Effect of Food and an Animal’s Sex on P-Glycoprotein Expression and Luminal Fluids in the Gastrointestinal Tract of Wistar Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Experimental Animals
2.3. Characterisation of Luminal Fluids in the GI Tract
2.4. Absolute Quantification of P-glycoprotein
2.4.1. Tissue Preparation
2.4.2. Total Protein Extraction
2.4.3. Protein Sample Digestion
2.4.4. LC-MS/MS Analysis
2.5. Statistical Analysis
3. Results
3.1. Sex Differences in Luminal Fluids Along the Rat GI Tract
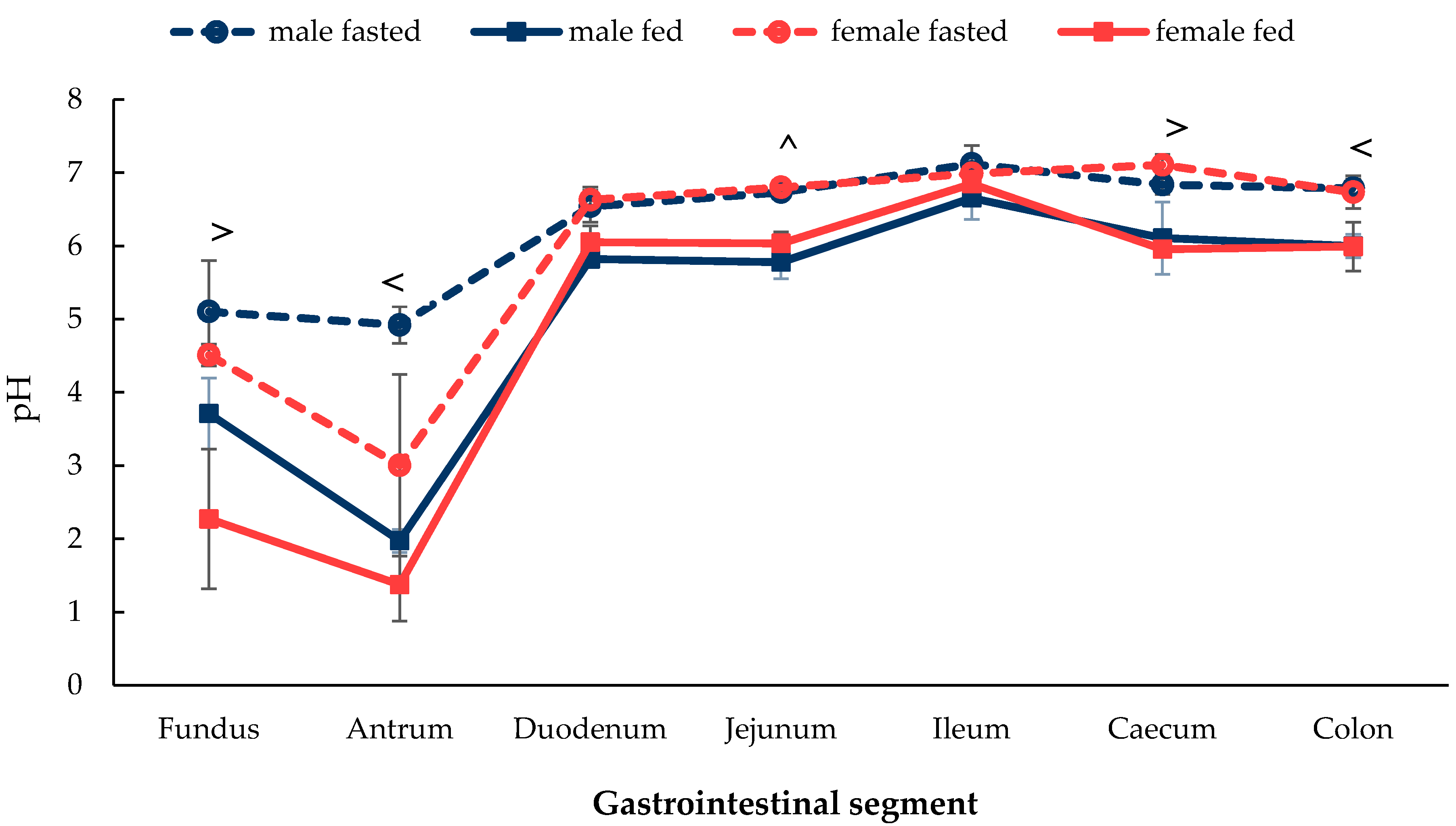
3.1.1. Gastrointestinal Fluid pH
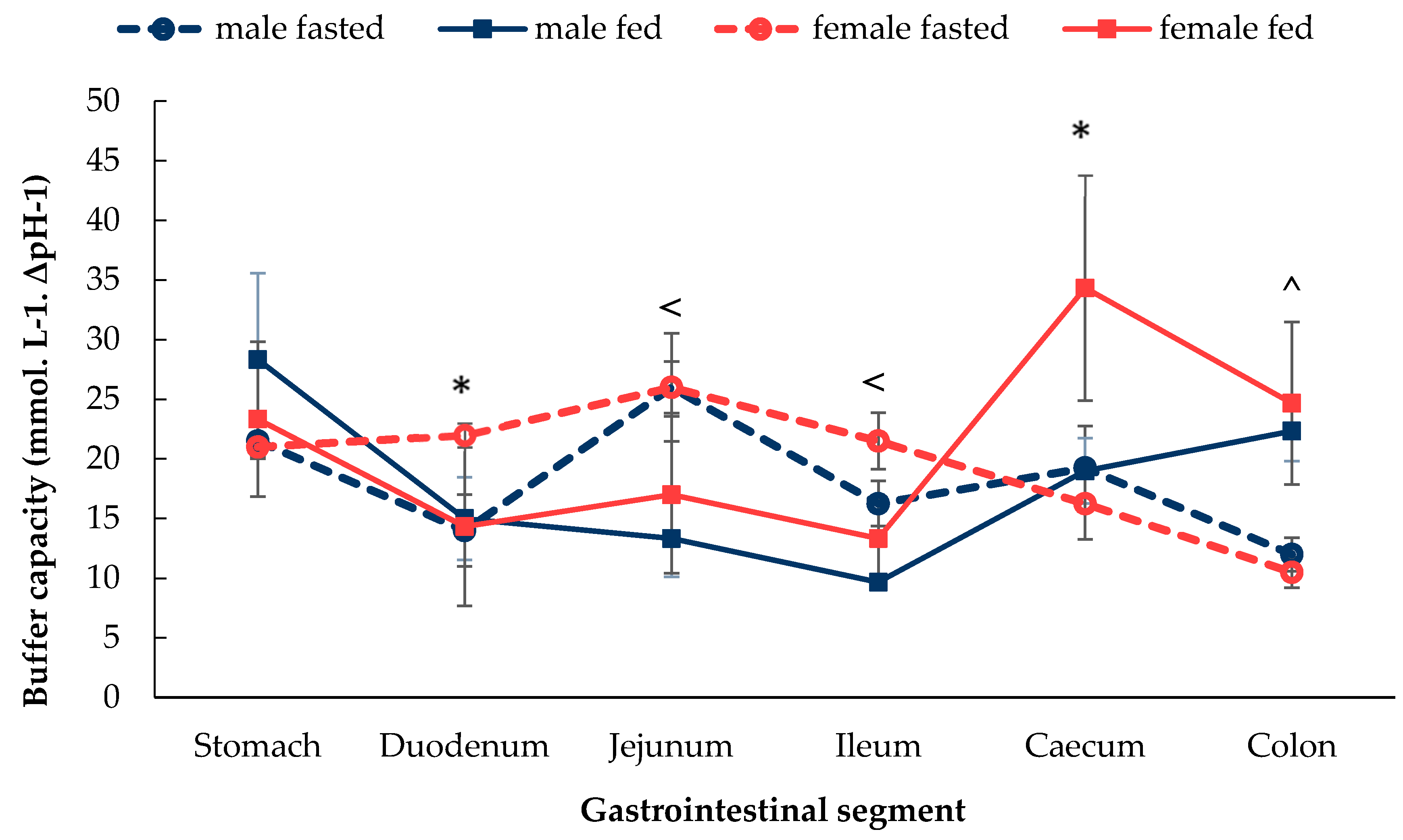
3.1.2. Gastrointestinal Fluid Buffer Capacity
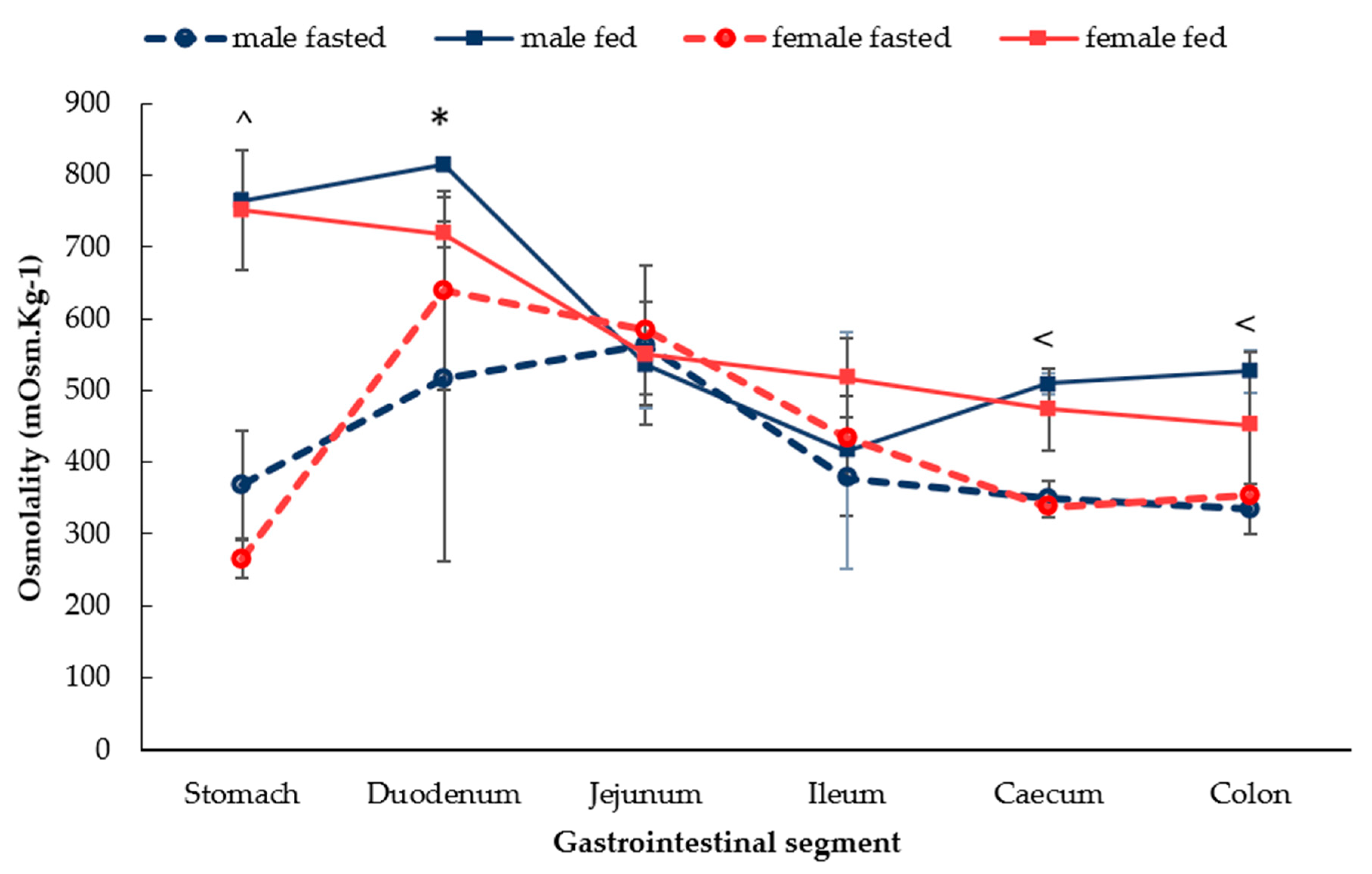
3.1.3. Gastrointestinal Fluid Osmolality
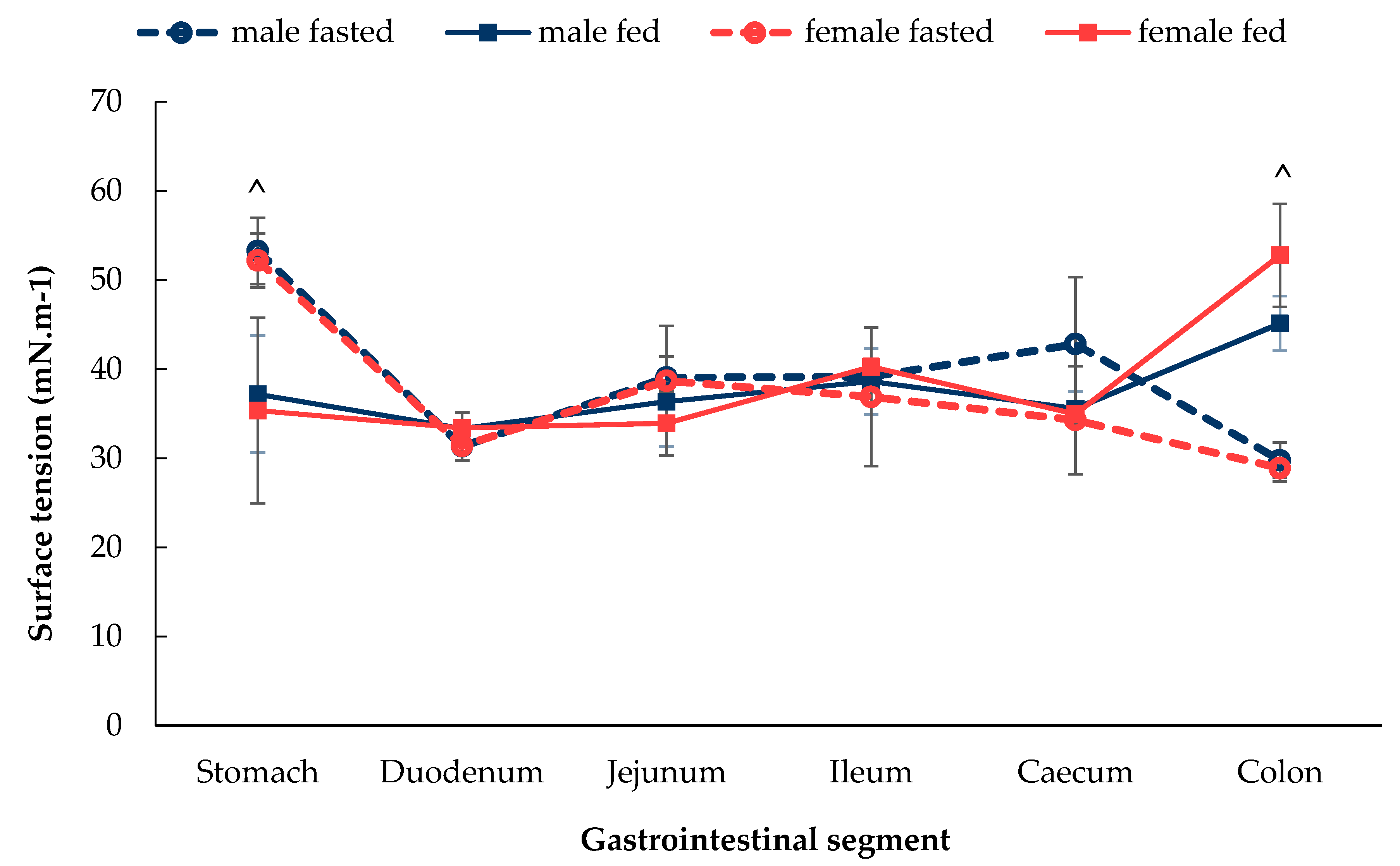
3.1.4. Gastrointestinal Fluid Surface Tension
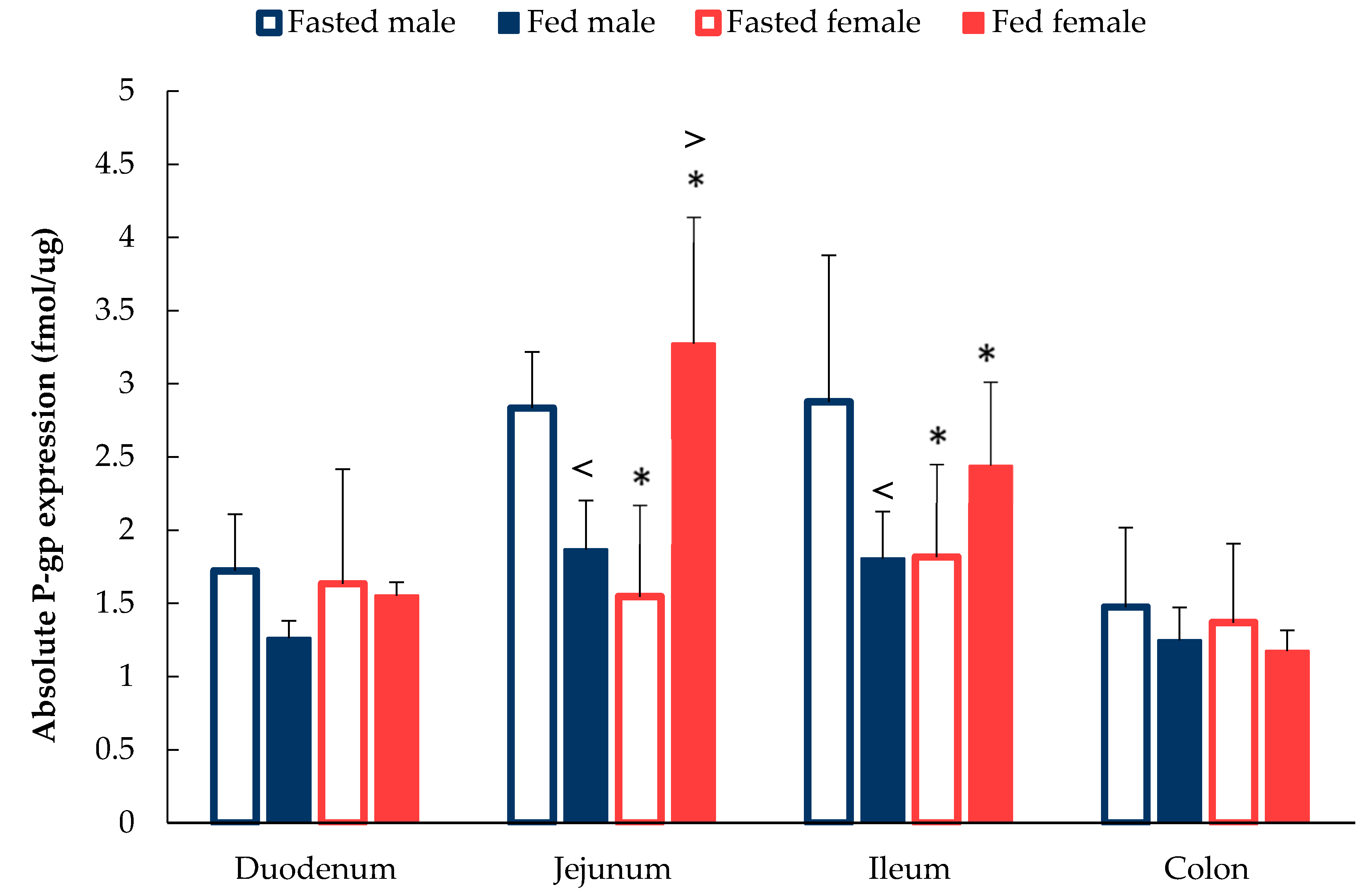
3.2. Sex Differences in P-gp expression Along the GI Tract
4. Discussion
4.1. Luminal Fluid Characterisation
4.2. Absolute P-gp Expression
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Harris, R.Z.; Jang, G.R.; Tsunoda, S. Dietary effects on drug metabolism and transport. Clin. Pharmacokinet. 2003, 42, 1071–1088. [Google Scholar] [CrossRef] [PubMed]
- FDA Guideline. Guidance for Industry: Food-Effect Bioavailability and Fed Bioequivalence Studies. 2002. Available online: http://www.fda.gov/cder/guidance/index.htm (accessed on 4 January 2020).
- Koziolek, M.; Alcaro, S.; Augustijns, P.; Basit, A.W.; Grimm, M.; Hens, B.; Hoad, C.L.; Jedamzik, P.; Madla, C.M.; Maliepaard, M.; et al. The mechanisms of pharmacokinetic food-drug interactions–A perspective from the UNGAP group. Eur. J. Pharm. Sci. 2019, 134, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Stillhart, C.; Vučićević, K.; Augustijns, P.; Basit, A.W.; Batchelor, H.; Flanagan, T.R.; Gesquiere, I.; Greupink, R.; Keszthelyi, D.; Koskinen, M.; et al. Impact of gastrointestinal physiology on drug absorption in special populations—An UNGAP review. Eur. J. Pharm. Sci. 2020, 105280. [Google Scholar] [CrossRef] [PubMed]
- Chu, T. Women’s Health: Gender Differences in Pharmacokinetics. US Pharm. 2014, 39, 40–43. [Google Scholar]
- Liu, K.A.; Dipietro Mager, N.A. Women’s involvement in clinical trials: Historical perspective and future implications. Pharm. Pract. 2016, 14, 5. [Google Scholar] [CrossRef]
- U. S. Food and Drug Administration. Center for Drug Evaluation and Research. Guideline for the study and evaluation of gender differences in the clinical evaluation of drugs: Health and human services notice. Federal Regist. 1993, 58, 39406–39416. [Google Scholar]
- Downing, J.R.; Allison, J.P.; Honjo, T. NIH to require both sexes in preclinical studies. Cancer Discov. 2014, 4, 860. [Google Scholar]
- Nature. Putting gender on the agenda. Nature 2010, 465, 665. [Google Scholar] [CrossRef][Green Version]
- Clayton, J.A.; Collins, F.S. Collins Policy: NIH to balance sex in cell and animal studies. Nature 2014, 509, 282–283. [Google Scholar] [CrossRef]
- European Commission. Guidance on Gender Equality in Horizon 2020. 2016. Available online: http://eige.europa.eu/sites/default/files/h2020-hi-guide-gender_en.pdf (accessed on 18 March 2019).
- Mogil, J.S.; Chanda, M.L. The case for the inclusion of female subjects in basic science studies of pain. Pain 2005, 117, 1–5. [Google Scholar] [CrossRef]
- Beery, A.K.; Zucker, I. Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 2011, 35, 565–572. [Google Scholar] [CrossRef]
- Hatton, G.B.; Yadav, V.; Basit, A.W.; Merchant, H.A. Animal Farm: Considerations in Animal Gastrointestinal Physiology and Relevance to Drug Delivery in Humans. J. Pharm. Sci. 2015, 104, 2747–2776. [Google Scholar] [CrossRef]
- Iannaccone, P.M.; Jacob, H.J. Rats! Dis. Models Mech. 2009, 2, 206–210. [Google Scholar] [CrossRef]
- Goyanes, A.; Fernández-Ferreiro, A.; Majeed, A.; Gomez-Lado, N.; Awad, A.; Luaces-Rodríguez, A.; Gaisford, S.; Aguiar, P.; Basit, A.W. PET/CT imaging of 3D printed devices in the gastrointestinal tract of rodents. Int. J. Pharm. 2018, 536, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Lado, N.; Seoane-Viaño, I.; Matiz, S.; Madla, C.M.; Yadav, V.; Aguiar, P.; Basit, A.W.; Goyanes, A. Gastrointestinal Tracking and Gastric Emptying of Coated Capsules in Rats with or without Sedation Using CT imaging. Pharmaceutics 2020, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Ashiru, D.A.; Patel, R.; Basit, A.W. Polyethylene glycol 400 enhances the bioavailability of a BCS class III drug (ranitidine) in male subjects but not females. Pharm. Res. 2008, 25, 2327–2333. [Google Scholar] [CrossRef] [PubMed]
- Afonso-Pereira, F.; Dou, L.; Trenfield, S.J.; Madla, C.M.; Murdan, S.; Sousa, J.; Veiga, F.; Basit, A.W. Sex differences in the gastrointestinal tract of rats and the implications for oral drug delivery. Eur. J. Pharm. Sci. 2018, 115, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Mai, Y.; Dou, L.; Murdan, S.; Basit, A.W. An animal’s sex influences the effects of the excipient PEG 400 on the intestinal P-gp protein and mRNA levels, which has implications for oral drug absorption. Eur. J. Pharm. Sci. 2018, 120, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Mai, Y.; Madla, C.M.; Orlu, M.; Basit, A.W. P-glycoprotein expression in the gastrointestinal tract of male and female rats is influenced differently by food. Eur. J. Pharm. Sci. 2018, 123, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Bebawy, M.; Chetty, M. Gender differences in p-glycoprotein expression and function: Effects on drug disposition and outcome. Curr. Drug Metab. 2009, 10, 322–328. [Google Scholar] [CrossRef]
- MacLean, C.; Moenning, U.; Reichel, A.; Fricker, G. Closing the gaps: A full scan of the intestinal expression of p-glycoprotein, breast cancer resistance protein, and multidrug resistance-associated protein 2 in male and female rats. Drug Metab. Dispos. 2008, 36, 1249–1254. [Google Scholar] [CrossRef]
- Gröer, C.; Brück, S.; Lai, Y.; Paulick, A.; Busemann, A.; Heidecke, C.D.; Siegmund, W.; Oswald, S. LC-MS/MS-based quantification of clinically relevant intestinal uptake and efflux transporter proteins. J. Pharm. Biomed. Anal. 2013, 85, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Horter, D.; Dressman, J.B. Influence of physicochemical properties on dissolution of drugs in the gastrointestinal tract. Adv. Drug Deliv. Rev. 2001, 46, 75–87. [Google Scholar] [CrossRef]
- McConnell, E.L.; Liu, F.; Basit, A.W. Colonic treatments and targets: Issues and opportunities. J. Drug Target. 2008, 17, 335–363. [Google Scholar] [CrossRef] [PubMed]
- Merchant, H.A.; Rabbie, S.C.; Varum, F.J.; Afonso-Pereira, F.; Basit, A.W. Influence of ageing on the gastrointestinal environment of the rat and its implications for drug delivery. Eur. J. Pharm. Sci. 2014, 62, 76–85. [Google Scholar] [CrossRef]
- Overhoff, K.A.; McConville, J.T.; Yang, W.; Johnston, K.P.; Peters, J.I.; Williams, R.O. Effect of stabilizer on the maximum degree and extent of supersaturation and oral absorption of tacrolimus made by ultra-rapid freezing. Pharm. Res. 2008, 25, 167–175. [Google Scholar] [CrossRef]
- Shore, R.; Björne, H.; Omoto, Y.; Siemiatkowska, A.; Gustafsson, J.Å.; Lindblad, M.; Holm, L. Sex differences and effects of oestrogen in rat gastric mucosal defence. World J. Gastroenterol. 2017, 23, 426–436. [Google Scholar] [CrossRef]
- Barnes, R.H.; Fiala, G.; McGehee, B.; Brown, A. Prevention of coprophagy in the rat. J. Nutr. 1957, 63, 489–498. [Google Scholar] [CrossRef]
- Barnes, R.H. Nutritional implications of coprophagy. Nutr. Rev. 1962, 20, 289–291. [Google Scholar] [CrossRef]
- Evans, D.F.; Pye, G.; Bramley, R.; Clark, A.G.; Dyson, T.J.; Hardcastle, J.D. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut 1988, 29, 1035–1041. [Google Scholar] [CrossRef]
- Mudie, D.M.; Amidon, G.L.; Amidon, G.E. Physiological parameters for oral delivery and in vitro testing. Mol. Pharm. 2010, 7, 1388–1405. [Google Scholar] [CrossRef]
- Oldham-Ott, C.K.; Gilloteaux, J. Comparative morphology of the gallbladder and biliary tract in vertebrates: Variation in structure, homology in function and gallstones. Microsc. Res. Tech. 1997, 38, 571–597. [Google Scholar] [CrossRef]
- Yousef, I.M.; Kakis, G.; Fisher, M.M. Bile acid metabolism in mammals. 3. Sex difference in the bile acid composition of rat bile. Can. J. Biochem. 1972, 50, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.; Kang, X.; Yuan, Y.; Du, M. Short chain fatty acids accumulation and microbial community succession during ultrasonic-pretreated sludge anaerobic fermentation process: Effect of alkaline adjustment. Int. Biodeterior. Biodegrad. 2014, 94, 128–133. [Google Scholar] [CrossRef]
- Vonk, R.J.; van Doorn, A.B.; Strubbe, J.H. Bile secretion and bile composition in the freely moving, unanaesthetized rat with a permanent biliary drainage: Influence of food intake on bile flow. Clin. Sci. Mol. Med. 1978, 55, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Strasberg, S.M.; Siminovitch, K.A.; Ilson, R.G. Bile production in fasted and fed primates. Ann. Surg. 1974, 180, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Riethorst, D.; Mols, R.; Duchateau, G.; Tack, J.; Brouwers, J.; Augustijns, P. Characterization of Human Duodenal Fluids in Fasted and Fed State Conditions. J. Pharm. Sci. 2016, 105, 673–681. [Google Scholar] [CrossRef]
- Vargaftik, N.B.; Volkov, B.N.; Voljak, L.D. International Tables of the Surface Tension of Water. J. Phys. Chem. Ref. Data 1983, 12, 817–820. [Google Scholar] [CrossRef]
- Oltra-Noguera, D.; Mangas-Sanjuan, V.; González-Álvarez, I.; Colon-Useche, S.; González-Álvarez, M.; Bermejo, M. Drug gastrointestinal absorption in rat: Strain and gender differences. Eur. J. Pharm. Sci. 2015, 78, 198–203. [Google Scholar] [CrossRef]
- Lifschitz, A.; Ballent, M.; Virkel, G.; Sallovitz, J.; Lanusse, C. Sex-related differences in the gastrointestinal disposition of ivermectin in the rat: P-glycoprotein involvement and itraconazole modulation. J. Pharm. Pharmacol. 2006, 58, 1055–1062. [Google Scholar] [CrossRef]
- Brady, J.M.; Cherrington, N.J.; Hartley, D.P.; Buist, S.C.; Li, N.; Klaassen, C.D. Tissue distribution and chemical induction of multiple drug resistance genes in rats. Drug Metab. Dispos. 2002, 30, 838–844. [Google Scholar] [CrossRef]
- Homma, H.; Hoy, E.; Xu, D.Z.; Lu, Q.; Feinman, R.; Deitch, E.A. The female intestine is more resistant than the male intestine to gut injury and inflammation when subjected to conditions associated with shock states. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G466–G472. [Google Scholar] [CrossRef] [PubMed]
- Dahan, A.; Amidon, G.L. Segmental dependent transport of low permeability compounds along the small intestine due to P-glycoprotein: The role of efflux transport in the oral absorption of BCS class III drugs. Mol. Pharm. 2009, 6, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, P.L.; Basson, M.D. The correlation between the expression of differentiation markers in rat small intestinal mucosa and the transcript levels of schlafen 3. JAMA Surg. 2013, 148, 1013–1019. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bruyere, A.; Decleves, X.; Bouzom, F.; Ball, K.; Marques, C.; Treton, X.; Pocard, M.; Valleur, P.; Bouhnik, Y.; Panis, Y.; et al. Effect of variations in the amounts of P-glycoprotein (ABCB1), BCRP (ABCG2) and CYP3A4 along the human small intestine on PBPK models for predicting intestinal first pass. Mol. Pharm. 2010, 7, 1596–1607. [Google Scholar] [CrossRef] [PubMed]
- Drozdzik, M.; Gröer, C.; Penski, J.; Lapczuk, J.; Ostrowski, M.; Lai, Y.; Prasad, B.; Unadkat, J.D.; Siegmund, W.; Oswald, S. Protein abundance of clinically relevant multidrug transporters along the entire length of the human intestine. Mol. Pharm. 2014, 11, 3547–3555. [Google Scholar] [CrossRef]
- Morris, M.E.; Lee, H.J.; Predko, L.M. Gender differences in the membrane transport of endogenous and exogenous compounds. Pharmacol. Rev. 2003, 55, 229–240. [Google Scholar] [CrossRef]
| Molecule Name | Peptide Sequence | Mass | Transition Number | Q1 m/z | Q3 m/z |
|---|---|---|---|---|---|
| ABCB1 (P-gp) | AGAVAEEVLAAIR (Standard) | 1268.7 | 1 | 635.3 | 771.3 |
| 2 | 635.3 | 900.5 | |||
| 3 | 635.3 | 971.6 | |||
| AGAVAEEVLAAIR (Internal standard) | 1278.6 | 1 | 640.3 | 781.4 | |
| 2 | 640.3 | 910.5 | |||
| 3 | 640.3 | 981.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, L.; Gavins, F.K.H.; Mai, Y.; Madla, C.M.; Taherali, F.; Orlu, M.; Murdan, S.; Basit, A.W. Effect of Food and an Animal’s Sex on P-Glycoprotein Expression and Luminal Fluids in the Gastrointestinal Tract of Wistar Rats. Pharmaceutics 2020, 12, 296. https://doi.org/10.3390/pharmaceutics12040296
Dou L, Gavins FKH, Mai Y, Madla CM, Taherali F, Orlu M, Murdan S, Basit AW. Effect of Food and an Animal’s Sex on P-Glycoprotein Expression and Luminal Fluids in the Gastrointestinal Tract of Wistar Rats. Pharmaceutics. 2020; 12(4):296. https://doi.org/10.3390/pharmaceutics12040296
Chicago/Turabian StyleDou, Liu, Francesca K. H. Gavins, Yang Mai, Christine M. Madla, Farhan Taherali, Mine Orlu, Sudaxshina Murdan, and Abdul W. Basit. 2020. "Effect of Food and an Animal’s Sex on P-Glycoprotein Expression and Luminal Fluids in the Gastrointestinal Tract of Wistar Rats" Pharmaceutics 12, no. 4: 296. https://doi.org/10.3390/pharmaceutics12040296
APA StyleDou, L., Gavins, F. K. H., Mai, Y., Madla, C. M., Taherali, F., Orlu, M., Murdan, S., & Basit, A. W. (2020). Effect of Food and an Animal’s Sex on P-Glycoprotein Expression and Luminal Fluids in the Gastrointestinal Tract of Wistar Rats. Pharmaceutics, 12(4), 296. https://doi.org/10.3390/pharmaceutics12040296








