Epidermal Delivery of Retinyl Palmitate Loaded Transfersomes: Penetration and Biodistribution Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Retinyl Palmitate-Loaded Transfersomes
2.3. Transfersome Incorporation in a Cream Formulation
2.4. Transfersomes Physic-Chemical Characterization
2.5. Cream Physic-Chemical Characterization
2.6. Diffusion Assay of RP-Loaded Transfersomes
2.7. RP HPLC Analysis and Encapsulation Efficiency
2.8. Pig Skin Penetration Assays
2.8.1. Skin Penetration Assay: Full Thickness Pig Ear Skin
2.8.2. Skin Layers Recovery
2.9. Fluorescence Biodistribution Assay
2.10. TEWL after In Vivo Topical Administration
3. Results and Discussion
3.1. Transfersomes Physico-Chemical Characterization
3.2. Transfersome Cream Physic-Chemical Characterization
3.3. Synthetic Membrane RP Difussion Assay
3.4. Franz-Cells Full-Thickness Pig Skin Penetration Assays
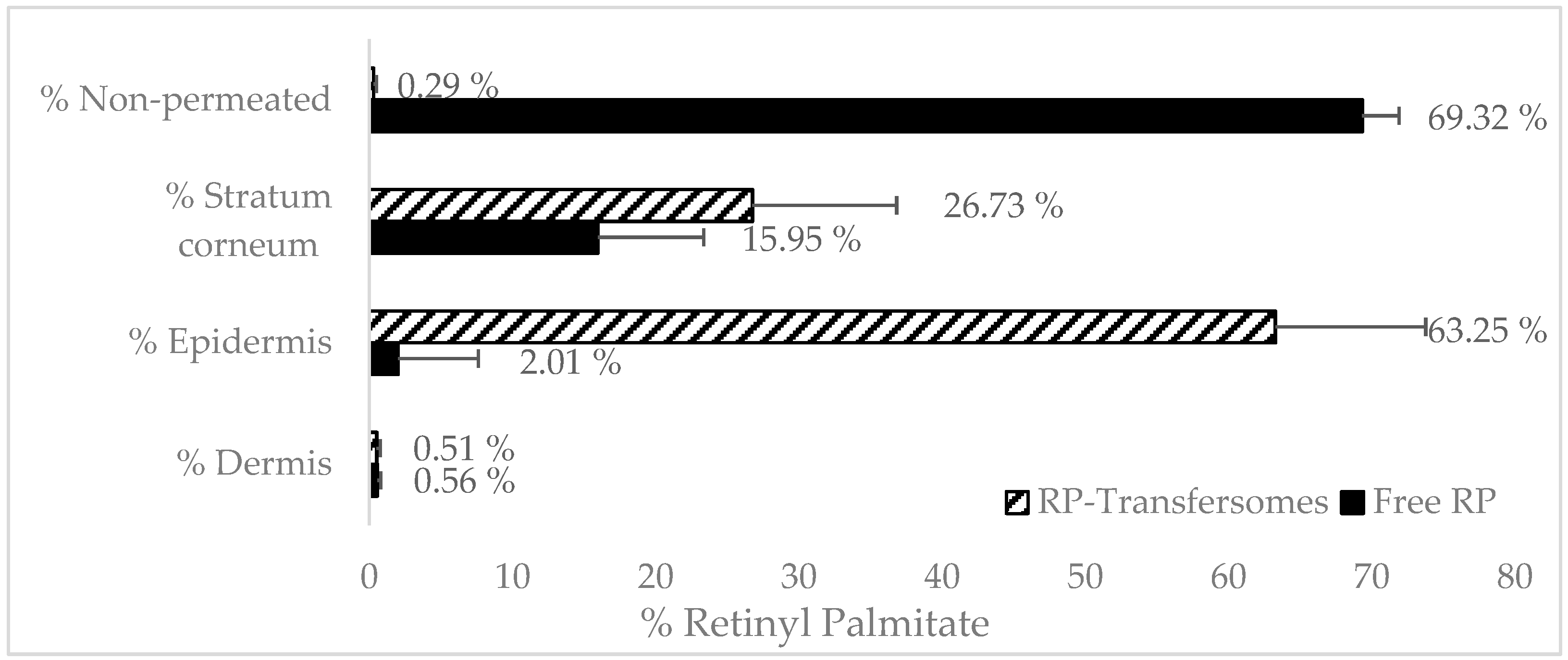
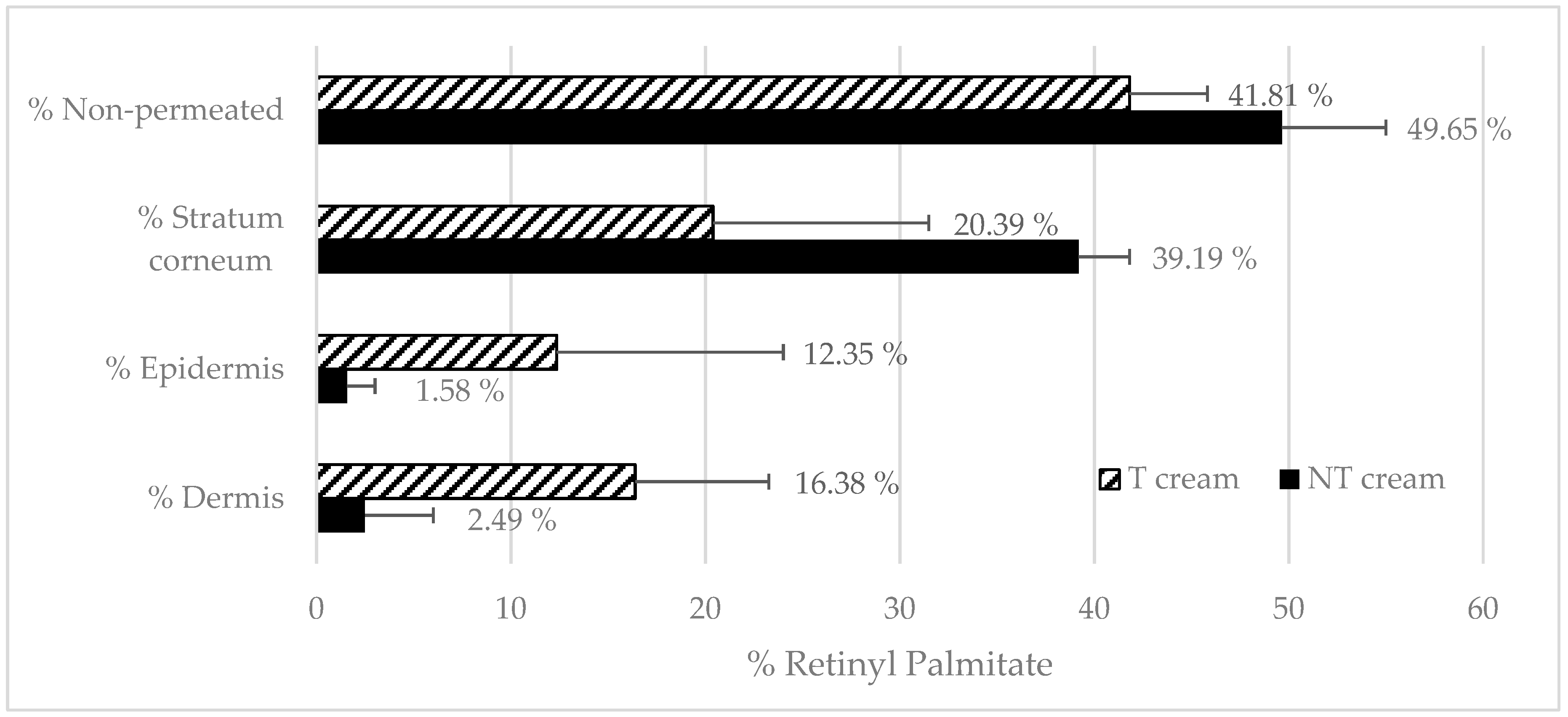
3.4.1. Transfersomes’ RP Penetration
3.4.2. Emulsions RP Penetration
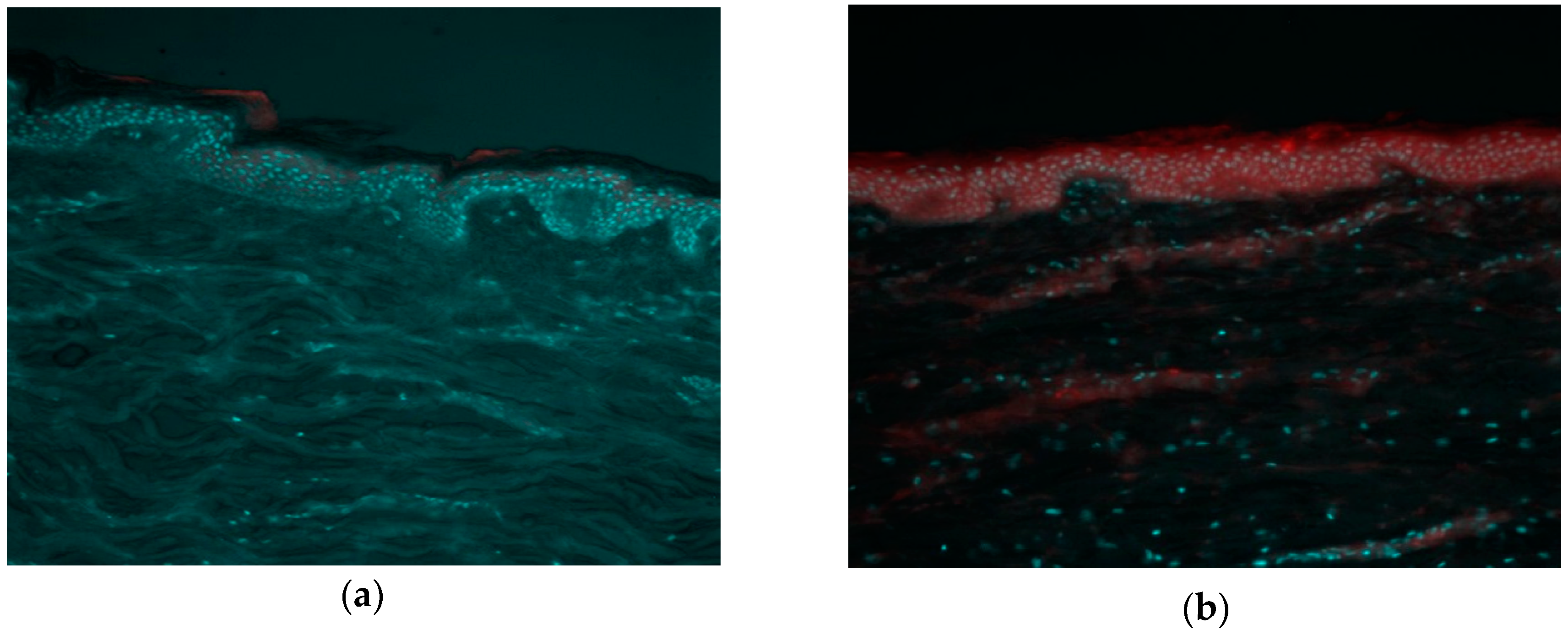
3.5. Fluorescence Biodistribution Assay
3.6. In Vivo Topical RP Penetration
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gerard, J.; James, G.; Mezick, A. Pharmacological effects of retinoids on skin cells. Ski. Pharm. 1993, 6, 24–34. [Google Scholar]
- Mukherjee, S.; Date, A.; Patravale, V.; Korting, H.C.; Roeder, A.; Weindl, G. Retinoids in the treatment of skin aging: An overview of clinical efficacy and safety. Clin. Interv. Aging. 2006, 1, 327–348. [Google Scholar] [CrossRef] [PubMed]
- Jean, J.; Soucy, J.; Roxane, P. Effects of Retinoic Acid on Keratinocyte Proliferation and Differentiation in a Psoriatic Skin Model. Tissue Eng. Part A 2011, 17, 13–14. [Google Scholar] [CrossRef] [PubMed]
- O’Byrne, S.M.; Blaner, W.S. Retinol and retinyl esters: Biochemistry and physiology. J. Lipid Res. 2013, 54, 1731–1743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scientific Committee on Consumer Safety. Opinion on Vitamin A (Retinol, Retinyl Acetate, Retinyl Palmitate); Scientific Committee on Consumer Safety: Brussels, Belgium, 2016. [Google Scholar]
- MacGregor, J.L.; Maibach, H.I. The Specificity of Retinoid-Induced Irritation and Its Role in Clinical Efficacy. Exog. Dermatol. 2002, 1, 68–73. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, Y.S.; Kang, K.S. The mechanism of retinol-induced irritation and its application to anti-irritant development. Toxicol. Lett. 2003, 146, 65–73. [Google Scholar] [CrossRef]
- Liu, J.C.; Wang, J.C.T.; Yusuf, M.; Yamamoto, N.; Kazama, S.; Stahl, C.R.; Holland, J.P.; Mather, K.; Aleles, M.A.; Hamada, S.; et al. Topical Oil in Water Emulsions Containing Retinoids. U.S. Patent No. 5976555, 2 November 1999. [Google Scholar]
- Oh, Y.; Kim, M.Y.; Shin, J.; Kim, T.W.; Yun, M.; Yang, S.J.; Choi, S.S.; Jung, W.; Kim, J.A.; Choi, H. Skin permeation of retinol in Tween 20-based deformable liposomes: In-vitro evaluation in human skin and keratinocyte models. J. Pharm. Pharmacol. 2006, 58, 161–166. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [Green Version]
- Braun-Falco, O.; Kortung, H.C.; Maibach, H.I. (Eds.) Griesb Ach Conference: Liposomes Dermatics; Springer: Berlin/Heidelberg, Germany, 1992. [Google Scholar]
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes–novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J. Control. Release 2000, 65, 403–418. [Google Scholar] [CrossRef]
- Jain, S.; Jain, P.; Umamaheshwari, R.B.; Jain, N.K. Transfersomes—A Novel Vesicular Carrier for Enhanced Transdermal Delivery: Development, Characterization, and Performance Evaluation. Drug Dev. Ind. Pharm. 2003, 29, 1013–1026. [Google Scholar] [CrossRef]
- Paul, A.; Cevc, G.; Bachhawat, B.K. Transdermal immunization with an integral membrane component gap junction protein, by means of ultradeformable drug carriers, transfersomes. Vaccine 1998, 16, 188–195. [Google Scholar] [CrossRef]
- Cevc, G. Transfersomes, liposomes and other lipid suspension on the skin, permeation enhancement, vesicles penetration and transdermal drug delivery. Crit. Rev. Ther. Drug Carr. Syst. 1996, 13, 257–388. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.M.; Hasan, O.A.; El Sisi, A.M. Preparation and optimization of lidocaine transferosomal gel containing permeation enhancers: A promising approach for enhancement of skin permeation. Int. J. Nanomed. 2019, 14, 1551–1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coderch, L.; López, O.; de la Maza, A.; Parra, J.L. Ceramides and skin function. Am. J. Clin. Dermatol. 2003, 4, 107–129. [Google Scholar] [CrossRef]
- Motta, S.; Sesana, S.; Monti, M.; Giuliani, A.; Caputo, R. Interlamellar lipid differences between normal and psoriatic stratum corneum. Acta Derm. Venereologica. Suppl. 1994, 186, 131–132. [Google Scholar]
- Matsumoto, N.U.M. Difference in Ceramide Composition between “Dry” and “Normal” Skin in Patients with Atopic Dermatitis. Acta Derm. Venereol. 1999, 79, 246–247. [Google Scholar] [CrossRef] [Green Version]
- Barbosa-Barros, L.; de la Maza, A.; López-Iglesias, C.; López, O. Ceramide effects in the bicelle structure. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 576–584. [Google Scholar] [CrossRef]
- Shabbits, J.A.; Mayer, L.D. Intracellular delivery of ceramide lipids via liposomes enhances apoptosis in vitro. Biochim. Biophys. Acta (BBA) Biomembr. 2003, 1612, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Woodbury, D.J.; Richardson, E.S.; Grigg, A.W.; Welling, R.D.; Knudson, B.H. Reducing Liposome Size with Ultrasound: Bimodal Size Distributions. J. Liposome Res. 2006, 16, 57–80. [Google Scholar] [CrossRef]
- Jesorka, A.; Orwar, O. Liposomes: Technologies and Analytical Applications. Annu. Rev. Anal. Chem. 2008, 1, 801–832. [Google Scholar] [CrossRef]
- Cevc, G.; Gebauer, D.; Stieber, J.; Schätzlein, A.; Blume, G. Ultraflexible vesicles, Transfersomes, have an extremely low pore penetration resistance and transport therapeutic amounts of insulin across the intact mammalian skin. Biochim. Et Biophys. Acta (BBA) Biomembr. 1998, 1368, 201–215. [Google Scholar] [CrossRef] [Green Version]
- European Centre for the Validation of Alternative Methods (CVAM). Test Guideline for Skin Absorption: In Vitro Method; Official Journal of the European Union Method B.45 of Annex to 440⁄2008⁄EC; European Commission: Paris, France, 2008. [Google Scholar]
- Nangia, A.; Berner, B.; Maibach, H.I. Transepidermal Water Loss Measurements for Assessing Skin Barrier Functions During in vitro Percutaneous Absortion Studies; Bronaugh, R.L., Maibach, H.I., Eds.; Percutaneous Absortion Drugs, Cosmetics, Mechanisms Methodology, Drugs and the Pharmaceutical Sciences, Marcel Dekker Inc.: New York, NY, USA, 1999; pp. 587–594. [Google Scholar]
- Dabboue, H.; Builles, N.; Frouin, E.; Scott, D.; Ramos, J.; Marti-Mestres, G. Assessing the Impact of Mechanical Damage on Full-Thickness Porcine and Human Skin Using an In Vitro Approach. BioMed Res. Int. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Wilhelm, K.P.; Elsner, P.; Berardesca, E.; Maibach, H.I. Bioengineering of the Skin: Skin Imaging and Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Elmahjoubi, E.; Frum, Y.; Eccleston, G.M.; Wilkinson, S.C.; Meidan, V.M. Transepidermal water loss for probing full-thickness skin barrier function: Correlation with tritiated water flux, sensitivity to punctures and diverse surfactant exposures. Toxicol. Vitr. 2009, 23, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Klang, V.; Schwarz, J.C.; Lenobel, B.; Nadj, M.; Auböck, J.; Wolzt, M.; Valenta, C. In vitro vs. in vivo tape stripping: Validation of the porcine ear model and penetration assessment of novel sucrose stearate emulsions. Eur. J. Pharm. Biopharm. 2012, 80, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Baber, N. International conference on harmorisation of technical requirements for registration of pharmaceutical for human use. Br. J. Clin. Pharmacol. 1994, 37, 401–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, Z.; Zanchetta, B.; Melo, B.A.; Oliveira, L.L.; Santana, M.H.; Paredes-Gamero, E.J.; Justo, G.Z.; Nader, H.B.; Guterres, S.S.; Durán, N. Retinyl palmitate flexible polymeric nanocapsules: Characterization and permeation studies. Colloids Surf. B Biointerfaces 2010, 81, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Benson, H.A.E.; Warkinson, A.C. Transdermal and Topical Drug Delivery, Principles and Practice; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; Chapter 1; p. 17. [Google Scholar]
- Singh, I.; Morris, A.P. Performance of transdermal therapeutic systems: Effects of biological factors. Int. J. Pharm. Investig. 2011, 1, 4–9. [Google Scholar] [CrossRef] [Green Version]
- Clares, B.; Calpena, A.C.; Parra, A.; Abrego, G.; Alvarado, H.; Fangueiro, J.F.; Souto, E.B. Nanoemulsions (NEs), liposomes (LPs) and solid lipid nanoparticles (SLNs) for retinyl palmitate: Effect on skin permeation. Int. J. Pharm. 2014, 473, 591–598. [Google Scholar] [CrossRef]
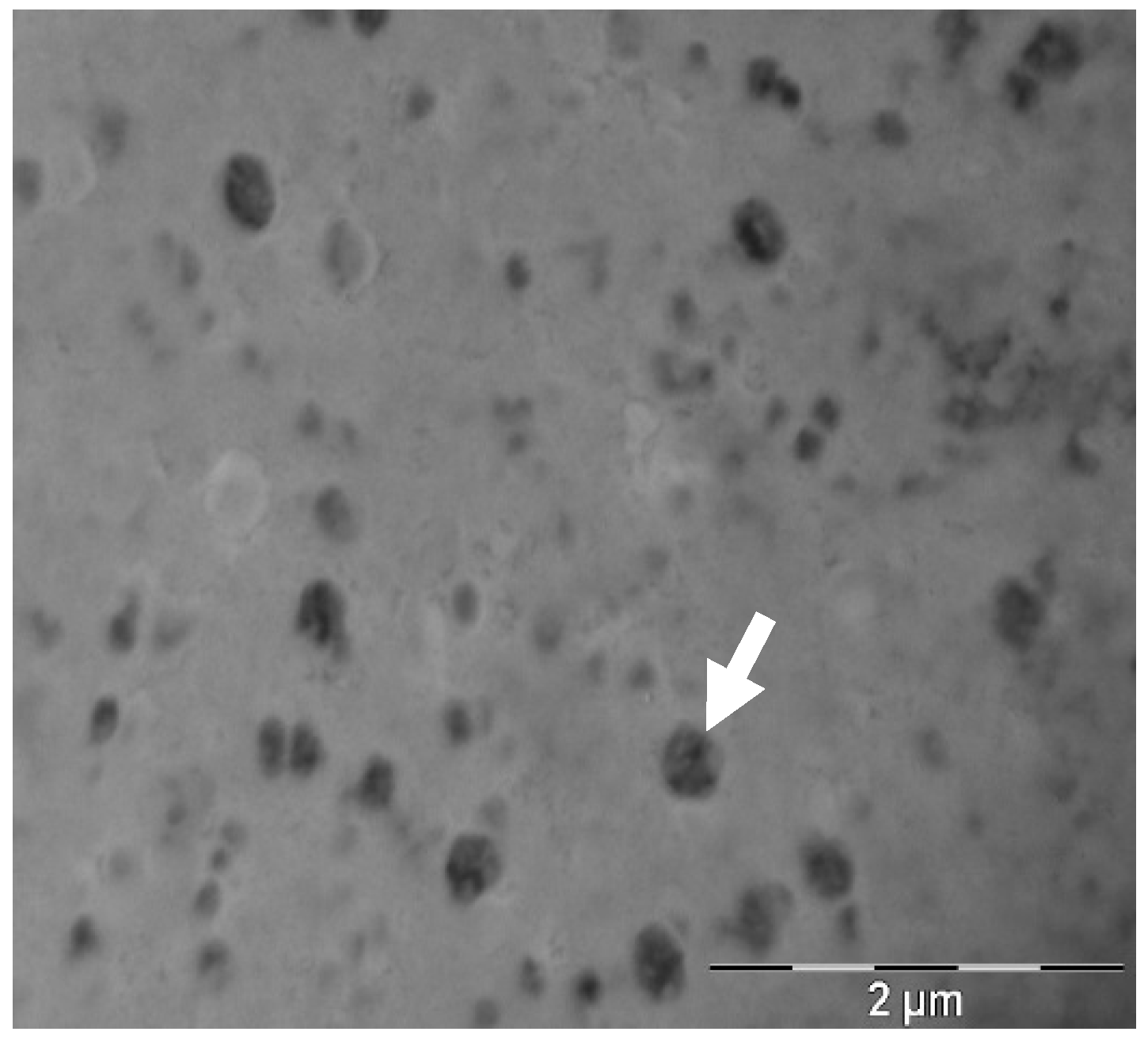


| Sample | Hydrodynamic Diameter (nm) | PDI | Z-Potential (mv) | Assay (%) | EE (%) |
|---|---|---|---|---|---|
| Transfersomes | 300.5 ± 10.9 | 0.471 ± 0.020 | −9.48 ± 1.50 | 102.63 ± 0.51 | 100 ± 0 |
| Condition | 25 °C/60% HR | 40 °C/75% HR | ||
|---|---|---|---|---|
| Response | Hydrodynamic Diameter | PDI | Hydrodynamic Diameter (nm) | PDI |
| Slope | 0.761 | −0.002 | 8.000 | −0.012 |
| p-value | 0.231 | 0.277 | 0.546 | 0.275 |
| Sample | Hydrodynamic Diameter (nm) | PDI | Deformability Index |
|---|---|---|---|
| Transfersomes | 300.5 ± 10.9 | 0.471 ± 0.020 | 8.12 |
| Extruded Transfersomes | 285.5 ± 9.7 | 0.247 ± 0.014 |
| Sample | Appearance | pH | Viscosity (cP) | Assay (%) |
|---|---|---|---|---|
| NT Cream | White-yellowish cream | 4.86 | 64.70 ± 0.18 | 98.07 ± 0.68 |
| T Cream | White-yellowish cream | 4.81 | 100.15 ± 2.35 | 96.87 ± 0.72 |
| Time (h) | Mean RP Released (%) | Standard Deviation (%) |
|---|---|---|
| 4 | 0 | 0 |
| 6 | 0.36 | 0.57 |
| 24 | 6.81 | 6.19 |
| 30 | 7.64 | 6.61 |
| Time 0 h TEWL (g/m2 h) | Standard Deviation (g/m2 h) | Time 2 h after Application TWEL (g/m2 h) | Standard Deviation (g/m2 h) | Ratio TWEL 2 h/0 h | p-Value vs. 1 |
|---|---|---|---|---|---|
| 11.17 | 1.25 | 10.63 | 0.99 | 0.95 | 0.388 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pena-Rodríguez, E.; Moreno, M.C.; Blanco-Fernandez, B.; González, J.; Fernández-Campos, F. Epidermal Delivery of Retinyl Palmitate Loaded Transfersomes: Penetration and Biodistribution Studies. Pharmaceutics 2020, 12, 112. https://doi.org/10.3390/pharmaceutics12020112
Pena-Rodríguez E, Moreno MC, Blanco-Fernandez B, González J, Fernández-Campos F. Epidermal Delivery of Retinyl Palmitate Loaded Transfersomes: Penetration and Biodistribution Studies. Pharmaceutics. 2020; 12(2):112. https://doi.org/10.3390/pharmaceutics12020112
Chicago/Turabian StylePena-Rodríguez, Eloy, Mari Carmen Moreno, Bárbara Blanco-Fernandez, Jordi González, and Francisco Fernández-Campos. 2020. "Epidermal Delivery of Retinyl Palmitate Loaded Transfersomes: Penetration and Biodistribution Studies" Pharmaceutics 12, no. 2: 112. https://doi.org/10.3390/pharmaceutics12020112
APA StylePena-Rodríguez, E., Moreno, M. C., Blanco-Fernandez, B., González, J., & Fernández-Campos, F. (2020). Epidermal Delivery of Retinyl Palmitate Loaded Transfersomes: Penetration and Biodistribution Studies. Pharmaceutics, 12(2), 112. https://doi.org/10.3390/pharmaceutics12020112







