In Vitro Performance of an Investigational Vibrating-Membrane Nebulizer with Surfactant under Simulated, Non-Invasive Neonatal Ventilation Conditions: Influence of Continuous Positive Airway Pressure Interface and Nebulizer Positioning on the Lung Dose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nebulizer Type and Surfactant Preparation
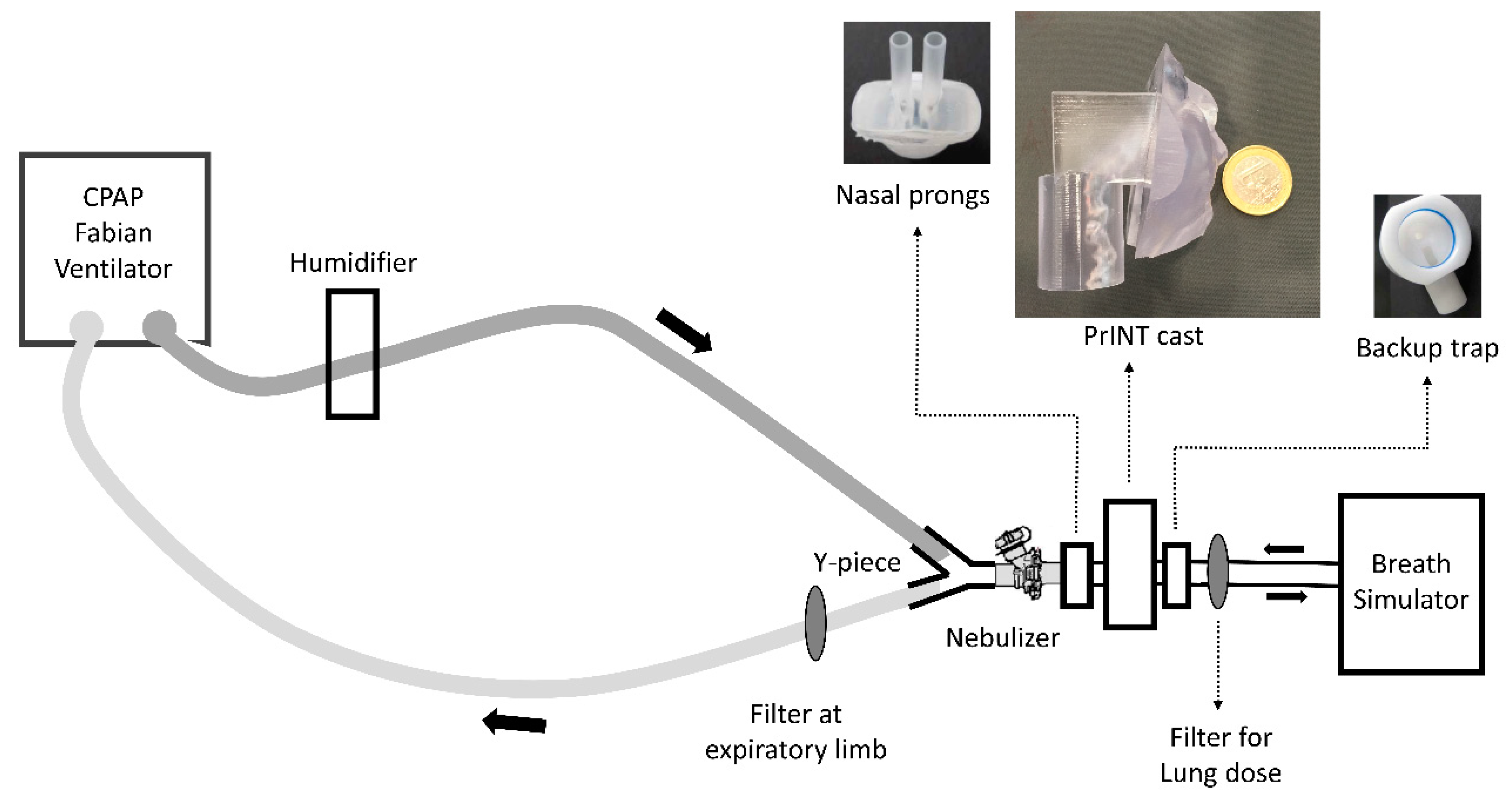
2.2. Surfactant Aerosol Deposition Experiments in a Simulated Neonatal CPAP Circuit
2.3. Dose-Dependent Deposition in a Simulated Neonatal CPAP Circuit
2.4. HPLC Analytical Method for Phosphatidylcholine
2.5. Particle Size Characterization
2.6. Statistical Analysis
3. Results
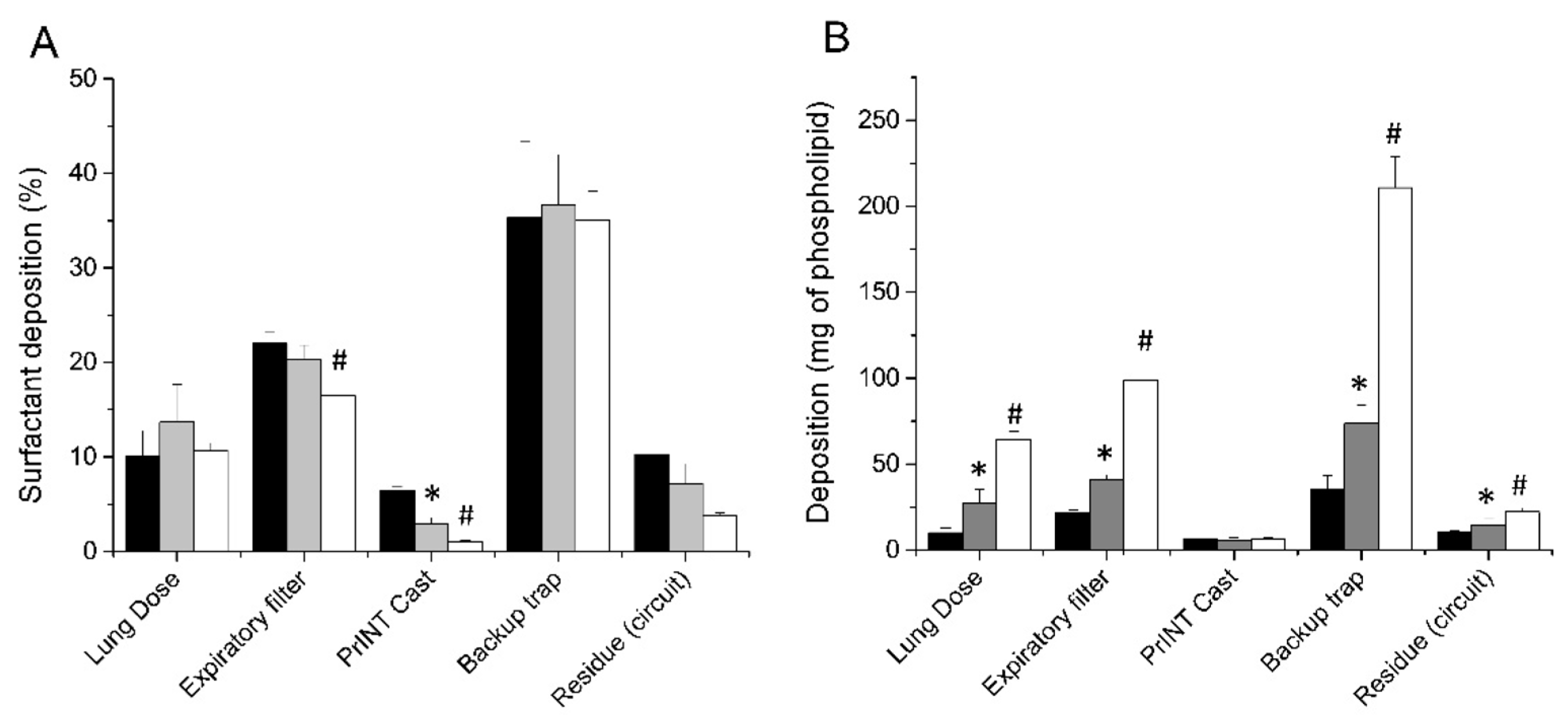
3.1. Nebulizer Positioning Significantly Affects the Lung Dose (LD)
3.2. The Choice of CPAP Interface Does not Affect the Surfactant Lung Dose
3.3. Lung Dose after Surfactant Nebulization Correlates with the Nominal Dose
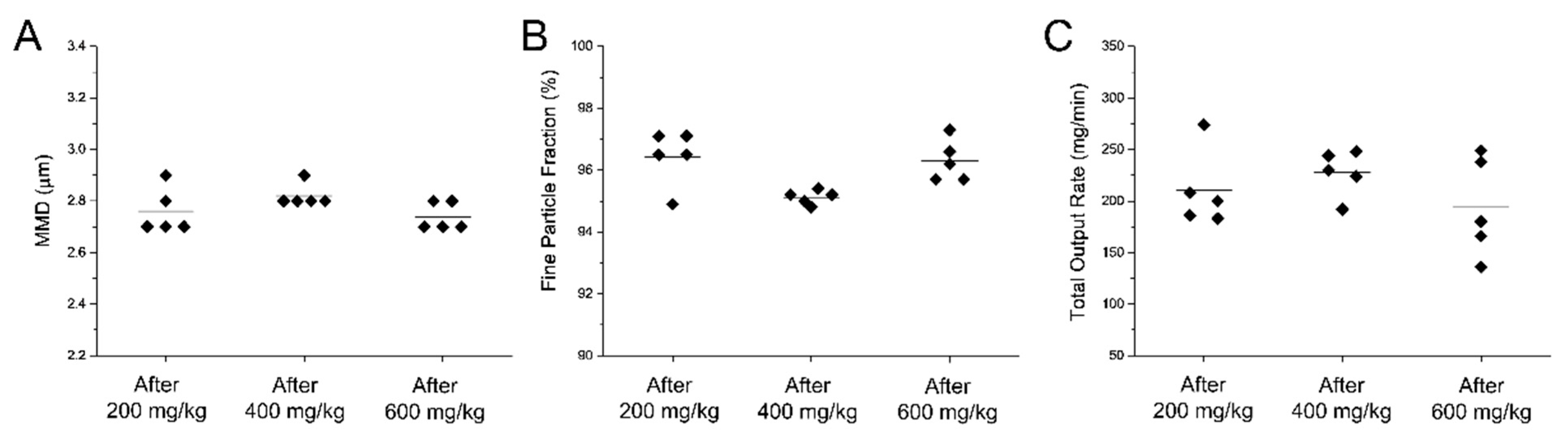
3.4. The Customized eFlow Neos Nebulizer Shows a Consistent Surfactant Aerosol Production Over Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Robillard, E.; Alarie, Y.; Dagenais-Perusse, P.; Baril, E.; Guilbeault, A. Microaerosol Administration of Synthetic β-γ-Dipalmitoyl-L-α-Lecithin in the Respiratory Distress Syndrome: A Preliminary Report. Can. Med. Assoc. J. 1964, 90, 55–57. [Google Scholar]
- Adams, F.H.; Towers, B.; Osher, A.B.; Ikegami, M.; Fujiwara, T.; Nozaki, M. Effects of tracheal instillation of natural surfactant in premature lambs. I. Clinical and autopsy findings. Pediatr. Res. 1978, 12, 841–848. [Google Scholar] [CrossRef] [Green Version]
- Sweet, D.G.; Carnielli, V.; Greisen, G.; Hallman, M.; Ozek, E.; Te Pas, A.; Plavka, R.; Roehr, C.C.; Saugstad, O.D.; Simeoni, U.; et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome—2019 Update. Neonatology 2019, 115, 432–450. [Google Scholar] [CrossRef] [Green Version]
- Verder, H.; Robertson, B.; Greisen, G.; Ebbesen, F.; Albertsen, P.; Lundstrom, K.; Jacobsen, T. Surfactant Therapy and Nasal Continuous Positive Airway Pressure for Newborns with Respiratory Distress Syndrome. N. Engl. J. Med. 1994, 331, 1051–1055. [Google Scholar] [CrossRef] [PubMed]
- Herting, E.; Härtel, C.; Göpel, W. Less invasive surfactant administration (LISA): Chances and limitations. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F655–F659. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.D.; Brown, R.; Lampland, A.L.; Leone, T.A.; Rudser, K.D.; Finer, N.N.; Rich, W.D.; Merritt, T.A.; Czynski, A.J.; Kessel, J.M.; et al. Laryngeal Mask Airway for Surfactant Administration in Neonates: A Randomized, Controlled Trial. J. Pediatr. 2018, 193, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Jorch, G.; Hartl, H.; Roth, B.; Kribs, A.; Gortner, L.; Schaible, T.; Hennecke, K.H.; Poets, C. To the editor: Surfactant aerosol treatment of respiratory distress syndrome in spontaneously breathing premature infants. Pediatr. Pulmonol. 1997, 24, 222–224. [Google Scholar] [CrossRef]
- Arroe, M.; Pedersen-Bjergaard, L.; Albertsen, P.; Bode, S.; Greison, G.; Jonsbo, F. Inhalation of aerosolized surfactant Exosurf to neonates treated with nasal continuous positive airway pressure. Prenat. Neonatal Med. 1998, 3, 346–352. [Google Scholar]
- Berggren, E.; Liljedahl, M.; Winbladh, B.; Andreasson, B.; Curstedt, T.; Robertson, B.; Schollin, J. Pilot study of nebulized surfactant therapy for neonatal respiratory distress syndrome. Acta Paediatr. 2000, 89, 460–464. [Google Scholar] [CrossRef]
- Finer, N.N.; Merritt, T.A.; Bernstein, G.; Job, L.; Mazela, J.; Segal, R. An Open Label, Pilot Study of Aerosurf ® Combined with nCPAP to Prevent RDS in Preterm Neonates. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 303–309. [Google Scholar] [CrossRef]
- Minocchieri, S.; Berry, C.A.; Pillow, J.J. Nebulised surfactant to reduce severity of respiratory distress: A blinded, parallel, randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 104, F313–F319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazela, J.; Polin, R.A. Aerosol delivery to ventilated newborn infants: Historical challenges and new directions. Eur. J. Pediatr. 2011, 170, 433–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazela, J.; Merritt, T.A.; Finer, N.N. Aerosolized surfactants. Curr. Opin. Pediatr. 2007, 19, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Ari, A.; Atalay, O.T.; Harwood, R.; Sheard, M.M.; Aljamhan, E.A.; Fink, J.B. Influence of nebulizer type, position, and bias flow on aerosol drug delivery in simulated pediatric and adult lung models during mechanical ventilation. Respir. Care 2010, 55, 845–851. [Google Scholar]
- Goikoetxea, E.; Murgia, X.; Serna-Grande, P.; Valls-i-Soler, A.; Rey-Santano, C.; Rivas, A.; Antón, R.; Basterretxea, F.J.; Miñambres, L.; Méndez, E.; et al. In vitro surfactant and perfluorocarbon aerosol deposition in a neonatal physical model of the upper conducting airways. PLoS ONE 2014, 9, e106835. [Google Scholar] [CrossRef]
- Murgia, X.; Gastiasoro, E.; Mielgo, V.; Alvarez-Diaz, F.; Lafuente, H.; Valls-i-Soler, A.; Gomez-Solaetxe, M.A.; Larrabe, J.L.; Rey-Santano, C. Surfactant and Perfluorocarbon Aerosolization by Means of Inhalation Catheters for the Treatment of Respiratory Distress Syndrome: An In Vitro Study. J. Aerosol Med. Pulm. Drug Deliv. 2011, 24, 81–87. [Google Scholar] [CrossRef]
- Bianco, F.; Ricci, F.; Catozzi, C.; Murgia, X.; Schlun, M.; Bucholski, A.; Hetzer, U.; Bonelli, S.; Lombardini, M.; Pasini, E.; et al. From bench to bedside: In vitro and in vivo evaluation of a neonate-focused nebulized surfactant delivery strategy. Respir. Res. 2019, 20, 134. [Google Scholar] [CrossRef]
- Minocchieri, S.; Burren, J.M.; Bachmann, M.A.; Stern, G.; Wildhaber, J.; Buob, S.; Schindel, R.; Kraemer, R.; Frey, U.P.; Nelle, M. Development of the premature infant nose throat-model (PrINT-Model)-an upper airway replica of a premature neonate for the study of aerosol delivery. Pediatr. Res. 2008, 64, 141–146. [Google Scholar] [CrossRef] [Green Version]
- Cole, C.H. Special problems in aerosol delivery: Neonatal and pediatric considerations. Respir. Care 2000, 45, 646–651. [Google Scholar]
- Fok, T.F.; Monkman, S.; Dolovich, M.; Gray, S.; Coates, G.; Paes, B.; Rashid, F.; Newhouse, M.; Kirpalani, H. Efficiency of aerosol medication delivery from a metered dose inhaler versus jet nebulizer in infants with bronchopulmonary dysplasia. Pediatr. Pulmonol. 1996, 21, 301–309. [Google Scholar] [CrossRef]
- Köhler, E.; Jilg, G.; Avenarius, S.; Jorch, G. Lung deposition after inhalation with various nebulisers in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2008, 93, F275–F279. [Google Scholar] [CrossRef] [PubMed]
- Dubus, J.C.; Vecellio, L.; De Monte, M.; Fink, J.B.; Grimbert, D.; Montharu, J.; Valat, C.; Behan, N.; Diot, P. Aerosol deposition in neonatal ventilation. Pediatr. Res. 2005, 58, 10–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linner, R.; Perez-de-Sa, V.; Cunha-Goncalves, D. Lung Deposition of Nebulized Surfactant in Newborn Piglets. Neonatology 2015, 107, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Minocchieri, S.; Knoch, S.; Schoel, W.M.; Ochs, M.; Nelle, M. Nebulizing poractant alfa versus conventional instillation: Ultrastructural appearance and preservation of surface activity. Pediatr. Pulmonol. 2014, 49, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Hütten, M.C.; Kuypers, E.; Ophelders, D.R.; Nikiforou, M.; Jellema, R.K.; Niemarkt, H.J.; Fuchs, C.; Tservistas, M.; Razetti, R.; Bianco, F.; et al. Nebulization of Poractant alfa via a vibrating membrane nebulizer in spontaneously breathing preterm lambs with binasal continuous positive pressure ventilation. Pediatr. Res. 2015, 78, 664–669. [Google Scholar] [CrossRef] [Green Version]
- Wiswell, T.E.; Courtney, S.E. Noninvasive Respiratory Support. In Assited Ventilation of the Neonate; Goldsmith, J.P., Karotkin, E.H., Eds.; Elsevier: St. Louis, MO, USA, 2011; p. 631. [Google Scholar]
- Nord, A.; Linner, R.; Salomone, F.; Bianco, F.; Ricci, F.; Murgia, X.; Schlun, M.; Cunha-Goncalves, D.; Perez-de-Sa, V. Lung deposition of nebulized surfactant in newborn piglets: Nasal CPAP vs Nasal IPPV. Pediatr. Pulmonol. 2020, 55, 514–520. [Google Scholar] [CrossRef]
- Ikegami, M.; Adams, F.H.; Towers, B.; Osher, A.B. The Quantity of Natural Surfactant Necessary to Prevent the Respiratory Distress Syndrome in Premature Lambs. Pediatr Res. 1980, 14, 1082–1085. [Google Scholar] [CrossRef] [Green Version]
- King, D.M.; Wang, Z.; Palmer, H.J.; Holm, B.A.; Notter, R.H. Bulk shear viscosities of endogenous and exogenous lung surfactants. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 282, L277–L284. [Google Scholar] [CrossRef] [Green Version]
- Ari, A. Jet, Ultrasonic, and Mesh Nebulizers: An Evaluation of Nebulizers for Better Clinical Outcomes. Eurasian J. Pulmonol. 2014, 16, 1–7. [Google Scholar] [CrossRef]
- Wagner, M.H.; Amthauer, H.; Sonntag, J.; Drenk, F.; Eichstädt, H.W.; Obladen, M. Endotracheal surfactant atomization: An alternative to bolus instillation? Crit. Care Med. 2000, 28, 2540–2544. [Google Scholar] [CrossRef]
- Milesi, I.; Tingay, D.G.; Zannin, E.; Bianco, F.; Tagliabue, P.; Mosca, F.; Lavizzari, A.; Ventura, M.L.; Zonneveld, C.E.; Perkins, E.J.; et al. Intratracheal atomized surfactant provides similar outcomes as bolus surfactant in preterm lambs with respiratory distress syndrome. Pediatr. Res. 2016, 80, 92–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rey-Santano, C.; Mielgo, V.E.; Andres, L.; Ruiz-del-Yerro, E.; Valls-i-Soler, A.; Murgia, X. Acute and sustained effects of aerosolized vs. bolus surfactant therapy in premature lambs with respiratory distress syndrome. Pediatr. Res. 2013, 73, 639–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milesi, I.; Tingay, D.G.; Lavizzari, A.; Bianco, F.; Zannin, E.; Tagliabue, P.; Mosca, F.; Ventura, M.L.; Rajapaksa, A.; Perkins, E.J.; et al. Supraglottic Atomization of Surfactant in Spontaneously Breathing Lambs Receiving Continuous Positive Airway Pressure. Pediatr. Crit. Care Med. 2017, 18, e428–e434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goikoetxea, E.; Rivas, A.; Murgia, X.; Antón, R. Mathematical modeling and numerical simulation of surfactant delivery within a physical model of the neonatal trachea for different aerosol characteristics. Aerosol Sci. Technol. 2017, 51, 168–177. [Google Scholar] [CrossRef] [Green Version]
- Aramendia, I.; Fernandez-Gamiz, U.; Lopez-Arraiza, A.; Rey-Santano, C.; Mielgo, V.; Basterretxea, F.J.; Sancho, J.; Gomez-Solaetxe, M.A. Experimental evaluation of perfluorocarbon aerosol generation with two novel nebulizer prototypes. Pharmaceutics 2019, 11, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nord, A.; Linner, R.; Milesi, I.; Zannin, E.; di Castri, M.; Bianco, F.; Dellacá, R.L.; Cunha-Goncalves, D.; Perez-de-Sa, V. A novel delivery system for supraglottic atomization allows increased lung deposition rates of pulmonary surfactant in newborn piglets. Pediatr. Res. 2019. [Google Scholar] [CrossRef]
- Gregory, T.J.; Irshad, H.; Chand, R.; Kuehl, P.J. Deposition of Aerosolized Lucinactant in Nonhuman Primates. J. Aerosol Med. Pulm. Drug Deliv. 2019, 33, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Pohlmann, G.; Iwatschenko, P.; Koch, W.; Windt, H.; Rast, M.; de Abreu, M.G.; Taut, F.J.H.; De Muynck, C. A Novel Continuous Powder Aerosolizer (CPA) for Inhalative Administration of Highly Concentrated Recombinant Surfactant Protein-C (rSP-C) Surfactant to Preterm Neonates. J. Aerosol Med. Pulm. Drug Deliv. 2013, 26, 370–379. [Google Scholar] [CrossRef]
- Rahmel, D.K.; Pohlmann, G.; Iwatschenko, P.; Volland, J.; Liebisch, S.; Kock, H.; Mecklenburg, L.; Maurer, C.; Kemkowski, J.; Taut, F.J.H. The non-intubated, spontaneously breathing, continuous positive airway pressure (CPAP) ventilated pre-term lamb: A unique animal model. Reprod. Toxicol. 2012, 34, 204–215. [Google Scholar] [CrossRef]
- Walther, F.J.; Gupta, M.; Lipp, M.M.; Chan, H.; Krzewick, J.; Gordon, L.M.; Waring, A.J. Aerosol delivery of dry powder synthetic lung surfactant to surfactant-deficient rabbits and preterm lambs on non-invasive respiratory support. Gates Open Res. 2019. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianco, F.; Pasini, E.; Nutini, M.; Murgia, X.; Stoeckl, C.; Schlun, M.; Hetzer, U.; Bonelli, S.; Lombardini, M.; Milesi, I.; et al. In Vitro Performance of an Investigational Vibrating-Membrane Nebulizer with Surfactant under Simulated, Non-Invasive Neonatal Ventilation Conditions: Influence of Continuous Positive Airway Pressure Interface and Nebulizer Positioning on the Lung Dose. Pharmaceutics 2020, 12, 257. https://doi.org/10.3390/pharmaceutics12030257
Bianco F, Pasini E, Nutini M, Murgia X, Stoeckl C, Schlun M, Hetzer U, Bonelli S, Lombardini M, Milesi I, et al. In Vitro Performance of an Investigational Vibrating-Membrane Nebulizer with Surfactant under Simulated, Non-Invasive Neonatal Ventilation Conditions: Influence of Continuous Positive Airway Pressure Interface and Nebulizer Positioning on the Lung Dose. Pharmaceutics. 2020; 12(3):257. https://doi.org/10.3390/pharmaceutics12030257
Chicago/Turabian StyleBianco, Federico, Elena Pasini, Marcello Nutini, Xabier Murgia, Carolin Stoeckl, Martin Schlun, Uwe Hetzer, Sauro Bonelli, Marta Lombardini, Ilaria Milesi, and et al. 2020. "In Vitro Performance of an Investigational Vibrating-Membrane Nebulizer with Surfactant under Simulated, Non-Invasive Neonatal Ventilation Conditions: Influence of Continuous Positive Airway Pressure Interface and Nebulizer Positioning on the Lung Dose" Pharmaceutics 12, no. 3: 257. https://doi.org/10.3390/pharmaceutics12030257
APA StyleBianco, F., Pasini, E., Nutini, M., Murgia, X., Stoeckl, C., Schlun, M., Hetzer, U., Bonelli, S., Lombardini, M., Milesi, I., Pertile, M., Minocchieri, S., Salomone, F., & Bucholski, A. (2020). In Vitro Performance of an Investigational Vibrating-Membrane Nebulizer with Surfactant under Simulated, Non-Invasive Neonatal Ventilation Conditions: Influence of Continuous Positive Airway Pressure Interface and Nebulizer Positioning on the Lung Dose. Pharmaceutics, 12(3), 257. https://doi.org/10.3390/pharmaceutics12030257




