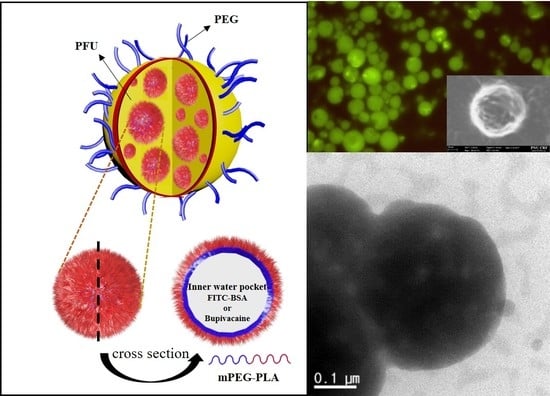
Synthesis and Characterization of Polyfumarateurethane Nanoparticles for Sustained Release of Bupivacaine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
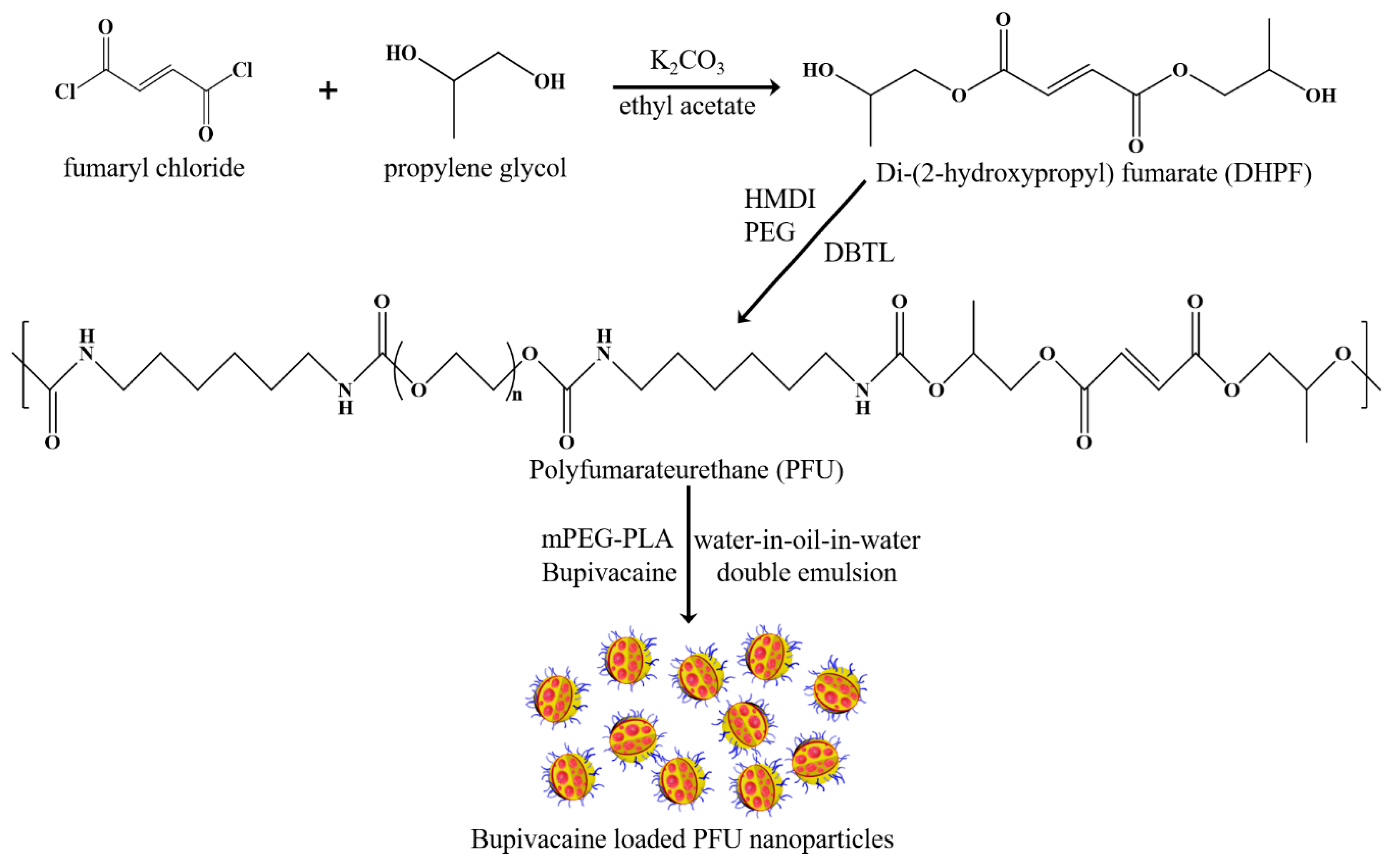
2.2. Synthesis of PFU
2.3. Synthesis of mPEG-PLA
2.4. Fabrication of Blank and FITC-Loaded PFU Nanoparticles
2.5. Fabrication of Bupivacaine-Loaded PFU Nanoparticles and Their Release Characteristics
2.6. Measurements
3. Results and Discussion
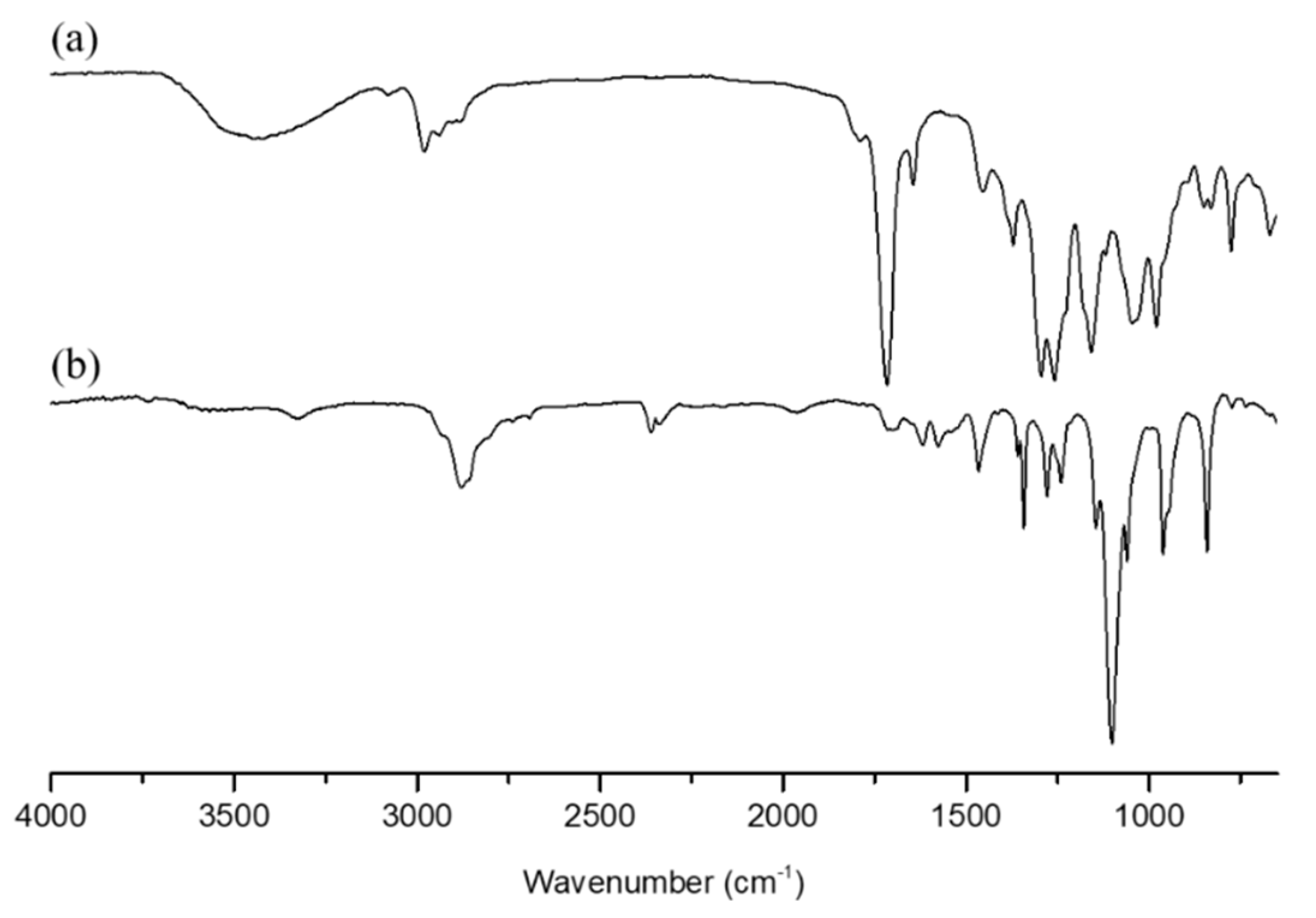
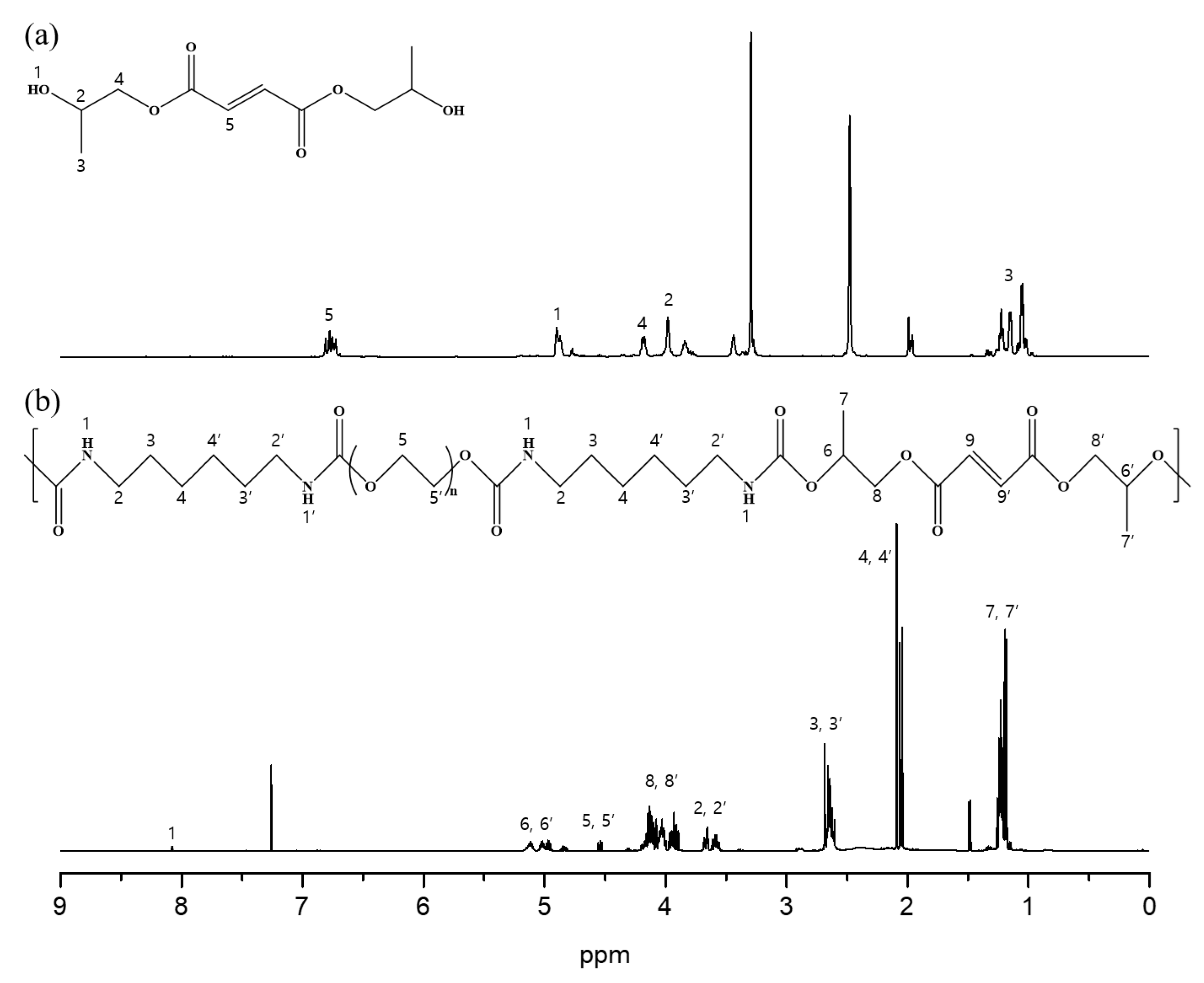
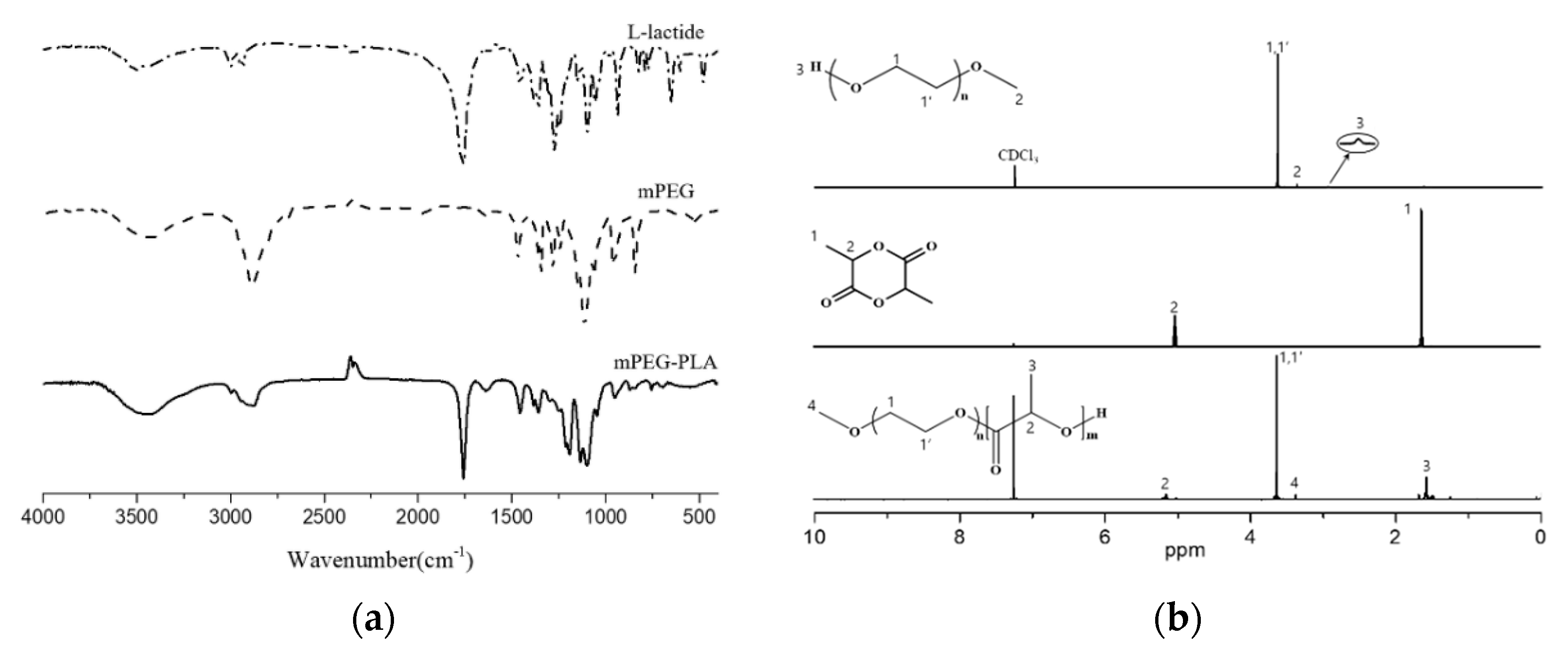
3.1. Characterization of DHPF, PFU, and mPEG-PLA
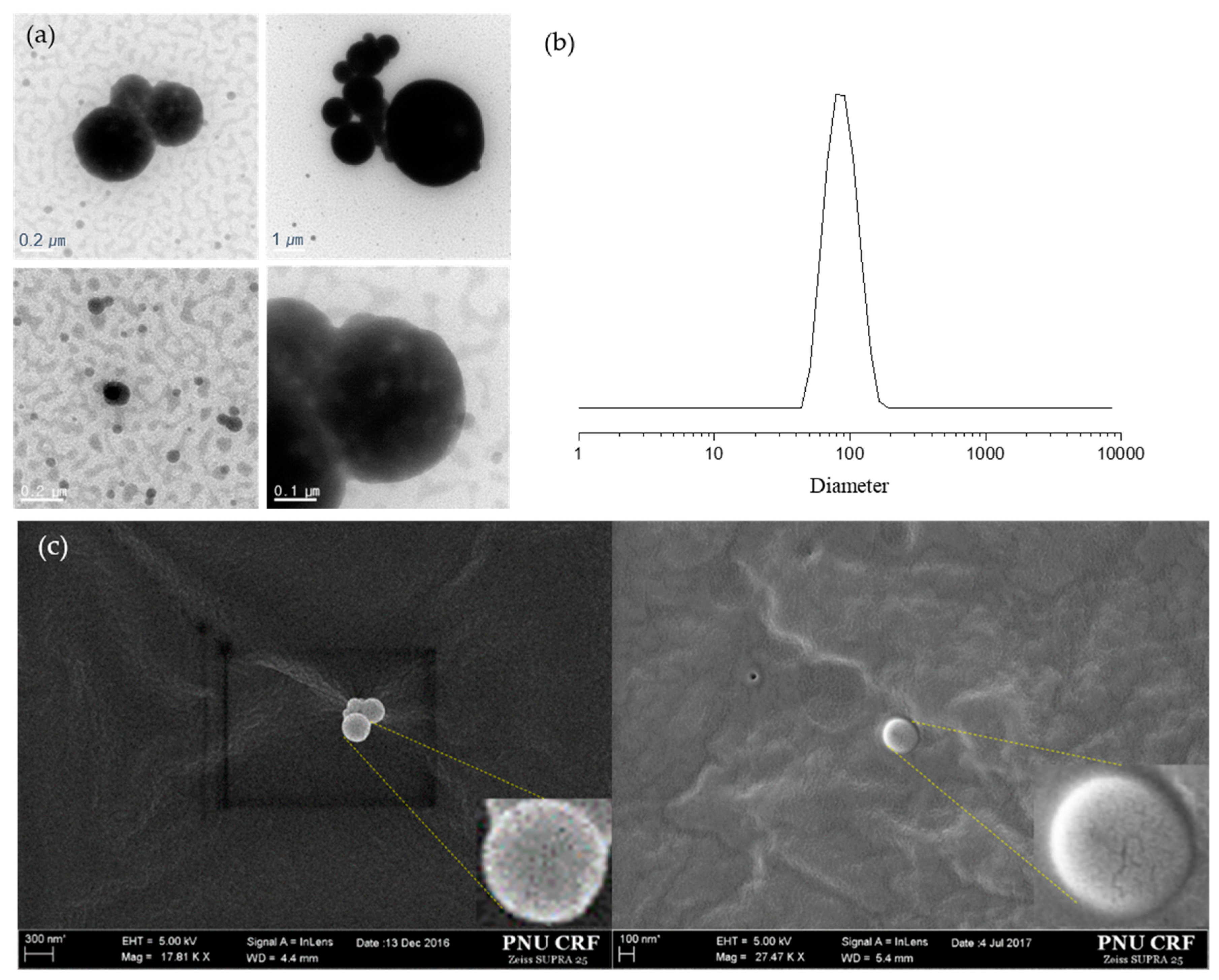
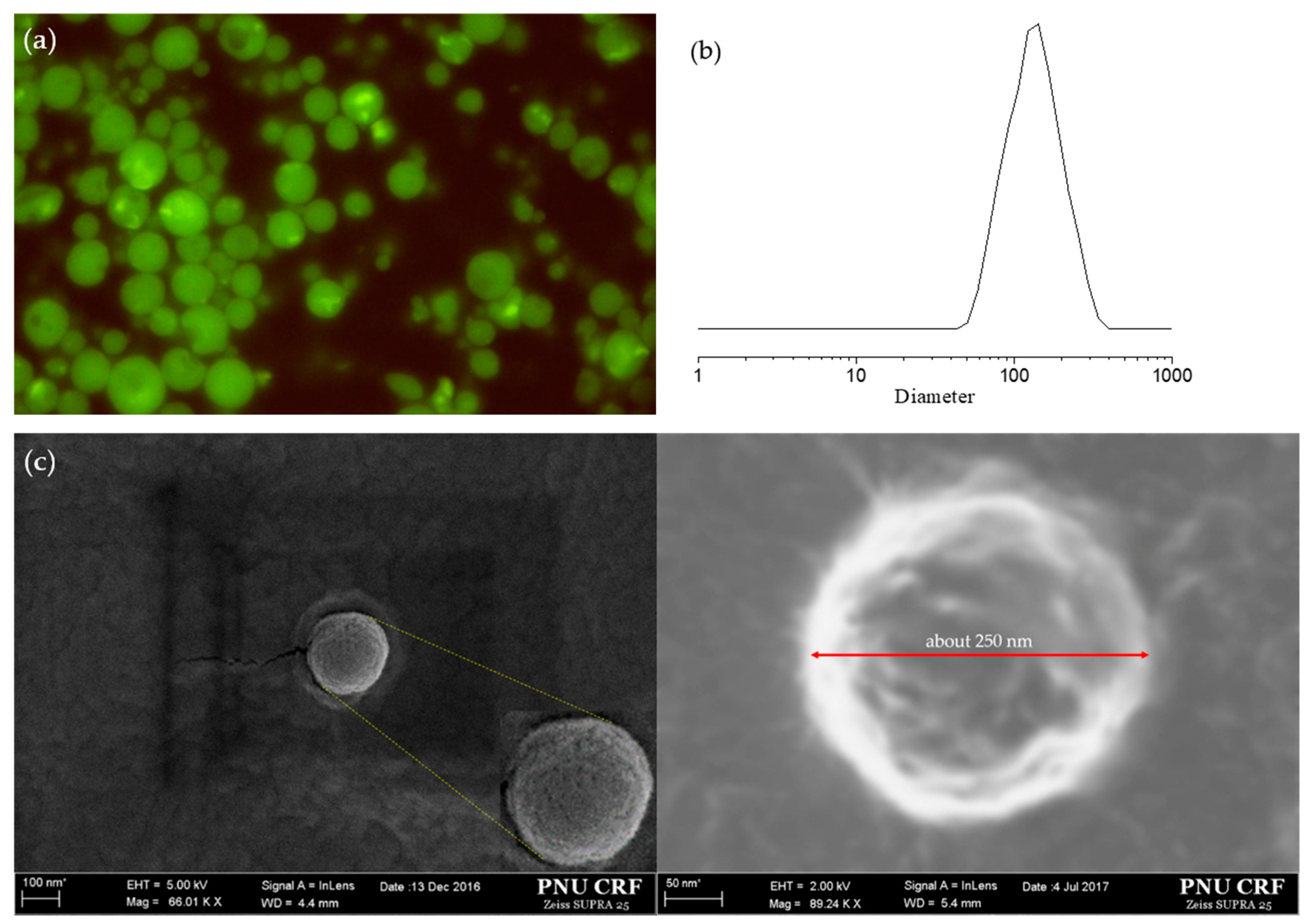
3.2. Particle Size Distribution and Encapsulation
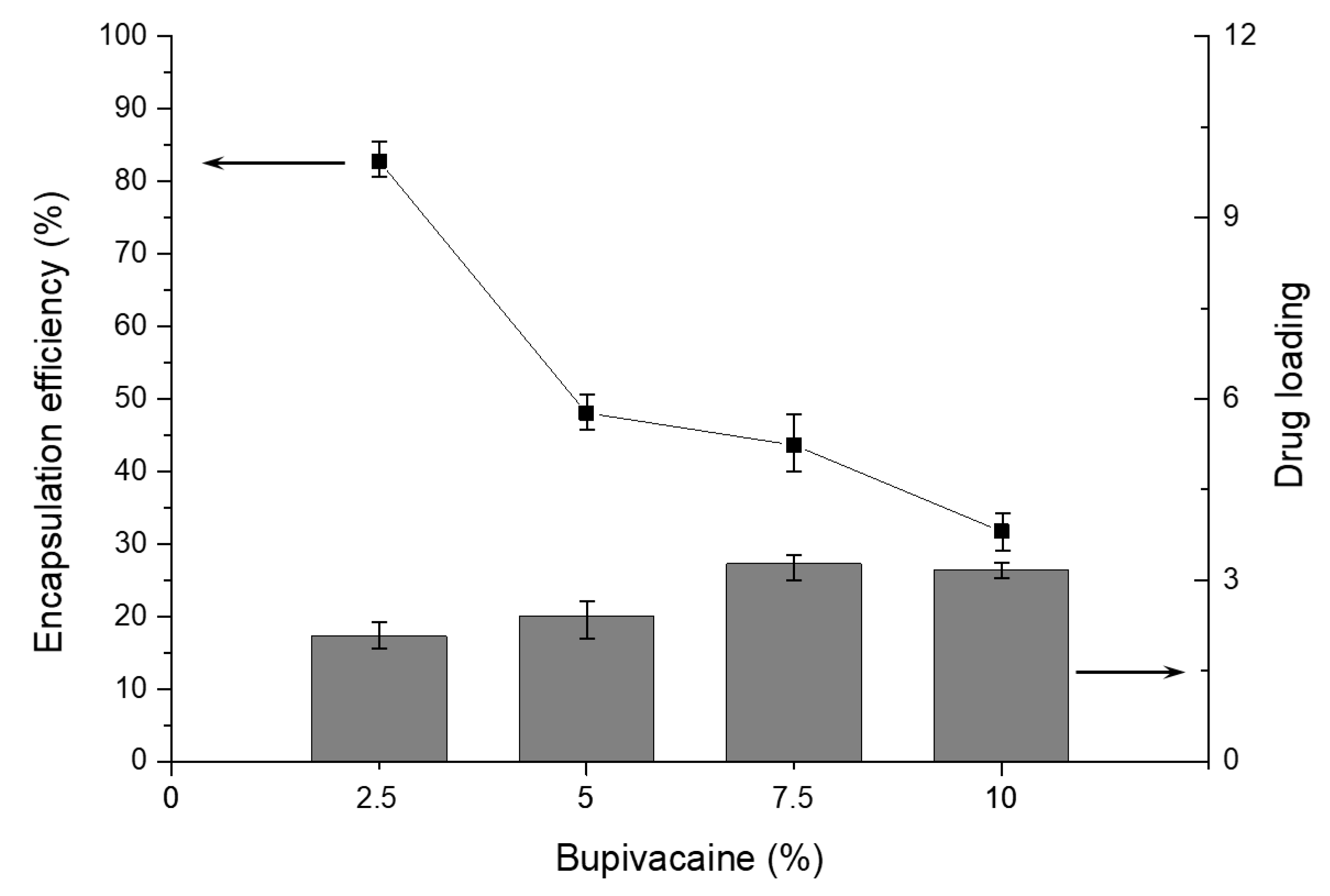
3.3. Encapsulation and Drug Loading Efficiency
- : concentration of extracted drug solution
- : volume of aqueous buffer used for the extraction
- : weight of nanoparticles used for the extraction
- : concentration of drug solution used in the encapsulation process
- : volume of drug solution used in the encapsulation process
- : weight of total nanoparticles yielded
- : concentration of the extracted drug solution
- : volume of aqueous buffer used for the extraction
- : weight of the nanoparticles used for the extraction
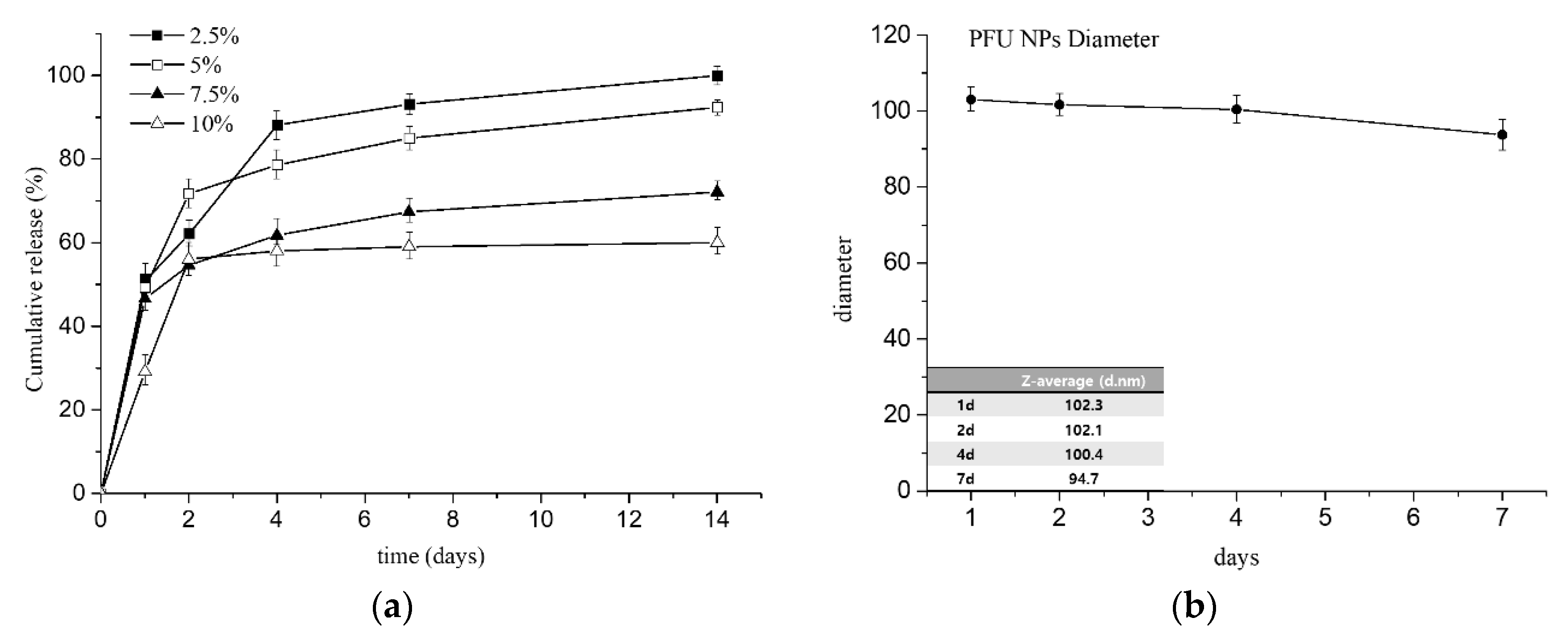
3.4. In Vitro Cumulative Drug Release with Stability of NPs in PBS
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Covino, B.G.; Vassallo, H.G. Local Anesthetics: Mechanics of Action and Clinical Use; Grune & Stratton: New York, NY, USA, 1976. [Google Scholar]
- Su, N.; Wang, H.; Zhang, S.; Liao, S.; Yang, S.; Huang, Y. Efficacy and safety of bupivacaine versus lidocaine in dental treatments: A meta-analysis of randomised controlled trials. Int. Dent. J. 2014, 64, 34–45. [Google Scholar] [CrossRef]
- Thakare, A.; Bhate, K.; Kathariya, R. Comparison of 4% articaine and 0.5% bupivacaine anesthetic efficacy in orthodontic extractions: Prospective, randomized crossover study. Acta Anaesthesiol. Taiwanica 2014, 52, 59–63. [Google Scholar] [CrossRef]
- Curley, J.; Castillo, J.; Hotz, J.; Uezono, M.; Hernandez, S.; Lim, J.-O.; Tigner, J.; Chasin, M.; Langer, R.; Berde, C. Prolonged regional nerve blockade injectable biodegradable bupivacaine/polyester microspheres. Anesthesiol. J. Am. Soc. Anesthesiol. 1996, 84, 1401–1410. [Google Scholar]
- Shah, J.; Johnston, J.; Krum, H.; Halladay, S.; Lissin, D.; Verity, N. (783): Pharmacokinetic characteristics of SABER™-Bupivacaine (Posidur™) formulation in humans. J. Pain 2007, 8, S46. [Google Scholar] [CrossRef]
- Mero, A.; Campisi, M. Hyaluronic acid bioconjugates for the delivery of bioactive molecules. Polymers 2014, 6, 346–369. [Google Scholar] [CrossRef]
- Anton, N.; Jakhmola, A.; Vandamme, T.F. Trojan microparticles for drug delivery. Pharmaceutics 2012, 4, 1–25. [Google Scholar] [CrossRef]
- Guarve, K.; Gupta, G.D. Asymmetric membrane capsules for extended delivery of the weakly basic drug carvedilol. Pharmaceutics 2010, 2, 199–208. [Google Scholar] [CrossRef]
- Alan, A.S.; Mohamed, H.E.; Arun, J.R.; Edward, A.I.; Ruvie, L.M. Early experience with lidocaine patch for postoperative pain control after laparoscopic ventral hernia repair. Int. J. Surg. 2009, 7, 36–38. [Google Scholar]
- Chen, D.W.-C.; Liao, J.-Y.; Liu, S.-J.; Chan, E.-C. Novel biodegradable sandwich-structured nanofibrous drug-eluting membranes for repair of infected wounds: An in vitro and in vivo study. Int. J. Nanomed. 2017, 7, 763–771. [Google Scholar]
- Valdes, B.G.; Serro, A.P.; Marto, J.; Dos Santos, R.G.; Gomez, E.C.; Otero-Espinar, F.J.; Bordado, J.M.; Ribeiro, H.M. Polyurethanes as new excipients in nail therapeutics. Pharmaceutics 2018, 10, 276. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, X.; Yang, J.; Wang, Y.; Zhang, Z.; Hou, Y.; Lin, F.; Li, Y. Influence of hard segments on the thermal, phase-separated morphology, mechanical, and biological properties of polycarbonate urethanes. Appl. Sci. 2017, 7, 306. [Google Scholar] [CrossRef]
- Gogolewski, S. Selected topics in biomedical polyurethanes. Colloid Polym. Sci. 1989, 267, 757–785. [Google Scholar] [CrossRef]
- Shah, P.N.; Manthe, R.L.; Lopina, S.T.; Yun, Y.H. Electrospinning of L-tyrosine polyurethanes for potential biomedical applications. Polymer 2009, 50, 2281–2289. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Salem, S.A.-D.; Mohamed, H.E.-N. Controlled release of 5-aminosalicylic acid (5-ASA) from new biodegradable polyurethane. Molecules 2010, 15, 2257–2268. [Google Scholar] [CrossRef]
- Dou, Y.; Zhang, B.; He, M.; Yin, G.; Cui, Y.; Savina, I.N. Keratin/polyvinyl alcohol blend films cross-linked by dialdehyde starch and their potential application for drug release. Polymers 2015, 7, 580–591. [Google Scholar] [CrossRef]
- Li, Y.-P.; Pei, Y.-Y.; Zhang, X.-Y.; Gu, Z.-H.; Zhou, Z.-H.; Yuan, W.-F.; Zhou, J.-J.; Zhu, J.-H.; Gao, X.-J. PEGylated PLGA nanoparticles as protein carriers: Synthesis, preparation and biodistribution in rats. J. Control. Release 2001, 71, 203–211. [Google Scholar] [CrossRef]
- Hiltunen, K.; Tuominen, J.; Seppälä, J.V. Hydrolysis of lactic acid based poly(ester-urethane)s. Polym. Int. 1998, 47, 186–192. [Google Scholar] [CrossRef]
- Diaz, A.; Kastsarava, R.; Puiggali, J. Synthesis, Properties and Applications of Biodegradable Polymers Derived from Diols and Dicarboxylic Acids: From Polyesters to Poly(ester amide)s. Int. J. Mol. Sci. 2014, 15, 7064–7123. [Google Scholar] [CrossRef]
- Storey, R.F.; Warren, S.C.; Allison, C.J.; Wiggins, J.S.; Puckett, A. Synthesis of bioabsorbable networks from methacrylate-endcapped polyesters. Polymer 1993, 34, 4365–4372. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, S.-Y.; Kim, T.; Ahn, H.; Chung, I. Syntheses of biodegradable polymer networks based on polycaprolactone and glutamic acid. Polym. Adv. Technol. 2019, 30, 872–878. [Google Scholar] [CrossRef]
- Reddy, N.S.; Rao, K.M.; Park, S.Y.; Kim, T.; Chung, I. Fabrication of aminosilanized halloysite based floating biopolymer composites for sustained gastro retentive release of curcumin. Macromol. Res. 2019, 27, 490–496. [Google Scholar] [CrossRef]
- Kim, T.; Park, S.Y.; Lee, M.-H.; Kim, D.-H.; Chung, I. Syntheses of polyrotaxane conjugated with 5-fluorouracil and vitamins with improved antitumor activities. J. Bioact. Compat. Polym. 2019, 34, 25–38. [Google Scholar] [CrossRef]
- Reddy, N.S.; Eswaramma, S.; Chung, I.; Krishna Rao, K.S.V.; Ramesh, P.; Sekhar, A.C. Chitosan/poly(dimethylaminoethylmethacrylate-co-hydroxyethylacrylate) based semi-IPN hydrogels and silver nanocomposites: Synthesis, evaluation, of amoxicillin release studies, and antibacterial studies. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 870–880. [Google Scholar] [CrossRef]
- Kim, T.; Mays, J.; Chung, I. Porous poly(ε-caprolactone) microspheres via UV photodegradation of block copolymers prepared by RAFT polymerization. Polymer 2018, 158, 198–203. [Google Scholar] [CrossRef]
- Hyun, J.; Wang, S.; Kim, J.; Rao, K.M.; Park, S.Y.; Chung, I.; Ha, C.-S.; Kim, S.-W.; Yun, Y.H.; Jung, Y. MicroRNA-378 limits activation of hepatic stellate cells and liver fibrosis by suppressing Gli3 expression. Nat. Commun. 2016, 7, 10993. [Google Scholar] [CrossRef]
- Lee, S.M.; Bala, Y.S.; Lee, W.K.; Jo, N.J.; Chung, I. Antitumor and antiangiogenic active dendrimer/5-fluorouracil conjugates. J. Biomed. Mater. Res. Part A 2013, 101A, 2306–2312. [Google Scholar] [CrossRef]
- Santerre, J.P.; Labow, R.S.; Duguay, D.G.; Erfle, D.; Adams, G.A. Biodegradation evaluation of polyether and polyester-urethanes with oxidative and hydrolytic enzymes. J. Biomed. Mater. Res. 1994, 28, 1187–1199. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, Y.; Zhang, G.; Gao, S.; Sun, D.; Zhong, Y. Bupivacaine-loaded biodegradable poly(lactic-co-glycolic) acid microspheres: I. Optimization of the drug incorporation into the polymer matrix and modelling of drug release. Int. J. Pharm. 2008, 351, 244–249. [Google Scholar] [CrossRef]
- Grillo, R.; de Melo, N.F.; de Araujo, D.R.; de Paula, E.; Rosa, A.H.; Fraceto, L.F. Polymeric alginate nanoparticles containing the local anesthetic bupivacaine. J. Drug Target. 2010, 18, 688–699. [Google Scholar] [CrossRef]
- Hu, D.S.G.; Liu, H.J.; Pan, I.L. Inhibition of bovine serum albumin adsorption by poly (ethylene glycol) soft segment in biodegradable poly(ethylene glycol)/poly(l-lactide) copolymers. J. Appl. Polym. Sci. 1993, 50, 1391–1396. [Google Scholar] [CrossRef]
- Sarkar, D.; Lopina, S.T. Oxidative and enzymatic degradations of L-tyrosine based polyurethanes. Polym. Degrad. Stab. 2007, 92, 1994–2004. [Google Scholar] [CrossRef]
- Kwon, G.S.; Kataoka, K. Block copolymer micelles as long-circulating drug vehicles. Adv. Drug Deliv. Rev. 1995, 16, 295–309. [Google Scholar] [CrossRef]
- Torchilinl, V.; Papisov, M. Why do polyethylene glycol-coated liposomes circulate so long? Molecular mechanism of liposome steric protection with polyethylene glycol: Role of polymer chain flexibility. J. Liposome Res. 1994, 4, 725–739. [Google Scholar] [CrossRef]
- Kyu, S.; Dukjoon, C. Drug-releasing behavior of MPEG/PLA block copolymer micelles and solid particles controlled by component block length. J. Appl. Polym. Sci. 2002, 83, 435–445. [Google Scholar] [CrossRef]
- Greenwald, R.B.; Choe, Y.H.; McGuire, J.; Conover, C.D. Effective drug delivery by PEGylated drug conjugates. Adv. Drug Deliv. Rev. 2003, 55, 217–250. [Google Scholar] [CrossRef]
- Ditto, A.J.; Shah, P.N.; Gump, L.R.; Yun, Y.H. Nanospheres formulated from L-tyrosine polyphosphate exhibiting sustained release of polyplexes and in vitro controlled transfection properties. Mol. Pharm. 2009, 6, 986–995. [Google Scholar] [CrossRef]
- Leal-Calderon, F.; Schmitt, V.; Bibette, J. Emulsion Science: Basic Principles; Springer: New York, NY, USA, 2007; pp. 173–188. [Google Scholar]
- Li, X.; Zhang, Y.; Yan, R.; Jia, W.; Yuan, M.; Deng, X.; Huang, Z. Influence of process parameters on the protein stability encapsulated in poly-dl-lactide–poly(ethylene glycol) microspheres. J. Control. Release 2000, 68, 41–52. [Google Scholar] [CrossRef]
- Li, X.; Deng, X.; Yuan, M.; Xiong, C.; Huang, Z.; Zhang, Y.; Jia, W. Investigation on process parameters involved in preparation of poly-dl-lactide-poly(ethylene glycol) microspheres containing Leptospira Interrogans antigens. Int. J. Pharm. 1999, 178, 245–255. [Google Scholar] [CrossRef]
- Shibata, H.; Nakagawa, S.; Tsutsumi, Y. Optimization of protein therapies by polymer-conjugation as an effective DDS. Molecules 2005, 10, 162–180. [Google Scholar] [CrossRef]
- Yang, Q.; Lai, S.K. Anti-PEG immunity: Emergence, characteristics, and unaddressed questions. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 655–677. [Google Scholar] [CrossRef]
- Chung, I.; Xie, D.; Puckett, A.D.; Mays, J.W. Synthesis and evaluation of biodegradable multifunctional polymer networks. Eur. Polym. J. 2003, 39, 1817–1822. [Google Scholar] [CrossRef]
- Winzenburg, G.; Schmidt, C.; Fuchs, S.; Kissel, T. Biodegradable polymers and their potential use in parenteral veterinary drug delivery systems. Adv. Drug Deliv. Rev. 2004, 56, 1453–1466. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Leong, K.-F.; Du, Z.; Chua, C.-K. The design of scaffolds for use in tissue engineering. Part 1. traditional factors. Tissue Eng. 2004, 7, 679–689. [Google Scholar] [CrossRef]
- Urbánek, T.; Jäger, E.; Jäger, A.; Hrubý, M. Selectively biodegradable polyesters: Nature-inspired construction materials for future biomedical applications. Polymers 2019, 11, 1061–1081. [Google Scholar] [CrossRef] [PubMed]
| Polymer | Concn (mg/mL) | Mass (mg) | Vol (mL) | Mass % (w/w) | Vol % (v/v) |
|---|---|---|---|---|---|
| Blank PFU Nanoparticles | |||||
| PFU | 24.8 | 198.0 | 8.0 | 99.0 | 7.2 |
| mPEG-PLA | 3.0 | 2.0 | 2.0 | 1.0 | 1.8 |
| FITC | - | - | 1.0 | - | 0.9 |
| 5% PVA | - | - | 100.0 | - | 90.1 |
| Total | - | 200.0 | 111.0 | 100.0 | 100.0 |
| FITC-loaded PFU Nanoparticles | |||||
| PFU | 24.8 | 198.0 | 8.0 | 98.9 | 8.1 |
| mPEG-PLA | 3.0 | 2.0 | 2.0 | 1.0 | 0.9 |
| FITC | 0.1 | 0.1 | 1.0 | 0.1 | 0.9 |
| 5% PVA | - | - | 100.0 | - | 90.1 |
| total | - | 200.1 | 111.0 | 100.0 | 100.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-Y.; Kang, J.; Yoon, J.-Y.; Chung, I. Synthesis and Characterization of Polyfumarateurethane Nanoparticles for Sustained Release of Bupivacaine. Pharmaceutics 2020, 12, 281. https://doi.org/10.3390/pharmaceutics12030281
Park S-Y, Kang J, Yoon J-Y, Chung I. Synthesis and Characterization of Polyfumarateurethane Nanoparticles for Sustained Release of Bupivacaine. Pharmaceutics. 2020; 12(3):281. https://doi.org/10.3390/pharmaceutics12030281
Chicago/Turabian StylePark, Soo-Yong, Jiin Kang, Ji-Young Yoon, and Ildoo Chung. 2020. "Synthesis and Characterization of Polyfumarateurethane Nanoparticles for Sustained Release of Bupivacaine" Pharmaceutics 12, no. 3: 281. https://doi.org/10.3390/pharmaceutics12030281
APA StylePark, S.-Y., Kang, J., Yoon, J.-Y., & Chung, I. (2020). Synthesis and Characterization of Polyfumarateurethane Nanoparticles for Sustained Release of Bupivacaine. Pharmaceutics, 12(3), 281. https://doi.org/10.3390/pharmaceutics12030281