Spray-Dried Amorphous Solid Dispersions of Griseofulvin in HPC/Soluplus/SDS: Elucidating the Multifaceted Impact of SDS as a Minor Component
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Spray-Dried Powders
2.3. Characterization Methods
2.3.1. Particle Sizing, Microscopy, and Solid-State Characterization
2.3.2. Assay Testing and Drug Release from the Powders Prepared via Spray Drying
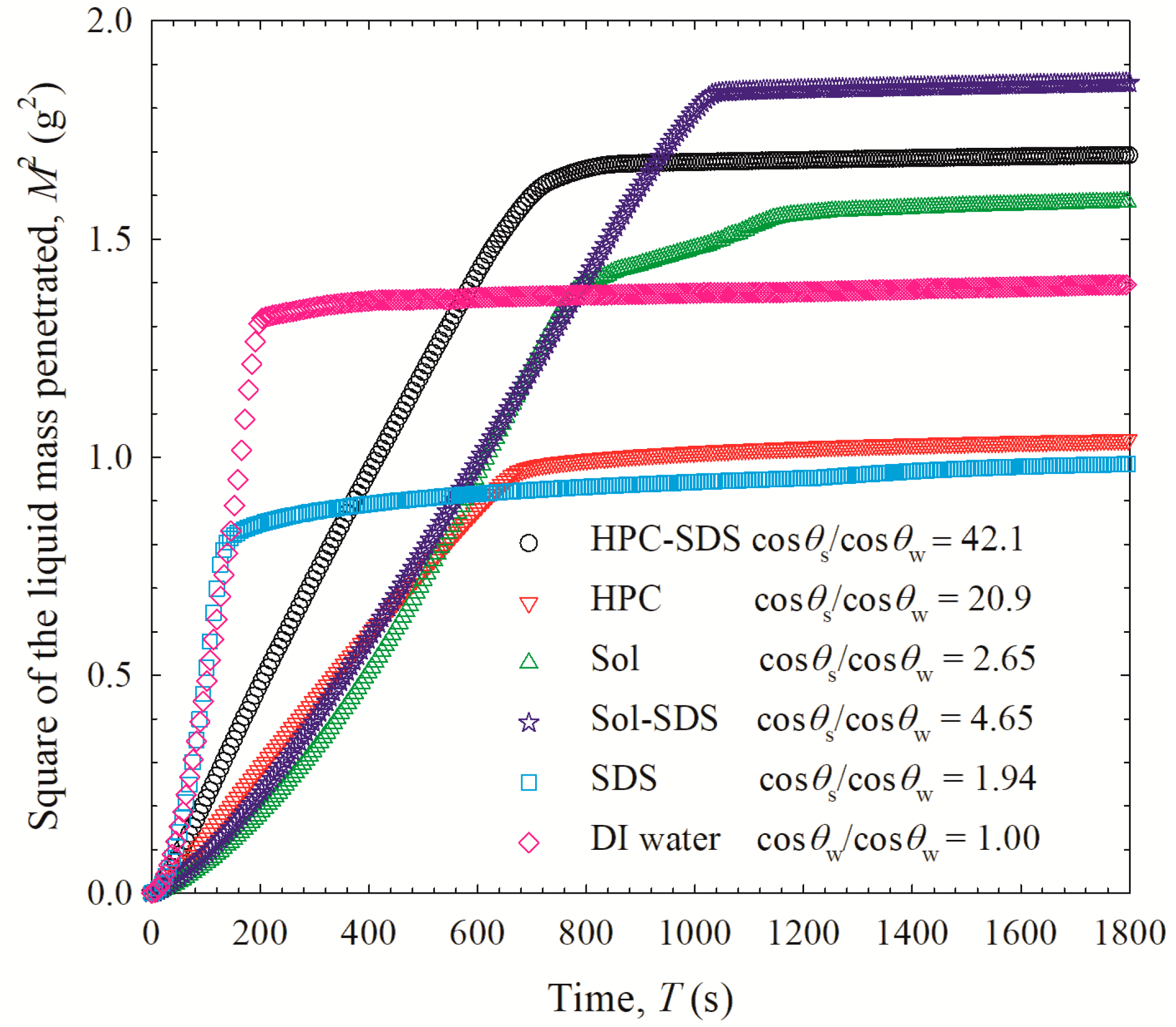
2.3.3. GF Wettability by Aqueous Polymer (Sol/HPC) Solutions with or w/o SDS
2.3.4. Impact of the Polymers and SDS on GF Recrystallization from Supersaturated Solutions
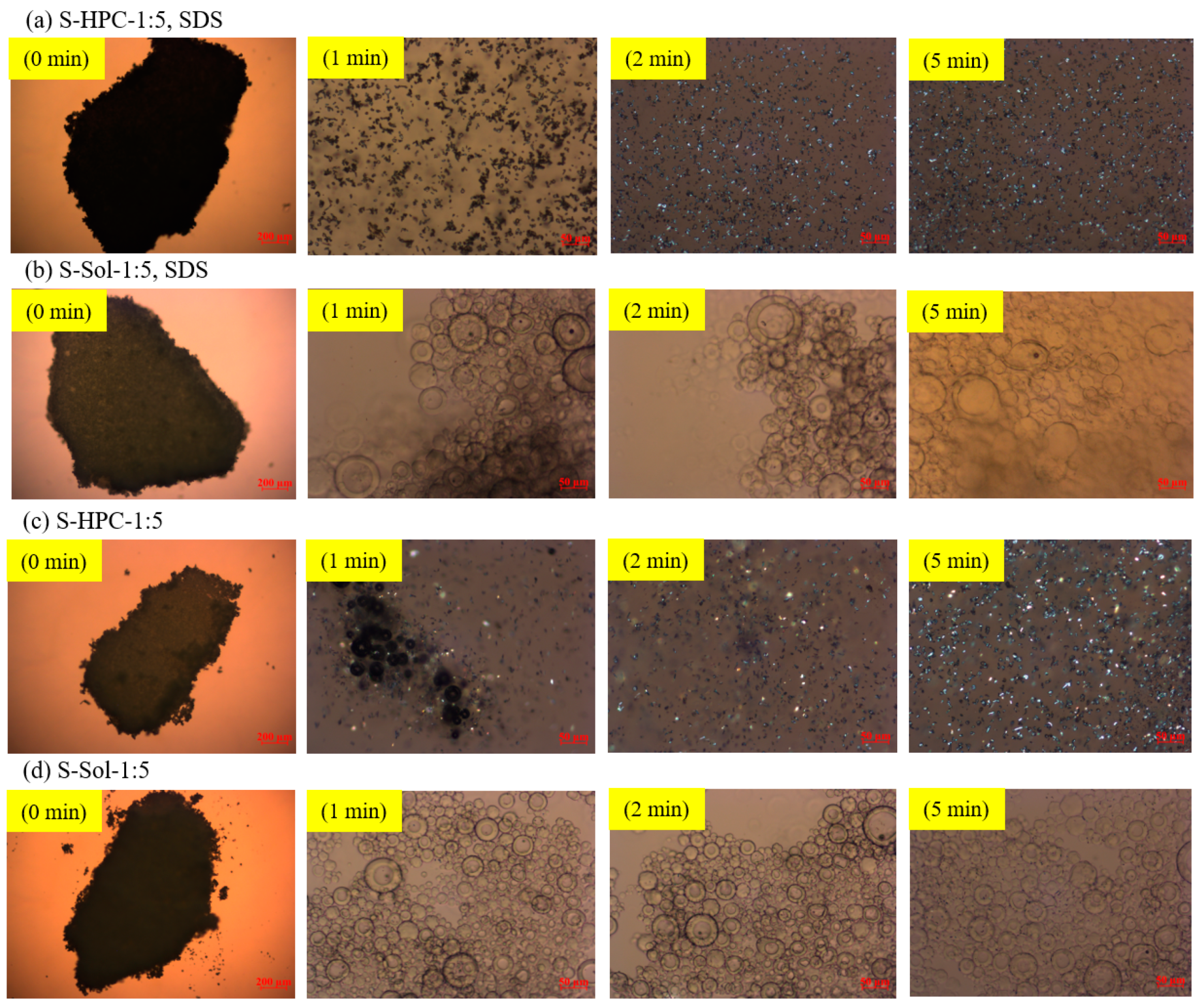
2.3.5. Visualization of Solution-Mediated Recrystallization of GF
3. Results and Discussion
3.1. Properties of the Spray-Dried Powders
3.2. Solid state Characterization of the Spray-Dried Powders
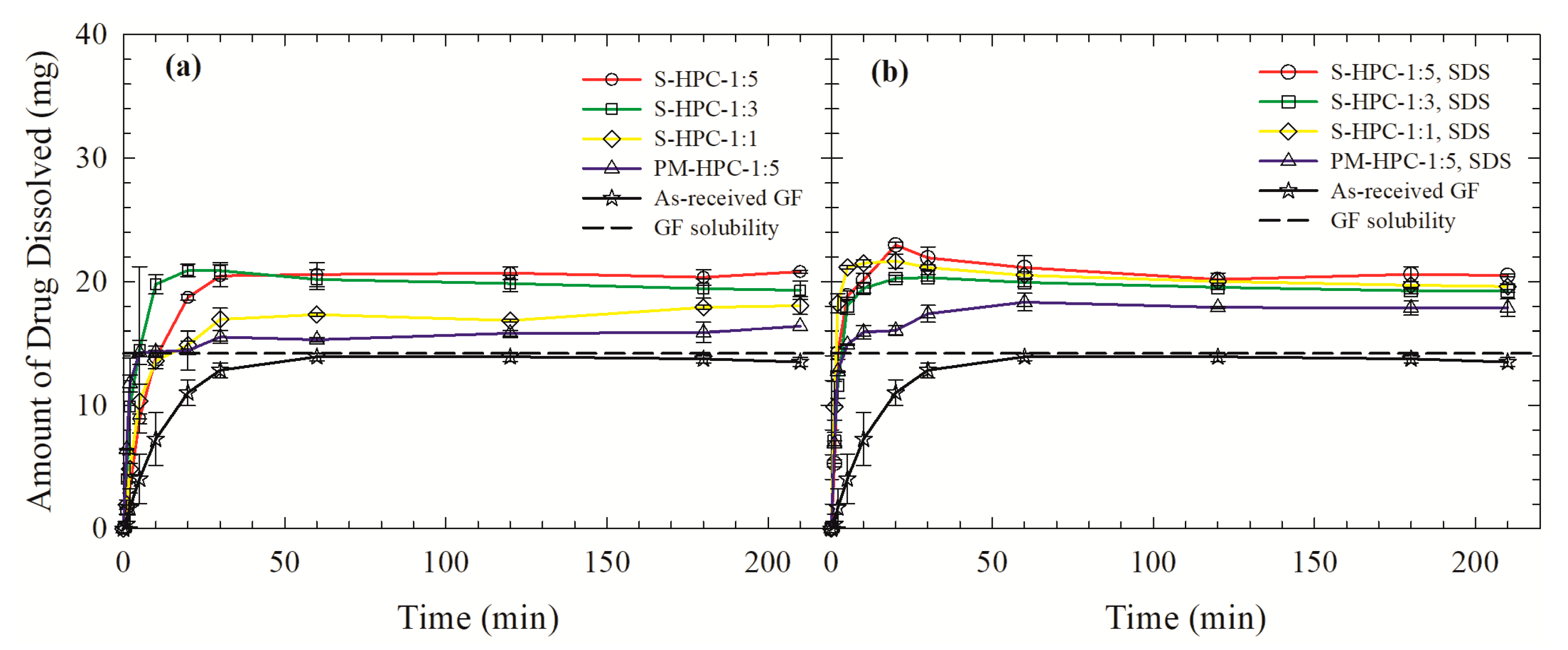
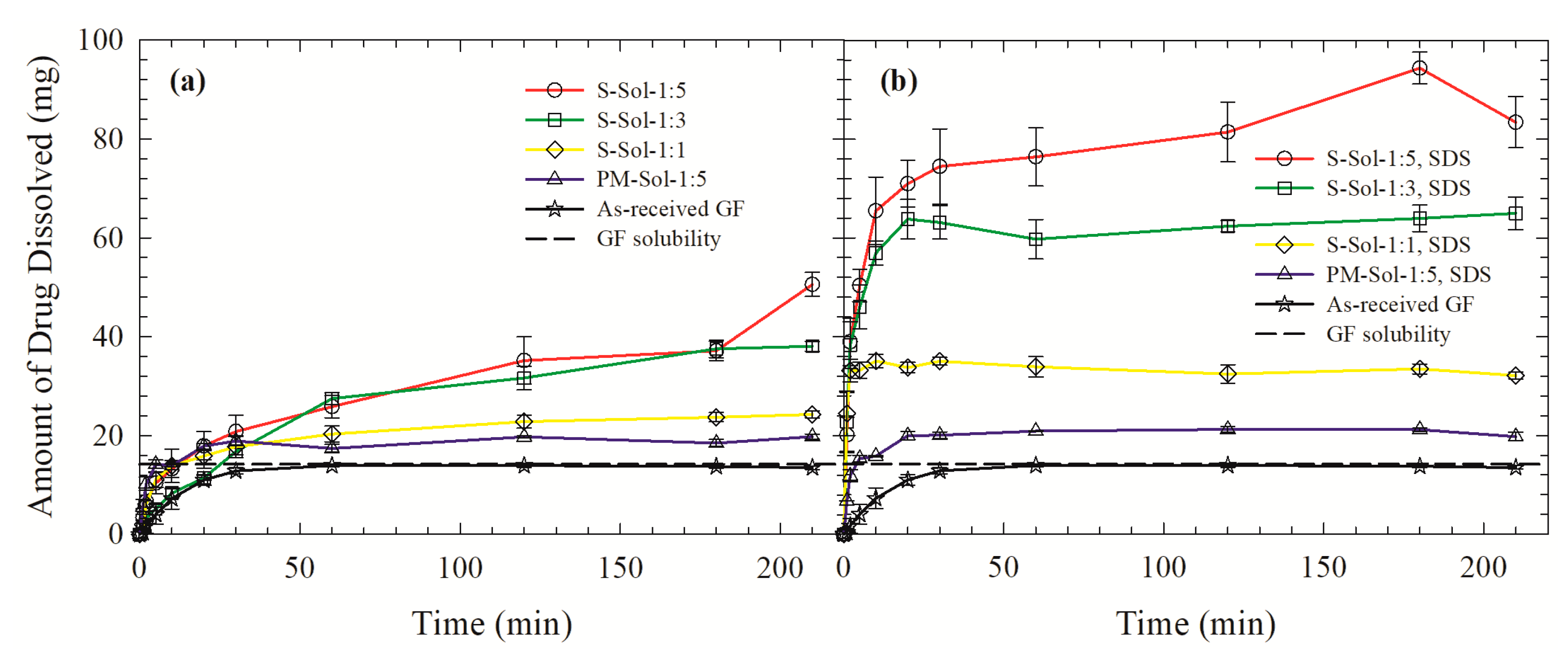
3.3. Drug Release from the Powders Prepared by Spray Drying
3.4. On the Roles of the Polymer/SDS in Recrystallization Inhibition/Promotion
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Teja, S.B.; Patil, S.P.; Shete, G.; Patel, S.; Bansal, A.K. Drug-excipient behavior in polymeric amorphous solid dispersions. J. Excip. Food Chem. 2013, 4, 70–94. [Google Scholar]
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins: Basic science and product development. J. Pharm. Pharmacol. 2010, 62, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Serajuddin, A. Solid dispersion of poorly water-soluble drugs: Early promises, subsequent problems, and recent breakthroughs. J. Pharm. Sci. 1999, 88, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.; Knipp, G.; Zografi, G. Assessing the performance of amorphous solid dispersions. J. Pharm. Sci. 2012, 101, 1355–1377. [Google Scholar] [CrossRef]
- Yamashita, K.; Nakate, T.; Okimoto, K.; Ohike, A.; Tokunaga, Y.; Ibuki, R.; Higaki, K.; Kimura, T. Establishment of new preparation method for solid dispersion formulation of tacrolimus. Int. J. Pharm. 2003, 267, 79–91. [Google Scholar] [CrossRef]
- Six, K.; Verreck, G.; Peeters, J.; Brewster, M.; Van den Mooter, G. Increased physical stability and improved dissolution properties of itraconazole, a class II drug, by solid dispersions that combine fast-and slow-dissolving polymers. J. Pharm. Sci. 2004, 93, 124–131. [Google Scholar] [CrossRef]
- Ambike, A.A.; Mahadik, K.; Paradkar, A. Spray-dried amorphous solid dispersions of simvastatin, a low T g drug: In vitro and in vivo evaluations. Pharm. Res. 2005, 22, 990–998. [Google Scholar] [CrossRef]
- Hancock, B.C.; Parks, M. What is the true solubility advantage for amorphous pharmaceuticals? Pharm. Res. 2000, 17, 397–404. [Google Scholar] [CrossRef]
- Marsac, P.J.; Konno, H.; Taylor, L.S. A comparison of the physical stability of amorphous felodipine and nifedipine systems. Pharm. Res. 2006, 23, 2306–2316. [Google Scholar] [CrossRef]
- Konno, H.; Handa, T.; Alonzo, D.E.; Taylor, L.S. Effect of polymer type on the dissolution profile of amorphous solid dispersions containing felodipine. Eur. J. Pharm. Biopharm. 2008, 70, 493–499. [Google Scholar] [CrossRef]
- Xie, T.; Taylor, L.S. Dissolution performance of high drug loading celecoxib amorphous solid dispersions formulated with polymer combinations. Pharm. Res. 2016, 33, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Ghebremeskel, A.N.; Vemavarapu, C.; Lodaya, M. Use of surfactants as plasticizers in preparing solid dispersions of poorly soluble API: Selection of polymer–surfactant combinations using solubility parameters and testing the processability. Int. J. Pharm. 2007, 328, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Joshi, H.N.; Tejwani, R.W.; Davidovich, M.; Sahasrabudhe, V.P.; Jemal, M.; Bathala, M.S.; Varia, S.A.; Serajuddin, A.T. Bioavailability enhancement of a poorly water-soluble drug by solid dispersion in polyethylene glycol–polysorbate 80 mixture. Int. J. Pharm. 2004, 269, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Goddeeris, C.; Willems, T.; Houthoofd, K.; Martens, J.; Van den Mooter, G. Dissolution enhancement of the anti-HIV drug UC 781 by formulation in a ternary solid dispersion with TPGS 1000 and Eudragit E100. Eur. J. Pharm. Biopharm. 2008, 70, 861–868. [Google Scholar] [CrossRef]
- Li, B.; Konecke, S.; Harich, K.; Wegiel, L.; Taylor, L.S.; Edgar, K.J. Solid dispersion of quercetin in cellulose derivative matrices influences both solubility and stability. Carbohydr. Polym. 2013, 92, 2033–2040. [Google Scholar] [CrossRef]
- Sotthivirat, S.; McKelvey, C.; Moser, J.; Rege, B.; Xu, W.; Zhang, D. Development of amorphous solid dispersion formulations of a poorly water-soluble drug, MK-0364. Int. J. Pharm. 2013, 452, 73–81. [Google Scholar] [CrossRef]
- Feng, D.; Peng, T.; Huang, Z.; Singh, V.; Shi, Y.; Wen, T.; Lu, M.; Quan, G.; Pan, X.; Wu, C. Polymer–Surfactant System Based Amorphous Solid Dispersion: Precipitation Inhibition and Bioavailability Enhancement of Itraconazole. Pharmaceutics 2018, 10, 53. [Google Scholar] [CrossRef]
- Lu, Y.; Tang, N.; Lian, R.; Qi, J.; Wu, W. Understanding the relationship between wettability and dissolution of solid dispersion. Int. J. Pharm. 2014, 465, 25–31. [Google Scholar] [CrossRef]
- Mura, P.; Moyano, J.; Gonzalez-Rodriguez, M.; Rabasco-Alvarez, A.; Cirri, M.; Maestrelli, F. Characterization and dissolution properties of ketoprofen in binary and ternary solid dispersions with polyethylene glycol and surfactants. Drug Dev. Ind. Pharm. 2005, 31, 425–434. [Google Scholar] [CrossRef]
- Waard, H.; Hinrichs, W.; Visser, M.; Bologna, C.; Frijlink, H. Unexpected differences in dissolution behavior of tablets prepared from solid dispersions with a surfactant physically mixed or incorporated. Int. J. Pharm. 2008, 349, 66–73. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, S.; Wang, S.; Liu, C.; Su, C.; Hageman, M.; Hussain, M.; Haskell, R.; Stefanski, K.; Qian, F. Sodium lauryl sulfate competitively interacts with HPMC-as and consequently reduces oral bioavailability of posaconazole/HPMC-as amorphous solid dispersion. Mol. Pharm. 2016, 13, 2787–2795. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Z.; Chen, Y.; Lu, J.; Li, Y.; Wang, S.; Wu, G.; Qian, F. Improving oral bioavailability of sorafenib by optimizing the “Spring” and “Parachute” based on molecular interaction mechanisms. Mol. Pharm. 2016, 13, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Mah, P.T.; Peltonen, L.; Novakovic, D.; Rades, T.; Strachan, C.J.; Laaksonen, T. The effect of surfactants on the dissolution behavior of amorphous formulations. Eur. J. Pharm. Biopharm. 2016, 103, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, T.M.; Shi, H.; Pietryka, J.; Hoag, S.W.; Medek, A. Investigation of polymer/surfactant interactions and their impact on itraconazole solubility and precipitation kinetics for developing spray-dried amorphous solid dispersions. Mol. Pharm. 2018, 15, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.C.; Sheskey, P.; Quinn, M. Handbook of Pharmaceutical Excipients; Pharmaceutical Press and American Pharmacists Association: Grayslake, IL, USA; Washington, DC, USA, 2009. [Google Scholar]
- Chaudhari, S.P.; Dugar, R.P. Application of surfactants in solid dispersion technology for improving solubility of poorly water soluble drugs. J. Drug Deliv. Sci. Technol. 2017, 41, 68–77. [Google Scholar] [CrossRef]
- Jung, H.J.; Ahn, H.I.; Park, J.Y.; Ho, M.J.; Lee, D.R.; Cho, H.R.; Park, J.S.; Choi, Y.S.; Kang, M.J. Improved oral absorption of tacrolimus by a solid dispersion with hypromellose and sodium lauryl sulfate. Int. J. Biol. Macromol. 2016, 83, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Yu, H.; Tao, J.; Zeng, J.; Zhu, Q.; Zhu, C.; Gan, Y. Supersaturated polymeric micelles for oral cyclosporine A delivery: The role of Soluplus–sodium dodecyl sulfate complex. Colloids Surf. B Biointerfaces 2016, 141, 301–310. [Google Scholar] [CrossRef]
- Rahman, M.; Ahmad, S.; Tarabokija, J.; Bilgili, E. Roles of surfactant and polymer in drug release from spray-dried hybrid nanocrystal-amorphous solid dispersions (HyNASDs). Powder Technol. 2020, 361, 663–678. [Google Scholar] [CrossRef]
- Park, Y.-J.; Ryu, D.-S.; Li, D.X.; Quan, Q.Z.; Oh, D.H.; Kim, J.O.; Seo, Y.G.; Lee, Y.-I.; Yong, C.S.; Woo, J.S. Physicochemical characterization of tacrolimus-loaded solid dispersion with sodium carboxylmethyl cellulose and sodium lauryl sulfate. Arch. Pharmacal Res. 2009, 32, 893–898. [Google Scholar] [CrossRef]
- Moes, J.; Koolen, S.; Huitema, A.; Schellens, J.; Beijnen, J.; Nuijen, B. Pharmaceutical development and preliminary clinical testing of an oral solid dispersion formulation of docetaxel (ModraDoc001). Int. J. Pharm. 2011, 420, 244–250. [Google Scholar] [CrossRef]
- Yan, Y.-D.; Sung, J.H.; Kim, K.K.; Kim, D.W.; Kim, J.O.; Lee, B.-J.; Yong, C.S.; Choi, H.-G. Novel valsartan-loaded solid dispersion with enhanced bioavailability and no crystalline changes. Int. J. Pharm. 2012, 422, 202–210. [Google Scholar] [CrossRef]
- Dave, R.H.; Patel, H.H.; Donahue, E.; Patel, A.D. To evaluate the change in release from solid dispersion using sodium lauryl sulfate and model drug sulfathiazole. Drug Dev. Ind. Pharm. 2013, 39, 1562–1572. [Google Scholar] [CrossRef]
- Truong, D.H.; Tran, T.H.; Ramasamy, T.; Choi, J.Y.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Preparation and characterization of solid dispersion using a novel amphiphilic copolymer to enhance dissolution and oral bioavailability of sorafenib. Powder Technol. 2015, 283, 260–265. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.; Huang, W.; Wang, H.; Du, Y.; Xiong, S. Bottom-up and top-down approaches to explore sodium dodecyl sulfate and Soluplus on the crystallization inhibition and dissolution of felodipine extrudates. J. Pharm. Sci. 2018, 107, 2366–2376. [Google Scholar] [CrossRef]
- Kim, D.S.; Choi, H.G.; Jin, S.G. Influence of Hydroxypropylmethylcellulose and Sodium Lauryl Sulfate on the Solubility and Dissolution of Sirolimus in Solvent-evaporated Solid Dispersions. Bull. Korean Chem. Soc. 2018, 39, 778–783. [Google Scholar] [CrossRef]
- Muralichand, G.; Bhikshapathi, D. Development and in vivo evaluation of solid dispersions containing nifedipine. Int. J. Pharm. Anal. Res. 2018, 7, 254–266. [Google Scholar]
- Mosquera-Giraldo, L.I.; Trasi, N.S.; Taylor, L.S. Impact of surfactants on the crystal growth of amorphous celecoxib. Int. J. Pharm. 2014, 461, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.B.; Kompella, U.B. Nanoparticle Technology for Drug Delivery; Taylor & Francis: New York, NY, USA, 2006; Volume 159. [Google Scholar]
- Kim, D.S.; Choi, J.S.; Kim, D.W.; Kim, K.S.; Seo, Y.G.; Cho, K.H.; Kim, J.O.; Yong, C.S.; Youn, Y.S.; Lim, S.-J. Comparison of solvent–wetted and kneaded l-sulpiride–loaded solid dispersions: Powder characterization and in vivo evaluation. Int. J. Pharm. 2016, 511, 351–358. [Google Scholar] [CrossRef]
- Baird, J.A.; Van Eerdenbrugh, B.; Taylor, L.S. A classification system to assess the crystallization tendency of organic molecules from undercooled melts. J. Pharm. Sci. 2010, 99, 3787–3806. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, G.G.; Law, D.; Grant, D.J.; Schmitt, E.A. Thermodynamics, molecular mobility and crystallization kinetics of amorphous griseofulvin. Mol. Pharm. 2008, 5, 927–936. [Google Scholar] [CrossRef]
- Sarode, A.; Wang, P.; Cote, C.; Worthen, D.R. Low-viscosity hydroxypropylcellulose (HPC) grades SL and SSL: Versatile pharmaceutical polymers for dissolution enhancement, controlled release, and pharmaceutical processing. AAPS PharmSciTech 2013, 14, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Terife, G.; Wang, P.; Faridi, N.; Gogos, C.G. Hot melt mixing and foaming of soluplus® and indomethacin. Polym. Eng. Sci. 2012, 52, 1629–1639. [Google Scholar] [CrossRef]
- Sharma, V.; Yadav, O.; Singh, J. Physicochemical studies of aqueous sodium dodecyl sulphate solutions in pyridine and isomeric picolines. Colloids Surf. A 1996, 110, 23–35. [Google Scholar] [CrossRef]
- Azad, M.; Arteaga, C.; Abdelmalek, B.; Davé, R.; Bilgili, E. Spray drying of drug-swellable dispersant suspensions for preparation of fast-dissolving, high drug-loaded, surfactant-free nanocomposites. Drug Dev. Ind. Pharm. 2015, 41, 1617–1631. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lopez, N.; Bilgili, E. A study of the impact of polymer–surfactant in drug nanoparticle coated pharmatose composites on dissolution performance. Adv. Powder Technol. 2016, 27, 1625–1636. [Google Scholar] [CrossRef]
- Rahman, M.; Arevalo, F.; Coelho, A.; Bilgili, E. Hybrid nanocrystal–amorphous solid dispersions (HyNASDs) as alternative to ASDs for enhanced release of BCS Class II drugs. Eur. J. Pharm. Biopharm. 2019, 145, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Bhakay, A.; Azad, M.; Bilgili, E.; Dave, R. Redispersible fast dissolving nanocomposite microparticles of poorly water-soluble drugs. Int. J. Pharm. 2014, 461, 367–379. [Google Scholar] [CrossRef]
- Shah, D.A.; Patel, M.; Murdande, S.B.; Dave, R.H. Influence of spray drying and dispersing agent on surface and dissolution properties of griseofulvin micro and nanocrystals. Drug Dev. Ind. Pharm. 2016, 42, 1842–1850. [Google Scholar] [CrossRef]
- Li, M.; Ioannidis, N.; Gogos, C.; Bilgili, E. A comparative assessment of nanocomposites vs. amorphous solid dispersions prepared via nanoextrusion for drug dissolution enhancement. Eur. J. Pharm. Biopharm. 2017, 119, 68–80. [Google Scholar] [CrossRef]
- Washburn, E.W. The dynamics of capillary flow. Phys. Rev. 1921, 17, 273–283. [Google Scholar] [CrossRef]
- Hołownia, D.; Kwiatkowska, I.; Hupka, J. An investigation on wetting of porous materials. Physicochem. Prob. Miner. Process. 2008, 42, 251–262. [Google Scholar]
- Bilgili, E.; Rahman, M.; Palacios, D.; Arevalo, F. Impact of polymers on the aggregation of wet-milled itraconazole particles and their dissolution from spray-dried nanocomposites. Adv. Powder Technol. 2018, 9, 2941–2956. [Google Scholar] [CrossRef]
- Korson, L.; Drost-Hansen, W.; Millero, F.J. Viscosity of water at various temperatures. J. Phys. Chem. 1969, 73, 34–39. [Google Scholar] [CrossRef]
- Kushner, L.M.; Duncan, B.C.; Hoffman, J.I. A viscometric study of the micelles of sodium dodecyl sulfate in dilute solutions. J. Res. Nat. Bur. Stand. 1952, 49, 85–90. [Google Scholar] [CrossRef]
- Li, M.; Suriel, I.; Vekaria, J.; Proske, J.; Orbe, P.; Armani, M.; Dave, R.; Bilgili, E. Impact of dispersants on dissolution of itraconazole from drug-loaded, surfactant-free, spray-dried nanocomposites. Powder Technol. 2018, 339, 281–295. [Google Scholar] [CrossRef]
- Poozesh, S.; Bilgili, E. Scale-up of pharmaceutical spray drying using scale-up rules: A review. Int. J. Pharm. 2019, 562, 271–292. [Google Scholar] [CrossRef]
- Baghel, S.; Cathcart, H.; O’Reilly, N.J. Polymeric amorphous solid dispersions: A review of amorphization, crystallization, stabilization, solid-state characterization, and aqueous solubilization of biopharmaceutical classification system class II drugs. J. Pharm. Sci. 2016, 105, 2527–2544. [Google Scholar] [CrossRef]
- Wlodarski, K.; Sawicki, W.; Kozyra, A.; Tajber, L. Physical stability of solid dispersions with respect to thermodynamic solubility of tadalafil in PVP-VA. Eur. J. Pharm. Biopharm. 2015, 96, 237–246. [Google Scholar] [CrossRef]
- Luebbert, C.; Huxoll, F.; Sadowski, G. Amorphous-amorphous phase separation in API/polymer formulations. Molecules 2017, 22, 296. [Google Scholar] [CrossRef]
- Newman, A.; Engers, D.; Bates, S.; Ivanisevic, I.; Kelly, R.C.; Zografi, G. Characterization of amorphous API: Polymer mixtures using X-ray powder diffraction. J. Pharm. Sci. 2008, 97, 4840–4856. [Google Scholar] [CrossRef]
- Baird, J.A.; Taylor, L.S. Evaluation of amorphous solid dispersion properties using thermal analysis techniques. Adv. Drug Deliv. Rev. 2012, 64, 396–421. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Pinal, R.; Carvajal, M.T. Process induced disorder in crystalline materials: Differentiating defective crystals from the amorphous form of griseofulvin. J. Pharm. Sci. 2008, 97, 3207–3221. [Google Scholar] [CrossRef] [PubMed]
- Żarów, A.; Zhou, B.; Wang, X.; Pinal, R.; Iqbal, Z. Spectroscopic and X-ray diffraction study of structural disorder in cryomilled and amorphous griseofulvin. Appl. Spectrosc. 2011, 65, 135–143. [Google Scholar] [CrossRef]
- Greenhalgh, D.J.; Williams, A.C.; Timmins, P.; York, P. Solubility parameters as predictors of miscibility in solid dispersions. J. Pharm. Sci. 1999, 88, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Forster, A.; Hempenstall, J.; Tucker, I.; Rades, T. Selection of excipients for melt extrusion with two poorly water-soluble drugs by solubility parameter calculation and thermal analysis. Int. J. Pharm. 2001, 226, 147–161. [Google Scholar] [CrossRef]
- Thakral, S.; Thakral, N.K. Prediction of drug–polymer miscibility through the use of solubility parameter based Flory–Huggins interaction parameter and the experimental validation: PEG as model polymer. J. Pharm. Sci. 2013, 102, 2254–2263. [Google Scholar] [CrossRef] [PubMed]
- Choi, P.; Kavassalis, T.A.; Rudin, A. Estimation of Hansen solubility parameters for (hydroxyethyl) and (hydroxypropyl) cellulose through molecular simulation. Ind. Eng. Chem. Res. 1994, 33, 3154–3159. [Google Scholar] [CrossRef]
- Kolter, K.; Karl, M.; Gryczke, A.; Ludwigshafen am Rhein, B. Hot-Melt Extrusion with BASF Pharma Polymers: Extrusion Compendium; BASF: Ludwigshafen, Germany, 2012. [Google Scholar]
- Meng, F.; Trivino, A.; Prasad, D.; Chauhan, H. Investigation and correlation of drug polymer miscibility and molecular interactions by various approaches for the preparation of amorphous solid dispersions. Eur. J. Pharm. Sci. 2015, 71, 12–24. [Google Scholar] [CrossRef]
- Matsui, K.; Tsume, Y.; Amidon, G.E.; Amidon, G.L. The evaluation of in vitro drug dissolution of commercially available oral dosage forms for itraconazole in gastrointestinal simulator with biorelevant media. J. Pharm. Sci. 2016, 105, 2804–2814. [Google Scholar] [CrossRef]
- Alonzo, D.E.; Gao, Y.; Zhou, D.; Mo, H.; Zhang, G.G.; Taylor, L.S. Dissolution and precipitation behavior of amorphous solid dispersions. J. Pharm. Sci. 2011, 100, 3316–3331. [Google Scholar] [CrossRef]
- Chen, J.; Ormes, J.D.; Higgins, J.D.; Taylor, L.S. Impact of surfactants on the crystallization of aqueous suspensions of celecoxib amorphous solid dispersion spray dried particles. Mol. Pharm. 2015, 12, 533–541. [Google Scholar] [CrossRef] [PubMed]
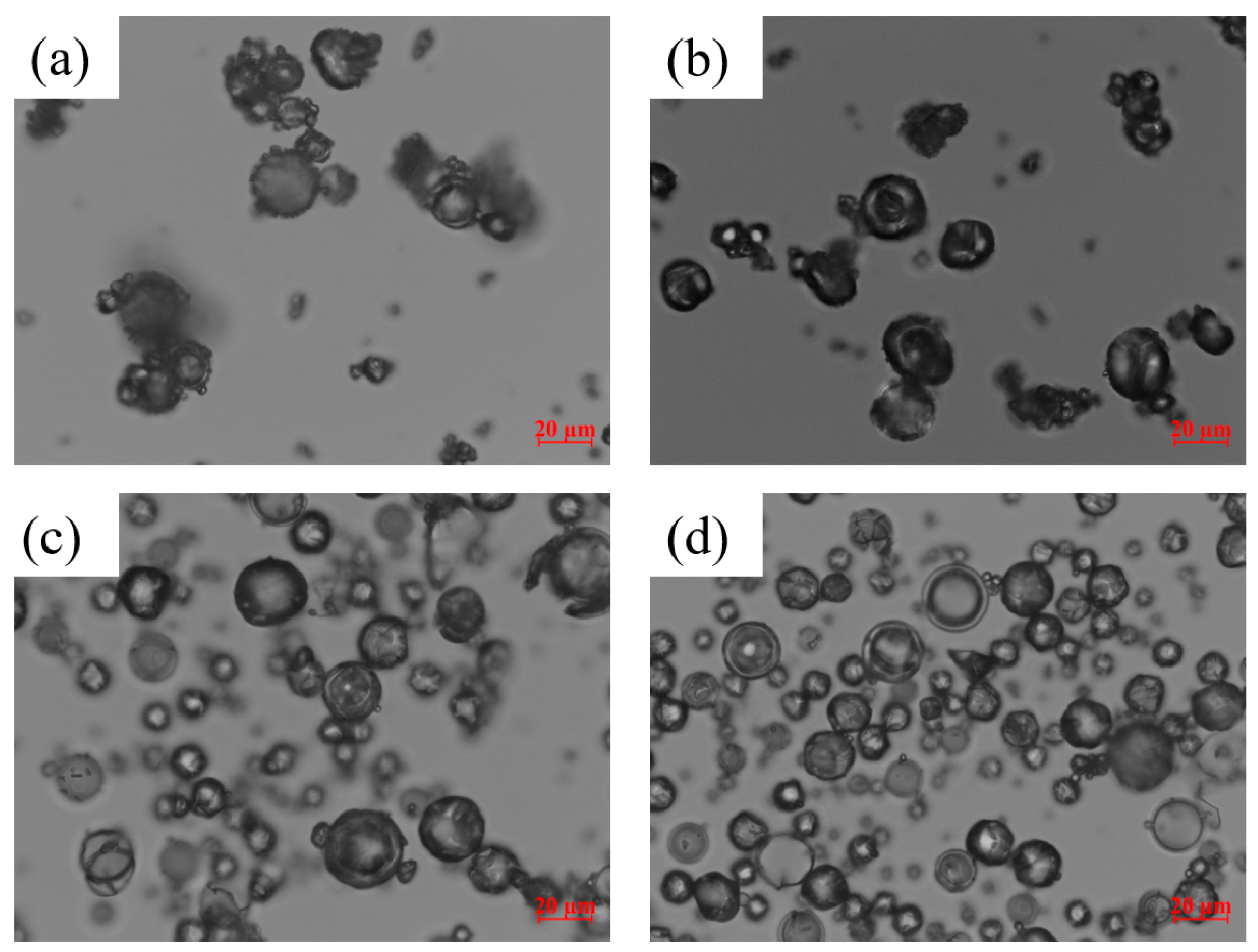
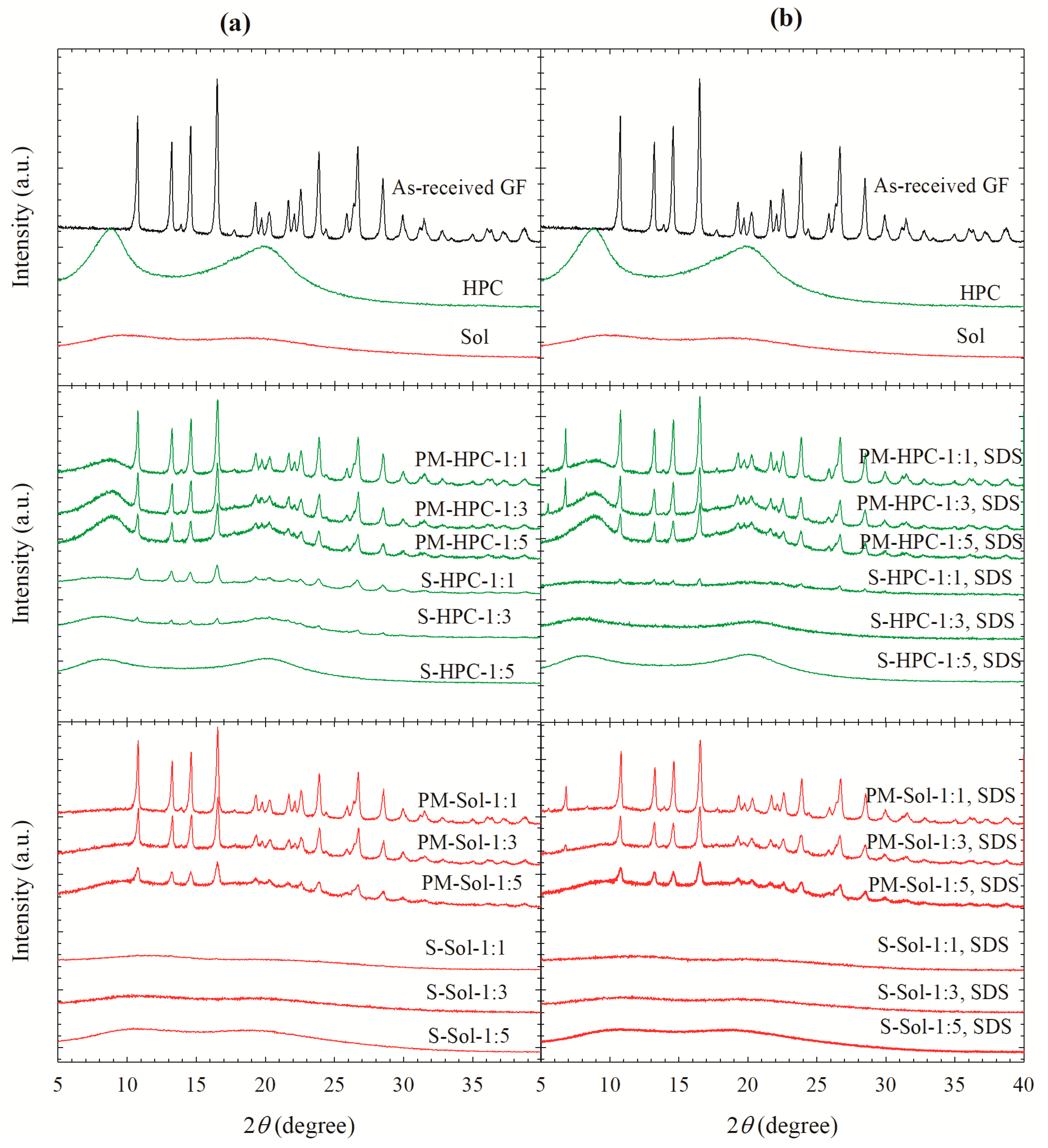
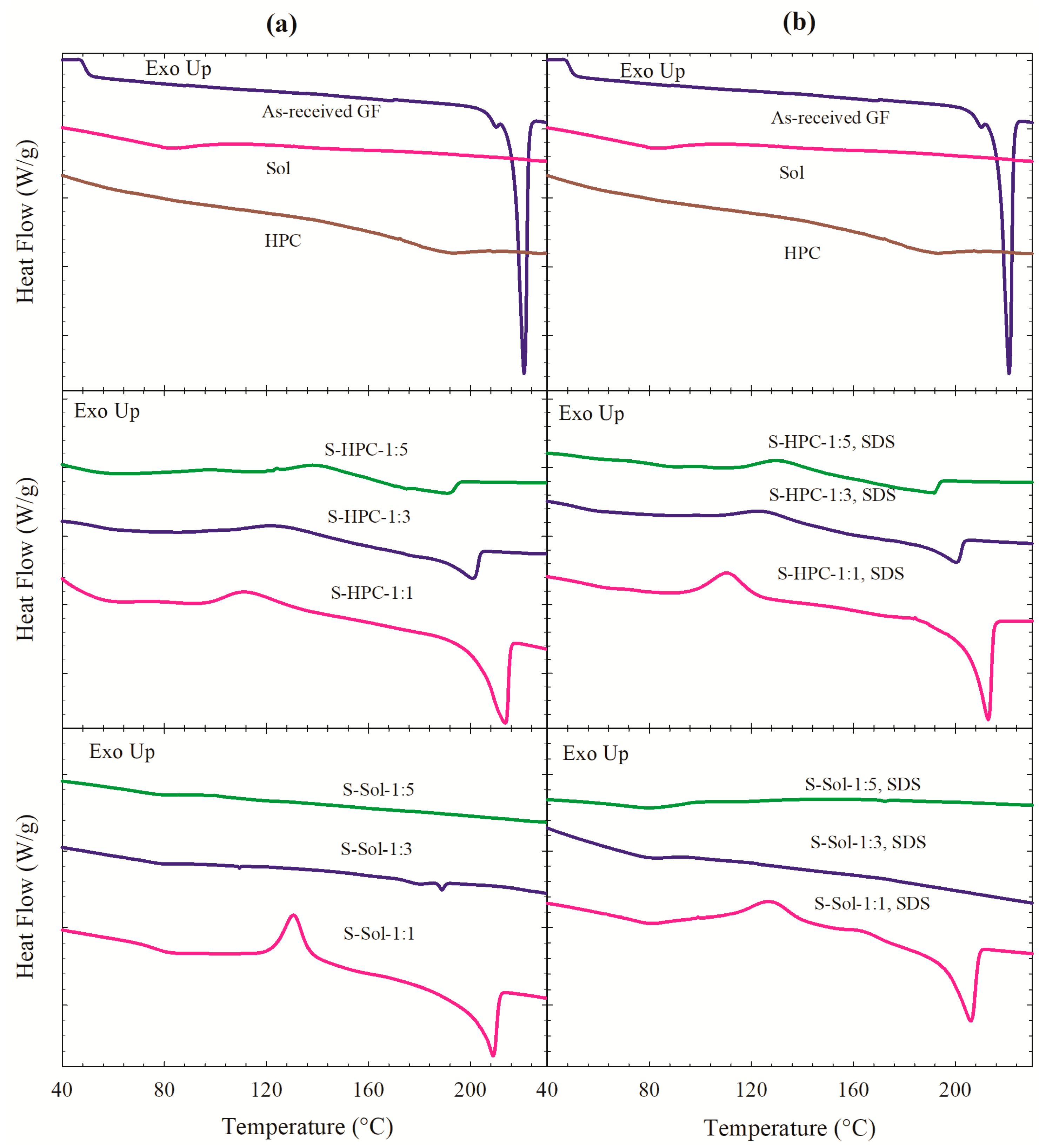
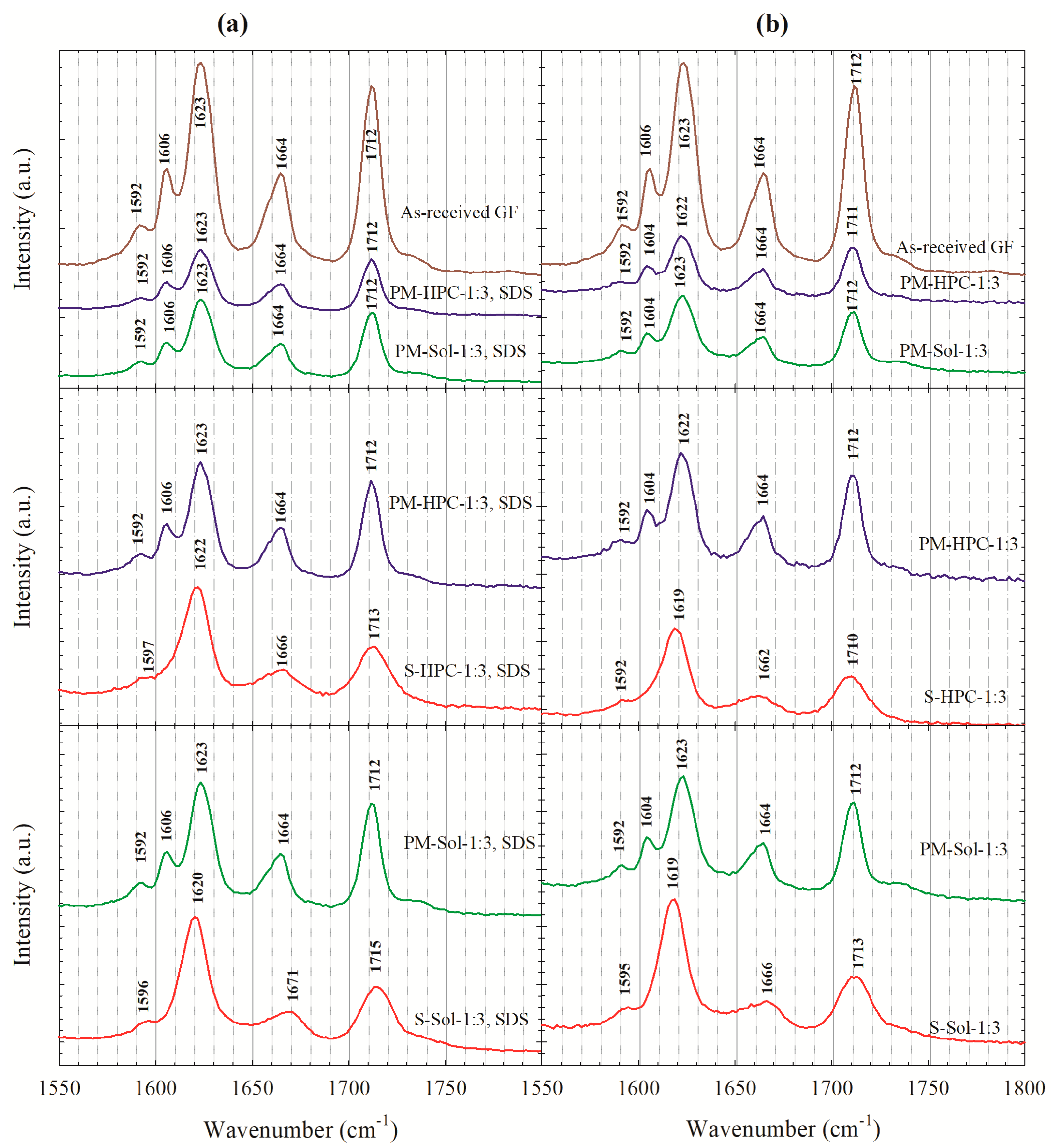
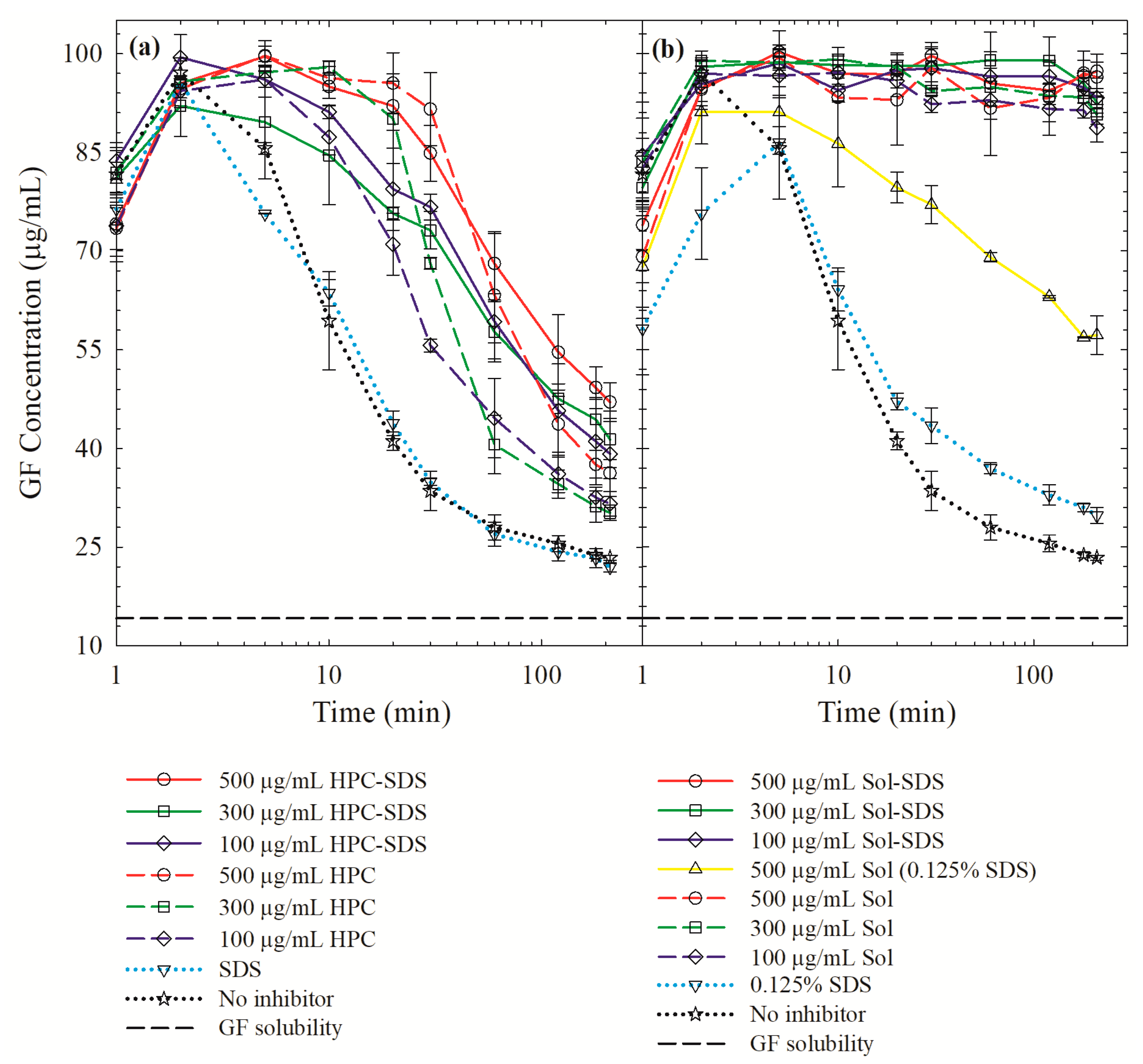
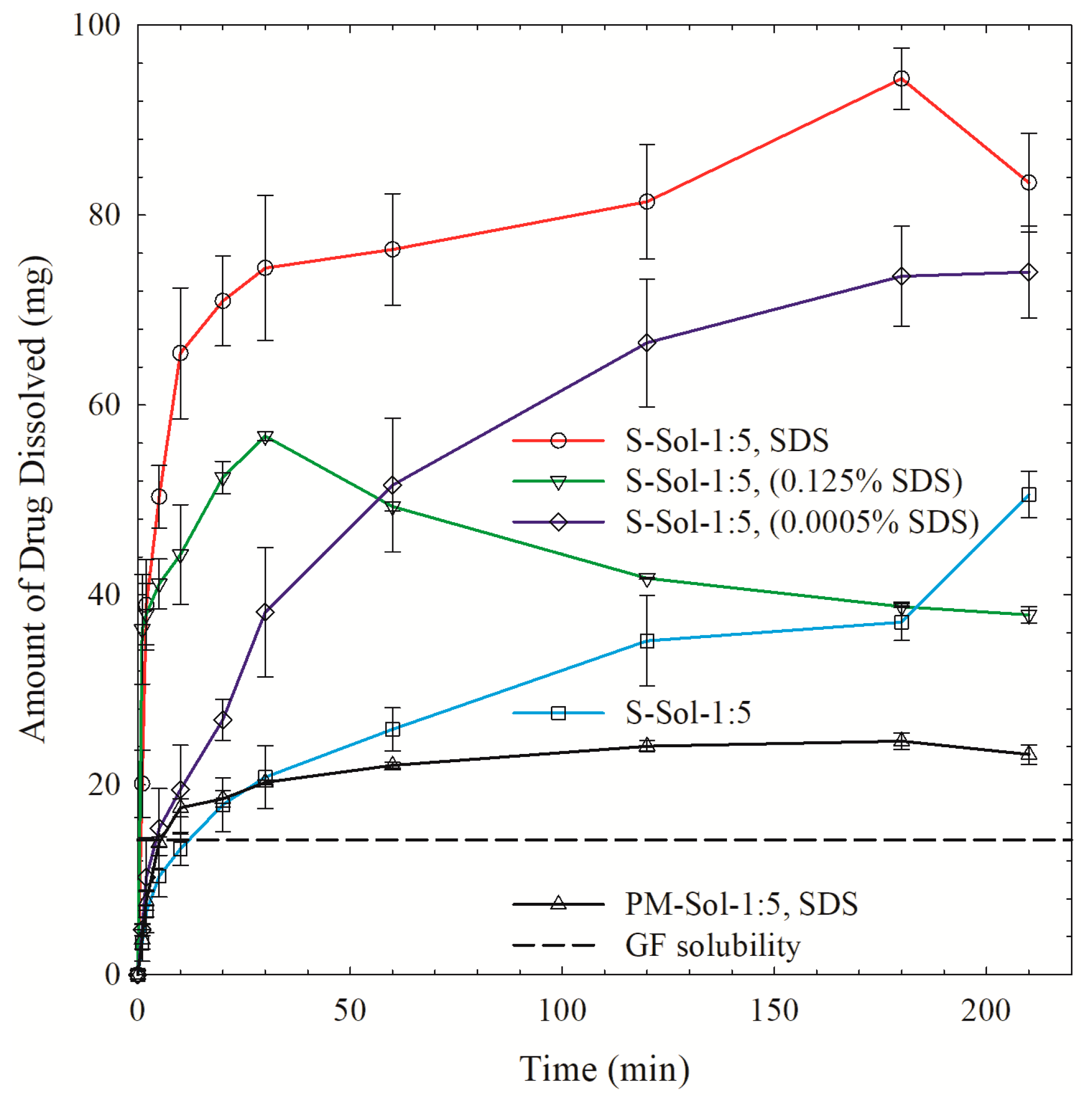
| Drug | Drug Loading (% w/w) | Polymer a | Drug:Polymer:SDS | Wettability Testing | DeS Testing b | References |
|---|---|---|---|---|---|---|
| Ketoprofen | 10% | PEG | 1:8:1 | No | No | Mura et al. [19] |
| Tacrolimus | 10% | CMC-Na | 1:8:1 | No | No | Park et al. [30] |
| Docetaxel | 5%–9% | PVP K30 | 1:9:1–1:19:1 | No | No | Moes et al. [31] |
| Valsartan | 50%–67% | HPMC | 1:0.42:0.08–1:0.67:0.33 | No | No | Yan et al. [32] |
| Sulfathiazole | 33%–50% | PVP K29/32 | 1:1:0.1–1:1:1 | No | No | Dave et al. [33] |
| Simvastatin | 20%–33% | PVP K29/32 | 1:3:0.02–1:3:0.06 | Yes | No | Lu et al. [18] |
| Sorafenib | 20%–50% | Soluplus | 1:0.9:0.1–1:4.5:0.5 | No | No | Truong et al. [34] |
| Tacrolimus | 20%–33% | HPMC | 1:1:1–1:1:3 | No | No | Jung et al. [27] |
| Felodipine | 23% | Soluplus | 1:3:0.2–1:3:0.4 | No | Yes | Chen et al. [35] |
| Itraconazole | 50% | Soluplus, PVP VA64 | 1:0.5:0.5 | No | Yes | Deshpande et al. [24] |
| Itraconazole | 20% | HPMC-AS | 1:3.75:0.25–1:2.75:1.25 | No | Yes | Feng et al. [17] |
| Sirolimus | 16%–48% | HPMC | 1:1:0.05–1:5:0.1 | No | No | Kim et al. [36] |
| Nifedipine | 14%–40% | Kolliphor, Soluplus | 1:1:0.5–1:4:2 | No | No | Muralichand and Bhikshapathi [37] |
| Formulation ID a | GF (% w/v) b | Polymers (% w/v) b | SDS (% w/v) b |
|---|---|---|---|
| S-Sol-1:5 | 2.5 | 12.5 | 0 |
| S-Sol-1:3 | 2.5 | 7.5 | 0 |
| S-Sol-1:1 | 2.5 | 2.5 | 0 |
| S-Sol-1:5, SDS | 2.5 | 12.5 | 0.125 |
| S-Sol-1:3, SDS | 2.5 | 7.5 | 0.125 |
| S-Sol-1:1, SDS | 2.5 | 2.5 | 0.125 |
| S-HPC-1:5 | 2.5 | 12.5 | 0 |
| S-HPC-1:3 | 2.5 | 7.5 | 0 |
| S-HPC-1:1 | 2.5 | 2.5 | 0 |
| S-HPC-1:5, SDS | 2.5 | 12.5 | 0.125 |
| S-HPC-1:3, SDS | 2.5 | 7.5 | 0.125 |
| S-HPC-1:1, SDS | 2.5 | 2.5 | 0.125 |
| Formulation ID | Characteristic Sizes from the Volume-Based Size Distribution (µm) | GF Content, RSD (% w/w, %) | ||
|---|---|---|---|---|
| d10 ± SD | d50 ± SD | d90 ± SD | ||
| S-Sol-1:5 | 7.03 ± 0.2 | 18.3 ± 0.2 | 38.3 ± 0.1 | 14.8, 1.79 |
| S-Sol-1:3 | 6.08 ± 0.1 | 14.3 ± 0.0 | 32.4 ± 0.1 | 22.1, 1.76 |
| S-Sol-1:1 | 3.46 ± 0.2 | 10.9 ± 0.1 | 21.5 ± 0.0 | 44.8, 3.46 |
| S-Sol-1:5, SDS | 6.23 ± 0.1 | 20.8 ± 0.1 | 40.1 ± 0.2 | 14.6, 4.45 |
| S-Sol-1:3, SDS | 4.11 ± 0.0 | 12.3 ± 0.0 | 33.2 ± 0.1 | 21.5, 2.02 |
| S-Sol-1:1, SDS | 5.03 ± 0.1 | 11.0 ± 0.1 | 20.2 ± 0.0 | 42.3, 2.21 |
| S-HPC-1:5 | 6.48 ± 0.2 | 21.5 ± 0.4 | 42.3 ± 0.2 | 15.0, 2.65 |
| S-HPC-1:3 | 5.87 ± 0.1 | 15.4 ± 0.3 | 33.5 ± 0.1 | 24.0, 1.51 |
| S-HPC-1:1 | 5.28 ± 0.1 | 12.7 ± 0.2 | 30.3 ± 1.2 | 44.9, 1.67 |
| S-HPC-1:5, SDS | 7.10 ± 0.2 | 22.6 ± 0.2 | 40.3 ± 0.3 | 15.1, 3.30 |
| S-HPC-1:3, SDS | 6.48 ± 0.0 | 15.8 ± 0.6 | 31.3 ± 1.0 | 24.4, 2.56 |
| S-HPC-1:1, SDS | 7.05 ± 0.2 | 13.0 ± 0.9 | 26.9 ± 0.8 | 42.7, 0.73 |
| Formulation ID a | Tg (°C) | Trc (°C) | ΔHrc (J/g) | Tm (°C) | ΔHf (J/g) | Crystallinity (%) |
|---|---|---|---|---|---|---|
| S-Sol-1:5 | 80.6 | — | — | — | — | — |
| S-Sol-1:3 | 80.4 | — | — | 189 | 0.64 | — |
| S-Sol-1:1 | 77.7 | 131 | −14.1 | 209 | 23.2 | — |
| S-Sol-1:5, SDS | 77.4 | — | — | — | — | — |
| S-Sol-1:3, SDS | 80.0 | — | — | — | — | — |
| S-Sol-1:1, SDS | 74.6 | 127 | −9.26 | 206 | 25.4 | — |
| S-HPC-1:5 | 52.9 | 139 | −3.35 | 192 | 6.64 | — |
| S-HPC-1:3 | 53.2 | 122 | −4.36 | 201 | 13.2 | 11.5 |
| S-HPC-1:1 | — | 111 | −9.70 | 213 | 34.8 | 27.7 |
| S-HPC-1:5, SDS | 51.7 | 130 | −2.43 | 191 | 7.43 | — |
| S-HPC-1:3, SDS | 57.7 | 124 | −3.86 | 200 | 11.6 | — |
| S-HPC-1:1, SDS | 58.9 | 109 | −15.8 | 213 | 38.9 | 6.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.; Ahmad, S.; Tarabokija, J.; Parker, N.; Bilgili, E. Spray-Dried Amorphous Solid Dispersions of Griseofulvin in HPC/Soluplus/SDS: Elucidating the Multifaceted Impact of SDS as a Minor Component. Pharmaceutics 2020, 12, 197. https://doi.org/10.3390/pharmaceutics12030197
Rahman M, Ahmad S, Tarabokija J, Parker N, Bilgili E. Spray-Dried Amorphous Solid Dispersions of Griseofulvin in HPC/Soluplus/SDS: Elucidating the Multifaceted Impact of SDS as a Minor Component. Pharmaceutics. 2020; 12(3):197. https://doi.org/10.3390/pharmaceutics12030197
Chicago/Turabian StyleRahman, Mahbubur, Stephanie Ahmad, James Tarabokija, Nathaniel Parker, and Ecevit Bilgili. 2020. "Spray-Dried Amorphous Solid Dispersions of Griseofulvin in HPC/Soluplus/SDS: Elucidating the Multifaceted Impact of SDS as a Minor Component" Pharmaceutics 12, no. 3: 197. https://doi.org/10.3390/pharmaceutics12030197
APA StyleRahman, M., Ahmad, S., Tarabokija, J., Parker, N., & Bilgili, E. (2020). Spray-Dried Amorphous Solid Dispersions of Griseofulvin in HPC/Soluplus/SDS: Elucidating the Multifaceted Impact of SDS as a Minor Component. Pharmaceutics, 12(3), 197. https://doi.org/10.3390/pharmaceutics12030197







