MiR-219a-5p Enriched Extracellular Vesicles Induce OPC Differentiation and EAE Improvement More Efficiently Than Liposomes and Polymeric Nanoparticles
Abstract
Highlights
- miR-219a-5p enriched EVs are more efficient than liposomes and polymeric nanoparticles inducing oligodendrocyte precursor cell differentiation.
- EVs are able to cross an in vitro model of the blood–brain barrier in an effective way.
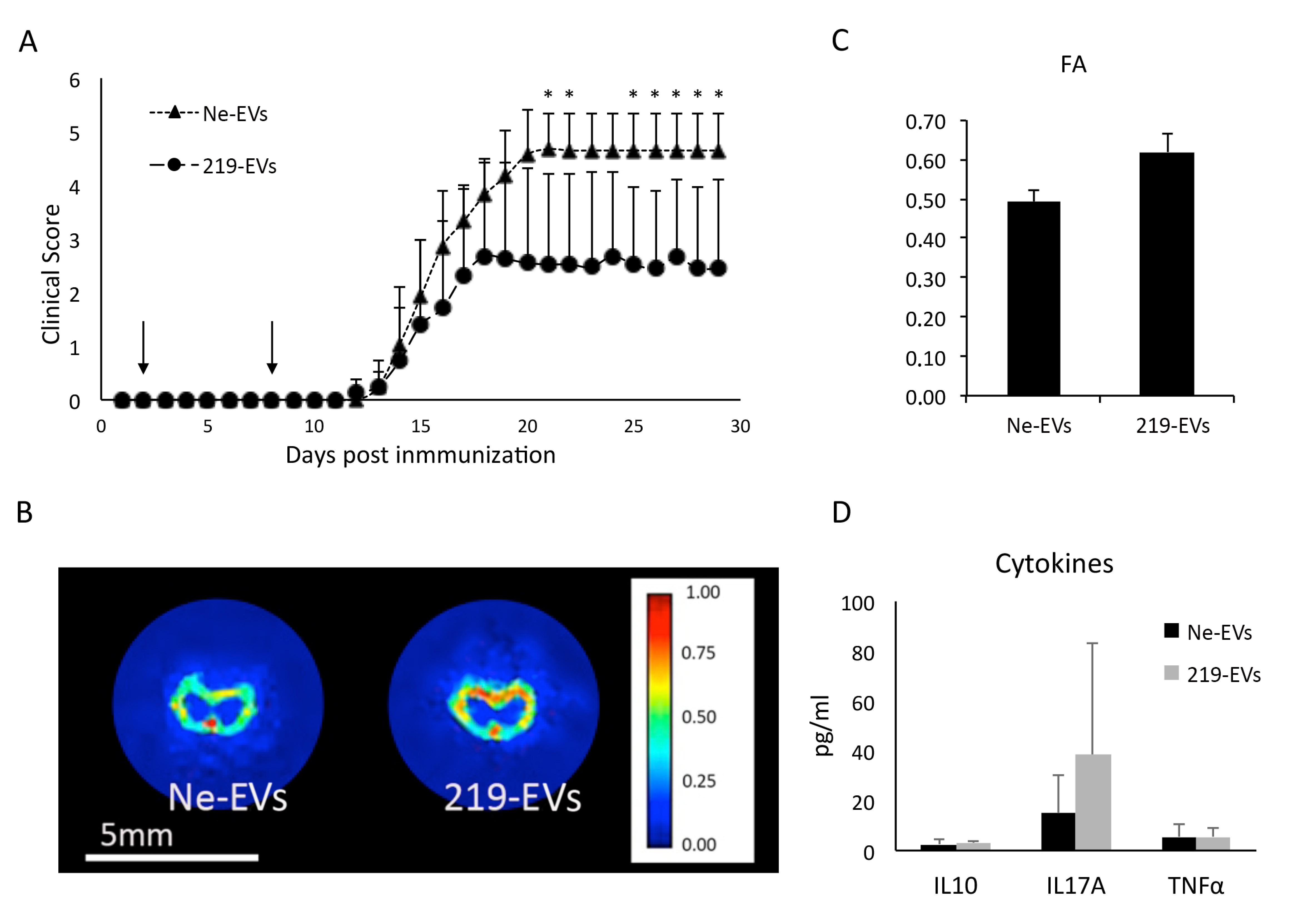
- miR-219a-5p enriched EVs are able to improve EAE clinical evolution.
1. Introduction
2. Materials and Methods
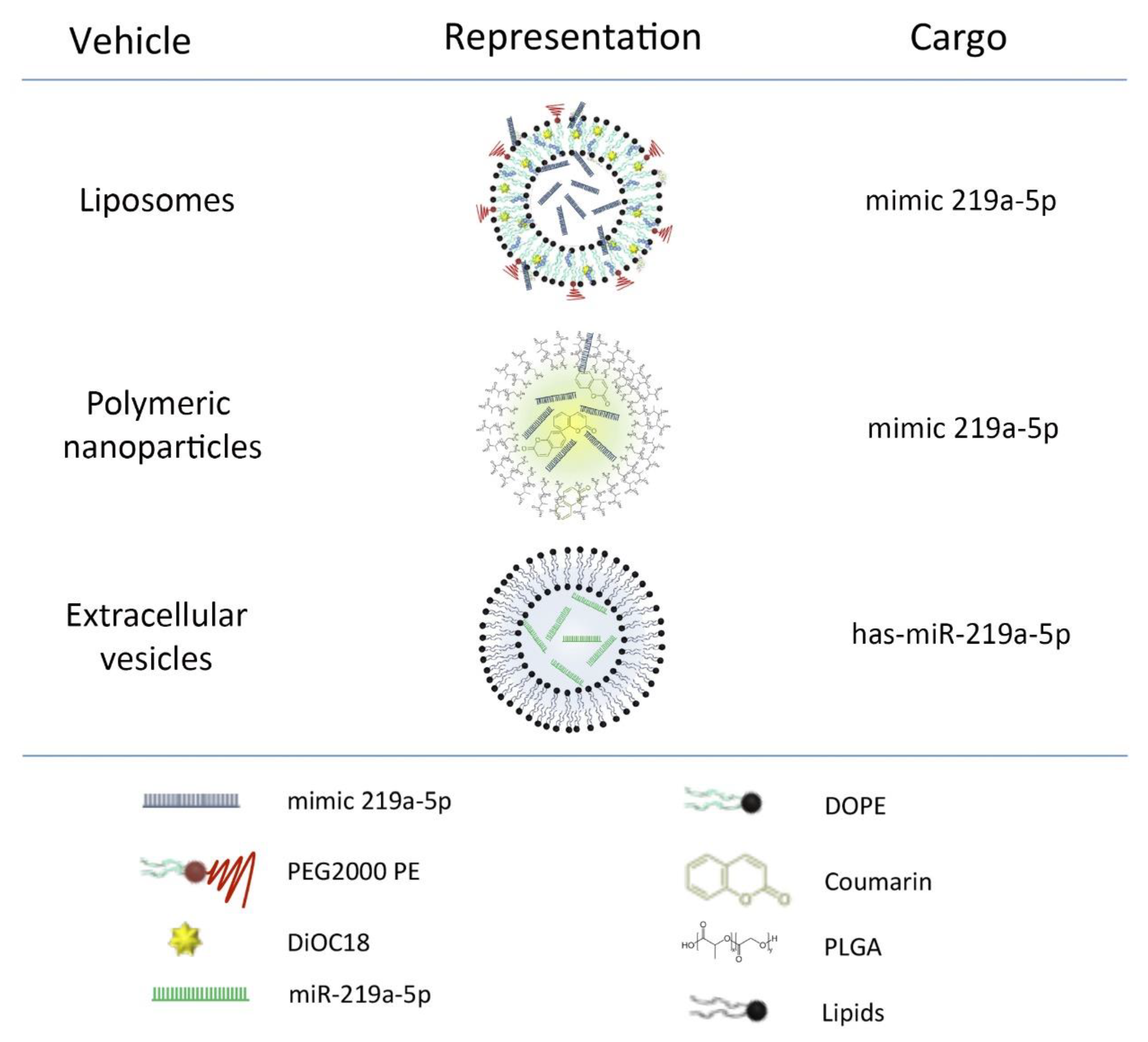
2.1. MicroRNA Carriers Synthesis and Loading
2.1.1. Liposomes
2.1.2. Polymeric Nanoparticles
2.1.3. Extracellular Vesicles
2.2. Characterization of microRNA Carriers
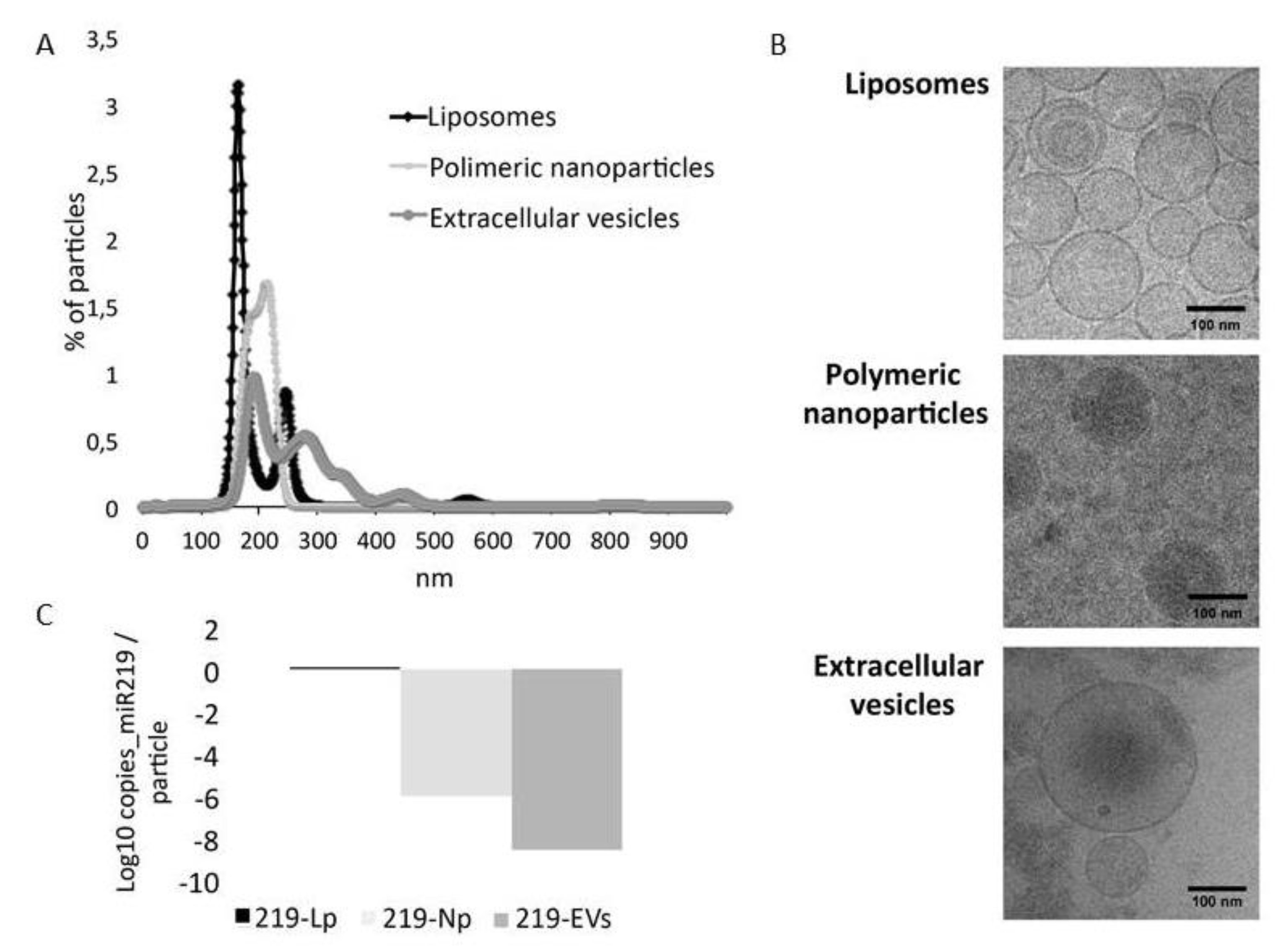
2.2.1. Nano Tracking Particle Analysis (NTA)
2.2.2. Droplet Digital PCR
2.2.3. CryoTEM
2.2.4. MicroRNA Microarray
2.3. Oligodendrocyte Precursor Cell Culture
2.3.1. Up-take Studies
2.3.2. Differentiation Studies
2.4. Blood–Brain Barrier Crossing Studies
Crossing Studies
2.5. Experimental Autoimmune Encephalomyelitis (EAE)
2.5.1. Induction and Clinical Evaluation
2.5.2. Extracellular Vesicles Administration
2.5.3. Sample Extraction
2.5.4. Plasma Derived Cytokine Measurement
2.5.5. Magnetic Resonance Imaging
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of microRNA Carriers
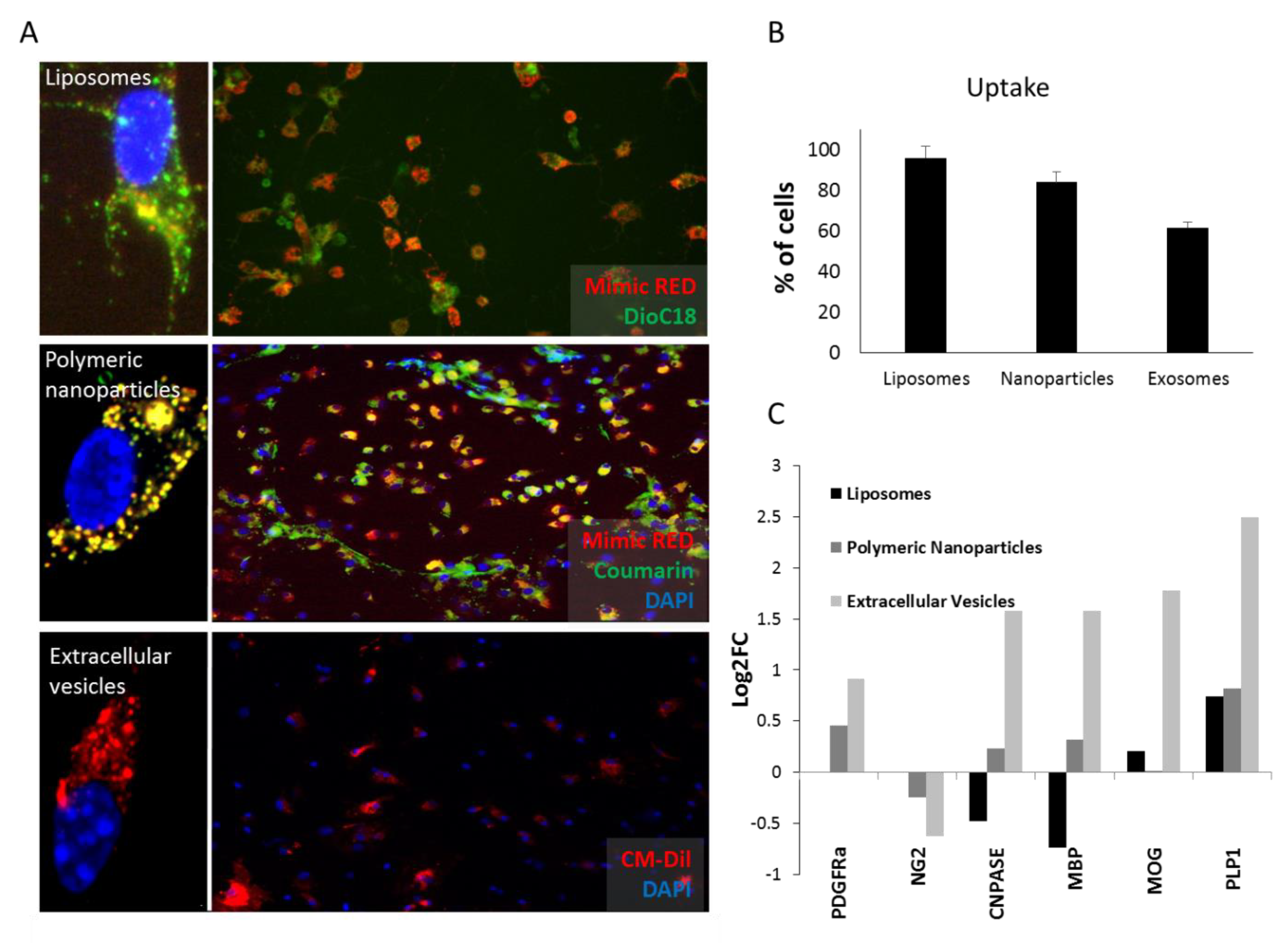
3.2. Liposomes and Polymeric Nanoparticles are More Efficiently Taken Up by OPCs. Extracellular Vesicles Induce Differentiation More Efficiently
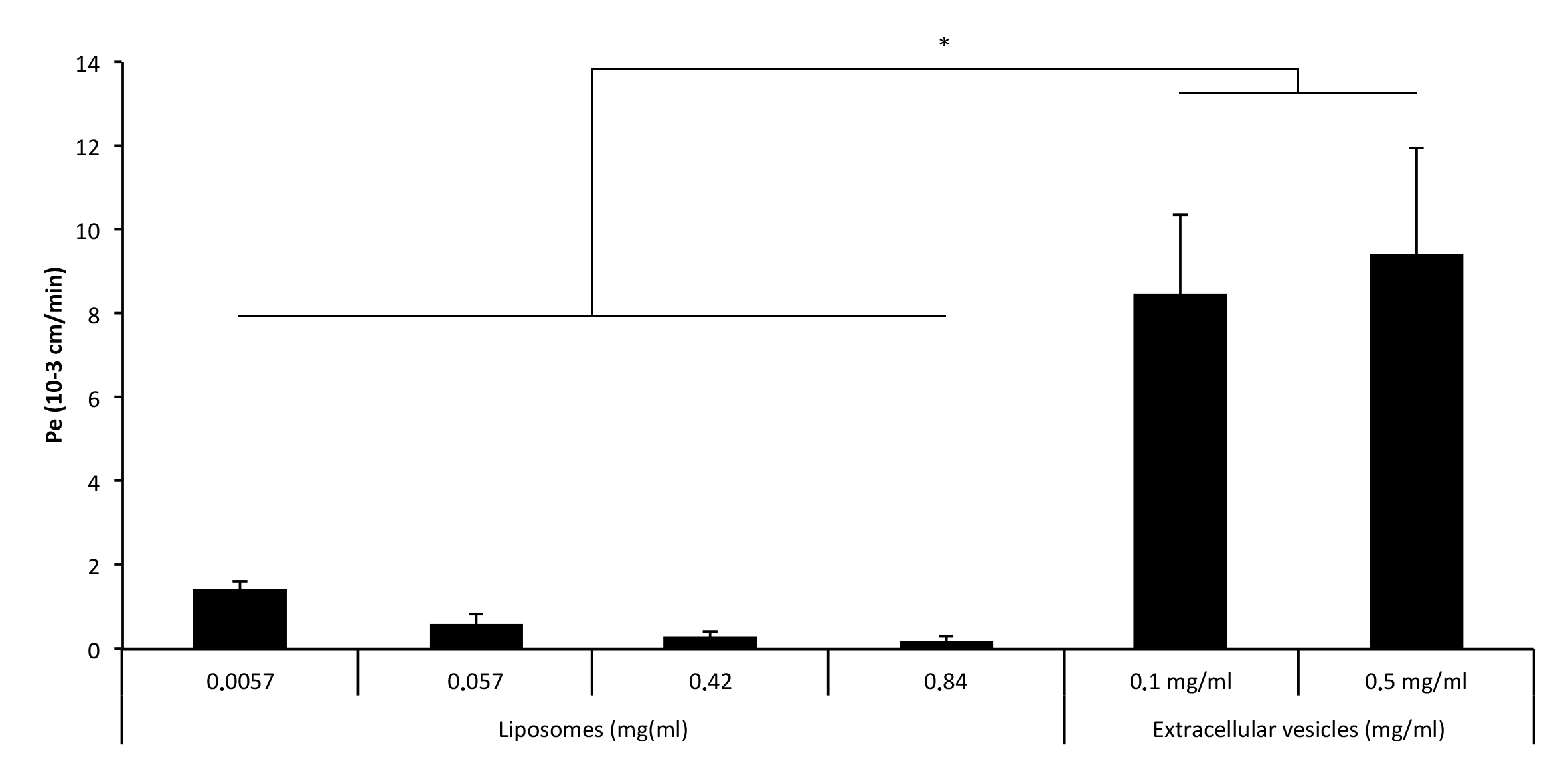
3.3. Extracellular Vesicles Cross the BBB More Efficiently than Liposomes and Nanoparticles
3.4. MiR-219a-5p Enriched Extracellular Vesicles Improve EAE Clinical Evolution
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Agulla, J.; Brea, D.; Campos, F.; Sobrino, T.; Argibay, B.; Al-Soufi, W.; Blanco, M.; Castillo, J.; Ramos-Cabrer, P. In vivo theranostics at the peri-infarct region in cerebral ischemia. Theranostics 2014, 4, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Alberro, A.; Sáenz-Cuesta, M.; Muñoz-Culla, M.; Mateo-Abad, M.; Gonzalez, E.; Carrasco-Garcia, E.; Araúzo-Bravo, M.J.; Matheu, A.; Vergara, I.; Otaegui, D. Inflammaging and frailty status do not result in an increased extracellular vesicle concentration in circulation. Int. J. Mol. Sci. 2016, 17, 1168. [Google Scholar] [CrossRef] [PubMed]
- Buyens, K.; Demeester, J.; De Smedt, S.S.; Sanders, N.N. Elucidating the encapsulation of short interfering RNA in PEGylated cationic liposomes. Langmuir 2009, 25, 4886–4891. [Google Scholar] [CrossRef] [PubMed]
- De Faria, O.; Cui, Q.-L.; Bin, J.M.; Bull, S.-J.; Kennedy, T.E.; Bar-Or, A.; Antel, J.P.; Colman, D.R.; Dhaunchak, A.S. Regulation of miRNA 219 and miRNA Clusters 338 and 17–92 in Oligodendrocytes. Front. Genet. 2012, 3, 46. [Google Scholar] [CrossRef]
- Dugas, J.C.; Cuellar, T.L.; Scholze, A.; Ason, B.; Ibrahim, A.; Emery, B.; Zamanian, J.L.; Foo, L.C.; McManus, M.T.; Barres, B.A. Dicer1 and miR-219 Are Required for Normal Oligodendrocyte Differentiation and Myelination. Neuron 2010, 65, 597–611. [Google Scholar] [CrossRef]
- Dulamea, A.O. The contribution of oligodendrocytes and oligodendrocyte progenitor cells to central nervous system repair in multiple sclerosis: Perspectives for remyelination therapeutic strategies. Neural Regen. Res. 2017, 12, 1939–1944. [Google Scholar] [CrossRef]
- Fan, H.-B.; Chen, L.-X.; Qu, X.-B.; Ren, C.-L.; Wu, X.-X.; Dong, F.-X.; Zhang, B.-L.; Gao, D.-S.; Yao, R.-Q. Transplanted miR-219-overexpressing oligodendrocyte precursor cells promoted remyelination and improved functional recovery in a chronic demyelinated model. Sci. Rep. 2017, 7, 41407. [Google Scholar] [CrossRef]
- Fletcher, J.M.; Lalor, S.J.; Sweeney, C.M.; Tubridy, N.; Mills, K.H.G. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin. Exp. Immunol. 2010, 162, 1–11. [Google Scholar] [CrossRef]
- Franklin, R.J.M. Why does remyelination fail in multiple sclerosis? Nat. Rev. Neurosci. 2002, 3, 705–714. [Google Scholar] [CrossRef]
- Franklin, R.J.M.; Ffrench-Constant, C. Regenerating CNS myelin—From mechanisms to experimental medicines. Nat. Rev. Neurosci. 2017, 18, 753–769. [Google Scholar] [CrossRef]
- Goldman, S.A.; Osorio, J. So many progenitors, so little myelin. Nat. Neurosci. 2014, 17, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Julián, P.; Milon, P.; Agirrezabala, X.; Lasso, G.; Gil, D.; Rodnina, M.V.; Valle, M. The Cryo-EM Structure of a Complete 30S Translation Initiation Complex from Escherichia coli. PLoS Biol. 2011, 9, e1001095. [Google Scholar] [CrossRef] [PubMed]
- Kurte, M.; Bravo-alegría, J.; Torres, A.; Carrasco, V.; Ibáñez, C.; Vega-letter, A.M.; Ryan, C.F.; Irarrázabal, C.E.; Figueroa, F.E.; Fuentealba, R.A.; et al. Intravenous Administration of Bone Marrow-Derived Mesenchymal Stem Cells Induces a Switch from Classical to Atypical Symptoms in Experimental Autoimmune Encephalomyelitis. Stem Cells Int. 2015, 2015, 140170. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ren, C.; Qu, X.; Wu, X.; Dong, F.; Chand, Y.K.; Fan, H.; Yao, R.; Geng, D. miR-219 attenuates demyelination in cuprizone-induced demyelinated mice by regulating monocarboxylate transporter 1. Eur. J. Neurosci. 2017, 45, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Lunavat, T.R.; Jang, S.C.; Nilsson, L.; Park, H.T.; Repiska, G.; Lässer, C.; Nilsson, J.A.; Gho, Y.S.; Lötvall, J. RNAi delivery by exosome-mimetic nanovesicles—Implications for targeting c-Myc in cancer. Biomaterials 2016, 102, 231–238. [Google Scholar] [CrossRef]
- Medina-Rodríguez, E.M.; Arenzana, F.J.; Bribián, A.; de Castro, F. Protocol to isolate a large amount of functional oligodendrocyte precursor cells from the cerebral cortex of adult mice and humans. PLoS ONE 2013, 8, e81620. [Google Scholar] [CrossRef]
- Melo, S.A.; Sugimoto, H.; Connell, J.T.O.; Kato, N.; Vidal, A.; Qiu, L.; Vitkin, E.; Perelman, L.T.; Melo, C.A.; Lucci, A.; et al. Cancer Exosomes Perform Cell-Independent MicroRNA Biogenesis and Promote Tumorigenesis. Cancer Cell 2014, 26, 707–721. [Google Scholar] [CrossRef]
- Nazari, B.; Soleimani, M.; Ebrahimi-Barough, S.; Enderami, S.E.; Kazemi, M.; Negahdari, B.; Sadroddiny, E.; Ai, J. Overexpression of miR-219 promotes differentiation of human induced pluripotent stem cells into pre-oligodendrocyte. J. Chem. Neuroanat. 2018, 91, 8–16. [Google Scholar] [CrossRef]
- Otero-Ortega, L.; Laso-García, F.; Gómez-de Frutos, M.D.C.; Rodríguez-Frutos, B.; Pascual-Guerra, J.; Fuentes, B.; Díez-Tejedor, E.; Gutiérrez-Fernández, M.; Ramos-Cejudo, J.; Rodríguez-Frutos, B.; et al. White matter injury restoration after stem cell administration in subcortical ischemic stroke. Sci. Rep. 2015, 6, 44433. [Google Scholar] [CrossRef]
- Pusic, A.D.; Kraig, R.P. Youth and environmental enrichment generate serum exosomes containing miR-219 that promote CNS myelination. Glia 2014, 62, 284–299. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Sork, H.; Corso, G.; Krjutskov, K.; Johansson, H.J.; Nordin, J.Z.; Wiklander, O.P.B.; Lee, Y.X.F.; Westholm, J.O.; Lehtiö, J.; Wood, M.J.A.; et al. Heterogeneity and interplay of the extracellular vesicle small RNA transcriptome and proteome. Sci. Rep. 2018, 8, 10813. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Boussac, M.; Véron, P.; Raposo, G.; Garin, J.; Amigorena, S. Proteomic Analysis of Dendritic Cell-Derived Exosomes: A Secreted Subcellular Compartment Distinct from Apoptotic Vesicles. J. Immunol. 2001, 166, 7309–7318. [Google Scholar] [CrossRef]
- Tian, Y.; Li, S.; Song, J.; Ji, T.; Zhu, M.; Anderson, G.J.; Wei, J.; Nie, G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014, 35, 2383–2390. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Ventola, C.L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Wang, H.; Moyano, A.L.; Ma, Z.Z.; Deng, Y.; Lin, Y.; Zhao, C.; Zhang, L.; Jiang, M.; He, X.; Ma, Z.Z.; et al. miR-219 Cooperates with miR-338 in Myelination and Promotes Myelin Repair in the CNS. Dev. Cell 2017, 40, 566–582.e5. [Google Scholar] [CrossRef]
- Willms, E.; Cabañas, C.; Mäger, I.; Wood, M.J.A. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front. Immunol. 2018, 9, 738. [Google Scholar] [CrossRef]
- Yu, X.; Odenthal, M.; Fries, J.W.U. Exosomes as miRNA carriers: Formation-function-future. Int. J. Mol. Sci. 2016, 17, 2028. [Google Scholar] [CrossRef]
- Zhuang, X.; Xiang, X.; Grizzle, W.; Sun, D.; Zhang, S.; Axtell, R.C.; Ju, S.; Mu, J.; Zhang, L.; Steinman, L.; et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol. Ther. 2011, 19, 1769–1779. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osorio-Querejeta, I.; Carregal-Romero, S.; Ayerdi-Izquierdo, A.; Mäger, I.; Nash, L.A.; Wood, M.; Egimendia, A.; Betanzos, M.; Alberro, A.; Iparraguirre, L.; et al. MiR-219a-5p Enriched Extracellular Vesicles Induce OPC Differentiation and EAE Improvement More Efficiently Than Liposomes and Polymeric Nanoparticles. Pharmaceutics 2020, 12, 186. https://doi.org/10.3390/pharmaceutics12020186
Osorio-Querejeta I, Carregal-Romero S, Ayerdi-Izquierdo A, Mäger I, Nash LA, Wood M, Egimendia A, Betanzos M, Alberro A, Iparraguirre L, et al. MiR-219a-5p Enriched Extracellular Vesicles Induce OPC Differentiation and EAE Improvement More Efficiently Than Liposomes and Polymeric Nanoparticles. Pharmaceutics. 2020; 12(2):186. https://doi.org/10.3390/pharmaceutics12020186
Chicago/Turabian StyleOsorio-Querejeta, Iñaki, Susana Carregal-Romero, Ana Ayerdi-Izquierdo, Imre Mäger, Leslie A. Nash, Matthew Wood, Ander Egimendia, M. Betanzos, Ainhoa Alberro, Leire Iparraguirre, and et al. 2020. "MiR-219a-5p Enriched Extracellular Vesicles Induce OPC Differentiation and EAE Improvement More Efficiently Than Liposomes and Polymeric Nanoparticles" Pharmaceutics 12, no. 2: 186. https://doi.org/10.3390/pharmaceutics12020186
APA StyleOsorio-Querejeta, I., Carregal-Romero, S., Ayerdi-Izquierdo, A., Mäger, I., Nash, L. A., Wood, M., Egimendia, A., Betanzos, M., Alberro, A., Iparraguirre, L., Moles, L., Llarena, I., Möller, M., Goñi-de-Cerio, F., Bijelic, G., Ramos-Cabrer, P., Muñoz-Culla, M., & Otaegui, D. (2020). MiR-219a-5p Enriched Extracellular Vesicles Induce OPC Differentiation and EAE Improvement More Efficiently Than Liposomes and Polymeric Nanoparticles. Pharmaceutics, 12(2), 186. https://doi.org/10.3390/pharmaceutics12020186






