholar]
- Méndez-Santiago, J.; Teja, A.S. The solubility of solids in supercritical fluids. Fluid Phase Equilib. 1999, 158, 501–510. [Google Scholar]
- Jana, S.K.; Nishida, R.; Shindo, K.; Kugita, T.; Namba, S. Pore size control of mesoporous molecular sieves using different organic auxiliary chemicals. Microporous Mesoporous Mater. 2004, 68, 133–142. [Google Scholar]
- Hussein, K.; Türk, M.; Wahl, M.A. Drug loading into β-cyclodextrin granules using a supercritical fluid process for improved drug dissolution. Eur. J. Pharm. Sci. 2008, 33, 306–312. [Google Scholar] [PubMed]
- Kumagai, S.; Noguchi, Y.; Kurimoto, Y.; Takeda, K. Oil adsorbent produced by the carbonization of rice husks. Waste Manag. 2007, 27, 554–561. [Google Scholar] [PubMed]
- Dolenc, A.; Govedarica, B.; Kocbek, P.; Srčič, S.; Kristl, J. Nanosized particles of orlistat with enhanced in vitro dissolution rate and lipase inhibition. Int. J. Pharm. 2010, 396, 149–155. [Google Scholar] [PubMed]
- Smedes, F. Determination of total lipid using non-chlorinated solvents. Analyst 1999, 124, 1711–1718. [Google Scholar]
- Gao, F.; Lu, Q.; Zhao, D. In situ adsorption method for synthesis of binary semiconductor CdS nanocrystals inside mesoporous SBA-15. Chem. Phys. Lett. 2002, 360, 585–591. [Google Scholar]
- Qiao, S.; Djojoputro, H.; Hu, Q.; Lu, G. Synthesis and lysozyme adsorption of rod-like large-pore periodic mesoporous organosilica. Prog. Solid State Chem. 2006, 34, 249–256. [Google Scholar]
- Wang, L.; Qi, T.; Zhang, Y.; Chu, J. Morphosynthesis route to large-pore SBA-15 microspheres. Microporous Mesoporous Mater. 2006, 91, 156–160. [Google Scholar]
- Van Speybroeck, M.; Barillaro, V.; Do Thi, T.; Mellaerts, R.; Martens, J.; Van Humbeeck, J.; Vermant, J.; Annaert, P.; Van Den Mooter, G.; Augustijns, P. Ordered mesoporous silica material SBA-15: A broad-spectrum formulation platform for poorly soluble drugs. J. Pharm. Sci. 2009, 98, 2648–2658. [Google Scholar] [PubMed]
- Numpilai, T.; Muenmee, S.; Witoon, T. Impact of pore characteristics of silica materials on loading capacity and release behavior of ibuprofen. Mater. Sci. Eng. C 2016, 59, 43–52. [Google Scholar]
- Bouchoucha, M.; C.-Gaudreault, R.; Fortin, M.A.; Kleitz, F. Mesoporous silica nanoparticles: Selective surface functionalization for optimal relaxometric and drug loading performances. Adv. Funct. Mater. 2014, 24, 5911–5923. [Google Scholar]
- Andersson, J.; Rosenholm, J.; Areva, S.; Lindén, M. Influences of material characteristics on ibuprofen drug loading and release profiles from ordered micro-and mesoporous silica matrices. Chem. Mater. 2004, 16, 4160–4167. [Google Scholar]
- Keri, V.; Csorvasi, A.; Aronhime, J. Preparation of Orlistat and Orlistat Crystalline Forms. U.S. Patent 6,734,314, 11 May 2004. [Google Scholar]
- Sliwinska-Bartkowiak, M.; Dudziak, G.; Gras, R.; Sikorski, R.; Radhakrishnan, R.; Gubbins, K.E. Freezing behavior in porous glasses and MCM-41. Colloids Surf. A Physicochem. Eng. Asp. 2001, 187, 523–529. [Google Scholar]
- Healy, A.M.; Worku, Z.A.; Kumar, D.; Madi, A.M. Pharmaceutical solvates, hydrates and amorphous forms: A special emphasis on cocrystals. Adv. Drug Deliv. Rev. 2017, 117, 25–46. [Google Scholar]
- Shen, S.-C.; Ng, W.K.; Chia, L.; Hu, J.; Tan, R.B.H. Physical state and dissolution of ibuprofen formulated by co-spray drying with mesoporous silica: Effect of pore and particle size. Int. J. Pharm. 2011, 410, 188–195. [Google Scholar]
- Sebban-Kreuzer, C.; Ayvazian, L.; Juhel, C.; Salles, J.P.; Chapus, C.; Kerfelec, B. Inhibitory effect of the pancreatic lipase C-terminal domain on intestinal lipolysis in rats fed a high-fat diet: Chronic study. Int. J. Obes. 2003, 27, 319–325. [Google Scholar]
- Zhao, J.; Ren, W.; Cheng, H.-M. Graphene sponge for efficient and repeatable adsorption and desorption of water contaminations. J. Mater. Chem. 2012, 22, 20197–20202. [Google Scholar]
- Sugimoto, M.; Okagaki, T.; Narisawa, S.; Koida, Y.; Nakajima, K. Improvement of dissolution characteristics and bioavailability of poorly water-soluble drugs by novel cogrinding method using water-soluble polymer. Int. J. Pharm. 1998, 160, 11–19. [Google Scholar]
- Vaughn, J.M.; McConville, J.T.; Crisp, M.T.; Johnston, K.P.; Williams, R.O. Supersaturation Produces High Bioavailability of Amorphous Danazol Particles Formed by Evaporative Precipitation into Aqueous Solution and Spray Freezing into Liquid Technologies. Drug Dev. Ind. Pharm. 2006, 32, 559–567. [Google Scholar] [PubMed]
- Yamashita, K.; Nakate, T.; Okimoto, K.; Ohike, A.; Tokunaga, Y.; Ibuki, R.; Higaki, K.; Kimura, T. Establishment of new preparation method for solid dispersion formulation of tacrolimus. Int. J. Pharm. 2003, 267, 79–91. [Google Scholar] [PubMed]








| Formulation | D:A (w/w)1 | Specific Surface Area (m2/g) | Total Pore Volume (cm3/g) | Pore Diameter (nm) | %DLmax_theo2 | RC3 (%) | Solid States4 | DE605 | Lipase Inhibition (%)6 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Pure adsorbent | Neusilin®UFL2 | 0:100 | 403.93 | 1.4137 | 17.01 | 58.1 | - | Amorphous | - | - |
| Neusilin®US2 | 0:100 | 287.65 | 0.9568 | 16.45 | 48.4 | - | Amorphous | - | - | |
| MCM_41 | 0:100 | 918.27 | 1.0333 | 4.09 | 50.3 | - | Amorphous | - | - | |
| SBA_15 | 0:100 | 687.65 | 0.9617 | 5.59 | 48.5 | - | Amorphous | - | - | |
| SBA_15_LP | 0:100 | 508.62 | 0.9385 | 37.21 | 47.9 | - | Amorphous | - | - | |
| SCMA processed with orlistat | UFL_1 | 20:80 | 105.21 | 0.6491 | - | - | 0 | Amorphous | 87.6 ± 0.6 | 88.8 ± 3.4 |
| UFL_2 | 40:60 | 19.91 | 0.2511 | - | - | 0 | Amorphous | 84.8 ± 3.1 | 87.5 ± 2.5 | |
| UFL_3 | 60:40 | 6.42 | 0.0531 | - | - | 32.0 | Amorphous+Crystal | 55.1 ± 1.0 | 83.2 ± 4.8 | |
| US_1 | 20:80 | 149.44 | 0.0690 | - | - | 0 | Amorphous | 70.3 ± 3.3 | 84.5 ± 1.7 | |
| US_2 | 40:60 | 49.70 | 0.0229 | - | - | 0 | Amorphous | 68.5 ± 1.2 | 88.4 ± 0.6 | |
| US_3 | 60:40 | 24.69 | 0.0111 | - | - | 52.0 | Amorphous+Crystal | 49.7 ± 0.7 | 74.5 ± 2.6 | |
| MCM_1 | 20:80 | 759.93 | 0.3506 | - | - | 0 | Amorphous | 44.9 ± 1.3 | 55.7 ± 2.5 | |
| MCM_2 | 40:60 | 34.35 | 0.0162 | - | - | 0 | Amorphous | 42.5 ± 1.3 | 53.2 ± 2.3 | |
| MCM_3 | 60:40 | 7.12 | 0.0030 | - | - | 41.4 | Amorphous+Crystal | 36.8 ± 1.0 | 53.5 ± 2.4 | |
| SBA_1 | 20:80 | 281.98 | 0.1358 | - | - | 0 | Amorphous | 76.9 ± 0.4 | 85.6 ± 5.5 | |
| SBA_2 | 40:60 | 45.51 | 0.0209 | - | - | 0 | Amorphous | 76.1 ± 1.9 | 84.6 ± 1.4 | |
| SBA_3 | 60:40 | 20.724 | 0.0097 | - | - | 50.3 | Amorphous+Crystal | 45.0 ± 1.0 | 38.5 ± 9.0 | |
| SBA_LP_1 | 20:80 | 127.92 | 0.0651 | - | - | 0 | Amorphous | 63.5 ± 1.4 | 55.5 ± 0.0 | |
| SBA_LP_2 | 40:60 | 22 | 0.0043 | - | - | 4.1 | Amorphous+Crystal | 59.2 ± 1.0 | 51.9 ± 1.4 | |
| SBA_LP_3 | 60:40 | 6.57 | 0.0036 | - | - | 68.8 | Amorphous+Crystal | 17.4 ± 0.3 | 15.3 ± 1.2 | |
| Commercial product | - | - | - | - | - | 99.2 | Crystal | 14.1 ± 1.3 | 16.8 ± 1.9 | |
| Raw orlistat | 100:0 | - | - | - | - | 100 | Crystal | 9.7 ± 1.1 | 5.4 ± 0.7 | |
| Formulation | PK Parameter of ΔTG | Relative PK Parameter (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| ΔTGmax (mg/dL) | AUC0→12 h (mg·hr/dL) | Compare to Control | Compare to Raw Orlistat | Compare to Commercial Product | ||||
| ΔTG%1 | AUC%2 | ΔTG%3 | AUC%4 | ΔTG%5 | AUC%6 | |||
| Control | 179.0 ± 18.9 | 1149.2 ± 219.4 | - | - | - | - | - | - |
| Raw orlistat | 140.7 ± 64.1 | 648.0 ± 203.2 | 78.6 | 56.4 | - | - | - | - |
| Commercial product | 128.0 ± 54.0 | 526.4 ± 138.9 | 71.5 | 45.8 | 91.0 | 81.2 | - | - |
| UFL_1 | 93.5 ± 27.5 | 345.9 ± 120.3 | 52.2 | 30.1 | 66.5 | 53.4 | 73.0 | 65.7 |
| US_1 | 83.3 ± 10.1 | 391.9 ± 164.9 | 46.5 | 34.1 | 59.2 | 60.5 | 65.1 | 74.4 |
| MCM_1 | 108.7 ± 18.3 | 410.2 ± 145.2 | 60.7 | 35.7 | 77.3 | 63.3 | 84.9 | 77.9 |
| SBA_1 | 87.8 ± 37.5 | 427.7 ± 144.8 | 49.1 | 37.2 | 62.4 | 66.0 | 68.6 | 81.3 |
| SBA_LP_1 | 93.4 ± 45.2 | 406.9 ± 136.5 | 52.2 | 35.4 | 66.4 | 62.8 | 73.0 | 77.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Cha, K.-H.; Hong, S.H.; Abuzar, S.M.; Lee, S.; Ha, E.-S.; Kim, J.-S.; Baek, I.-H.; Kim, M.-S.; Hwang, S.-J. Pharmaceutical Characterization and In Vivo Evaluation of Orlistat Formulations Prepared by the Supercritical Melt-Adsorption Method Using Carbon Dioxide: Effects of Mesoporous Silica Type. Pharmaceutics 2020, 12, 333. https://doi.org/10.3390/pharmaceutics12040333
Park H, Cha K-H, Hong SH, Abuzar SM, Lee S, Ha E-S, Kim J-S, Baek I-H, Kim M-S, Hwang S-J. Pharmaceutical Characterization and In Vivo Evaluation of Orlistat Formulations Prepared by the Supercritical Melt-Adsorption Method Using Carbon Dioxide: Effects of Mesoporous Silica Type. Pharmaceutics. 2020; 12(4):333. https://doi.org/10.3390/pharmaceutics12040333
Chicago/Turabian StylePark, Heejun, Kwang-Ho Cha, Seung Hyeon Hong, Sharif Md Abuzar, Seungyeol Lee, Eun-Sol Ha, Jeong-Soo Kim, In-Hwan Baek, Min-Soo Kim, and Sung-Joo Hwang. 2020. "Pharmaceutical Characterization and In Vivo Evaluation of Orlistat Formulations Prepared by the Supercritical Melt-Adsorption Method Using Carbon Dioxide: Effects of Mesoporous Silica Type" Pharmaceutics 12, no. 4: 333. https://doi.org/10.3390/pharmaceutics12040333
APA StylePark, H., Cha, K.-H., Hong, S. H., Abuzar, S. M., Lee, S., Ha, E.-S., Kim, J.-S., Baek, I.-H., Kim, M.-S., & Hwang, S.-J. (2020). Pharmaceutical Characterization and In Vivo Evaluation of Orlistat Formulations Prepared by the Supercritical Melt-Adsorption Method Using Carbon Dioxide: Effects of Mesoporous Silica Type. Pharmaceutics, 12(4), 333. https://doi.org/10.3390/pharmaceutics12040333