Development of Lipomer Nanoparticles for the Enhancement of Drug Release, Anti-Microbial Activity and Bioavailability of Delafloxacin
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Lipid-Polymer Hybrid Nanoparticles
2.3. Particle Characterization of DFL Loaded L-P-NPs
2.4. Measurement of Drug Encapsulation Efficiency
2.5. Differential Scanning Calorimetry (DSC) Studies
2.6. Fourier Transform Infrared Spectroscopy (FTIR)
2.7. In-Vitro Release Studies
2.8. Morphology
2.9. In-Vitro Antimicrobial Activity
2.9.1. Bacterial Isolates
2.9.2. Culture Conditions and Media
2.9.3. McFarland Standard Preparation and Inoculum Size
2.9.4. Minimum Inhibitory Concentration (MIC)
2.10. Bio-Analytical Methods
2.11. Pharmacokinetic Study in Rats
3. Results and Discussion
3.1. Particle Characterization of DFL-Loaded L-P-NPs
3.2. Measurement of Drug Encapsulation Efficiency
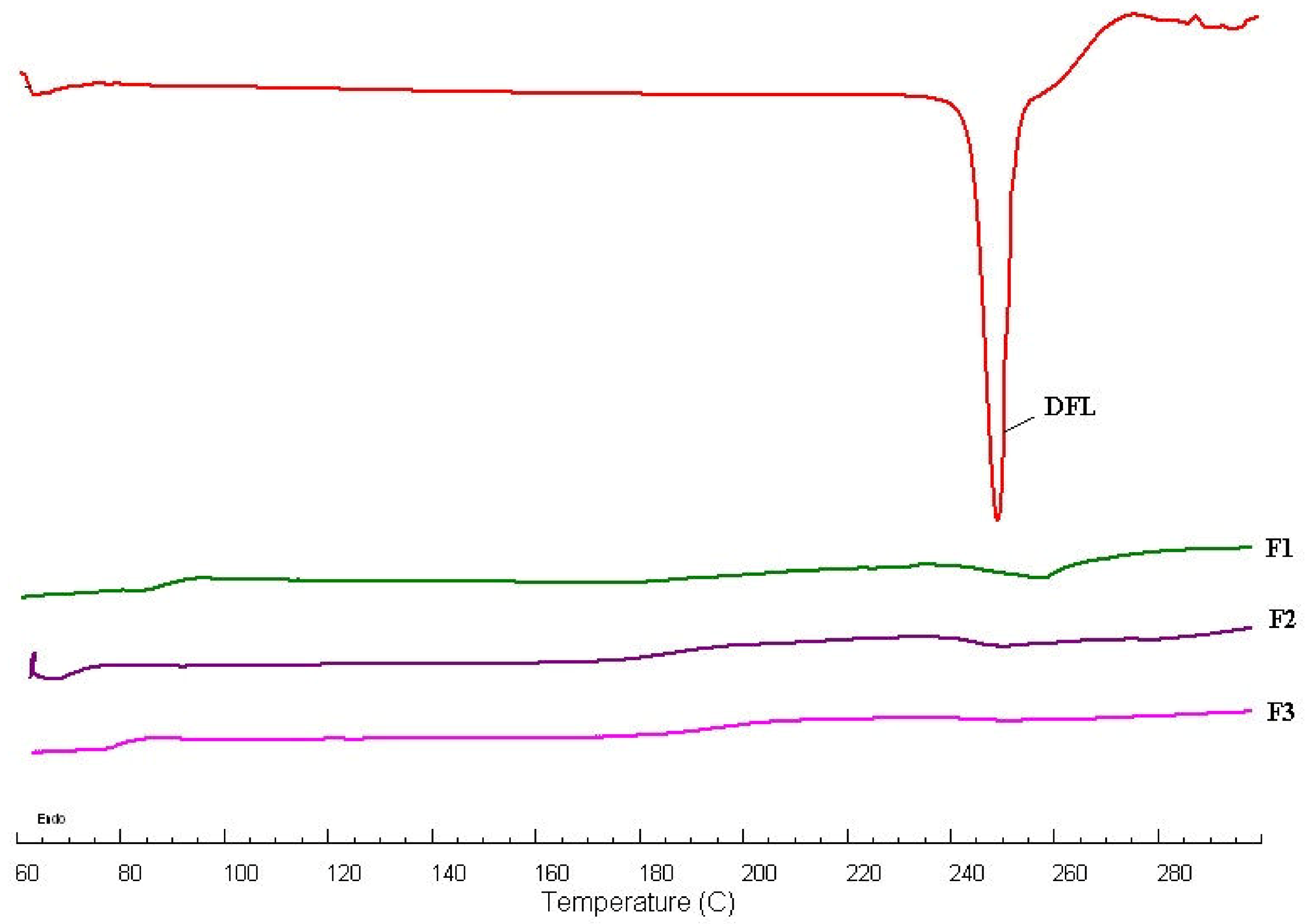
3.3. DSC Studies
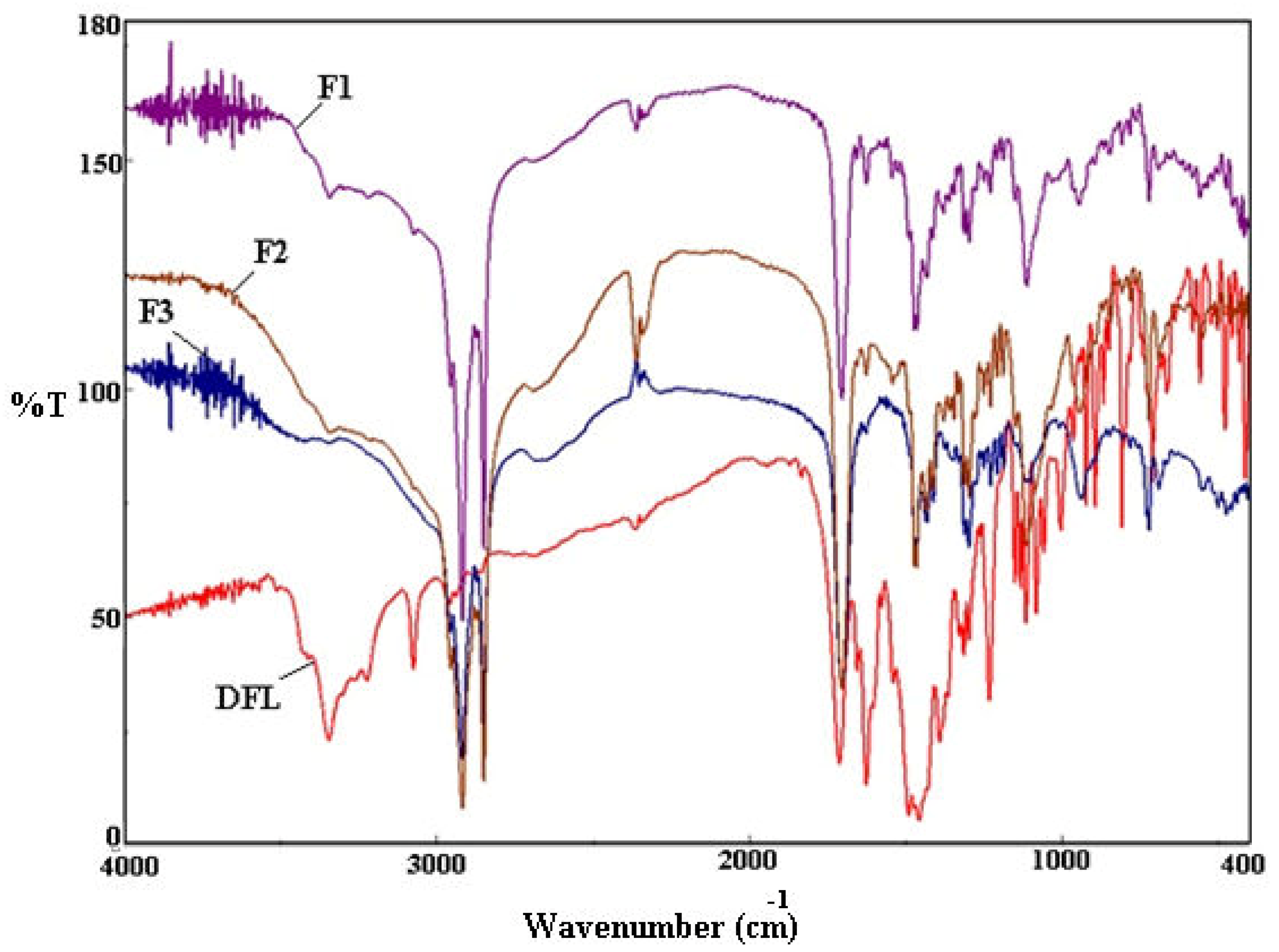
3.4. FTIR Analysis
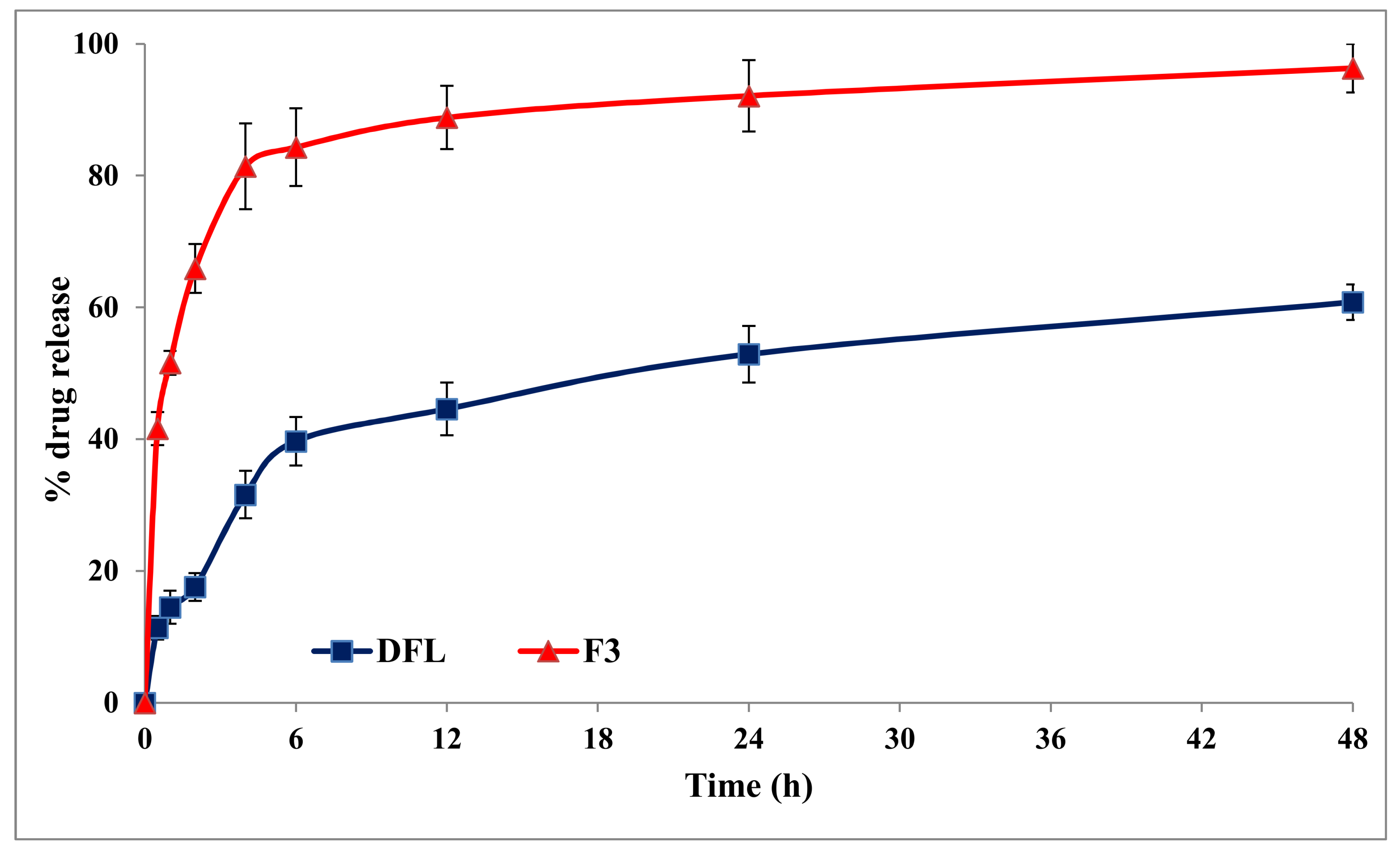
3.5. In-Vitro Release Studies
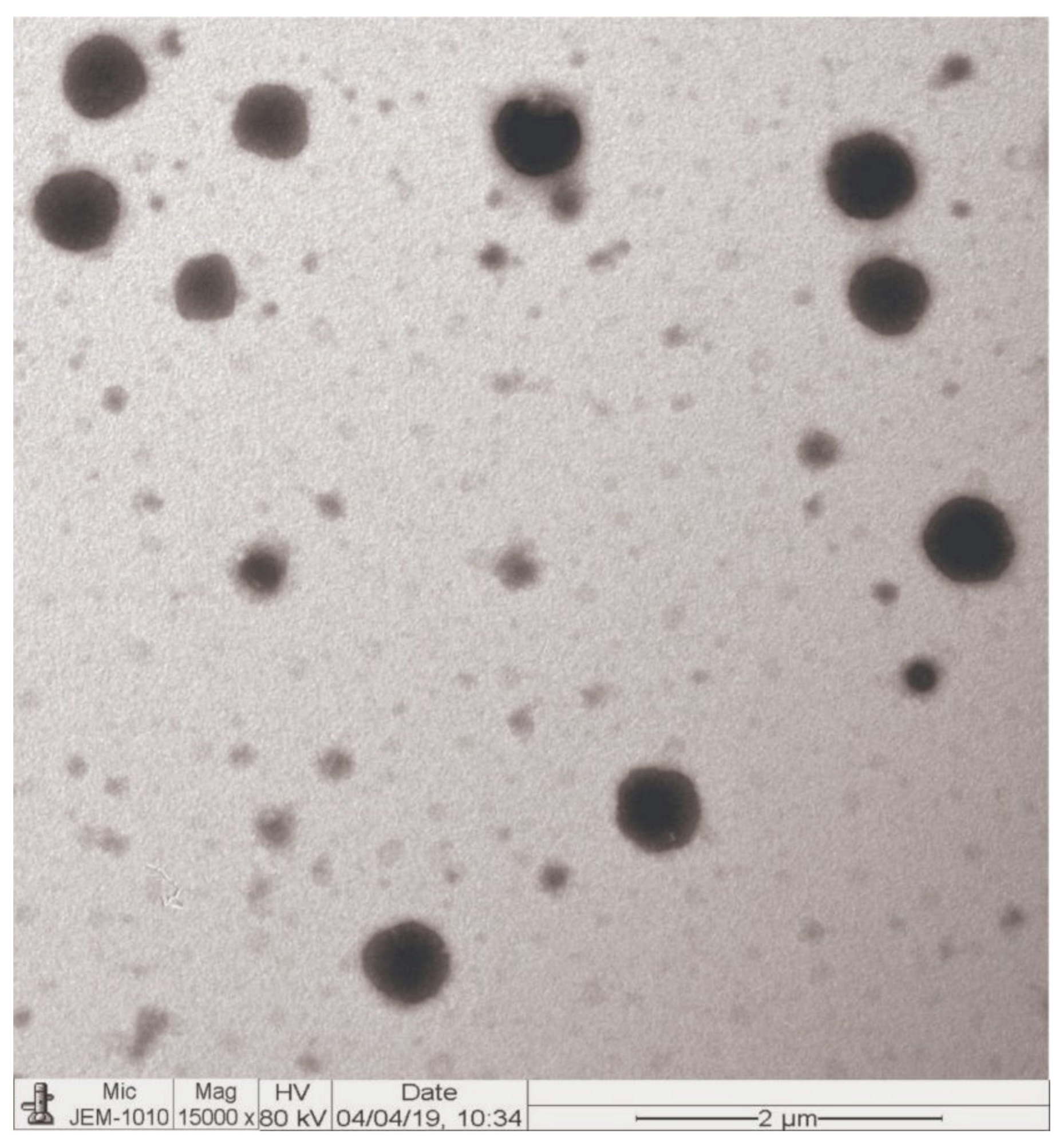
3.6. Morphology
3.7. In Vitro Antibacterial Activity
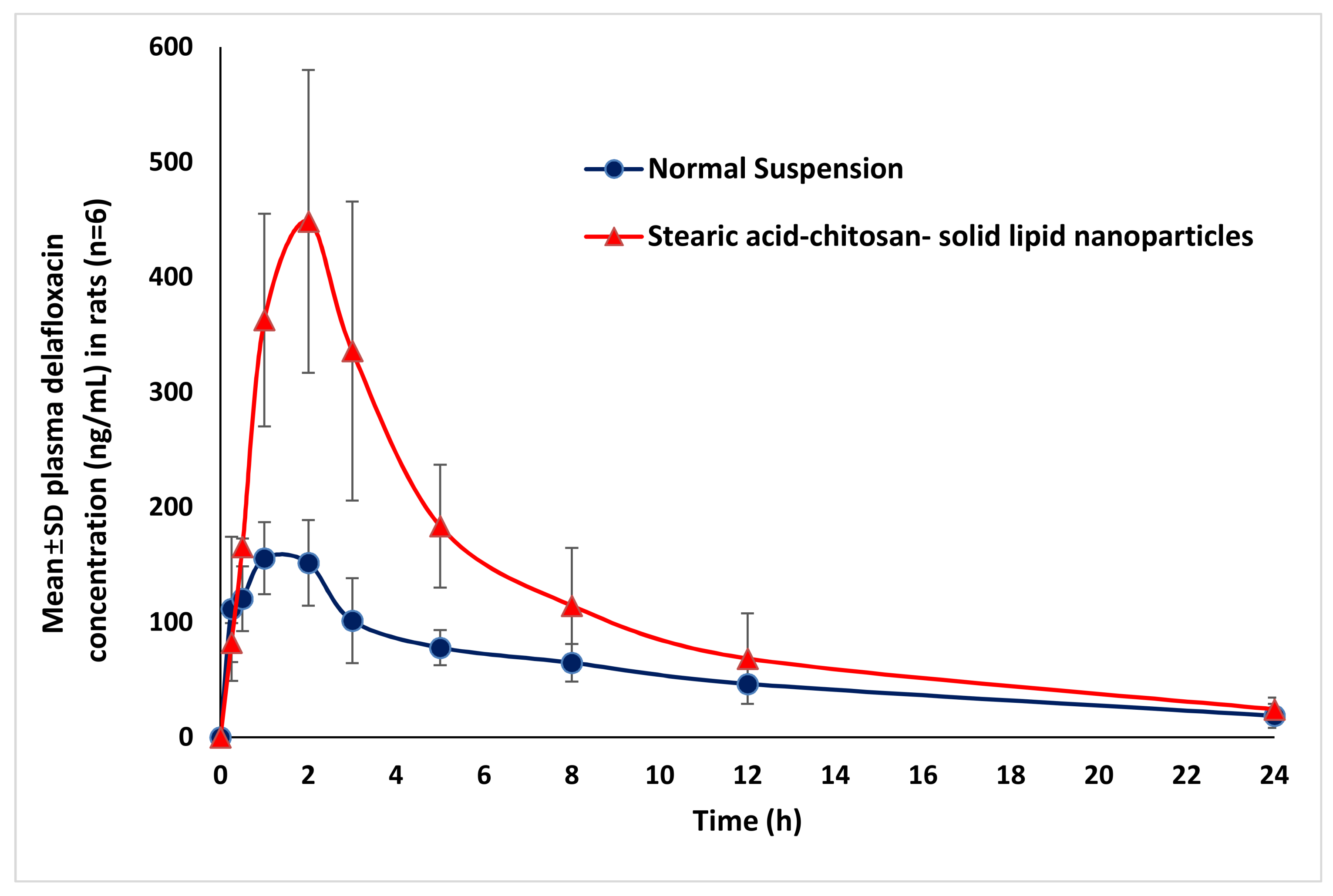
3.8. Pharmacokinetic Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Falcone, M.; Concia, E.; Giusti, M.; Mazzone, A.; Santini, C.; Stefani, S.; Violi, F. Acute bacterial skin and skin structure infections in internal medicine wards: Old and new drugs. Intern. Emerg. Med. 2016, 11, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Ray, G.T.; Suaya, J.A.; Baxter, R. Incidence, microbiology, and patient characteristics of skin and soft-tissue infections in a U. S. population: A retrospective population-based study. BMC Infect. Dis. 2013, 13, 252. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Self, W.H.; Wunderink, R.G.; Fakhran, S.; Balk, R.; Bramley, A.M.; Reed, C.; Grijalva, C.G.; Anderson, E.J.; Courtney, D.M.; et al. CDC EPIC Study Team. Community-Acquired Pneumonia Requiring Hospitalization among U. S. Adults. N. Engl. J. Med. 2015, 373, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Markham, A. Delafloxacin: First Global Approval. Drugs 2017, 77, 1481–1486. [Google Scholar] [CrossRef]
- US Prescribing Information of BAXDELA™ (Delafloxacin); Melinta Therapeutics, Inc.: New Haven, CT, USA, 2017.
- Bassetti, M.; Pecori, D.; Cojutti, P.; Righi, E.; Pea, F. Clinical and pharmacokinetic drug evaluation of delafloxacin for the treatment of acute bacterial skin and skin structure infections. Expert. Opin. Drug Metab. Toxicol. 2017, 13, 1193–1200. [Google Scholar] [CrossRef][Green Version]
- Lemaire, S.; Tulkens, P.M.; van Bambeke, F. Contrasting effects of acidic pH on the extracellular and intracellular activities ofthe anti-Gram-positive fluoroquinolonesmoxifloxacin and delafloxacin against Staphylococcus aureus. Antimicrob. Agents. Chemother. 2011, 55, 649–658. [Google Scholar] [CrossRef]
- Siala, W.; Mingeot-Leclercq, M.P.; Tulkens, P.M.; Hallin, M.; Denis, O.; Van Bambeke, F. Comparison of the antibiotic activities of daptomycin, vancomycin, and the investigational fluoroquinolonedelafloxacin against biofilms from Staphylococcus aureus clinical isolates. Antimicrob. Agents. Chemother. 2014, 58, 6385–6397. [Google Scholar] [CrossRef]
- Remy, J.M.; Tow-Keogh, C.A.; McConnell, T.S.; Dalton, J.M.; Devito, J.A. Activity of delafloxacin against methicillin-resistant Staphylococcus aureus: Resistance selection and characterization. J. Antimicrob. Chemother. 2012, 67, 2814–2820. [Google Scholar] [CrossRef]
- Smith, H.J.; Nichol, K.A.; Hoban, D.J.; Zhanel, G.G. Dual activity of fluoroquinolones against Streptococcus pneumoniae: The facts behind the claims. J. Antimicrob. Chemother. 2002, 49, 893–895. [Google Scholar] [CrossRef]
- Shiu, J.; Ting, G.; Kiang, T.K. Clinical Pharmacokinetics and Pharmacodynamics of Delafloxacin. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 305–317. [Google Scholar] [CrossRef]
- Wu, K.; Yan, Z. FDA: Center for Drug Evaluation and Research- 208610Orig1s000. 2016. Available online: https://www.accessdata.fda.gov/drugsatfdadocs/nda/2017/208610Orig1s000,208611Orig1s000ClinPharmR.pdf (accessed on 18 August 2019).
- Cho, J.C.; Crotty, M.P.; White, B.P.; Worley, M.V. What Is Old Is New Again: Delafloxacin, a Modern Fluoroquinolone. Pharmacotherapy 2018, 38, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Hoover, R.; Hunt, T.; Benedict, M.; Paulson, S.K.; Lawrence, L.; Cammarata, S.; Sun, E. Single and Multiple Ascending-dose Studies of Oral Delafloxacin: Effects of Food, Sex, and Age. Clin. Ther. 2016, 38, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Hoover, R.; Hunt, T.; Benedict, M.; Paulson, S.K.; Lawrence, L.; Cammarata, S.; Sun, E. Safety, tolerability, and pharmacokinetic properties of intravenous delafloxacin after single and multiple doses in healthy volunteers. Clin. Ther. 2016, 38, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Kardas, P. Patient compliance with antibiotic treatment for respiratory tract infections. J. Antimicrob. Chemother. 2002, 49, 897–903. [Google Scholar] [CrossRef] [PubMed]
- López-López, M.; Fernández-Delgado, A.; Moyá, M.L.; Blanco-Arévalo, D.; Carrera, C.; de la Haba, R.R.; Ventosa, A.; Bernal, E.; López-Cornejo, P. Optimized Preparation of Levofloxacin Loaded Polymeric Nanoparticles. Pharmaceutics 2019, 11, 57. [Google Scholar] [CrossRef]
- Mukherjee, A.; Waters, A.K.; Kalyan, P.; Achrol, A.S.; Kesari, S.; Yenugonda, V.M. Lipid-polymer hybrid nanoparticles as a next-generation drug delivery platform: State of the art, emerging technologies, and perspectives. Int. J. Nanomed. 2019, 14, 1937–1952. [Google Scholar] [CrossRef]
- Wang, L.; Li, L.; Sun, Y.; Ding, J.; Li, J.; Duan, X.; Li, Y.; Junyaprasert, V.B.; Mao, S. In vitro and in vivo evaluation of chitosan graft glycerylmonooleate as peroral delivery carrier of enoxaparin. Int. J. Pharm. 2014, 471, 391–399. [Google Scholar] [CrossRef]
- Harde, H.; Das, M.; Jain, S. Solid lipid nanoparticles: An oral bioavailability enhancer vehicle. Expert Opin. Drug Deliv. 2011, 8, 1407–1424. [Google Scholar] [CrossRef]
- Zhang, L.; Chan, J.M.; Gu, F.X.; Rhee, J.W.; Wang, A.Z.; Radovic-Moreno, A.F.; Alexis, F.; Langer, R.; Farokhzad, O.C. Self-assembled lipid--polymer hybrid nanoparticles: A robust drug delivery platform. ACS Nano 2008, 2, 1696–1702. [Google Scholar] [CrossRef]
- Jain, A.S.; Shah, S.; Nagarsenker, M.S.; Nikam, Y.; Gude, R.P.; Steiniger, F.; Thamm, J.; Fahr, A. Lipid colloidal carriers for improvement of anticancer activity of orally delivered quercetin: Formulation, characterization and establishing in vitro-in vivo advantage. J. Biomed. Nanotechnol. 2013, 9, 1230–1240. [Google Scholar] [CrossRef]
- Sonvico, F.; Cagnani, A.; Rossi, A.; Motta, S.; Di Bari, M.; Cavatorta, F.; Alonso, M.J.; Deriu, A.; Colombo, P. Formation of self-organized nanoparticles by lecithin/chitosan ionic interaction. Int. J. Pharm. 2006, 324, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Madni, A.; Torchilin, V.; Filipczak, N.; Pan, J.; Tahir, N.; Shah, H. Lipid-chitosan hybrid nanoparticles for controlled delivery of cisplatin. Drug Deliv. 2019, 26, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Wang, X.; Liu, C.; Zhang, X.; Zhang, X.; Chen, X.; Kou, Y.; Mao, S. Chitosan based polymer-lipid hybrid nanoparticles for oral delivery of enoxaparin. Int. J. Pharm. 2018, 547, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Cheow, W.S.; Chang, M.W.; Hadinoto, K. The roles of lipid in anti-biofilm efficacy of lipid–polymer hybrid nanoparticles encapsulating antibiotics. Colloids Surfaces A Physicochem. Eng. Asp. 2011, 389, 158–165. [Google Scholar] [CrossRef]
- Vieira, A.C.; Chaves, L.; Pinheiro, S.; Pinto, S.; Pinheiro, M.; Lima, S.; Ferreira, D.; Sarmento, B.; Reis, S. Mucoadhesive chitosan-coated solid lipid nanoparticles for better management of tuberculosis. Int. J. Pharm. 2018, 536, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Anwer, M.K.; Mohammad, M.; Ezzeldin, E.; Fatima, F.; Alalaiwe, A.; Iqbal, M. Preparation of sustained release apremilast-loaded PLGA nanoparticles: In vitro characterization and in vivo pharmacokinetic study in rats. Int. J. Nanomed. 2019, 14, 1587–1595. [Google Scholar] [CrossRef]
- Anwer, K.; Mohammad, M.; Iqbal, M.; Ansari, M.N.; Ezzeldin, E.; Fatima, F.; Alshahrani, S.M.; Aldawsari, M.F.; Alalaiwe, A.; Alzahrani, A.A.; et al. Sustained release and enhanced oral bioavailability of rivaroxaban by PLGA nanoparticles with no food effect. J. Thromb. Thrombolysis. 2020, 1–9. [Google Scholar] [CrossRef]
- Mohammed, M.; Alnafisah, M.S.; Anwer, K.; Fatima, F.; Almutairy, B.K.; Alshahrani, S.M.; Alshetaili, A.S.; Alalaiwe, A.; Fayed, M.H.; Alanazi, A.Z.; et al. Chitosan surface modified PLGA nanoparticles loaded with brigatinib for the treatment of non-small cell lung cancer. J. Polym. Engn. 2019, 39, 909–916. [Google Scholar] [CrossRef]
- Cockerill, F.R.; Wikler, M.A.; Alder, J.; Dudley, M.N.; Eliopoulos, G.M.; Ferraro, M.J.; Hardy, D.J.; Hecht, D.W.; Hindler, J.A.; Patel, J.B.; et al. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grown Aerobically; Approved Standards, 9th ed.; CLSI: Wayne, PA, USA, 2012; p. 12. ISBN 1-56238-784-7. [Google Scholar]
- Van Bambeke, F.; Michot, J.M.; Van Eldere, J.; Tulkens, P.M. Quinolones in 2005: An update. Clin. Microbiol. Infect. 2005, 11, 256–280. [Google Scholar] [CrossRef]
- Brook, I.; Wexler, H.M.; Goldstein, E.J. Antianaerobic antimicrobials: Spectrum and susceptibility testing. Clin. Microbiol. Rev. 2013, 26, 526–546. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Sixteenth Informational Supplement, CLSI Document M100-S16CLSI; CLSI: Wayne, PA, USA, 2006. [Google Scholar]
- Iqbal, M.; Ezzeldin, E.; Herqash, R.N.; Anwer, M.K.; Azam, F. Development and validation of a novel UPLC-MS/MS method for quantification of delafloxacin in plasma and aqueous humour for pharmacokinetic analyses. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2020, 1138, 121961. [Google Scholar] [CrossRef] [PubMed]
- Hanselmann, R.; Reeve, M.M. Crystalline Forms of d-Glucitol, 1-Deoxy-1-(Methylamino)-1-(6-Amino 3,5-Difluoropyridine-2-yl)-8-Chloro 6-Fluoro-1,4-Dihydro-7-(3-hy Droxyazetidin-1-yl)-4-oxo-3-Quinolinecarboxylate. U.S. Patent US2016/0046603A1, 18 February 2016. [Google Scholar]
- Anwer, M.K.; Al-Shdefat, R.; Ezzeldin, E.; Alshahrani, S.M.; Alshetaili, A.S.; Iqbal, M. Preparation, Evaluation and Bioavailability Studies of Eudragit Coated PLGA Nanoparticles for Sustained Release of Eluxadoline for the Treatment of Irritable Bowel Syndrome. Front. Pharmacol. 2017, 8, 844. [Google Scholar] [CrossRef] [PubMed]
| Formulae | DFL (mg) | Stearic Acid (mg) | Chitosan (mg) | Pluronic 127 (mg) |
|---|---|---|---|---|
| F1 | 10 | 100 | 10 | 50 |
| F2 | 10 | 200 | 10 | 50 |
| F3 | 10 | 400 | 10 | 50 |
| Formulae | Stearic Acid (mg) | Size (nm) | PDI | ZP (mV) | EE% | LC% |
|---|---|---|---|---|---|---|
| F1 | 100 | 299 ± 2.7 | 0.269 ± 0.042 | +27.8 ± 3.2 | 64.9 ± 2.3 | 3.8 ± 0.3 |
| F2 | 200 | 345 ± 3.9 | 0.230 ± 0.018 | +20.1 ± 1.5 | 76.1 ± 1.6 | 2.8 ± 0.5 |
| F3 | 400 | 368 ± 5.2 | 0.215 ± 0.015 | +19.2 ± 1.4 | 80.4 ± 3.1 | 1.7 ± 0.1 |
| Bacterial Strain | DFL | F3 | Levofloxacin |
|---|---|---|---|
| Escherichia coil (ATCC25922) | 0.1250 | 0.0312 | 1.0 |
| Escherichia coil (ATCC 11229) | 0.1250 | 0.0625 | 1.0 |
| Escherichia coil (Clinical isolate) | 0.50 | 0.1250 | 1.5 |
| Klebsiella pneumonia (ATCC 9633) | 0.1250 | 0.0625 | 0.50 |
| Klebsiella pneumonia (Clinical isolate) | 0.0625 | 0.0312 | 0.125 |
| Pseudomonas aeruginosa (ATCC 27853) | 0.50 | 0.125 | 2.5 |
| Pseudomonas aeruginosa (ATCC 10145) | 0.50 | 0.125 | 2.0 |
| Escherichia aerogenes (ATCC13048) | 0.50 | 0.125 | 1.0 |
| Escherichia aerogenes (NCIMB 10102) | 1.0 | 0.250 | 1.5 |
| Haemophilus influenza (ATCC 10211) | 0.0312 | 0.0156 | 0.125 |
| Pseudomonas mirabilis (NCIMB13283) | 1.0 | 0.250 | 2.0 |
| Salmonella typhimurium (ATCC 14028) | 0.1250 | 0.0312 | 0.50 |
| Salmonella typhimurium (Clinical isolate) | 0.1250 | 0.0312 | 0.50 |
| Salmonella aureus (ATCC 29213) | 0.0156 | 0.0078 | 0.50 |
| Salmonella aureus (ATCC 25923) | 0.1250 | 0.0625 | 0.50 |
| Salmonella aureus (NCTC 6571) | 0.0156 | 0.0078 | 0.50 |
| Salmonella aureus (ATCC 29737) | 0.0156 | 0.0078 | 0.250 |
| Salmonella aureus (Clinical isolate) | 0.0156 | 0.0078 | 0.50 |
| Salmonella epidermidis (ATCC 12228) | 0.0078 | 0.0039 | 0.0312 |
| Salmonella pneumonia (ATCC 49619) | 0.0156 | 0.0078 | 0.50 |
| Salmonella haemolyticus (ATCC 29970) | 0.250 | 0.0625 | 1.0 |
| Bacillus subtilis (ATCC 11774) | 0.0156 | 0.0039 | 0.0312 |
| Bacillus cereus (ATCC 10876) | 0.0312 | 0.0078 | 0.0625 |
| Escherichia faecalis (ATCC 29212) | 0.0625 | 0.0312 | 0.50 |
| Escherichia faecalis (ATCC 19433) | 0.1250 | 0.0312 | 0.50 |
| Escherichia faecalis (ATCC 49532) | 0.250 | 0.0625 | 1.0 |
| Parameters | Normal Suspension (Mean ± SD, n = 6) | DFL-Loaded L-P-NPs (F3) (Mean ± SD, n = 6) |
|---|---|---|
| Cmax (ng/mL) | 231 ± 67 | 597 ± 228 ** |
| Tmax (h) | 1 | 2 |
| AUClast (ng/h/mL) | 1618 ± 301 | 3717 ± 1600 * |
| AUCtot (ng/h/mL) | 2084 ± 106 | 3895 ± 1508 * |
| Kel (h) | 0.087 ± 0.038 | 0.130 ± 0.038 |
| T1/2 (h) | 6.17 ± 0.27 | 5.70 ± 1.94 |
| MRT (h) | 9.72 ± 2.04 | 7.77 ± 2.81 |
| Relative Bioavailability (%) | 100 | 230% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anwer, M.K.; Iqbal, M.; Muharram, M.M.; Mohammad, M.; Ezzeldin, E.; Aldawsari, M.F.; Alalaiwe, A.; Imam, F. Development of Lipomer Nanoparticles for the Enhancement of Drug Release, Anti-Microbial Activity and Bioavailability of Delafloxacin. Pharmaceutics 2020, 12, 252. https://doi.org/10.3390/pharmaceutics12030252
Anwer MK, Iqbal M, Muharram MM, Mohammad M, Ezzeldin E, Aldawsari MF, Alalaiwe A, Imam F. Development of Lipomer Nanoparticles for the Enhancement of Drug Release, Anti-Microbial Activity and Bioavailability of Delafloxacin. Pharmaceutics. 2020; 12(3):252. https://doi.org/10.3390/pharmaceutics12030252
Chicago/Turabian StyleAnwer, Md. Khalid, Muzaffar Iqbal, Magdy M. Muharram, Muqtader Mohammad, Essam Ezzeldin, Mohammed F. Aldawsari, Ahmed Alalaiwe, and Faisal Imam. 2020. "Development of Lipomer Nanoparticles for the Enhancement of Drug Release, Anti-Microbial Activity and Bioavailability of Delafloxacin" Pharmaceutics 12, no. 3: 252. https://doi.org/10.3390/pharmaceutics12030252
APA StyleAnwer, M. K., Iqbal, M., Muharram, M. M., Mohammad, M., Ezzeldin, E., Aldawsari, M. F., Alalaiwe, A., & Imam, F. (2020). Development of Lipomer Nanoparticles for the Enhancement of Drug Release, Anti-Microbial Activity and Bioavailability of Delafloxacin. Pharmaceutics, 12(3), 252. https://doi.org/10.3390/pharmaceutics12030252








