Mucoadhesive Buccal Films for Local Delivery of Lactobacillus brevis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of HPMC Buccal Films
2.3. Characterization of Buccal Films
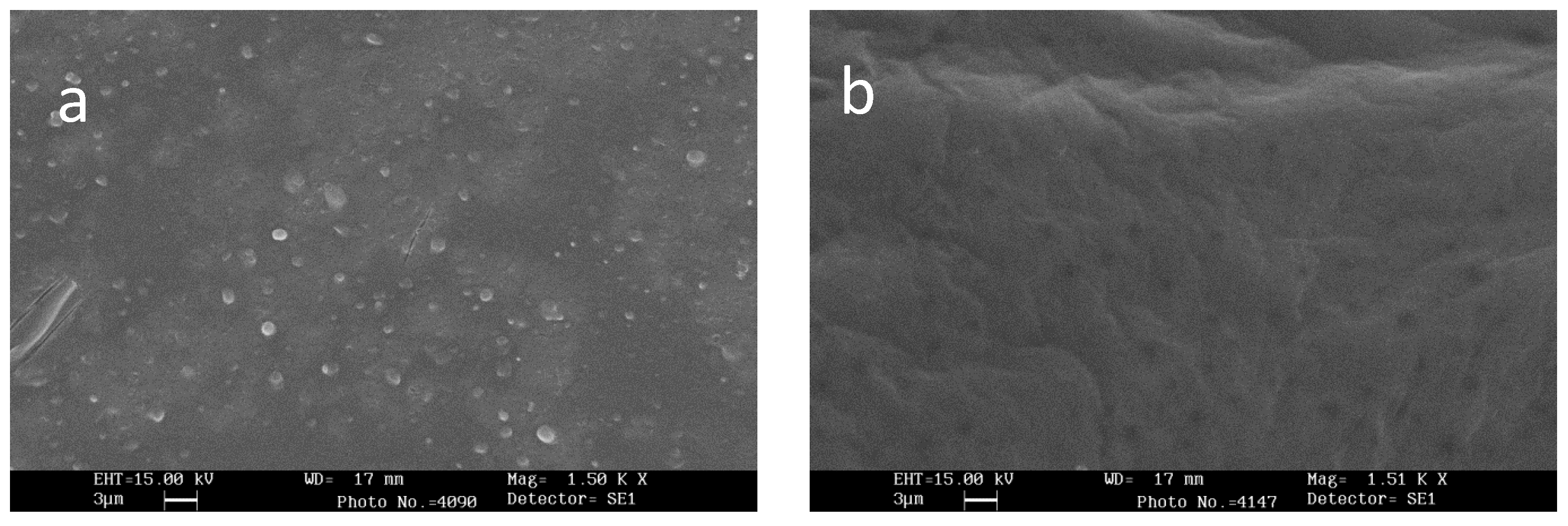
2.4. Scanning Electron Microscopy (SEM)
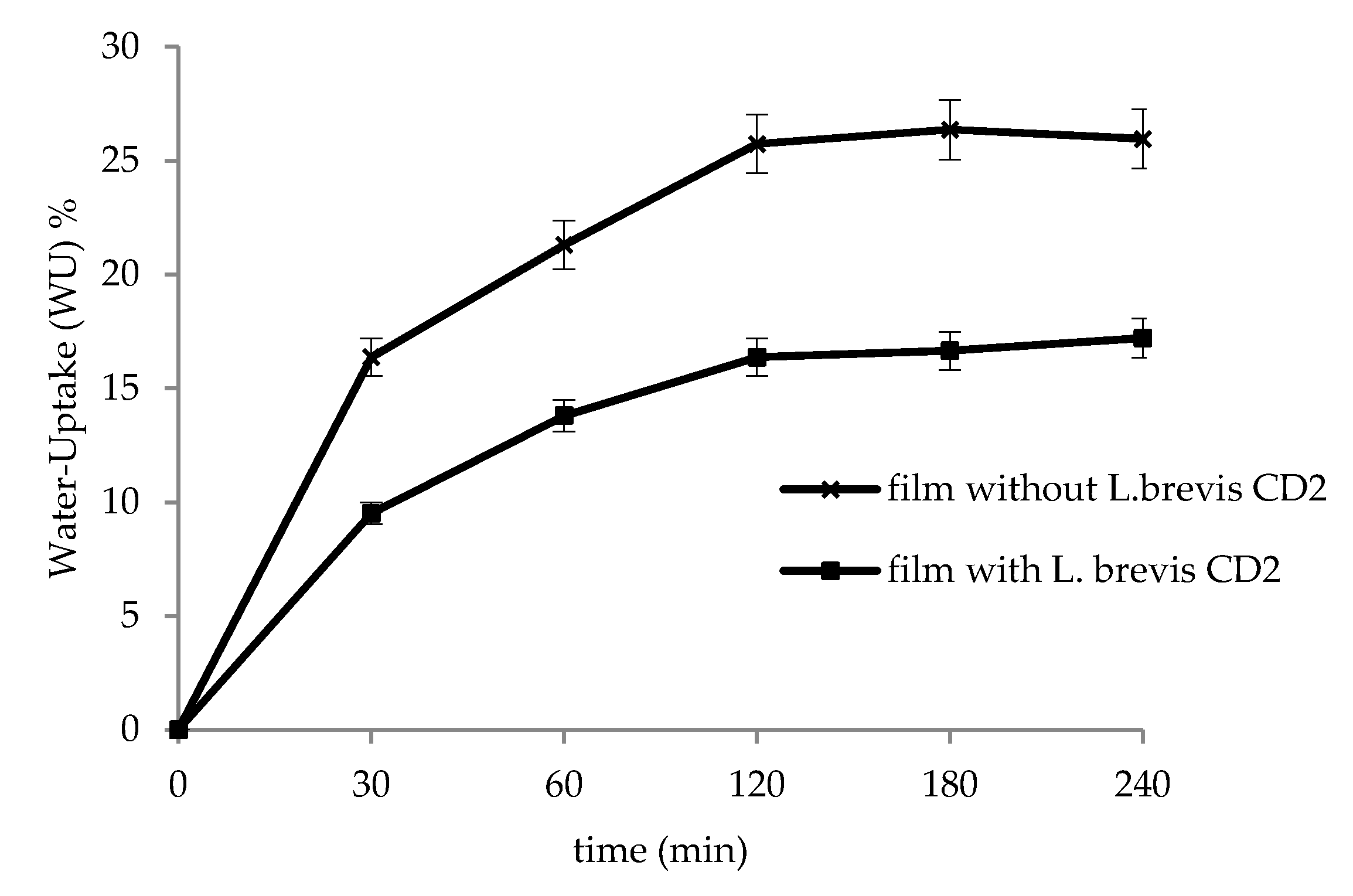
2.5. In Vitro Water-Uptake Studies
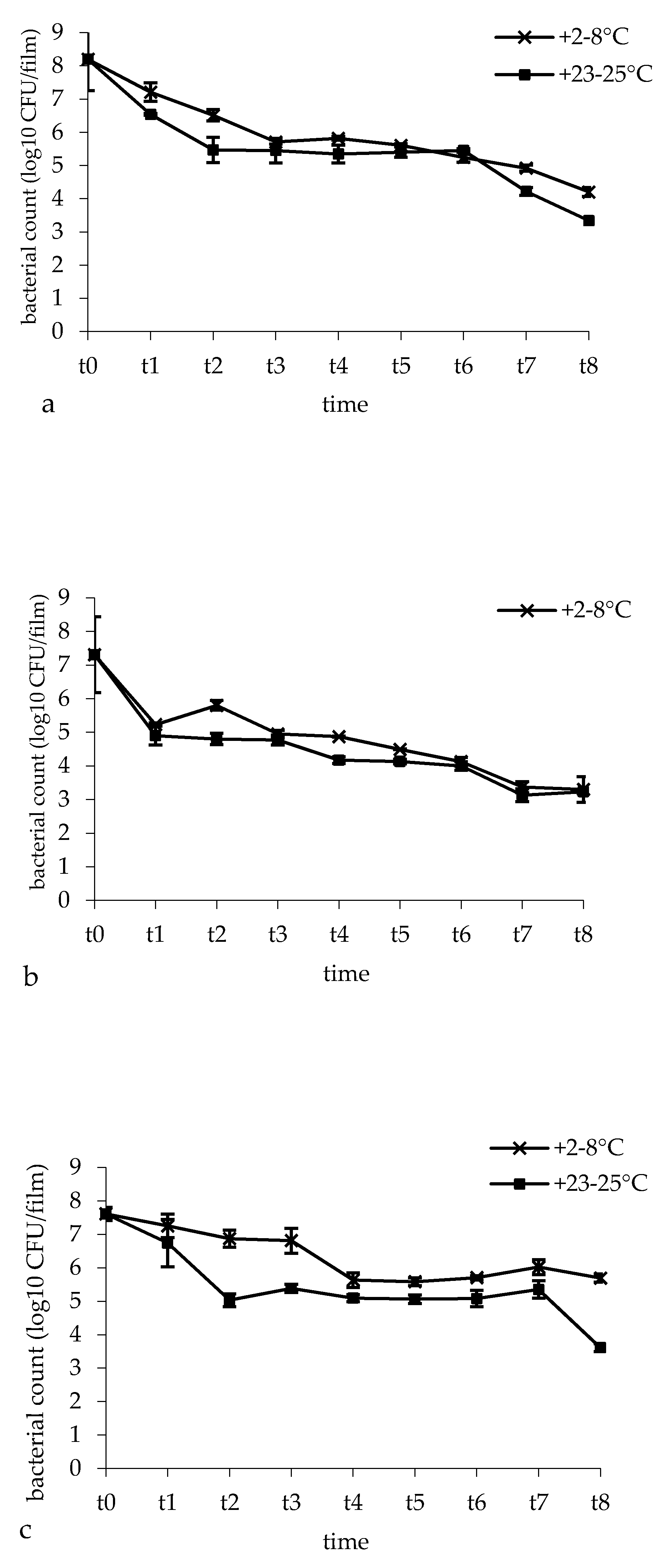
2.6. Microbiological Analysis
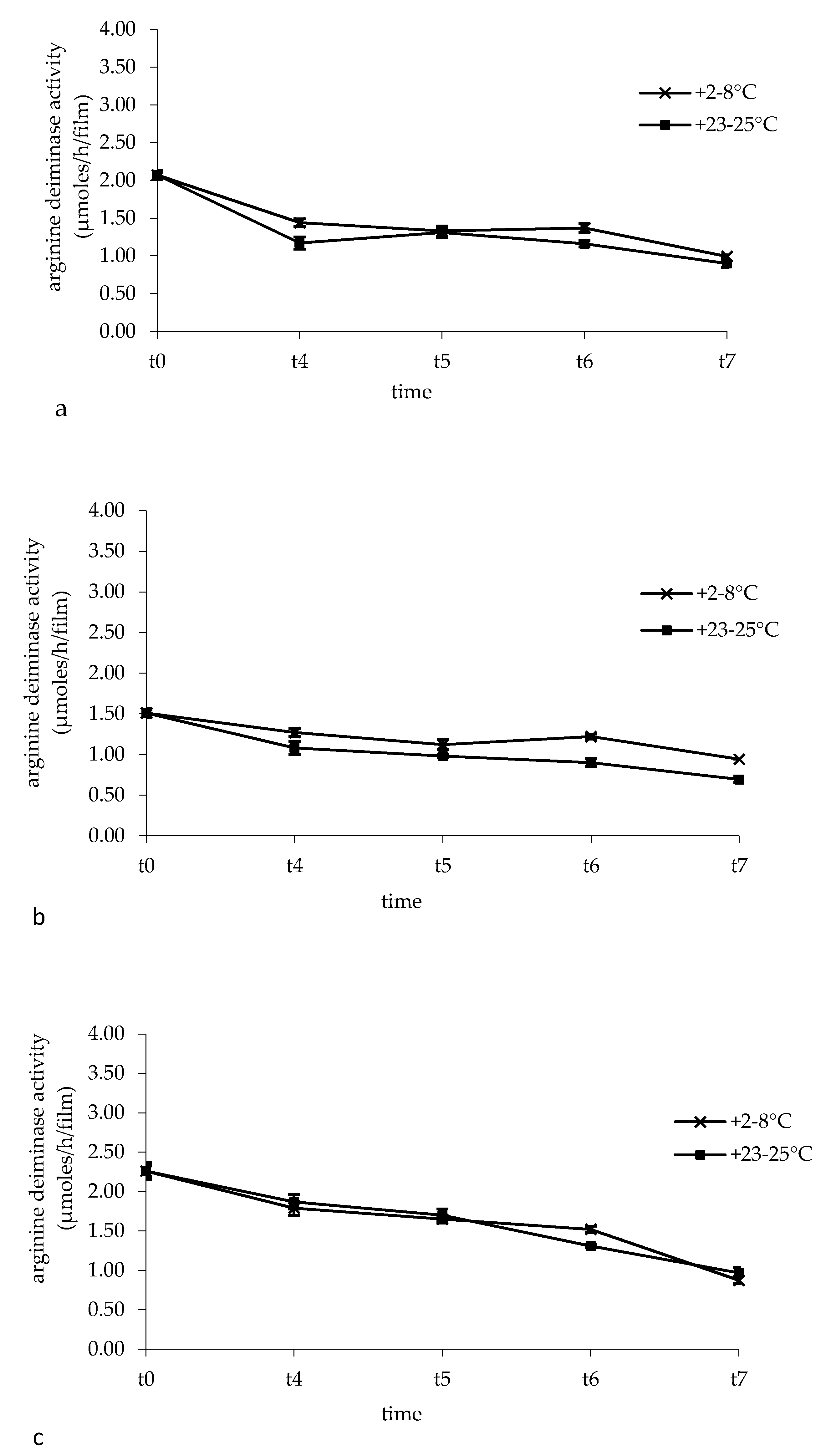
2.7. Arginine Deiminase Activity In Buccal Films
2.8. In Vitro Residence Time and Mucoadhesive Ability of Buccal Films
2.9. In Vitro Release of L. Brevis
2.10. Statistical Analysis
3. Results
3.1. Characterization of Buccal Films
3.2. Scanning Electron Microscopy (SEM)
3.3. In Vitro Water-Uptake Studies
3.4. Bacterial Survival and Film Microbial Contamination
3.5. Arginine Deiminase Activity in Buccal Films
3.6. In Vitro Residence Time and Mucoadhesive Ability of Buccal Films
3.7. In Vitro Release of Lactobacilli
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arweiler, N.B.; Netuschil, L. The Oral Microbiota. Adv. Exp. Med. Biol. 2016, 902, 45–60. [Google Scholar]
- Chen, H.; Jiang, W. Application of high-throughput sequencing in understanding human oral microbiome related with health and disease. Front. Microbiol. 2014, 13, 508. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Palmer, R.J. Human oral bacterial biofilms. In Microbial Biofilms; Ghannoum, M., O’Toole, G.A., Eds.; ASM Press: Washington, DC, USA, 2004; pp. 85–117. [Google Scholar]
- Badet, C.; Thebaud, N.B. Ecology of lactobacilli in the oral cavity: A review of literature. Open Microbiol. J. 2008, 2, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef]
- Hoare, A.; Soto, C.; Rojas-Celis, V.; Bravo, D. Chronic inflammation as a link between periodontitis and carcinogenesis. Mediat. Inflamm. 2019, 2019, 1029857. [Google Scholar] [CrossRef] [PubMed]
- Houle, M.A.; Grenier, D. Maladies parodontales: connaissances actuelles. Current concepts in periodontal diseases. Med. Maladies Infect. 2003, 33, 331–340. (In French) [Google Scholar] [CrossRef]
- Vasconcelos, R.M.; Sanfilippo, N.; Paster, B.J.; Kerr, A.R.; Li, Y.; Ramalho, L.; Queiroz, E.L.; Smith, B.; Sonis, S.T.; Corby, P.M. Host-microbiome cross-talk in oral mucositis. J. Dent. Res. 2016, 95, 725–733. [Google Scholar] [CrossRef]
- Naidu, M.U.; Ramana, G.V.; Rani, P.U.; Mohan, I.K.; Suman, A.; Roy, P. Chemotherapy-induced and/or radiation therapy-induced oral mucositis complicating the treatment of cancer. Neoplasia 2004, 6, 423–431. [Google Scholar] [CrossRef]
- Sonis, S.T. The pathobiology of mucositis. Nat. Rev. Cancer 2004, 4, 277–284. [Google Scholar] [CrossRef]
- Tsai, S.J.; Anderson, L.E.; Juengel, J.L.; Niswender, G.D.; Wiltbank, M.C. Regulation of prostaglandin F2 alpha and E receptor mRNA by prostaglandin F2 alpha in ovine corpora lutea. J. Reprod. Fertil. 1998, 114, 69–75. [Google Scholar] [CrossRef][Green Version]
- Matejka, M.; Ulm, C.; Partyka, L.; Ulm, M.; Sinzinger, H. Nitric oxide synthesis is increased in periodontitis. Adv. Exp. Med. Biol. 1999, 469, 419–424. [Google Scholar] [PubMed]
- Takeichi, O.; Haber, J.; Kawai, T. Cytokine profiles of T-lymphocytes from gingival tissues with pathological pocketing. J. Dent. Res. 2000, 79, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Schenk, K.; Poppelsdorf, D.; Denis, C. Levels of salivary IgA antibodies reactive with bacteria from dental plaque are associated with susceptibility to experimental gingivitis. J. Clin. Period. 1993, 20, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Reid, G. Probiotics: Definition, scope and mechanisms of action. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 17–25. [Google Scholar] [CrossRef]
- Meurman, J.H. Probiotics: Do they have a role in oral medicine and dentistry? Eur. J. Oral Sci. 2005, 113, 188–196. [Google Scholar] [CrossRef]
- Erickson, K.L.; Hubbard, NE. Probiotic immunomodulation in health and disease. J. Nutr. 2000, 130, 403S–409S. [Google Scholar] [CrossRef]
- Marco, M.L.; Pavan, S.; Kleerebezem, M. Towards understanding molecular modes of probiotic action. Curr. Opin. Biotech. 2006, 17, 204–210. [Google Scholar] [CrossRef]
- Della Riccia, N.; Bizzini, F.; Perilli, M.G.; Polimeni, A.; Trinchieri, V.; Amicosante, G.; Cifone, M.G. Anti-inflammatory effects of Lactobacillus brevis (CD2) on periodontal disease. Oral Dis. 2007, 13, 376–385. [Google Scholar] [CrossRef]
- Vuotto, C.; Barbanti, F.; Mastrantonio, P.; Donelli, G. Lactobacillus brevis CD2 inhibits Prevotella melaninogenica biofilm. Oral Dis. 2014, 20, 668–674. [Google Scholar] [CrossRef]
- Di Marzio, L.; Russo, F.P.; D’Alò, S.; Biordi, L.; Ulisse, S.; Amicosante, G.; De Simone, C.; Cifone, M.G. Apoptotic effects of selected strains of lactic acid bacteria on a human T leukemia cell line are associated with bacterial arginine deiminase and/or sphingomyelinase activities. Nutr. Cancer 2001, 40, 185–196. [Google Scholar] [CrossRef]
- Duan, R.D. Alkaline sphingomyelinase: An old enzyme with novel implications. BBA Mol. Cell Biol. Lipids 2006, 1761, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Rath, G.K.; Chaudhary, S.P.; Thakar, A.; Mohanti, B.K.; Bahadur, S. Lactobacillus brevis CD2 lozenges reduce radiation- and chemotherapy-induced mucositis in patients with head and neck cancer: A randomized double-blind placebo-controlled study. Eur. J. Cancer 2012, 48, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Tasli, M.L.; Mat, C.; De Simone, C.; Yazici, H. Lactobacillus lozenges in the management of oral ulcers of Behcet’s syndrome. Clin. Exp. Rheumatol. 2006, 24, 83–86. [Google Scholar]
- Campus, G.; Cocco, G.; Cocco, F.; Carta, G.; Cagetti, M.G.; Simark-Mattson, C.; Strohmenger, L.; Lingström, P. Effect of a daily dose of Lactobacillus brevis CD2 lozenges in high caries risk schoolchildren. Clin. Oral Investig. 2014, 18, 555–561. [Google Scholar] [CrossRef]
- Abruzzo, A.; Bigucci, F.; Cerchiara, T.; Cruciani, F.; Vitali, B.; Luppi, B. Mucoadhesive chitosan/gelatin films for buccal delivery of propranolol hydrochloride. Carbohydr. Polym. 2012, 87, 581–588. [Google Scholar] [CrossRef]
- Salamat-Miller, N.; Chittchang, M.; Johnston, T.P. The use of mucoadhesive polymers in buccal drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1666–1691. [Google Scholar] [CrossRef]
- Borges, A.F.; Silva, C.; Coelho, J.F.; Simões, S. Oral films: Current status and future perspectives: I-Galenical development and quality attributes. J. Control. Release 2015, 206, 1–19. [Google Scholar] [CrossRef]
- Saha, S.; Tomaro-Duchesneau, C.; Daoud, J.T.; Tabrizian, M.; Prakash, S. Novel probiotic dissolvable carboxymethyl cellulose films as oral health biotherapeutics: In vitro preparation and characterization. Expert Opin. Drug Deliv. 2013, 10, 1–12. [Google Scholar] [CrossRef]
- Heinemann, R.J.B.; Carvalho, R.A.; Favaro-Trindade, C.S. Orally disintegrating film (ODF) for delivery of probiotics in the oral cavity- Development of a novel product for oral health. Innov. Food Sci. Emerg. Technol. 2013, 19, 227–232. [Google Scholar] [CrossRef]
- Marques, M.R.C.; Loebenberg, R.; Almukainzi, M. Simulated biological fluids with possible application in dissolution testing. Dissol. Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Zúñiga, M.; Champomier-Verges, M.; Zagorec, M.; Pèrez-Martinez, G. Structural and functional analysis of the gene cluster encoding the enzymes of the arginine deiminase pathway of Lactobacillus sake. J. Bacteriol. 1998, 180, 4154–4159. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Mariotti, L.; Rossi, J.; Servili, M.; Fox, P.F.; Rollán, G.; Gobbetti, M. Arginine catabolism by sourdough lactic acid bacteria: purification and characterization of the arginine deiminase pathway enzymes from Lactobacillus sanfranciscensis CB1. Appl. Environ. Microbiol. 2002, 68, 6193–6201. [Google Scholar] [CrossRef] [PubMed]
- Del Consuelo, I.D.; Pizzolato, G.P.; Falson, F.; Guy, R.H.; Jacques, Y. Evaluation of pig esophageal mucosa as a permeability barrier model for buccal tissue. J. Pharm. Sci. 2005, 94, 2777–2788. [Google Scholar] [CrossRef] [PubMed]
- Abruzzo, A.; Nicoletta, F.P.; Dalena, F.; Cerchiara, T.; Luppi, B.; Bigucci, F. Bilayered buccal films as child-appropriate dosage form for systemic administration of propranolol. Int. J. Pharm. 2017, 531, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Kumria, R.; Harsha, S.; Attimarad, M.; Al-Dhubiab, B.E.; Alhaider, I.A. In vitro techniques to evaluate buccal films. J. Control. Release 2013, 166, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Vitali, B.; Abruzzo, A.; Parolin, C.; Ñahui Palomino, R.A.; Dalena, F.; Bigucci, F.; Cerchiara, T.; Luppi, B. Association of Lactobacillus crispatus with fructo-oligosaccharides and ascorbic acid in hydroxypropyl methylcellulose vaginal insert. Carbohydr. Polym. 2016, 136, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Williams, H. D.; Ward, R.; Hardy, I.J.; Melia, C.D. The extended release properties of HPMC matrices in the presence of dietary sugars. J. Control. Release 2009, 138, 251–259. [Google Scholar] [CrossRef]
- Trastullo, R.; Abruzzo, A.; Saladini, B.; Gallucci, M.C.; Cerchiara, T.; Luppi, B.; Bigucci, F. Design and evaluation of buccal films as paediatric dosage form for transmucosal delivery of ondansetron. Eur. J. Pharm. Biopharm. 2016, 105, 115–121. [Google Scholar] [CrossRef]
- Luppi, B.; Bigucci, F.; Baldini, M.; Abruzzo, A.; Cerchiara, T.; Corace, G.; Zecchi, V. Hydroxypropylmethylcellulose films for prolonged delivery of the antipsychotic drug chlorpromazine. J. Pharm. Pharmacol. 2010, 62, 305–309. [Google Scholar] [CrossRef]
- Li, C.L.; Martini, L.G.; Ford, J.L.; Roberts, M. The use of hypromellose in oral drug delivery. J. Pharm. Pharmacol. 2005, 57, 533–546. [Google Scholar] [CrossRef]
- Bertram, U.; Bodmeier, R. In situ gelling, bioadhesive nasal inserts for extended drug delivery: In vitro characterization of a new nasal dosage form. Eur. J. Pharm. Sci. 2006, 27, 62–71. [Google Scholar] [CrossRef] [PubMed]

| Film | BATCH I | BATCH II | BATCH III |
|---|---|---|---|
| A | 88 ± 5 | 85 ± 5 | 80 ± 6 |
| B | 83 ± 6 | 82 ± 5 | 86 ± 4 |
| C | 83 ± 6 | 84 ± 6 | 85 ± 6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abruzzo, A.; Vitali, B.; Lombardi, F.; Guerrini, L.; Cinque, B.; Parolin, C.; Bigucci, F.; Cerchiara, T.; Arbizzani, C.; Gallucci, M.C.; et al. Mucoadhesive Buccal Films for Local Delivery of Lactobacillus brevis. Pharmaceutics 2020, 12, 241. https://doi.org/10.3390/pharmaceutics12030241
Abruzzo A, Vitali B, Lombardi F, Guerrini L, Cinque B, Parolin C, Bigucci F, Cerchiara T, Arbizzani C, Gallucci MC, et al. Mucoadhesive Buccal Films for Local Delivery of Lactobacillus brevis. Pharmaceutics. 2020; 12(3):241. https://doi.org/10.3390/pharmaceutics12030241
Chicago/Turabian StyleAbruzzo, Angela, Beatrice Vitali, Francesca Lombardi, Luca Guerrini, Benedetta Cinque, Carola Parolin, Federica Bigucci, Teresa Cerchiara, Catia Arbizzani, Maria Caterina Gallucci, and et al. 2020. "Mucoadhesive Buccal Films for Local Delivery of Lactobacillus brevis" Pharmaceutics 12, no. 3: 241. https://doi.org/10.3390/pharmaceutics12030241
APA StyleAbruzzo, A., Vitali, B., Lombardi, F., Guerrini, L., Cinque, B., Parolin, C., Bigucci, F., Cerchiara, T., Arbizzani, C., Gallucci, M. C., & Luppi, B. (2020). Mucoadhesive Buccal Films for Local Delivery of Lactobacillus brevis. Pharmaceutics, 12(3), 241. https://doi.org/10.3390/pharmaceutics12030241












