Synergistic Mechanisms of Constituents in Herbal Extracts during Intestinal Absorption: Focus on Natural Occurring Nanoparticles
Abstract
1. Introduction
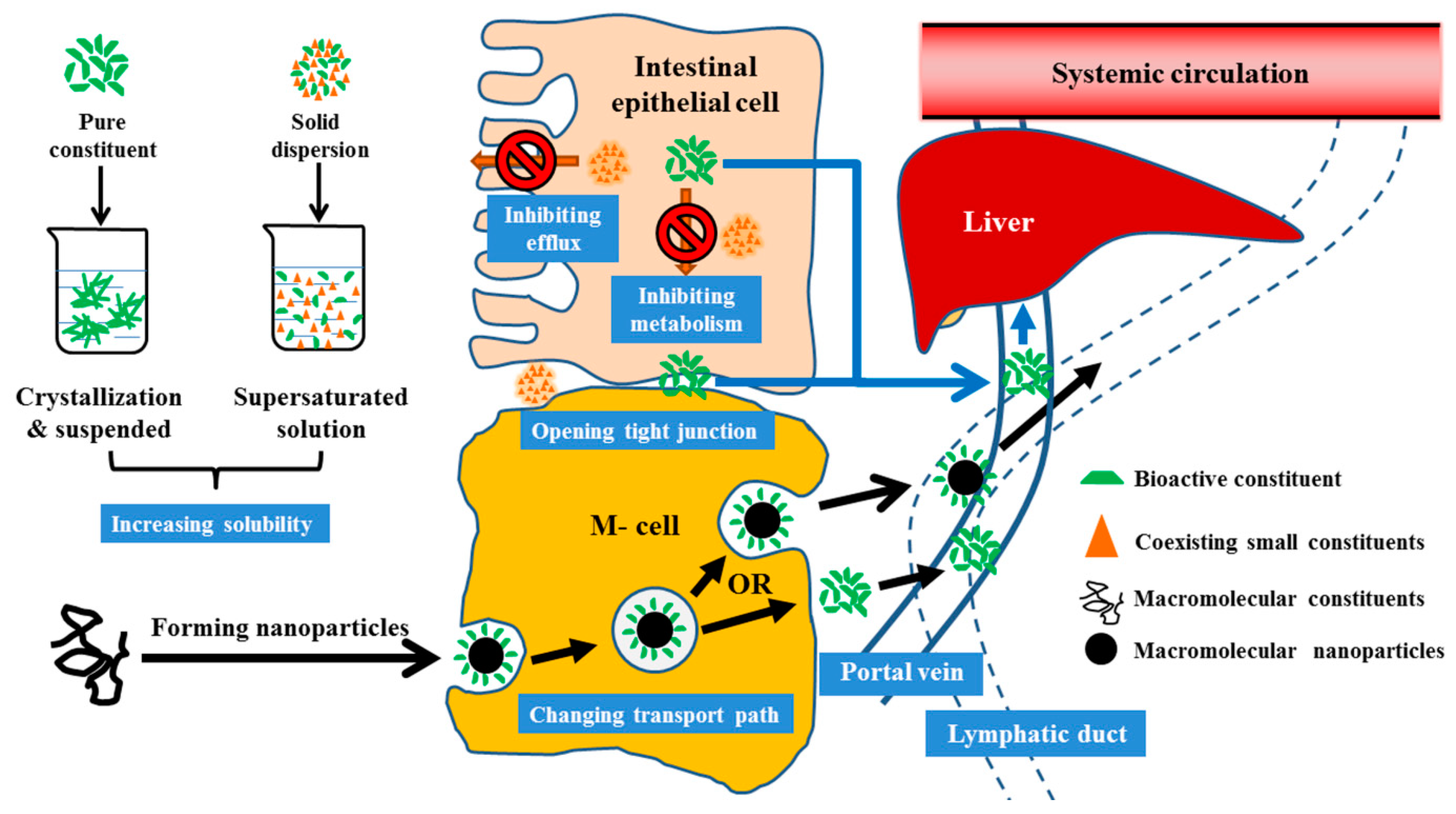
2. Synergy Mechanisms in the Absorption Process
2.1. Synergies in Improving Water Solubility
2.2. Synergies in Inhibiting Intestinal Metabolism
2.3. Synergies in Reducing Intestinal Efflux
2.4. Synergies in Increasing Enterocyte Membrane Permeability
2.5. Synergies in Opening Paracellular Tight Junctions Between Enterocytes
2.6. Synergies in Forming Naturally Occurring Nanoparticles
2.6.1. Ubiquity of Naturally Occurring Nanoparticles in Herbal Extracts
2.6.2. Isolation, Identification, and Composition of Natural Nanoparticles
2.6.3. Factors Affecting Natural Nanoparticle Formation
2.6.4. Pharmacological Effects of Natural Nanoparticles
2.6.5. Pharmacokinetics of Natural Nanoparticles
2.6.6. Natural Nanoparticles in the Delivery of Active Constituents
3. Causes of Pharmacokinetic Synergies in Herbal Extracts: A Botanical Perspective
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AFM | atomic force microscopy |
| AUC | area under the curve |
| BCA | bicinchoninic acid |
| BCRP | breast cancer resistance protein |
| CPE | chemical permeation enhancers |
| CYPs | cytochrome P450 enzymes |
| DLS/ELS | dynamic light scattering/electrophoretic light scattering |
| DOX | doxorubicin |
| G-CSF | granulocyte colony stimulating factor |
| HPLC | high-performance liquid chromatography |
| IL-6 | interleukin 6 |
| INPs | ivy nanoparticles |
| IP-10 | IFN-γ-induced protein 10 |
| LC-MS/MS | liquid chromatography tandem mass spectrometry |
| MDC | macrophage derived chemokine |
| MRP2 | multidrug resistance protein 2 |
| NADES | natural deep eutectic solvent |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| PBS | phosphate buffer solution |
| P-gp | p-glycoprotein |
| PMs | primary metabolites |
| RANTES | regulated upon activation normal T cell expressed and secreted factor |
| Rh | hydrodynamic radius |
| SEM | scanning electron microscopy |
| SMs | secondary metabolites |
| TCMs | traditional Chinese medicines |
| TJ | tight junction |
| TNF-α | tumor necrosis factor-α |
| TNP | nanoparticles isolated from green tea |
| UGTs | UDP-glucuronosyltransferases |
References
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Sheridan, H.; Kopp, B.; Krenn, L.; Guo, D.; Sendker, J. Traditional Chinese herbal medicine preparation: Invoking the butterfly effect. Science 2015, 350, S64–S66. [Google Scholar]
- Li, J.W.; Vederas, J.C. Drug discovery and natural products: End of an era or an endless frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef]
- Jiang, Y.; David, B.; Tu, P.F.; Barbin, Y. Recent analytical approaches in quality control of traditional Chinese medicines—A review. Anal. Chim. Acta 2010, 657, 9–18. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.M.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Elfawal, M.A.; Towler, M.J.; Reich, N.G.; Weathers, P.J.; Rich, S.M. Dried whole-plant Artemisia annua slows evolution of malaria drug resistance and overcomes resistance to artemisinin. Proc. Natl. Acad. Sci. USA 2015, 112, 821–826. [Google Scholar] [CrossRef]
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Weathers, P.J.; Arsenault, P.R.; Covello, P.S.; McMickle, A.; Teoh, K.H.; Reed, D.W. Artemisinin production in Artemisia annua: Studies in planta and results of a novel delivery method for treating malaria and other neglected diseases. Phytochem. Rev. 2011, 10, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, H.; Sun, S.; Sun, F.F.; Chen, J.; Zhao, L.; Zhang, G.Q. Comparative pharmacokinetics of hypaconitine after oral administration of pure hypaconitine, Aconitum carmichaelii extract and Sini Decoction to rats. Molecules 2015, 20, 1560–1570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yan, R.; Su, R.; Yang, C.; Liu, S.; Yu, X.; Chang, X.; Zhang, S.; Liu, C.; Xu, M.; et al. Bioavailability enhancement of osthole after oral administration of Bushen Yizhi prescription extract to rats followed by Cnidium monnieri (L.) Cusson fruits extract in comparison to pure osthole at different doses. J. Ethnopharmacol. 2014, 152, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.L.; Yin, C.; Zhang, B.K.; Dai, Y.; Jia, Y.Q.; Yang, Y.; Li, Q.; Shi, R.; Wang, T.M.; Wu, J.S.; et al. Naturally occurring proteinaceous nanoparticles in Coptidis Rhizoma extract act as concentration-dependent carriers that facilitate berberine absorption. Sci. Rep. 2016, 6, 20110. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Cheng, X.M.; Bligh, S.W.; White, K.N.; Branford-White, C.J.; Wang, Z.T. Pharmacokinetics and bioavailability of gentiopicroside from decoctions of Gentianae and Longdan Xiegan Tang after oral administration in rats--comparison with gentiopicroside alone. J. Pharm. Biomed. Anal. 2007, 44, 1113–1117. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Ye, M.; Xiang, C.; Wang, Q.; Liu, C.F.; Miao, W.J.; Guo, D.A. Analytical strategy to reveal the in vivo process of multi-component herbal medicine: A pharmacokinetic study of licorice using liquid chromatography coupled with triple quadrupole mass spectrometry. J. Chromatogr. A 2012, 1258, 84–93. [Google Scholar] [CrossRef]
- Joo, K.M.; Lee, J.H.; Jeon, H.Y.; Park, C.W.; Hong, D.K.; Jeong, H.J.; Lee, S.J.; Lee, S.Y.; Lim, K.M. Pharmacokinetic study of ginsenoside Re with pure ginsenoside Re and ginseng berry extracts in mouse using ultra performance liquid chromatography/mass spectrometric method. J. Pharm. Biomed. Anal. 2010, 51, 278–283. [Google Scholar] [CrossRef]
- Song, M.; Hang, T.J.; Zhang, Z.; Chen, H.Y. Effects of the coexisting diterpenoid tanshinones on the pharmacokinetics of cryptotanshinone and tanshinone IIA in rat. Eur. J. Pharm. Sci. 2007, 32, 247–253. [Google Scholar] [CrossRef]
- Xu, M.; Wang, G.; Xie, H.; Huang, Q.; Wang, W.; Jia, Y. Pharmacokinetic comparisons of schizandrin after oral administration of schizandrin monomer, Fructus Schisandrae aqueous extract and Sheng-Mai-San to rats. J. Ethnopharmacol. 2008, 115, 483–488. [Google Scholar] [CrossRef]
- Stermitz, F.R.; Lorenz, P.; Tawara, J.N.; Zenewicz, L.A.; Lewis, K. Synergy in a medicinal plant: Antimicrobial action of berberine potentiated by 5‘-methoxyhydnocarpin, a multidrug pump inhibitor. Proc. Natl. Acad. Sci. USA 2000, 97, 1433–1437. [Google Scholar] [CrossRef]
- Svensson, U.S.H.; Ashton, M. Identification of the human cytochrome P450 enzymes involved in the in vitro metabolism of artemisinin. Br. J. Clin. Pharm. 1999, 48, 528–535. [Google Scholar] [CrossRef]
- Cai, T.Y.; Zhang, Y.R.; Ji, J.B.; Xing, J. Investigation of the component in Artemisia annua L. leading to enhanced antiplasmodial potency of artemisinin via regulation of its metabolism. J. Ethnopharmacol. 2017, 207, 86–91. [Google Scholar] [CrossRef]
- Li, Q.; Yang, Y.; Zhou, T.; Wang, R.; Li, N.; Zheng, M.; Li, Y.-Y.; Zhang, J.-Q.; Wu, F.; Yang, B.-C.; et al. A Compositive Strategy to Study the Pharmacokinetics of TCMs: Taking Coptidis Rhizoma, and Coptidis Rhizoma-Glycyrrhizae Radix et Rhizoma as Examples. Molecules 2018, 23, 2042. [Google Scholar] [CrossRef] [PubMed]
- Thanki, K.; Gangwal, R.P.; Sangamwar, A.T.; Jain, S. Oral delivery of anticancer drugs: Challenges and opportunities. J. Control. Release 2013, 170, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Henkel, T. Traditional Chinese medicine (TCM): Are polyphenols and saponins the key ingredients triggering biological activities? Curr. Med. Chem. 2002, 9, 1483–1485. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wen, Q.; Jiang, J.; Li, H.-L.; Tan, Y.-F.; Li, Y.-H.; Zeng, N.-K. Could the gut microbiota reconcile the oral bioavailability conundrum of traditional herbs? J. Ethnopharmacol. 2016, 179, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.D.; Trevaskis, N.L.; Charman, S.A.; Shanker, R.M.; Charman, W.N.; Pouton, C.W.; Porter, C.J. Strategies to address low drug solubility in discovery and development. Pharmacol. Rev. 2013, 65, 315–499. [Google Scholar] [CrossRef] [PubMed]
- Keung, W.M.; Lazo, O.; Kunze, L.; Vallee, B.L. Potentiation of the bioavailability of daidzin by an extract of Radix puerariae. Proc. Natl. Acad. Sci. USA 1996, 93, 4284–4288. [Google Scholar] [CrossRef] [PubMed]
- Jurgenliemk, G.; Nahrstedt, A. Dissolution, solubility and cooperativity of phenolic compounds from Hypericum perforatum L. in aqueous systems. Pharmazie 2003, 58, 200–203. [Google Scholar] [PubMed]
- Kornievskaya, V.S.; Kruppa, A.I.; Polyakov, N.E.; Leshina, T.V. Effect of glycyrrhizic acid on lappaconitine phototransformation. J. Phys. Chem. B 2007, 111, 11447–11452. [Google Scholar] [CrossRef] [PubMed]
- Kong, R.P.; Zhu, X.Y.; Meteleva, E.S.; Chistyachenko, Y.S.; Suntsova, L.P.; Polyakov, N.E.; Khvostov, M.V.; Baev, D.S.; Tolstikova, T.G.; Yu, J.M.; et al. Enhanced solubility and bioavailability of simvastatin by mechanochemically obtained complexes. Int. J. Pharm. 2017, 534, 108–118. [Google Scholar] [CrossRef]
- Petrova, S.S.; Schlotgauer, A.A.; Kruppa, A.I.; Leshina, T.V. Self-Association of Glycyrrhizic Acid. NMR Study. Z. Phys. Chem. 2017, 231, 839–855. [Google Scholar] [CrossRef]
- Matsuoka, K.; Miyajima, R.; Ishida, Y.; Karasawa, S.; Yoshimura, T. Aggregate formation of glycyrrhizic acid. Colloid Surf. A 2016, 500, 112–117. [Google Scholar] [CrossRef]
- Taylor, L.S.; Zhang, G.G.Z. Physical chemistry of supersaturated solutions and implications for oral absorption. Adv. Drug Deliv. Rev. 2016, 101, 122–142. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Dai, W.G. Drug precipitation inhibitors in supersaturable formulations. Int. J. Pharm. 2013, 453, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.; Witkamp, G.J.; Verpoorte, R. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Faggian, M.; Sut, S.; Perissutti, B.; Baldan, V.; Grabnar, I.; Dall’Acqua, S. Natural Deep Eutectic Solvents (NADES) as a Tool for Bioavailability Improvement: Pharmacokinetics of Rutin Dissolved in Proline/Glycine after Oral Administration in Rats: Possible Application in Nutraceuticals. Molecules 2016, 21, 1531. [Google Scholar] [CrossRef]
- Sut, S.; Faggian, M.; Baldan, V.; Poloniato, G.; Castagliuolo, I.; Grabnar, I.; Perissutti, B.; Brun, P.; Maggi, F.; Voinovich, D.; et al. Natural Deep Eutectic Solvents (NADES) to Enhance Berberine Absorption: An In Vivo Pharmacokinetic Study. Molecules 2017, 22, 1921. [Google Scholar] [CrossRef]
- Heikkinen, A.T.; Friedlein, A.; Matondo, M.; Hatley, O.J.D.; Petsalo, A.; Juvonen, R.; Galetin, A.; Rostami-Hodjegan, A.; Aebersold, R.; Lamerz, J.; et al. Quantitative ADME Proteomics-CYP and UGT Enzymes in the Beagle Dog Liver and Intestine. Pharm. Res. 2015, 32, 74–90. [Google Scholar] [CrossRef]
- Ferreira, J.F.; Luthria, D.L.; Sasaki, T.; Heyerick, A. Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules 2010, 15, 3135–3170. [Google Scholar] [CrossRef]
- Li, C.R.; Zhang, L.; Wo, S.K.; Zhou, L.M.; Lin, G.; Zuo, Z. Pharmacokinetic interactions among major bioactive components in Radix Scutellariae via metabolic competition. Biopharm. Drug Dispos. 2012, 33, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.L.; Ma, Y.M. Pharmacokinetic herb-drug interactions with traditional Chinese medicine: Progress, causes of conflicting results and suggestions for future research. Drug Metab. Rev. 2016, 48, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Feng, W.; Tang, F.; Ao, H.; Peng, C. Gut microbial transformation, a potential improving factor in the therapeutic activities of four groups of natural compounds isolated from herbal medicines. Fitoterapia 2019, 138, 104293. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-S.; Xu, J.; Zhu, H.; Wu, J.; Xu, J.-D.; Yan, R.; Li, X.-Y.; Liu, H.-H.; Duan, S.-M.; Wang, Z.; et al. Gut microbiota-involved mechanisms in enhancing systemic exposure of ginsenosides by coexisting polysaccharides in ginseng decoction. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chang, X.; Luo, X.; Su, M.; Xu, R.; Chen, J.; Ding, Y.; Shi, Y. An Integrated Approach to Characterize Intestinal Metabolites of Four Phenylethanoid Glycosides and Intestinal Microbe-Mediated Antioxidant Activity Evaluation In Vitro Using UHPLC-Q-Exactive High-Resolution Mass Spectrometry and a 1,1-Diphenyl-2-picrylhydrazyl-Based Assay. Front. Pharmacol. 2019, 10, 826. [Google Scholar]
- Shen, H.; Gao, X.-J.; Li, T.; Jing, W.-H.; Han, B.-L.; Jia, Y.-M.; Hu, N.; Yan, Z.-X.; Li, S.-L.; Yan, R. Ginseng polysaccharides enhanced ginsenoside Rb1 and microbial metabolites exposure through enhancing intestinal absorption and affecting gut microbial metabolism. J. Ethnopharmacol. 2018, 216, 47–56. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Guo, S.-C.; Peng, Z.-T.; Wang, C.; Duan, R.; Dong, T.T.X.; Tsim, K.W.K. Ophiopogon Polysaccharide Promotes the In Vitro Metabolism of Ophiopogonins by Human Gut Microbiota. Molecules 2019, 24, 2886. [Google Scholar] [CrossRef]
- Giacomini, K.M.; Huang, S.M.; Tweedie, D.J.; Benet, L.Z.; Brouwer, K.L.; Chu, X.; Dahlin, A.; Evers, R.; Fischer, V.; Hillgren, K.M.; et al. Membrane transporters in drug development. Nat. Rev. Drug Discov. 2010, 9, 215–236. [Google Scholar]
- Yasuda, K.; Lan, L.B.; Sanglard, D.; Furuya, K.; Schuetz, J.D.; Schuetz, E.G. Interaction of cytochrome P450 3A inhibitors with P-glycoprotein. J. Pharmacol. Exp. Ther. 2002, 303, 323–332. [Google Scholar] [CrossRef]
- Cummins, C.L.; Jacobsen, W.; Benet, L.Z. Unmasking the dynamic interplay between intestinal P-glycoprotein and CYP3A4. J. Pharmacol. Exp. Ther. 2002, 300, 1036–1045. [Google Scholar] [CrossRef]
- Ducharme, M.P.; Warbasse, L.H.; Edwards, D.J. Disposition of intravenous and oral cyclosporine after administration with grapefruit juice. Clin. Pharmacol. Ther. 1995, 57, 485–491. [Google Scholar] [CrossRef]
- Jin, M.J.; Han, H.K. Effect of piperine, a major component of black pepper, on the intestinal absorption of fexofenadine and its implication on food-drug interaction. J. Food Sci. 2010, 75, H93–H96. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, Z.; Wang, C.; Meng, Q.; Huo, X.; Liu, Q.; Sun, H.; Sun, P.; Yang, X.; Ma, X.; et al. P-gp, MRP2 and OAT1/OAT3 mediate the drug-drug interaction between resveratrol and methotrexate. Toxicol. Appl. Pharmacol. 2016, 306, 27–35. [Google Scholar] [CrossRef]
- Bedada, S.K.; Appani, R.; Boga, P.K. Capsaicin pretreatment enhanced the bioavailability of fexofenadine in rats by P-glycoprotein modulation: In vitro, in situ and in vivo evaluation. Drug Dev. Ind. Pharm. 2017, 43, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Cai, D.; Bi, H.; Zhong, G.; Zeng, H.; Gu, L.; Huang, Z.; Huang, M. Comparative pharmacokinetics of paclitaxel after oral administration of Taxus yunnanensis extract and pure paclitaxel to rats. Fitoterapia 2013, 90, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Xie, Y.; Luo, H.; Li, G.; Wu, T.; Zhang, T. Transport characteristics of isorhamnetin across intestinal Caco-2 cell monolayers and the effects of transporters on it. Food Chem. Toxicol. 2014, 66, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y.; Zhou, J.; Gao, X.; Qu, D.; Liu, C. Study on the mechanism of intestinal absorption of epimedins a, B and C in the Caco-2 cell model. Molecules 2014, 19, 686–698. [Google Scholar] [CrossRef]
- Feng, X.; Ding, L.; Qiu, F. Potential drug interactions associated with glycyrrhizin and glycyrrhetinic acid. Drug Metab. Rev. 2015, 47, 229–238. [Google Scholar] [CrossRef]
- To, K.K.W.; Wu, X.; Yin, C.; Chai, S.; Yao, S.; Kadioglu, O.; Efferth, T.; Ye, Y.; Lin, G. Reversal of multidrug resistance by Marsdenia tenacissima and its main active ingredients polyoxypregnanes. J. Ethnopharmacol. 2017, 203, 110–119. [Google Scholar] [CrossRef]
- Yokooji, T.; Kida, M.; Mori, M.; Akashi, H.; Mori, N.; Yoshihara, S.; Murakami, T. Interaction of Rhei Rhizoma extract with cytochrome P450 3A and efflux transporters in rats. Pharmazie 2010, 65, 367–374. [Google Scholar]
- Bi, X.; Yuan, Z.; Qu, B.; Zhou, H.; Liu, Z.; Xie, Y. Piperine enhances the bioavailability of silybin via inhibition of efflux transporters BCRP and MRP2. Phytomedicine 2019, 54, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Augustin, J.M.; Kuzina, V.; Andersen, S.B.; Bak, S. Molecular activities, biosynthesis and evolution of triterpenoid saponins. Phytochemistry 2011, 72, 435–457. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Igarashi, Y.; Nagano, T.; Nishida, K.; Nakamura, J. Different Effects of Absorption Promoters on Corneal and Conjunctival Penetration of Ophthalmic Beta-Blockers. Pharm. Res. 1995, 12, 1146–1150. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Yamamura, K.; Tei, C.Y.; Nishida, K.; Nakamura, J. Ocular Permeability of Fitc-Dextran with Absorption Promoter for Ocular Delivery of Peptide Drug. J. Drug Target. 1995, 3, 129–135. [Google Scholar] [CrossRef]
- Gee, J.M.; Wortley, G.M.; Johnson, I.T.; Price, K.R.; Rutten, A.A.J.J.L.; Houben, G.F.; Penninks, A.H. Effects of saponins and glycoalkaloids on the permeability and viability of mammalian intestinal cells and on the integrity of tissue preparations in vitro. Toxicol. In Vitro 1996, 10, 117–128. [Google Scholar] [CrossRef]
- Selyutina, O.Y.; Polyakov, N.E.; Korneev, D.V.; Zaitsev, B.N. Influence of glycyrrhizin on permeability and elasticity of cell membrane: Perspectives for drugs delivery. Drug Deliv. 2016, 23, 858–865. [Google Scholar] [CrossRef]
- Selyutina, O.Y.; Apanasenko, I.E.; Shilov, A.G.; Khalikov, S.S.; Polyakov, N.E. Effect of natural polysaccharides and oligosaccharides on the permeability of cell membranes. Russ. Chem. Bull. 2017, 66, 129–135. [Google Scholar] [CrossRef]
- Selyutina, O.Y.; Apanasenko, I.E.; Kim, A.V.; Shelepova, E.A.; Khalikov, S.S.; Polyakov, N.E. Spectroscopic and molecular dynamics characterization of glycyrrhizin membrane-modifying activity. Colloid Surf. B 2016, 147, 459–466. [Google Scholar] [CrossRef]
- Verstraeten, S.L.; Deleu, M.; Janikowska-Sagan, M.; Claereboudt, E.J.S.; Lins, L.; Tyteca, D.; Mingeot-Leclercq, M.P. The activity of the saponin ginsenoside Rh2 is enhanced by the interaction with membrane sphingomyelin but depressed by cholesterol. Sci. Rep. 2019, 9, 7285. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, J.; Zou, X.H.; Zhao, F.; Guo, M.Q.; Wang, H.B.; Zhang, T.; Zhang, C.L.; Feng, W.; Pessah, IN.; et al. Saikosaponin d causes apoptotic death of cultured neocortical neurons by increasing membrane permeability and elevating intracellular Ca2+ concentration. Neurotoxicology 2019, 70, 112–121. [Google Scholar] [CrossRef]
- Sudji, I.R.; Subburaj, Y.; Frenkel, N.; Garcia-Saez, A.J.; Wink, M. Membrane Disintegration Caused by the Steroid Saponin Digitonin Is Related to the Presence of Cholesterol. Molecules 2015, 20, 20146–20160. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.K.; Wu, W.B.; Zhang, M.; Ma, X.Y.; Cui, Q.X.; Tang, Z.Y.; Huang, H.; Tong, T.T.; Yau, L.; Jiang, Z.H.; et al. Micro-PET Imaging Demonstrates 3-O-beta-D-Glucopyranosyl Platycodigenin as an Effective Metabolite Affects Permeability of Cell Membrane and Improves Dosimetry of [F-18]-Phillygenin in Lung Tissue. Front. Pharmacol. 2019, 10, 1020. [Google Scholar] [CrossRef]
- Deli, M.A. Potential use of tight junction modulators to reversibly open membranous barriers and improve drug delivery. Biochim. Biophys. Acta 2009, 1788, 892–910. [Google Scholar] [CrossRef] [PubMed]
- Kosinska, A.; Andlauer, W. Modulation of tight junction integrity by food components. Food Res. Int. 2013, 54, 951–960. [Google Scholar] [CrossRef]
- Chen, W.; Lu, Z.; Viljoen, A.; Hamman, J. Intestinal drug transport enhancement by Aloe vera. Planta Med. 2009, 75, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Haasbroek, A.; Willers, C.; Glyn, M.; du Plessis, L.; Hamman, J. Intestinal Drug Absorption Enhancement by Aloe vera Gel and Whole Leaf Extract: In Vitro Investigations into the Mechanisms of Action. Pharmaceutics 2019, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Narai, A.; Arai, S.; Shimizu, M. Rapid decrease in transepithelial electrical resistance of human intestinal caco-2 cell monolayers by cytotoxic membrane perturbents. Toxicol. In Vitro 1997, 11, 347–354. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, W.; Viljoen, A.; Hamman, J.H. Effect of sinomenine on the in vitro intestinal epithelial transport of selected compounds. Phytother. Res. 2010, 24, 211–218. [Google Scholar]
- Li, Y.; Duan, Z.; Tian, Y.; Liu, Z.; Wang, Q. A novel perspective and approach to intestinal octreotide absorption: Sinomenine-mediated reversible tight junction opening and its molecular mechanism. Int. J. Mol. Sci. 2013, 14, 12873–12892. [Google Scholar] [CrossRef]
- Watari, A.; Hashegawa, M.; Yagi, K.; Kondoh, M. Homoharringtonine increases intestinal epithelial permeability by modulating specific claudin isoforms in Caco-2 cell monolayers. Eur. J. Pharm. Biopharm. 2015, 89, 232–238. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, N. Nanocarriers for the delivery of active ingredients and fractions extracted from natural products used in traditional Chinese medicine (TCM). Adv. Colloid Interface Sci. 2015, 221, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Groning, R.; Baroth, V.; Breitkreuz, J. Nanoparticles in plant extracts -investigations into the colloidal structure of aqueous infusions of black tea. Pharm. Pharmacol. Lett. 1995, 5, 77–79. [Google Scholar]
- Groning, R.; Breitkreutz, J.; Muller, R.S. Physico-chemical interactions between extracts of Hypericum perforatum L. and drugs. Eur. J. Pharm. Biopharm. 2003, 56, 231–236. [Google Scholar] [CrossRef]
- Zhuang, Y.; Yan, J.; Zhu, W.; Chen, L.; Liang, D.; Xu, X. Can the aggregation be a new approach for understanding the mechanism of Traditional Chinese Medicine? J. Ethnopharmacol. 2008, 117, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Wang, Y.; Huang, Y.; Xia, L.; Sun, L.; Lenaghan, S.C.; Zhang, M. Tea nanoparticles for immunostimulation and chemo-drug delivery in cancer treatment. J. Biomed. Nanotechnol. 2014, 10, 1016–1029. [Google Scholar] [CrossRef] [PubMed]
- Lenaghan, S.C.; Zhu, Q.; Xia, L.; Zhang, M. Extraction of organic nanoparticles from plants. Methods Mol. Biol. 2012, 906, 381–391. [Google Scholar] [PubMed]
- Hu, J.; Wu, Z.; Yan, J.; Pang, W.; Liang, D.; Xu, X. A promising approach for understanding the mechanism of Traditional Chinese Medicine by the aggregation morphology. J. Ethnopharmacol. 2009, 123, 267–274. [Google Scholar] [CrossRef]
- Groning, R.; Adesina, S.; Muller, R.S. Formation of particles in aqueous infusions of the medical plant Harungana madagascariensis. Pharmazie 2004, 59, 279–281. [Google Scholar] [PubMed]
- Zhou, J.; Gao, G.; Chu, Q.; Wang, H.; Rao, P.; Ke, L. Chromatographic isolation of nanoparticles from Ma-Xing-Shi-Gan-Tang decoction and their characterization. J. Ethnopharmacol. 2014, 151, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Seidler, J.; McGovern, S.L.; Doman, T.N.; Shoichet, B.K. Identification and prediction of promiscuous aggregating inhibitors among known drugs. J. Med. Chem. 2003, 46, 4477–4486. [Google Scholar] [CrossRef] [PubMed]
- Pohjala, L.; Tammela, P. Aggregating behavior of phenolic compounds—A source of false bioassay results? Molecules 2012, 17, 10774–10790. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Doak, A.K.; Nedyalkova, L.; Shoichet, B.K. Colloidal aggregation and the in vitro activity of traditional Chinese medicines. ACS Chem. Biol. 2015, 10, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, P.; Guo, W.; Huang, X.; Tian, X.; Wu, G.; Xu, B.; Li, F.; Yan, C.; Liang, X.J.; et al. Natural Berberine-Based Chinese Herb Medicine Assembled Nanostructures with Modified Antibacterial Application. ACS Nano 2019. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.Y.; Shoichet, B.K. Synergy and antagonism of promiscuous inhibition in multiple-compound mixtures. J. Med. Chem. 2006, 49, 2151–2154. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Grzybowski, B. Self-assembly at all scales. Science 2002, 295, 2418–2421. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Z.; Haque, S.S.; Zhang, M.; Xia, L. Localized Surface Plasmon Resonance Effects by Naturally Occurring Chinese Yam Particles. J. Chem. Phys. 2010, 108, 123502. [Google Scholar] [CrossRef]
- Lenaghan, S.C.; Burris, J.N.; Chourey, K.; Huang, Y.; Xia, L.; Lady, B.; Sharma, R.; Pan, C.; LeJeune, Z.; Foister, S.; et al. Isolation and chemical analysis of nanoparticles from English ivy (Hedera helix L.). J. R. Soc. Interface 2013, 10, 20130392. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, J.; Lin, D.; Gao, G.; Wang, H.; Guo, J.; Rao, P.; Ke, L. Boiling-induced nanoparticles and their constitutive proteins from Isatis indigotica Fort. root decoction: Purification and identification. J. Tradit Complement. Med. 2017, 7, 178–187. [Google Scholar] [CrossRef]
- Hasson, T.H.; Takaoka, A.; de la Rica, R.; Matsui, H.; Smeureanu, G.; Drain, C.M.; Kawamura, A. Immunostimulatory lipid nanoparticles from herbal medicine. Chem. Biol. Drug Des. 2014, 83, 493–497. [Google Scholar] [CrossRef]
- Zhuang, X.; Deng, Z.B.; Mu, J.; Zhang, L.; Yan, J.; Miller, D.; Feng, W.; McClain, C.J.; Zhang, H.G. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J. Extracell. Vesicles 2015, 4, 28713. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.J.; Wang, Y.; Yi, S.; Fan, Z.; Sun, L.; Lin, D.; Anreddy, N.; Zhu, H.; Schmidt, M.; et al. Exploring naturally occurring ivy nanoparticles as an alternative biomaterial. Acta Biomater. 2015, 25, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Ke, L.J.; Gao, G.Z.; Shen, Y.; Zhou, J.W.; Rao, P.F. Encapsulation of Aconitine in Self-Assembled Licorice Protein Nanoparticles Reduces the Toxicity In Vivo. Nanoscale Res. Lett. 2015, 10, 449. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Joung, H.J.; Lee, W.; Chen, X.; Park, H.J. The influence of different water types and brewing durations on the colloidal properties of green tea infusion. Int. J. Food Sci. Technol. 2015, 50, 2483–2489. [Google Scholar] [CrossRef]
- Wang, G.; Yang, C.; Zhang, K.; Hu, J.; Pang, W. Molecular clusters size of Puerariae thomsonii radix aqueous decoction and relevance to oral absorption. Molecules 2015, 20, 12376–12388. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, J.; Zhu, H.; Jiang, X.; Liu, J.; Xu, W.; Ma, H.; Feng, Q.; Wu, J.; Zhao, M.; et al. Aqueous extract of Rabdosia rubescens leaves: Forming nanoparticles, targeting P-selectin, and inhibiting thrombosis. Int. J. Nanomed. 2015, 10, 6905–6918. [Google Scholar]
- Li, Q.; Xia, L.; Zhang, Z.; Zhang, M. Ultraviolet Extinction and Visible Transparency by Ivy Nanoparticles. Nanoscale Res. Lett. 2010, 5, 1487–1491. [Google Scholar] [CrossRef]
- Lenaghan, S.C.; Xia, L.; Zhang, M. Identification of nanofibers in the Chinese herbal medicine: Yunnan Baiyao. J. Biomed. Nanotechnol. 2009, 5, 472–476. [Google Scholar] [CrossRef]
- Doak, A.K.; Wille, H.; Prusiner, S.B.; Shoichet, B.K. Colloid formation by drugs in simulated intestinal fluid. J. Med. Chem. 2010, 53, 4259–4265. [Google Scholar] [CrossRef]
- Gao, L.; Liu, G.; Ma, J.; Wang, X.; Zhou, L.; Li, X.; Wang, F. Application of drug nanocrystal technologies on oral drug delivery of poorly soluble drugs. Pharm. Res. 2013, 30, 307–324. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Xiao, B.; Wang, H.; Han, M.K.; Zhang, Z.; Viennois, E.; Xu, C.L.; Merlin, D. Edible Ginger-derived Nano-lipids Loaded with Doxorubicin as a Novel Drug-delivery Approach for Colon Cancer Therapy. Mol. Ther. 2016, 24, 1783–1796. [Google Scholar] [CrossRef]
- Acosta, E. Bioavailability of nanoparticles in nutrient and nutraceutical delivery. Curr. Opin. Colloid Interface Sci. 2009, 14, 3–15. [Google Scholar] [CrossRef]
- Kohli, K.; Chopra, S.; Dhar, D.; Arora, S.; Khar, R.K. Self-emulsifying drug delivery systems: An approach to enhance oral bioavailability. Drug Discov. Today 2010, 15, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, Y.V.; Clark, A.D., Jr.; Das, K.; Wang, Y.H.; Lewi, P.J.; Janssen, P.A.; Arnold, E. Concentration and pH dependent aggregation of hydrophobic drug molecules and relevance to oral bioavailability. J. Med. Chem. 2005, 48, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Coan, K.E.; Shoichet, B.K. Stability and equilibria of promiscuous aggregates in high protein milieus. Mol. Biosyst. 2007, 3, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Cao, J.; Naeem, M.; Noh, J.; Hasan, N.; Choi, H.K.; Yoo, J.W. Size-controlled biodegradable nanoparticles: Preparation and size-dependent cellular uptake and tumor cell growth inhibition. Colloids Surf. B Biointerfaces 2014, 122, 545–551. [Google Scholar] [CrossRef]
- Su, B.; Kan, Y.; Xie, J.; Hu, J.; Pang, W. Relevance of the Pharmacokinetic and Pharmacodynamic Profiles of Puerariae lobatae Radix to Aggregation of Multi-Component Molecules in Aqueous Decoctions. Molecules 2016, 21, 845. [Google Scholar] [CrossRef]
- Liu, Y.T.; Hao, H.P.; Xie, H.G.; Lai, L.; Wang, Q.; Liu, C.X.; Wang, G.J. Extensive intestinal first-pass elimination and predominant hepatic distribution of berberine explain its low plasma levels in rats. Drug Metab. Dispos. 2010, 38, 1779–1784. [Google Scholar] [CrossRef]
- Pan, G.Y.; Wang, G.J.; Liu, X.D.; Fawcett, J.P.; Xie, Y.Y. The involvement of P-glycoprotein in berberine absorption. Pharmacol. Toxicol. 2002, 91, 193–197. [Google Scholar] [CrossRef]
- Tsai, P.L.; Tsai, T.H. Hepatobiliary excretion of berberine. Drug Metab. Dispos. 2004, 32, 405–412. [Google Scholar] [CrossRef]
- Yang, C.H.; Zhang, M.Z.; Merlin, D. Advances in plant-derived edible nanoparticle-based lipid nano-drug delivery systems as therapeutic nanomedicines. J. Mater. Chem. B 2018, 6, 1312–1321. [Google Scholar] [CrossRef]
- Wang, Y.J.; Huang, Y.J.; Anreddy, N.; Zhang, G.N.; Zhang, Y.K.; Xie, M.N.; Lin, D.; Yang, D.H.; Zhang, M.J.; Chen, Z.S. Tea nanoparticle, a safe and biocompatible nanocarrier, greatly potentiates the anticancer activity of doxorubicin. Oncotarget 2016, 7, 5877–5891. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [PubMed]
- Selmar, D.; Kleinwachter, M. Stress Enhances the Synthesis of Secondary Plant Products: The Impact of Stress-Related Over-Reduction on the Accumulation of Natural Products. Plant Cell Physiol. 2013, 54, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Mithofer, A.; Boland, W. Plant Defense against Herbivores: Chemical Aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef] [PubMed]
- Piasecka, A.; Jedrzejczak-Rey, N.; Bednarek, P. Secondary metabolites in plant innate immunity: Conserved function of divergent chemicals. New Phytol. 2015, 206, 948–964. [Google Scholar] [CrossRef] [PubMed]
- Bartwal, A.; Mall, R.; Lohani, P.; Guru, S.K.; Arora, S. Role of Secondary Metabolites and Brassinosteroids in Plant Defense Against Environmental Stresses. J. Plant Growth Regul. 2013, 32, 216–232. [Google Scholar] [CrossRef]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of Antioxidant Potential of Plants and its Relevance to Therapeutic Applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Cushnie, B.; Lamb, A.J. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agents 2014, 44, 377–386. [Google Scholar] [CrossRef]
- Hussain, M.; Debnath, B.; Qasim, M.; Bamisile, B.S.; Islam, W.; Hameed, M.S.; Wang, L.D.; Qiu, D.L. Role of Saponins in Plant Defense Against Specialist Herbivores. Molecules 2019, 24, 2067. [Google Scholar] [CrossRef]
- Kazan, K.; Lyons, R. Intervention of Phytohormone Pathways by Pathogen Effectors. Plant Cell 2014, 26, 2285–2309. [Google Scholar] [CrossRef]
- Seki, H.; Tamura, K.; Muranaka, T. P450s and UGTs: Key Players in the Structural Diversity of Triterpenoid Saponins. Plant Cell Physiol. 2015, 56, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Takanashi, K.; Yamada, Y.; Sasaki, T.; Yamamoto, Y.; Sato, F.; Yazaki, K. A multidrug and toxic compound extrusion transporter mediates berberine accumulation into vacuoles in Coptis japonica. Phytochemistry 2017, 138, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Shitan, N.; Sawada, K.; Van Montagu, M.C.; Inze, D.; Rischer, H.; Goossens, A.; Oksman-Caldentey, K.M.; Moriyama, Y.; Yazaki, K. Vacuolar transport of nicotine is mediated by a multidrug and toxic compound extrusion (MATE) transporter in Nicotiana tabacum. Proc. Natl. Acad. Sci. USA 2009, 106, 2447–2452. [Google Scholar] [CrossRef] [PubMed]
- Miresmailli, S.; Isman, M.B. Botanical insecticides inspired by plant-herbivore chemical interactions. Trends Plant Sci. 2014, 19, 29–35. [Google Scholar] [CrossRef]
- Yazaki, K. ABC transporters involved in the transport of plant secondary metabolites. FEBS Lett. 2006, 580, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Pichersky, E.; Raguso, R.A. Why do plants produce so many terpenoid compounds? New Phytol. 2018, 220, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Z.; Nikaido, H. Efflux-Mediated Drug Resistance in Bacteria: An Update. Drugs 2009, 69, 1555–1623. [Google Scholar] [CrossRef]
- Stavri, M.; Piddock, L.J.V.; Gibbons, S. Bacterial efflux pump inhibitors from natural sources. J. Antimicrob. Chemoth. 2007, 59, 1247–1260. [Google Scholar] [CrossRef]
- Abdallah, H.M.; Al-Abd, A.M.; El-Dine, R.S.; El-Halawany, A.M. P-glycoprotein inhibitors of natural origin as potential tumor chemo-sensitizers: A review. J. Adv. Res. 2015, 6, 45–62. [Google Scholar] [CrossRef]
- Junio, H.A.; Sy-Cordero, A.A.; Ettefagh, K.A.; Burns, J.T.; Micko, K.T.; Graf, T.N.; Richter, S.J.; Cannon, R.E.; Oberlies, N.H.; Cech, N.B. Synergy-Directed Fractionation of Botanical Medicines: A Case Study with Goldenseal (Hydrastis canadensis). J. Nat. Prod. 2011, 74, 1621–1629. [Google Scholar] [CrossRef]
- Britton, E.R.; Kellogg, J.J.; Kvalheim, O.M.; Cech, N.B. Biochemometrics to Identify Synergists and Additives from Botanical Medicines: A Case Study with Hydrastis canadensis (Goldenseal). J. Nat. Prod. 2018, 81, 484–493. [Google Scholar] [CrossRef] [PubMed]

| Plants | TCM Names | Active Constituents | AUC0–t extract/AUC0–t pure constituent | References |
|---|---|---|---|---|
| Aconitum carmichaelii Debx. | Aconiti Lateralis Radix Praeparata | hypaconitine | 2.7 | [10] |
| Artemisia annua L. | Artemisiae Annuae Herba | artemisinin | >40 | [9] |
| Cnidium monnieri (L.) Cuss. | Cnidii Fructus | osthole | >13.5 | [11] |
| Coptis chinensis Franch. | Coptidis Rhizoma | berberine | 15.3 | [12] |
| Gentiana manshurica Kitag. | Gentianae Radix et Rhizoma | gentiopicroside | 2.2 | [13] |
| Glycyrrhiza uralensis Fisch. | Glycyrrhizae Radix et Rhizoma | liquiritigenin | 133 | [14] |
| isoliquiritigenin | 109 | |||
| Panax ginseng C. A. Mey. | Ginseng Radix et Rhizoma | ginsenoside Re | 3.9 | [15] |
| Salvia miltiorrhiza Bge. | Salviae Miltiorrhizae Radix et Rhizoma | cryptotanshinone | 4.1 | [16] |
| tanshinone IIA | 19.1 | |||
| Schisandra chinensis (Turcz.) Baill | Schisandrae Chinensis Fructus | schizandrin | 2.2 | [17] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Luan, X.; Zheng, M.; Tian, X.-H.; Zhao, J.; Zhang, W.-D.; Ma, B.-L. Synergistic Mechanisms of Constituents in Herbal Extracts during Intestinal Absorption: Focus on Natural Occurring Nanoparticles. Pharmaceutics 2020, 12, 128. https://doi.org/10.3390/pharmaceutics12020128
Zhao Q, Luan X, Zheng M, Tian X-H, Zhao J, Zhang W-D, Ma B-L. Synergistic Mechanisms of Constituents in Herbal Extracts during Intestinal Absorption: Focus on Natural Occurring Nanoparticles. Pharmaceutics. 2020; 12(2):128. https://doi.org/10.3390/pharmaceutics12020128
Chicago/Turabian StyleZhao, Qing, Xin Luan, Min Zheng, Xin-Hui Tian, Jing Zhao, Wei-Dong Zhang, and Bing-Liang Ma. 2020. "Synergistic Mechanisms of Constituents in Herbal Extracts during Intestinal Absorption: Focus on Natural Occurring Nanoparticles" Pharmaceutics 12, no. 2: 128. https://doi.org/10.3390/pharmaceutics12020128
APA StyleZhao, Q., Luan, X., Zheng, M., Tian, X.-H., Zhao, J., Zhang, W.-D., & Ma, B.-L. (2020). Synergistic Mechanisms of Constituents in Herbal Extracts during Intestinal Absorption: Focus on Natural Occurring Nanoparticles. Pharmaceutics, 12(2), 128. https://doi.org/10.3390/pharmaceutics12020128






