The Phospholipid Research Center: Current Research in Phospholipids and Their Use in Drug Delivery
Abstract
:1. Introduction
2. Phospholipids—General Aspects
2.1. Natural Phospholipids
2.2. Synthetic Phospholipids
2.3. Industrial Production of Phospholipids
2.4. Regulatory and Safety Aspects
2.5. Use of Phospholipids in Pharmaceutical Formulations
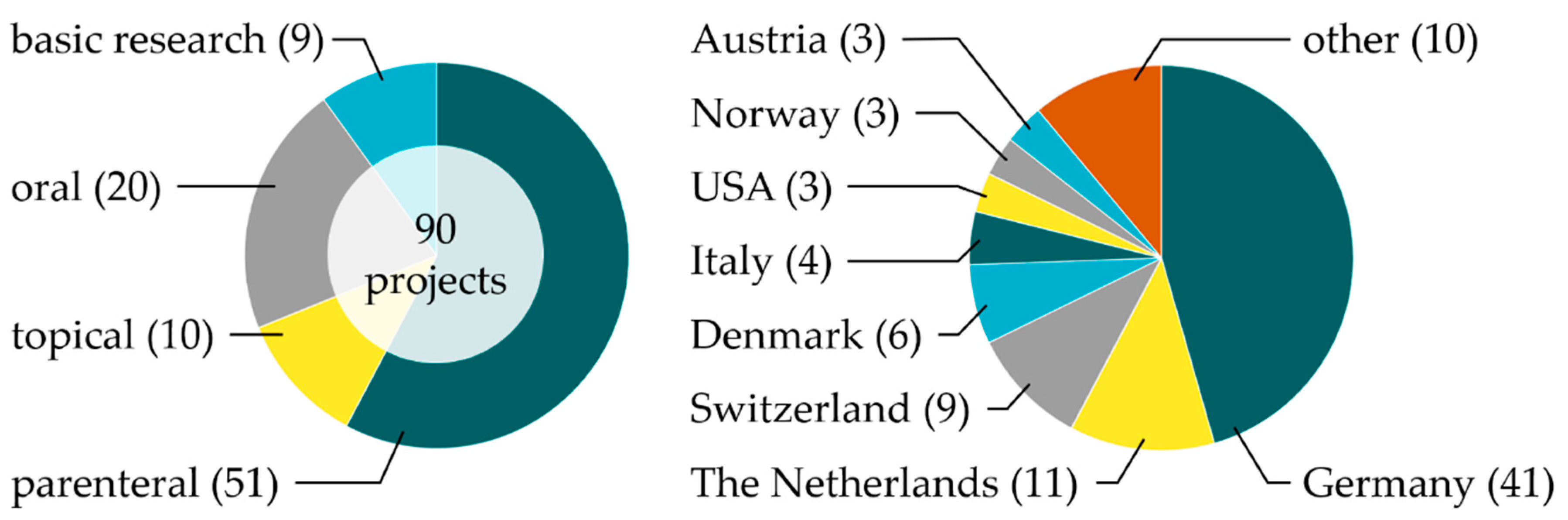
3. Phospholipids in Research—Project Overview
3.1. Parenteral Administration
3.1.1. Stimuli-Responsive Liposomes
3.1.2. Targeted Liposomes
3.1.3. Exosomes
3.1.4. Other Liposomal Approaches for Parenteral Administration
3.1.5. Further Lipid-Based Formulations for Parenteral Administration
3.2. Oral Administration
3.3. Topical Administration
3.4. Basic Research
3.5. Upcoming Projects
4. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACT | artemisinin combination therapy |
| ADI | acceptable daily intake |
| ALA | alpha-linolenic acid; |
| API | active pharmaceutical ingredients |
| ARA | arachidonic acid |
| ATP | adenosine triphosphate |
| BBB | blood–brain barrier |
| Cas9 | CRISPR-associated protein 9 |
| CD44 | cluster of differentiation 44; |
| CMC | critical micellar concentration |
| CPP | cell penetrating peptides |
| CRISPR | clustered regularly interspaced short palindromic repeats |
| DCC | dicyclohexylcarbodiimide |
| DHA | docosahexaenoic acid |
| DMAP | 4-(dimethylamino)pyridine |
| DOTAP | 1,2-dioleoyl-3-trimethylammonium-propane chloride |
| DPA | docosapentaenoic acid |
| DPPC | 1,2-dipalmitoyl-sn-glycero-3-phosphocholine |
| DSC | differential scanning calorimetry |
| DSPE-PEG2000 | 1,2-distearoyl-sn-glycero-3-phosphoethanol-amine-N-amino(polyethylene glycol)2000 |
| ELISA | enzyme-linked immunosorbent assay |
| EPR | enhanced permeability and retention |
| ESR | electron spin resonance |
| EV | extracellular vesicles |
| FDA | US Food and Drug Administration |
| FTIR | Fourier-transform infrared |
| GI | gastrointestinal |
| GIXD | grazing incidence X-ray diffraction |
| GPC | glycerophosphocholine |
| GRAS | generally recognized as safe |
| HA | hyaluronic acid |
| HLD | hydrophilic–lipophilic deviation |
| HSC | hepatic stellate cells |
| HSPC | hydrogenated soybean phosphatidylcholine |
| i.m. | intramuscular |
| i.v. | intravenous |
| ITC | isothermal titration calorimetry |
| LLOD | lower limit of detection |
| LPE | lyso-phosphatidylethanolamine |
| miRNA | micro ribonucleic acid |
| ML-I | mistletoe-lectin-I |
| MM | mixed micelles |
| MM-DDS | mixed micellar drug delivery systems |
| MNBA | 2-methyl-6-nitrobenzoic anhydride |
| MPS | mononuclear phagocytic system |
| mRNA | messenger ribonucleic acid |
| MTP-PE | muramyl tripeptide |
| NAFLD | non-alcoholic fatty liver disease |
| NASH | non-alcoholic steatohepatitis |
| NIR | near-infrared |
| NSAID | non-steroidal anti-inflammatory drugs |
| o/w | oil-in-water |
| OXIL | oxidation-responsive liposomes |
| OxPL | oxidized phospholipids |
| PA | phosphatic acid |
| PC | phosphatidylcholine |
| PE | phosphatidylethanolamine |
| PEG | polyethylene glycol |
| PG | phosphatidylglycerol |
| PI | phosphatidylinositol |
| PLA1 | phospholipase A1 (alternatively A2, B, C, D) |
| PLGA | poly(lactide-co-glycolide) |
| POPC | 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine |
| PRC | Phospholipid Research Center Heidelberg |
| PRR | pattern-recognition-receptor |
| PS | phosphatidylserine |
| PUFA | polyunsaturated fatty acids |
| RNA | ribonucleic acid |
| s.c. | subcutaneous |
| SANP | self-assembling nanoparticle |
| SAXS | small angle X-ray scattering |
| SEDDS | self-emulsifying drug delivery systems |
| siRNA | small interfering ribonucleic acid |
| SLN | solid-lipid nanoparticles |
| SM | sphingomyelin |
| SNEDDS | self nano-emulsifying drug delivery systems |
| SPM | specialized pro-resolving mediators |
| TAM | tumor-associated macrophages |
| TB | tuberculosis |
| TEL | tetraether lipid |
| TRXF | total reflection X-ray fluorescence |
| TSL | temperature-sensitive liposomes |
| USL | ultrasound-sensitive liposomes |
| USP | United States Pharmacopeia |
| w/o | water-in-oil |
| WHO | World Health Organization |
References
- IUPAC-IUB. Nomenclature of phosphorus-containing compounds of biochemical importance (Recommendations1976). Proc. Natl. Acad. Sci. USA 1977, 74, 2222–2230. [Google Scholar] [CrossRef] [Green Version]
- Cevc, G. Phospholipids Handbook; Taylor & Francis Inc.: London, UK, 1993; Volume 1, p. 1004. [Google Scholar]
- Vertzoni, M.; Markopoulos, C.; Symillides, M.; Goumas, C.; Imanidis, G.; Reppas, C. Luminal lipid phases after administration of a triglyceride solution of danazol in the fed state and their contribution to the flux of danazol across Caco-2 cell monolayers. Mol. Pharm. 2012, 9, 1189–1198. [Google Scholar] [CrossRef]
- Hanin, I.; Pepeu, G. Phospholipids. Biochemical, Pharmaceutical, and Analytical Considerations; Plenum Press: New York, NY, USA, 1990; Volume 1. [Google Scholar]
- Merolli, A.; Santin, M. Role of phosphatidyl-serine in bone repair and its technological exploitation. Molecules 2009, 14, 5367–5381. [Google Scholar] [CrossRef]
- Lentz, B.R. Exposure of platelet membrane phosphatidylserine regulates blood coagulation. Prog. Lipid Res. 2003, 42, 423–438. [Google Scholar] [CrossRef]
- Devitt, A.; Pierce, S.; Oldreive, C.; Shingler, W.H.; Gregory, C.D. CD14-dependent clearance of apoptotic cells by human macrophages: The role of phosphatidylserine. Cell Death Differ. 2003, 10, 371–382. [Google Scholar] [CrossRef] [PubMed]
- van Hoogevest, P.; Tiemessen, H.; Metselaar, J.M.; Drescher, S.; Fahr, A. The Use of Phospholipids to make Pharmaceutical Form Line Extensions. Eur. J. Lipid Sci. Technol. 2021. revision submitted. [Google Scholar]
- van Hoogevest, P.; Wendel, A. The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur. J. Lipid Sci. Technol. 2014, 116, 1088–1107. [Google Scholar] [CrossRef] [Green Version]
- van Hoogevest, P. Review—An update on the use of oral phospholipid excipients. Eur. J. Pharm. Sci. 2017, 108, 1–12. [Google Scholar] [CrossRef]
- van Hoogevest, P.; Luciani, P. Recent Advances in the Use of Phospholipid Excipients in Local or Injectable Depot Formulations. Pharm. Ind. 2018, 8, 1104. [Google Scholar]
- van Hoogevest, P.; Fahr, A. Phospholipids in Cosmetic Carriers (Phospholipids, Liposomes, Emulsions, Lamellar structures, Solubilizers, Biocompatibility). In Nanocosmetics—From Ideas to Products; Cornier, J., Keck, C., Van de Voorde, M., Eds.; Springer: Cham, Switzerland, 2019; Volume 6, pp. 97–140. [Google Scholar]
- van Hoogevest, P. Non-Aqueous Phospholipid Concentrates for Increasing the Bioavailability of Poorly Soluble Compounds. Eur. J. Lipid Sci. Technol. 2020, 122, 1900411. [Google Scholar] [CrossRef]
- PRC. Phospholipid Research Center (The PRC—Vision and Mission). Available online: https://www.phospholipid-institute.com/about/the-prc/ (accessed on 13 August 2020).
- PRC. Phospholipid Research Center (Funded Projects). Available online: https://www.phospholipid-institute.com/funding/funded-projects/ (accessed on 18 August 2020).
- Analytical Data; Lipoid GmbH: Ludwigshafen, Germany. Available online: https://www.lipoid.com/en/headquarter-and-manufacturing (accessed on 16 December 2020).
- Phospholipid Research Center, Analytics Newsletter, Number 1. Egg Lecithins for Pharmaceutical Application; Heidelberg, Germany. 2008. Available online: https://www.phospholipid-research-center.com/contact/contact-person/ (accessed on 16 December 2020).
- Senior, J.; Gregoriadis, G. Stability of small unilamellar liposomes in serum and clearance from the circulation: The effect of the phospholipid and cholesterol components. Life Sci. 1982, 30, 2123–2136. [Google Scholar] [CrossRef]
- Pryde, E.H. Chemical Reactions of Phosphatides. In Lecithins; Szuhaj, B.F., List, G.R., Eds.; American Oil Chemists’ Society: Champaign, IL, USA, 1985; Volume 1, pp. 213–246. [Google Scholar]
- Xu, X.; Vikbjerg, A.F.; Guo, Z.; Zhang, L.; Acharya, A.K. Chapter 3—Enzymatic modification of phospholipids. In Phospholipid Technology and Applications; Gunstone, F.D., Ed.; Woodhead Publishing: Bridgewater, CT, USA, 2012; pp. 41–82. [Google Scholar] [CrossRef]
- Paltauf, F.; Hermetter, A. Strategies for the synthesis of glycerophospholipids. Prog. Lipid Res. 1994, 33, 239–328. [Google Scholar] [CrossRef]
- Koo, O.M.; Rubinstein, I.; Onyuksel, H. Role of nanotechnology in targeted drug delivery and imaging: A concise review. Nanomedicine 2005, 1, 193–212. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, R.C.; Rakhmanova, V.A.; Choi, K.L.; Rosenzweig, H.S.; Lahiri, M.K. O-ethylphosphatidylcholine: A metabolizable cationic phospholipid which is a serum-compatible DNA transfection agent. J. Pharm. Sci. 1999, 88, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Eibl, H. Phospholipide als funktionelle Bausteine biologischer Membranen. Angew. Chem. 1984, 96, 247–262. [Google Scholar] [CrossRef]
- Ohno, M.; Fujita, K.; Nakai, H.; Kobayashi, S.; Inoue, K.; Nojima, S. An enantioselective synthesis of platelet-activating factors, their enantiomers, and their analogues from D- and L-tartaric acids. Chem. Pharm. Bull. 1985, 33, 572–582. [Google Scholar] [CrossRef] [Green Version]
- Virtanen, J.A.; Brotherus, J.R.; Renkonen, O.; Kates, M. Synthesis of monoacid 2,3-diacyl-sn-glycerols via 1,6-ditrityl-d-mannitol. Chem. Phys. Lipids 1980, 27, 185–190. [Google Scholar] [CrossRef]
- Crans, D.C.; Whitesides, G.M. Glycerol Kinase: Synthesis of Dihydroxyacetone Phosphate, sn-Glycerol-3-Phosphate, and Chiral Analogues. J. Am. Chem. Soc. 1985, 107, 7019–7027. [Google Scholar] [CrossRef]
- Warner, T.G.; Benson, A.A. An improved method for the preparation of unsaturated phosphatidylcholines: Acylation of sn-glycero-3-phosphorylcholine in the presence of sodium methylsulfinylmethide. J. Lipid Res. 1977, 18, 548–552. [Google Scholar]
- Neises, B.; Steglich, W. Einfaches Verfahren zur Veresterung von Carbonsäuren. Angew. Chem. 1978, 90, 556–557. [Google Scholar] [CrossRef]
- Yasuda, T.; Kinoshita, M.; Murata, M.; Matsumori, N. Detailed Comparison of Deuterium Quadrupole Profiles between Sphingomyelin and Phosphatidylcholine Bilayers. Biophys. J. 2014, 106, 631–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wendel, A. Kirk-Othmer Encyclopedia of Chemical Technology; Kirk, R.E., Othmer, D.F., Eds.; John Wiley & Sons: New York, NY, USA; Chichester, UK; Weinheim, Germany; Brisbane, Australia; Singapore; Toronto, ON, Canada, 1995; pp. 191–210. [Google Scholar]
- WHO. WHO Food Additives Series No. 5. Available online: http://www.inchem.org/documents/jecfa/jecmono/v05je42.htm (accessed on 13 August 2020).
- EMA. COMMISSION REGULATION (EU) No 231/2012 of 9 March 2012 Laying down Specifications for Food Additives Listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council European Commission. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:32012R0231 (accessed on 13 August 2020).
- Publications Office of the EU (Report from the Commission on Dietary Food Additive Intake in the European Union). Available online: https://op.europa.eu/en/publication-detail/-/publication/26105dba-6d8f-4515-a641-0e43fe3f5498/language-en (accessed on 20 August 2020).
- FDA. 21CFR184.1400. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1400 (accessed on 20 August 2020).
- FDA. 21CFR184.1063. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1063 (accessed on 20 August 2020).
- Singh, T.P.; Singh, P.; Kumar, P. Egg allergy: An emerging foodborne problem. J. Foodborne Zoonotic Dis. 2014, 2, 19–26. [Google Scholar]
- Warner, J.; Meyer, R. SoyaAllergy: The Facts (PDF File). Available online: https://www.allergywise.org.uk/wp-content/uploads/2017/12/Soya.pdf (accessed on 10 August 2020).
- Analytical Data; Phospholipid GmbH: Cologne, Germany. Available online: https://www.lipoid.com/en/headquarter-and-manufacturing (accessed on 16 December 2020).
- Study of the Allergenicity and Protein Content in Egg Lecithin; IFP Institut für Produktqualität: Berlin, Germany. Available online: https://www.produktqualitaet.com/de/ (accessed on 16 December 2020).
- Sommerfield, D.L.; Lucas, M.; Schilling, A.; Drake-Brockman, T.F.E.; Sommerfield, A.; Arnold, A.; von Ungern-Sternberg, B.S. Propofol use in children with allergies to egg, peanut, soybean or other legumes. Anaesthesia 2019, 74, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Fricker, G.; Kromp, T.; Wendel, A.; Blume, A.; Zirkel, J.; Rebmann, H.; Setzer, C.; Quinkert, R.-O.; Martin, F.; Müller-Goymann, C.C. Phospholipids and Lipid-Based Formulations in Oral Drug Delivery. Pharm. Res. 2010, 27, 1469–1486. [Google Scholar] [CrossRef]
- van Hoogevest, P.; Liu, X.; Fahr, A.; Leigh, M.L.S. Role of phospholipids in the oral and parenteral delivery of poorly water soluble drugs. J. Drug Delivery Sci. Technol. 2011, 21, 5–16. [Google Scholar] [CrossRef]
- Lipoid. (Products). Available online: https://www.lipoid.com/en/node/10 (accessed on 10 August 2020).
- Driscoll, D.F. Lipid injectable emulsions: Pharmacopeial and safety issues. Pharm. Res. 2006, 23, 1959–1969. [Google Scholar] [CrossRef]
- Shah, P.; Bhalodia, D.; Shelat, P. Nanoemulsion: A pharmaceutical review. Syst. Rev. Pharm. 2013, 1, 24–32. [Google Scholar] [CrossRef]
- Hammad, M.A.; Müller, B.W. Increasing drug solubility by means of bile salt-phosphatidylcholine-based mixed micelles. Eur. J. Pharm. Biopharm. 1998, 46, 361–367. [Google Scholar] [CrossRef]
- Gregory, T.J.; Steinberg, K.P.; Spragg, R.; Gadek, J.E.; Hyers, T.M.; Longmore, W.J.; Moxley, M.A.; Cai, G.Z.; Hite, R.D.; Smith, R.M.; et al. Bovine surfactant therapy for patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1997, 155, 1309–1315. [Google Scholar] [CrossRef]
- Vandevanter, D.R.; Geller, D.E. Tobramycin administered by the TOBI® Podhaler® for persons with cystic fibrosis: A review. Med. Devices (Auckl.) 2011, 4, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Barenholz, Y. Doxil—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Crommelin, D.J.A.; van Hoogevest, P.; Storm, G. The role of liposomes in clinical nanomedicine development. What now? Now what? J. Control. Release 2020, 318, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Drummond, D.C.; Meyer, O.; Hong, K.; Kirpotin, D.B.; Papahadjopoulos, D. Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol. Rev. 1999, 51, 691–743. [Google Scholar] [PubMed]
- Fanciullino, R.; Ciccolini, J. Liposome-Encapsulated Anticancer Drugs: Still Waiting for the Magic Bullet? Curr. Med. Chem. 2009, 16, 4361–4373. [Google Scholar] [CrossRef]
- Kneidl, B.; Peller, M.; Winter, G.; Lindner, L.H.; Hossann, M. Thermosensitive liposomal drug delivery systems: State of the art review. Int. J. Nanomed. 2014, 9, 4387–4398. [Google Scholar] [CrossRef] [Green Version]
- de Matos, M.B.C.; Beztsinna, N.; Heyder, C.; Fens, M.; Mastrobattista, E.; Schiffelers, R.M.; Leneweit, G.; Kok, R.J. Thermosensitive liposomes for triggered release of cytotoxic proteins. Eur. J. Pharm. Biopharm. 2018, 132, 211–221. [Google Scholar] [CrossRef]
- Javadi, M.; Pitt, W.G.; Tracy, C.M.; Barrow, J.R.; Willardson, B.M.; Hartley, J.M.; Tsosie, N.H. Ultrasonic gene and drug delivery using eLiposomes. J. Control. Release 2013, 167, 92–100. [Google Scholar] [CrossRef]
- de Matos, M.B.C.; Deckers, R.; van Elburg, B.; Lajoinie, G.; de Miranda, B.S.; Versluis, M.; Schiffelers, R.; Kok, R.J. Ultrasound-Sensitive Liposomes for Triggered Macromolecular Drug Delivery: Formulation and in Vitro Characterization. Front. Pharmacol. 2019, 10, 1463. [Google Scholar] [CrossRef]
- Lajunen, T.; Kontturi, L.-S.; Viitala, L.; Manna, M.; Cramariuc, O.; Róg, T.; Bunker, A.; Laaksonen, T.; Viitala, T.; Murtomäki, L.; et al. Indocyanine Green-Loaded Liposomes for Light-Triggered Drug Release. Mol. Pharm. 2016, 13, 2095–2107. [Google Scholar] [CrossRef]
- Lajunen, T.; Nurmi, R.; Wilbie, D.; Ruoslahti, T.; Johansson, N.G.; Korhonen, O.; Rog, T.; Bunker, A.; Ruponen, M.; Urtti, A. The effect of light sensitizer localization on the stability of indocyanine green liposomes. J. Control. Release 2018, 284, 213–223. [Google Scholar] [CrossRef]
- Mattheolabakis, G.; Milane, L.; Singh, A.; Amiji, M.M. Hyaluronic acid targeting of CD44 for cancer therapy: From receptor biology to nanomedicine. J. Drug Target. 2015, 23, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Kari, O.K.; Tavakoli, S.; Parkkila, P.; Baan, S.; Savolainen, R.; Ruoslahti, T.; Johansson, N.G.; Ndika, J.; Alenius, H.; Viitala, T.; et al. Light-Activated Liposomes Coated with Hyaluronic Acid as a Potential Drug Delivery System. Pharmaceutics 2020, 12, 763. [Google Scholar] [CrossRef] [PubMed]
- Bobyk, L.; Edouard, M.; Deman, P.; Vautrin, M.; Pernet-Gallay, K.; Delaroche, J.; Adam, J.F.; Estève, F.; Ravanat, J.L.; Elleaume, H. Photoactivation of gold nanoparticles for glioma treatment. Nanomedicine 2013, 9, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Bulin, A.L.; Broekgaarden, M.; Chaput, F.; Baisamy, V.; Garrevoet, J.; Busser, B.; Brueckner, D.; Youssef, A.; Ravanat, J.L.; Dujardin, C.; et al. Radiation Dose-Enhancement Is a Potent Radiotherapeutic Effect of Rare-Earth Composite Nanoscintillators in Preclinical Models of Glioblastoma. Adv. Sci. 2020, 7, 2001675. [Google Scholar] [CrossRef]
- Broekgaarden, M.; de Kroon, A.I.; Gulik, T.M.; Heger, M. Development and in vitro proof-of-concept of interstitially targeted zinc-phthalocyanine liposomes for photodynamic therapy. Curr. Med. Chem. 2014, 21, 377–391. [Google Scholar] [CrossRef] [Green Version]
- Le Guével, X.; Henry, M.; Motto-Ros, V.; Longo, E.; Montañez, M.I.; Pelascini, F.; de La Rochefoucauld, O.; Zeitoun, P.; Coll, J.L.; Josserand, V.; et al. Elemental and optical imaging evaluation of zwitterionic gold nanoclusters in glioblastoma mouse models. Nanoscale 2018, 10, 18657–18664. [Google Scholar] [CrossRef]
- Broekgaarden, M.; Bulin, A.L.; Porret, E.; Musnier, B.; Chovelon, B.; Ravelet, C.; Sancey, L.; Elleaume, H.; Hainaut, P.; Coll, J.L.; et al. Surface functionalization of gold nanoclusters with arginine: A trade-off between microtumor uptake and radiotherapy enhancement. Nanoscale 2020, 12, 6959–6963. [Google Scholar] [CrossRef]
- Obaid, G.; Bano, S.; Mallidi, S.; Broekgaarden, M.; Kuriakose, J.; Silber, Z.; Bulin, A.L.; Wang, Y.; Mai, Z.; Jin, W.; et al. Impacting Pancreatic Cancer Therapy in Heterotypic in Vitro Organoids and in Vivo Tumors with Specificity-Tuned, NIR-Activable Photoimmunonanoconjugates: Towards Conquering Desmoplasia? Nano Lett. 2019, 19, 7573–7587. [Google Scholar] [CrossRef]
- Holme, M.N.; Fedotenko, I.A.; Abegg, D.; Althaus, J.; Babel, L.; Favarger, F.; Reiter, R.; Tanasescu, R.; Zaffalon, P.L.; Ziegler, A.; et al. Shear-stress sensitive lenticular vesicles for targeted drug delivery. Nat. Nanotechnol. 2012, 7, 536–543. [Google Scholar] [CrossRef]
- Fedotenko, I.A.; Zaffalon, P.-L.; Favarger, F.; Zumbuehl, A. The synthesis of 1,3-diamidophospholipids. Tetrahedron Lett. 2010, 51, 5382–5384. [Google Scholar] [CrossRef]
- Weinberger, A.; Tanasescu, R.; Stefaniu, C.; Fedotenko, L.A.; Favarger, F.; Ishikawa, T.; Brezesinski, G.; Marques, C.M.; Zumbuehl, A. Bilayer Properties of 1,3-Diamidophospholipids. Langmuir 2015, 31, 1879–1884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Che, Z.; Mazhar, K.; Price, T.J.; Qin, Z. Ultrafast Near-Infrared Light-triggered Intracellular Uncaging to Probe Cell Signaling. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, H.; Li, X.; Kang, P.; Perish, J.; Neuhaus, F.; Ploski, J.E.; Kroener, S.; Ogunyankin, M.O.; Shin, J.E.; Zasadzinski, J.A.; et al. Near-infrared Light Triggered-release in Deep Brain Regions Using Ultra-photosensitive Nanovesicles. Angew. Chem. Int. Ed. 2020, 59, 8608–8615. [Google Scholar] [CrossRef] [PubMed]
- Tobe, M.; Suto, T.; Saito, S. The history and progress of local anesthesia: Multiple approaches to elongate the action. J. Anesth. 2018, 32, 632–636. [Google Scholar] [CrossRef]
- Cullion, K.; Kohane, D.S. Externally triggered patient-controlled local anesthesia. Pain Manag. 2018, 8, 313–315. [Google Scholar] [CrossRef]
- Wang, D.; Sun, Y.; Liu, Y.; Meng, F.; Lee, R.J. Clinical translation of immunoliposomes for cancer therapy: Recent perspectives. Expert Opin. Drug Deliv. 2018, 15, 893–903. [Google Scholar] [CrossRef]
- Belfiore, L.; Saunders, D.N.; Ranson, M.; Thurecht, K.J.; Storm, G.; Vine, K.L. Towards clinical translation of ligand-functionalized liposomes in targeted cancer therapy: Challenges and opportunities. J. Control. Release 2018, 277, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Andresen, T.L.; Jensen, S.S.; Jørgensen, K. Advanced strategies in liposomal cancer therapy: Problems and prospects of active and tumor specific drug release. Prog. Lipid Res. 2005, 44, 68–97. [Google Scholar] [CrossRef]
- Barenholz, Y. Liposome application: Problems and prospects. Curr. Opin. Colloid Interface Sci. 2001, 6, 66–77. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [Green Version]
- Rampes, S.; Ma, D. Hepatic ischemia-reperfusion injury in liver transplant setting: Mechanisms and protective strategies. J. Biomed. Res. 2019, 33, 221–234. [Google Scholar] [CrossRef]
- Corvo, M.L.; Marinho, H.S.; Marcelino, P.; Lopes, R.M.; Vale, C.A.; Marques, C.R.; Martins, L.C.; Laverman, P.; Storm, G.; Martins, M.B. Superoxide dismutase enzymosomes: Carrier capacity optimization, in vivo behaviour and therapeutic activity. Pharm. Res. 2015, 32, 91–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcelino, P.; Marinho, H.S.; Campos, M.C.; Neves, A.R.; Real, C.; Fontes, F.S.; Carvalho, A.; Feio, G.; Martins, M.B.F.; Corvo, M.L. Therapeutic activity of superoxide dismutase-containing enzymosomes on rat liver ischaemia-reperfusion injury followed by magnetic resonance microscopy. Eur. J. Pharm. Sci. 2017, 109, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Poh, A.R.; Ernst, M. Targeting Macrophages in Cancer: From Bench to Bedside. Front. Oncol. 2018, 8, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binnemars-Postma, K.A.; Storm, G.; Prakash, J. Nanomedicine Strategies to Target Tumor-Associated Macrophages. Int. J. Mol. Sci. 2017, 18, 979. [Google Scholar] [CrossRef] [PubMed]
- Binnemars-Postma, K.A.; Bansal, R.; Storm, G.; Prakash, J. Targeting the Stat6 pathway in tumor-associated macrophages reduces tumor growth and metastatic niche formation in breast cancer. FASEB J. 2018, 32, 969–978. [Google Scholar] [CrossRef] [Green Version]
- Abumanhal-Masarweh, H.; da Silva, D.; Poley, M.; Zinger, A.; Goldman, E.; Krinsky, N.; Kleiner, R.; Shenbach, G.; Schroeder, J.E.; Shklover, J.; et al. Tailoring the lipid composition of nanoparticles modulates their cellular uptake and affects the viability of triple negative breast cancer cells. J. Control. Release 2019, 307, 331–341. [Google Scholar] [CrossRef]
- Zinger, A.; Koren, L.; Adir, O.; Poley, M.; Alyan, M.; Yaari, Z.; Noor, N.; Krinsky, N.; Simon, A.; Gibori, H.; et al. Collagenase Nanoparticles Enhance the Penetration of Drugs into Pancreatic Tumors. ACS Nano 2019, 13, 11008–11021. [Google Scholar] [CrossRef]
- Abumanhal-Masarweh, H.; Koren, L.; Zinger, A.; Yaari, Z.; Krinsky, N.; Kaneti, G.; Dahan, N.; Lupu-Haber, Y.; Suss-Toby, E.; Weiss-Messer, E.; et al. Sodium bicarbonate nanoparticles modulate the tumor pH and enhance the cellular uptake of doxorubicin. J. Control. Release 2019, 296, 1–13. [Google Scholar] [CrossRef]
- Fernandez-Pineda, I.; Hudson, M.M.; Pappo, A.S.; Bishop, M.W.; Klosky, J.L.; Brinkman, T.M.; Srivastava, D.K.; Neel, M.D.; Rao, B.N.; Davidoff, A.M.; et al. Long-term functional outcomes and quality of life in adult survivors of childhood extremity sarcomas: A report from the St. Jude Lifetime Cohort Study. J. Cancer Surviv. 2017, 11, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Hajdin, K.; D’Alessandro, V.; Niggli, F.K.; Schäfer, B.W.; Bernasconi, M. Furin Targeted Drug Delivery for Treatment of Rhabdomyosarcoma in a Mouse Model. PLoS ONE 2010, 5, e10445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roveri, M.; Pfohl, A.; Jaaks, P.; Alijaj, N.; Leroux, J.C.; Luciani, P.; Bernasconi, M. Prolonged circulation and increased tumor accumulation of liposomal vincristine in a mouse model of rhabdomyosarcoma. Nanomedicine (Lond. UK) 2017, 12, 1135–1151. [Google Scholar] [CrossRef] [PubMed]
- Roveri, M.; Bernasconi, M.; Leroux, J.C.; Luciani, P. Peptides for tumor-specific drug targeting: State of the art and beyond. J. Mater. Chem. B 2017, 5, 4348–4364. [Google Scholar] [CrossRef] [PubMed]
- Alijaj, N.; Moutel, S.; Gouveia, Z.L.; Gray, M.; Roveri, M.; Dzhumashev, D.; Weber, F.; Meier, G.; Luciani, P.; Rossler, J.K.; et al. Novel FGFR4-Targeting Single-Domain Antibodies for Multiple Targeted Therapies against Rhabdomyosarcoma. Cancers 2020, 12, 3313. [Google Scholar] [CrossRef] [PubMed]
- Skapek, S.X.; Ferrari, A.; Gupta, A.A.; Lupo, P.J.; Butler, E.; Shipley, J.; Barr, F.G.; Hawkins, D.S. Rhabdomyosarcoma. Nat. Rev. Dis. Primers 2019, 5, 1. [Google Scholar] [CrossRef]
- Trujillo, J.A.; Sweis, R.F.; Bao, R.; Luke, J.J. T Cell-Inflamed versus Non-T Cell-Inflamed Tumors: A Conceptual Framework for Cancer Immunotherapy Drug Development and Combination Therapy Selection. Cancer Immunol. Res. 2018, 6, 990–1000. [Google Scholar] [CrossRef] [Green Version]
- Bol, K.F.; Schreibelt, G.; Gerritsen, W.R.; de Vries, I.J.; Figdor, C.G. Dendritic Cell-Based Immunotherapy: State of the Art and Beyond. Clin. Cancer Res. 2016, 22, 1897–1906. [Google Scholar] [CrossRef] [Green Version]
- Crocker, P.R.; Kelm, S.; Dubois, C.; Martin, B.; McWilliam, A.S.; Shotton, D.M.; Paulson, J.C.; Gordon, S. Purification and properties of sialoadhesin, a sialic acid-binding receptor of murine tissue macrophages. EMBO J. 1991, 10, 1661–1669. [Google Scholar] [CrossRef]
- van Dinther, D.; Veninga, H.; Iborra, S.; Borg, E.G.F.; Hoogterp, L.; Olesek, K.; Beijer, M.R.; Schetters, S.T.T.; Kalay, H.; Garcia-Vallejo, J.J.; et al. Functional CD169 on Macrophages Mediates Interaction with Dendritic Cells for CD8(+) T Cell Cross-Priming. Cell Rep. 2018, 22, 1484–1495. [Google Scholar] [CrossRef] [Green Version]
- Müller, U.C.; Deller, T.; Korte, M. Not just amyloid: Physiological functions of the amyloid precursor protein family. Nat. Rev. Neurosci. 2017, 18, 281–298. [Google Scholar] [CrossRef]
- Uhl, P.; Helm, F.; Hofhaus, G.; Brings, S.; Kaufman, C.; Leotta, K.; Urban, S.; Haberkorn, U.; Mier, W.; Fricker, G. A liposomal formulation for the oral application of the investigational hepatitis B drug Myrcludex B. Eur. J. Pharm. Biopharm. 2016, 103, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuhrmann, G.; Herrmann, I.K.; Stevens, M.M. Cell-derived vesicles for drug therapy and diagnostics: Opportunities and challenges. Nano Today 2015, 10, 397–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gould, S.J.; Raposo, G. As we wait: Coping with an imperfect nomenclature for extracellular vesicles. J. Extracell. Vesicles 2013, 2. [Google Scholar] [CrossRef]
- Valentino, G.; Zivko, C.; Weber, F.; Brülisauer, L.; Luciani, P. Synergy of Phospholipid-Drug Formulations Significantly Deactivates Profibrogenic Human Hepatic Stellate Cells. Pharmaceutics 2019, 11, 676. [Google Scholar] [CrossRef] [Green Version]
- Zivko, C.; Fuhrmann, G.; Luciani, P. Liver-derived extracellular vesicles: A cell by cell overview to isolation and characterization practices. Biochim. Biophys. Acta Gen. Subj. 2020, 129559. [Google Scholar] [CrossRef]
- Dowdy, S.F. Overcoming cellular barriers for RNA therapeutics. Nat. Biotechnol. 2017, 35, 222–229. [Google Scholar] [CrossRef]
- Lunavat, T.R.; Jang, S.C.; Nilsson, L.; Park, H.T.; Repiska, G.; Lässer, C.; Nilsson, J.A.; Gho, Y.S.; Lötvall, J. RNAi delivery by exosome-mimetic nanovesicles—Implications for targeting c-Myc in cancer. Biomaterials 2016, 102, 231–238. [Google Scholar] [CrossRef]
- O’Loughlin, A.J.; Mager, I.; de Jong, O.G.; Varela, M.A.; Schiffelers, R.M.; El Andaloussi, S.; Wood, M.J.A.; Vader, P. Functional Delivery of Lipid-Conjugated siRNA by Extracellular Vesicles. Mol. Ther. 2017, 25, 1580–1587. [Google Scholar] [CrossRef] [Green Version]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef]
- Hettich, B.F.; Ben-Yehuda Greenwald, M.; Werner, S.; Leroux, J.-C. Exosomes for Wound Healing: Purification Optimization and Identification of Bioactive Components. Adv. Sci. 2020, 2002596. [Google Scholar] [CrossRef] [PubMed]
- Lener, T.; Gimona, M.; Aigner, L.; Borger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.T.; Umezaki, K.; Sawada, S.; Mukai, S.A.; Sasaki, Y.; Harada, N.; Shiku, H.; Akiyoshi, K. Engineering hybrid exosomes by membrane fusion with liposomes. Sci. Rep. 2016, 6, 21933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, J.P.; Holme, M.N.; Stevens, M.M. Re-Engineering Extracellular Vesicles as Smart Nanoscale Therapeutics. ACS Nano 2017, 11, 69–83. [Google Scholar] [CrossRef] [Green Version]
- Piffoux, M.; Silva, A.K.A.; Wilhelm, C.; Gazeau, F.; Tareste, D. Modification of Extracellular Vesicles by Fusion with Liposomes for the Design of Personalized Biogenic Drug Delivery Systems. ACS Nano 2018, 12, 6830–6842. [Google Scholar] [CrossRef]
- Bordi, F.; Sennato, S.; Truzzolillo, D. Polyelectrolyte-induced aggregation of liposomes: A new cluster phase with interesting applications. J. Phys. Condens. Matter 2009, 21, 203102. [Google Scholar] [CrossRef]
- Agrati, C.; Marianecci, C.; Sennato, S.; Carafa, M.; Bordoni, V.; Cimini, E.; Tempestilli, M.; Pucillo, L.P.; Turchi, F.; Martini, F.; et al. Multicompartment vectors as novel drug delivery systems: Selective activation of Tγδ lymphocytes after zoledronic acid delivery. Nanomedicine 2011, 7, 153–161. [Google Scholar] [CrossRef]
- Hildebrandt, E.; Sommerling, J.-H.; Guthausen, G.; Zick, K.; Stürmer, J.; Nirschl, H.; Leneweit, G. Phospholipid adsorption at oil in water versus water in oil interfaces: Implications for interfacial densities and bulk solubilities. Colloids Surf. A 2016, 505, 56–63. [Google Scholar] [CrossRef]
- Hildebrandt, E.; Dessy, A.; Sommerling, J.-H.; Guthausen, G.; Nirschl, H.; Leneweit, G. Interactions between Phospholipids and Organic Phases: Insights into Lipoproteins and Nanoemulsions. Langmuir 2016, 32, 5821–5829. [Google Scholar] [CrossRef]
- Sommerling, J.-H.; Uhlenbruck, N.; Leneweit, G.; Nirschl, H. Transfer of colloidal particles between two non-miscible liquid phases. Colloids Surf. A 2017, 535, 257–264. [Google Scholar] [CrossRef]
- Sommerling, J.-H.; de Matos, M.B.C.; Hildebrandt, E.; Dessy, A.; Kok, R.J.; Nirschl, H.; Leneweit, G. Instability Mechanisms of Water-in-Oil Nanoemulsions with Phospholipids: Temporal and Morphological Structures. Langmuir 2018, 34, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, E.; Nirschl, H.; Kok, R.J.; Leneweit, G. Adsorption of phospholipids at oil/water interfaces during emulsification is controlled by stress relaxation and diffusion. Soft Matter 2018, 14, 3730–3737. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, K.; Poggemann, L.; Nirschl, H.; Leneweit, G. Adsorption process for phospholipids of different chain lengths at a fluorocarbon/water interface studied by Du Noüy ring and spinning drop. Colloid Polym. Sci. 2020, 298, 407–417. [Google Scholar] [CrossRef] [Green Version]
- de Matos, M.B.C.; Miranda, B.S.; Rizky Nuari, Y.; Storm, G.; Leneweit, G.; Schiffelers, R.M.; Kok, R.J. Liposomes with asymmetric bilayers produced from inverse emulsions for nucleic acid delivery. J. Drug Target. 2019, 27, 681–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Cassader, M.; Paschetta, E.; Gambino, R. Bioactive Lipid Species and Metabolic Pathways in Progression and Resolution of Nonalcoholic Steatohepatitis. Gastroenterology 2018, 155, 282–302. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Cassader, M.; Gambino, R. Non-alcoholic steatohepatitis: Emerging molecular targets and therapeutic strategies. Nat. Rev. Drug Discov. 2016, 15, 249–274. [Google Scholar] [CrossRef]
- Serbulea, V.; Upchurch, C.M.; Schappe, M.S.; Voigt, P.; DeWeese, D.E.; Desai, B.N.; Meher, A.K.; Leitinger, N. Macrophage phenotype and bioenergetics are controlled by oxidized phospholipids identified in lean and obese adipose tissue. Proc. Natl. Acad. Sci. USA 2018, 115, E6254–E6263. [Google Scholar] [CrossRef] [Green Version]
- ten Hove, M.; Pater, L.; Storm, G.; Weiskirchen, S.; Weiskirchen, R.; Lammers, T.; Bansal, R. The hepatic lipidome: From basic science to clinical translation. Adv. Drug Deliv. Rev. 2020. [Google Scholar] [CrossRef]
- Salzano, G.; Marra, M.; Porru, M.; Zappavigna, S.; Abbruzzese, A.; La Rotonda, M.I.; Leonetti, C.; Caraglia, M.; De Rosa, G. Self-assembly nanoparticles for the delivery of bisphosphonates into tumors. Int. J. Pharm. 2011, 403, 292–297. [Google Scholar] [CrossRef]
- Marra, M.; Salzano, G.; Leonetti, C.; Porru, M.; Franco, R.; Zappavigna, S.; Liguori, G.; Botti, G.; Chieffi, P.; Lamberti, M.; et al. New self-assembly nanoparticles and stealth liposomes for the delivery of zoledronic acid: A comparative study. Biotechnol. Adv. 2012, 30, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Salzano, G.; Zappavigna, S.; Luce, A.; D’Onofrio, N.; Balestrieri, M.L.; Grimaldi, A.; Lusa, S.; Ingrosso, D.; Artuso, S.; Porru, M.; et al. Transferrin-Targeted Nanoparticles Containing Zoledronic Acid as a Potential Tool to Inhibit Glioblastoma Growth. J. Biomed. Nanotechnol. 2016, 12, 811–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campani, V.; Zappavigna, S.; Scotti, L.; Abate, M.; Porru, M.; Leonetti, C.; Caraglia, M.; De Rosa, G. Hybrid lipid self-assembling nanoparticles for brain delivery of microRNA. Int. J. Pharm. 2020, 588, 119693. [Google Scholar] [CrossRef] [PubMed]
- Koeberle, A.; Shindou, H.; Koeberle, S.C.; Laufer, S.A.; Shimizu, T.; Werz, O. Arachidonoyl-phosphatidylcholine oscillates during the cell cycle and counteracts proliferation by suppressing Akt membrane binding. Proc. Natl. Acad. Sci. USA 2013, 110, 2546–2551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pein, H.; Koeberle, S.C.; Voelkel, M.; Schneider, F.; Rossi, A.; Thürmer, M.; Loeser, K.; Sautebin, L.; Morrison, H.; Werz, O.; et al. Vitamin A regulates Akt signaling through the phospholipid fatty acid composition. FASEB J. 2017, 31, 4566–4577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glatzel, D.K.; Koeberle, A.; Pein, H.; Löser, K.; Stark, A.; Keksel, N.; Werz, O.; Müller, R.; Bischoff, I.; Fürst, R. Acetyl-CoA carboxylase 1 regulates endothelial cell migration by shifting the phospholipid composition. J. Lipid Res. 2018, 59, 298–311. [Google Scholar] [CrossRef] [Green Version]
- Kolbina, M.; Bodmeier, R.; Körber, M. Saturated phosphatidylcholine as matrix former for oral extended release dosage forms. Eur. J. Pharm. Sci. 2017, 108, 86–92. [Google Scholar] [CrossRef]
- Kolbina, M.; Schulte, A.; van Hoogevest, P.; Körber, M.; Bodmeier, R. Evaluation of Hydrogenated Soybean Phosphatidylcholine Matrices Prepared by Hot Melt Extrusion for Oral Controlled Delivery of Water-Soluble Drugs. AAPS PharmSciTech 2019, 20, 159. [Google Scholar] [CrossRef]
- Klein, M.E.; Mauch, S.; Rieckmann, M.; Martinez, D.G.; Hause, G.; Noutsias, M.; Hofmann, U.; Lucas, H.; Meister, A.; Ramos, G.; et al. Phosphatidylserine (PS) and phosphatidylglycerol (PG) nanodispersions as potential anti-inflammatory therapeutics: Comparison of in vitro activity and impact of pegylation. Nanomedicine 2020, 23, 102096. [Google Scholar] [CrossRef]
- Zlomke, C.; Albrecht, J.; Mäder, K. Nicardipine Loaded Solid Phospholipid Extrudates for the Prevention of Cerebral Vasospasms: In Vitro Characterization. Pharmaceutics 2020, 12, 817. [Google Scholar] [CrossRef]
- Ramos, G.C.; Fernandes, D.; Charão, C.T.; Souza, D.G.; Teixeira, M.M.; Assreuy, J. Apoptotic mimicry: Phosphatidylserine liposomes reduce inflammation through activation of peroxisome proliferator-activated receptors (PPARs) in vivo. Br. J. Pharmacol. 2007, 151, 844–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, D.; Choudhary, V.; Seremwe, M.; Edwards, J.G.; Wang, A.; Emmons, A.C.; Bollag, K.A.; Johnson, M.H.; Bollag, W.B. Soy Phosphatidylglycerol Reduces Inflammation in a Contact Irritant Ear Edema Mouse Model in Vivo. J. Pharmacol. Exp. Ther. 2018, 366, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yeom, M.; Hahm, D.H.; Sur, B.J.; Han, J.J.; Lee, H.J.; Yang, H.I.; Kim, K.S. Phosphatidylserine inhibits inflammatory responses in interleukin-1β-stimulated fibroblast-like synoviocytes and alleviates carrageenan-induced arthritis in rat. Nutr. Res. 2013, 33, 242–250. [Google Scholar] [CrossRef] [PubMed]
- van Rooij, T.; Daeichin, V.; Skachkov, I.; de Jong, N.; Kooiman, K. Targeted ultrasound contrast agents for ultrasound molecular imaging and therapy. Int. J. Hyperth. 2015, 31, 90–106. [Google Scholar] [CrossRef] [Green Version]
- Kooiman, K.; Vos, H.J.; Versluis, M.; de Jong, N. Acoustic behavior of microbubbles and implications for drug delivery. Adv. Drug Deliv. Rev. 2014, 72, 28–48. [Google Scholar] [CrossRef]
- Kooiman, K.; Roovers, S.; Langeveld, S.A.G.; Kleven, R.T.; Dewitte, H.; O’Reilly, M.A.; Escoffre, J.M.; Bouakaz, A.; Verweij, M.D.; Hynynen, K.; et al. Ultrasound-Responsive Cavitation Nuclei for Therapy and Drug Delivery. Ultrasound Med. Biol. 2020, 46, 1296–1325. [Google Scholar] [CrossRef] [Green Version]
- Beekers, I.; Lattwein, K.R.; Kouijzer, J.J.P.; Langeveld, S.A.G.; Vegter, M.; Beurskens, R.; Mastik, F.; Verduyn Lunel, R.; Verver, E.; van der Steen, A.F.W.; et al. Combined Confocal Microscope and Brandaris 128 Ultra-High-Speed Camera. Ultrasound Med. Biol. 2019, 45, 2575–2582. [Google Scholar] [CrossRef] [Green Version]
- Langeveld, S.A.G.; Schwieger, C.; Beekers, I.; Blaffert, J.; van Rooij, T.; Blume, A.; Kooiman, K. Ligand Distribution and Lipid Phase Behavior in Phospholipid-Coated Microbubbles and Monolayers. Langmuir 2020, 36, 3221–3233. [Google Scholar] [CrossRef]
- Zetterberg, M.M.; Ahlgren, S.; Agmo Hernández, V.; Parveen, N.; Edwards, K. Optimization of lipodisk properties by modification of the extent and density of the PEG corona. J. Colloid Interface Sci. 2016, 484, 86–96. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, H.; Chen, J.; Wang, S.; Xin, Y.; Qu, Y.; Zhang, Q.; Ji, W.; Yamashita, F.; Rui, M.; et al. Ratiometric co-encapsulation and co-delivery of doxorubicin and paclitaxel by tumor-targeted lipodisks for combination therapy of breast cancer. Int. J. Pharm. 2019, 560, 191–204. [Google Scholar] [CrossRef]
- Ahlgren, S.; Fondell, A.; Gedda, L.; Edwards, K. EGF-targeting lipodisks for specific delivery of poorly water-soluble anticancer agents to tumour cells. RSC Adv. 2017, 7, 22178–22186. [Google Scholar] [CrossRef] [Green Version]
- Ahlgren, S.; Reijmar, K.; Edwards, K. Targeting lipodisks enable selective delivery of anticancer peptides to tumor cells. Nanomedicine 2017, 13, 2325–2328. [Google Scholar] [CrossRef] [PubMed]
- Lundsten, S.; Hernández, V.A.; Gedda, L.; Sarén, T.; Brown, C.J.; Lane, D.P.; Edwards, K.; Nestor, M. Tumor-Targeted Delivery of the p53-Activating Peptide VIP116 with PEG-Stabilized Lipodisks. Nanomaterials 2020, 10, 783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, J.; Hussain, M.M. Intestinal lipid absorption. Am. J. Physiol. 2009, 296, E1183–E1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuhrhop, J.-H.; Wang, T. Bolaamphiphiles. Chem. Rev. 2004, 104, 2901–2937. [Google Scholar] [CrossRef] [PubMed]
- Meister, A.; Blume, A. Self-assembly of bipolar amphiphiles. Curr. Opin. Colloid Interface Sci. 2007, 12, 138–147. [Google Scholar] [CrossRef]
- Parmentier, J.; Becker, M.M.M.; Heintz, U.; Fricker, G. Stability of liposomes containing bio-enhancers and tetraether lipids in simulated gastro-intestinal fluids. Int. J. Pharm. 2011, 405, 210–217. [Google Scholar] [CrossRef]
- Parmentier, J.; Thewes, B.; Gropp, F.; Fricker, G. Oral peptide delivery by tetraether lipid liposomes. Int. J. Pharm. 2011, 415, 150–157. [Google Scholar] [CrossRef]
- Uhl, P.; Pantze, S.; Storck, P.; Parmentier, J.; Witzigmann, D.; Hofhaus, G.; Huwyler, J.; Mier, W.; Fricker, G. Oral delivery of vancomycin by tetraether lipid liposomes. Eur. J. Pharm. Sci. 2017, 108, 111–118. [Google Scholar] [CrossRef]
- Jacobsen, A.C.; Jensen, S.M.; Fricker, G.; Brandl, M.; Treusch, A.H. Archaeal lipids in oral delivery of therapeutic peptides. Eur. J. Pharm. Sci. 2017, 108, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Markowski, T.; Müller, S.; Dobner, B.; Meister, A.; Blume, A.; Drescher, S. An Asymmetrical Glycerol Diether Bolalipid with Protonable Phosphodimethylethanolamine Headgroup: The Impact of pH on Aggregation Behavior and Miscibility with DPPC. Polymers 2017, 9, 573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drescher, S.; Otto, C.; Müller, S.; Garamus, V.M.; Garvey, C.J.; Grünert, S.; Lischka, A.; Meister, A.; Blume, A.; Dobner, B. Impact of Headgroup Asymmetry and Protonation State on the Aggregation Behavior of a New Type of Glycerol Diether Bolalipid. Langmuir 2018, 34, 4360–4373. [Google Scholar] [CrossRef] [PubMed]
- Gruhle, K.; Müller, S.; Meister, A.; Drescher, S. Synthesis and aggregation behaviour of single-chain, 1,32-alkyl branched bis(phosphocholines): Effect of lateral chain length. Org. Biomol. Chem. 2018, 16, 2711–2724. [Google Scholar] [CrossRef] [PubMed]
- Gruhle, K.; Tuchtenhagen, M.; Müller, S.; Hause, G.; Meister, A.; Drescher, S. Synthesis and aggregation behaviour of single-chain, 1,32-alkyl-branched bis(phosphocholines)—Part 2: Lateral chain length triggers self-assembling from sheets to fibres to vesicles. Org. Biomol. Chem. 2020, 18, 3585–3598. [Google Scholar] [CrossRef]
- Müller, S.; Meister, A.; Otto, C.; Hause, G.; Drescher, S. Mixing behaviour of asymmetrical glycerol diether bolalipids with saturated and unsaturated phosphatidylcholines. Biophys. Chem. 2018, 238, 39–48. [Google Scholar] [CrossRef]
- Müller, S.; Kind, M.; Gruhle, K.; Hause, G.; Meister, A.; Drescher, S. Mixing behaviour of bilayer-forming phosphatidylcholines with single-chain alkyl-branched bolalipids: Effect of lateral chain length. Biophys. Chem. 2019, 244, 1–10. [Google Scholar] [CrossRef]
- Müller, S.; Gruhle, K.; Meister, A.; Hause, G.; Drescher, S. Bolalipid-Doped Liposomes: Can Bolalipids Increase the Integrity of Liposomes Exposed to Gastrointestinal Fluids? Pharmaceutics 2019, 11, 646. [Google Scholar] [CrossRef] [Green Version]
- Dong, F.; Xie, Y.; Qi, J.; Hu, F.; Lu, Y.; Li, S.; Wu, W. Bile salt/phospholipid mixed micelle precursor pellets prepared by fluid-bed coating. Int. J. Nanomed. 2013, 8, 1653–1663. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Cai, J.; Li, P.; Xu, D.; Ni, X.; Wen, H.; Liu, D.; Lin, S.; Hu, H. Comparison of bile salt/phosphatidylcholine mixed micelles in solubilization to sterols and stability. Drug Des. Dev. Ther. 2016, 10, 3789–3798. [Google Scholar] [CrossRef] [Green Version]
- Lv, Q.; Li, X.; Shen, B.; Xu, H.; Shen, C.; Dai, L.; Bai, J.; Yuan, H.; Han, J. Application of spray granulation for conversion of mixed phospholipid-bile salt micelles to dry powder form: Influence of drug hydrophobicity on nanoparticle reagglomeration. Int. J. Nanomed. 2014, 9, 505–515. [Google Scholar] [CrossRef] [Green Version]
- Anagu, O.L.; Attama, A.A.; Okore, V.C.; Gugu, H.T.; Ngene, A.A.; Esimone, C.O. Azadirachta indica extract–artesunic acid combination produces an increased cure rate of Plasmodium berghei-infected mice. Pharm. Biol. 2014, 52, 883–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attama, A.A.; Kenechulwu, F.C.; Onuigbo, E.B.; Nnamani, P.O.; Obitte, N.; Finke, J.H.; Pretor, S.; Müller-Goymann, C.C. Solid lipid nanoparticles encapsulating a fluorescent marker (coumarin 6) and antimalarials—Artemether and lumefantrine: Evaluation of cellular uptake and antimalarial activity. Eur. J. Nanomed. 2016, 8, 129–138. [Google Scholar] [CrossRef]
- Shi, C.; Tong, Q.; Fang, J.; Wang, C.; Wu, J.; Wang, W. Preparation, characterization and in vivo studies of amorphous solid dispersion of berberine with hydrogenated phosphatidylcholine. Eur. J. Pharm. Sci. 2015, 74, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Fong, S.Y.K.; Brandl, M.; Bauer-Brandl, A. Phospholipid-based solid drug formulations for oral bioavailability enhancement: A meta-analysis. Eur. J. Pharm. Sci. 2015, 80, 89–110. [Google Scholar] [CrossRef] [PubMed]
- Fong, S.Y.K.; Martins, S.M.; Brandl, M.; Bauer-Brandl, A. Solid Phospholipid Dispersions for Oral Delivery of Poorly Soluble Drugs: Investigation into Celecoxib Incorporation and Solubility-in Vitro Permeability Enhancement. J. Pharm. Sci. 2016, 105, 1113–1123. [Google Scholar] [CrossRef]
- Gautschi, N.; van Hoogevest, P.; Kuentz, M. Molecular insights into the formation of drug-monoacyl phosphatidylcholine solid dispersions for oral delivery. Eur. J. Pharm. Sci. 2017, 108, 93–100. [Google Scholar] [CrossRef]
- Jacobsen, A.C.; Elvang, P.A.; Bauer-Brandl, A.; Brandl, M. A dynamic in vitro permeation study on solid mono- and diacyl-phospholipid dispersions of celecoxib. Eur. J. Pharm. Sci. 2019, 127, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Nielsen, H.M.; Fano, M.; Müllertz, A. Preparation and characterization of insulin-surfactant complexes for loading into lipid-based drug delivery systems. J. Pharm. Sci. 2013, 102, 2689–2698. [Google Scholar] [CrossRef]
- Li, P.; Tan, A.; Prestidge, C.A.; Nielsen, H.M.; Müllertz, A. Self-nanoemulsifying drug delivery systems for oral insulin delivery: In vitro and in vivo evaluations of enteric coating and drug loading. Int. J. Pharm. 2014, 477, 390–398. [Google Scholar] [CrossRef]
- Welling, S.H.; Hubálek, F.; Jacobsen, J.; Brayden, D.J.; Rahbek, U.L.; Buckley, S.T. The role of citric acid in oral peptide and protein formulations: Relationship between calcium chelation and proteolysis inhibition. Eur. J. Pharm. Biopharm. 2014, 86, 544–551. [Google Scholar] [CrossRef]
- Liu, J.; Werner, U.; Funke, M.; Besenius, M.; Saaby, L.; Fanø, M.; Mu, H.; Müllertz, A. SEDDS for intestinal absorption of insulin: Application of Caco-2 and Caco-2/HT29 co-culture monolayers and intra-jejunal instillation in rats. Int. J. Pharm. 2019, 560, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hirschberg, C.; Fanø, M.; Mu, H.; Müllertz, A. Evaluation of self-emulsifying drug delivery systems for oral insulin delivery using an in vitro model simulating the intestinal proteolysis. Eur. J. Pharm. Sci. 2020, 147, 105272. [Google Scholar] [CrossRef] [PubMed]
- Patil, H.; Feng, X.; Ye, X.; Majumdar, S.; Repka, M.A. Continuous production of fenofibrate solid lipid nanoparticles by hot-melt extrusion technology: A systematic study based on a quality by design approach. AAPS J. 2015, 17, 194–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ditzinger, F.; Scherer, U.; Schönenberger, M.; Holm, R.; Kuentz, M. Modified Polymer Matrix in Pharmaceutical Hot Melt Extrusion by Molecular Interactions with a Carboxylic Coformer. Mol. Pharm. 2019, 16, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Klang, V.; Valenta, C. Lecithin-based nanoemulsions. J. Drug Delivery Sci. Technol. 2011, 21, 55–76. [Google Scholar] [CrossRef]
- Sahle, F.F.; Metz, H.; Wohlrab, J.; Neubert, R.H.H. Lecithin-Based Microemulsions for Targeted Delivery of Ceramide AP into the Stratum Corneum: Formulation, Characterizations, and In Vitro Release and Penetration Studies. Pharm. Res. 2013, 30, 538–551. [Google Scholar] [CrossRef]
- Shah, S.M.; Ashtikar, M.; Jain, A.S.; Makhija, D.T.; Nikam, Y.; Gude, R.P.; Steiniger, F.; Jagtap, A.A.; Nagarsenker, M.S.; Fahr, A. LeciPlex, invasomes, and liposomes: A skin penetration study. Int. J. Pharm. 2015, 490, 391–403. [Google Scholar] [CrossRef]
- Lauterbach, A.; Müller-Goymann, C.C. Design of lipid microparticle dispersions based on the physicochemical properties of the lipid and aqueous phase. Int. J. Pharm. 2015, 494, 445–452. [Google Scholar] [CrossRef]
- Wolf, M.; Klang, V.; Halper, M.; Stix, C.; Heuser, T.; Kotisch, H.; Valenta, C. Monoacyl-phosphatidylcholine nanostructured lipid carriers: Influence of lipid and surfactant content on in vitro skin permeation of flufenamic acid and fluconazole. J. Drug Delivery Sci. Technol. 2017, 41, 419–430. [Google Scholar] [CrossRef]
- Ebeling, S.; Naumann, K.; Pollok, S.; Wardecki, T.; Vidal, Y.S.S.; Nascimento, J.M.; Boerries, M.; Schmidt, G.; Brandner, J.M.; Merfort, I. From a traditional medicinal plant to a rational drug: Understanding the clinically proven wound healing efficacy of birch bark extract. PLoS ONE 2014, 9, e86147. [Google Scholar] [CrossRef] [Green Version]
- Scheffler, A. The Wound Healing Properties of Betulin from Birch Bark from Bench to Bedside. Planta Med. 2019, 85, 524–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.-O.; Jafari, S.-H.; Supaphol, P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Adv. Technol. 2010, 21, 77–95. [Google Scholar] [CrossRef]
- Mwiiri, F.K.; Daniels, R. Optimized Birch Bark Extract-Loaded Colloidal Dispersion Using Hydrogenated Phospholipids as Stabilizer. Pharmaceutics 2020, 12, 832. [Google Scholar] [CrossRef]
- Mwiiri, F.K.; Brandner, J.M.; Daniels, R. Electrospun Bioactive Wound Dressing Containing Colloidal Dispersions of Birch Bark Dry Extract. Pharmaceutics 2020, 12, 770. [Google Scholar] [CrossRef]
- Vater, C.; Adamovic, A.; Ruttensteiner, L.; Steiner, K.; Tajpara, P.; Klang, V.; Elbe-Bürger, A.; Wirth, M.; Valenta, C. Cytotoxicity of lecithin-based nanoemulsions on human skin cells and ex vivo skin permeation: Comparison to conventional surfactant types. Int. J. Pharm. 2019, 566, 383–390. [Google Scholar] [CrossRef]
- Vater, C.; Hlawaty, V.; Werdenits, P.; Cichon, M.A.; Klang, V.; Elbe-Burger, A.; Wirth, M.; Valenta, C. Effects of lecithin-based nanoemulsions on skin: Short-time cytotoxicity MTT and BrdU studies, skin penetration of surfactants and additives and the delivery of curcumin. Int. J. Pharm. 2020, 580, 119209. [Google Scholar] [CrossRef]
- Agra, L.C.; Ferro, J.N.; Barbosa, F.T.; Barreto, E. Triterpenes with healing activity: A systematic review. J. Dermatol. Treat. 2015, 26, 465–470. [Google Scholar] [CrossRef]
- Jokinen, J.J.; Sipponen, A. Refined Spruce Resin to Treat Chronic Wounds: Rebirth of an Old Folkloristic Therapy. Adv. Wound Care (New Rochelle) 2016, 5, 198–207. [Google Scholar] [CrossRef] [Green Version]
- Otto, F.; Brezesinski, G.; van Hoogevest, P.; Neubert, R.H.H. Physicochemical characterization of natural phospholipid excipients with varying PC content. Colloids Surf. A 2018, 558, 291–296. [Google Scholar] [CrossRef]
- Todosijević, M.N.; Brezesinski, G.; Savić, S.D.; Neubert, R.H.H. Sucrose esters as biocompatible surfactants for penetration enhancement: An insight into the mechanism of penetration enhancement studied using stratum corneum model lipids and Langmuir monolayers. Eur. J. Pharm. Sci. 2017, 99, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.; Caspi, Y.; Meijering, A.E.; Dekker, C. Octanol-assisted liposome assembly on chip. Nat. Commun. 2016, 7, 10447. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.; Dekker, C. On-chip microfluidic production of cell-sized liposomes. Nat. Protoc. 2018, 13, 856–874. [Google Scholar] [CrossRef] [PubMed]
- Al Nahas, K.; Cama, J.; Schaich, M.; Hammond, K.; Deshpande, S.; Dekker, C.; Ryadnov, M.G.; Keyser, U.F. A microfluidic platform for the characterisation of membrane active antimicrobials. Lab Chip 2019, 19, 837–844. [Google Scholar] [CrossRef] [Green Version]
- Bossa, G.V.; Gunderson, S.; Downing, R.; May, S. Role of Transmembrane Proteins for Phase Separation and Domain Registration in Asymmetric Lipid Bilayers. Biomolecules 2019, 9, 303. [Google Scholar] [CrossRef] [Green Version]
- Downing, R.; Volpe Bossa, G.; May, S. Saddle-curvature instability of lipid bilayer induced by amphipathic peptides: A molecular model. Soft Matter 2020, 16, 5032–5043. [Google Scholar] [CrossRef]
- Rufeil Fiori, E.; Downing, R.; Bossa, G.V.; May, S. Influence of spontaneous curvature on the line tension of phase-coexisting domains in a lipid monolayer: A Landau-Ginzburg model. J. Chem. Phys. 2020, 152, 054707. [Google Scholar] [CrossRef]
- EMA. Reflection Paper on the Pharmaceutical Development of Intravenous Medicinal Products Containing Active Substances Solubilised in Micellar Systems. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-pharmaceutical-development-intravenous-medicinal-products-containing-active_en.pdf (accessed on 16 October 2020).
- Rahnfeld, L.; Thamm, J.; Steiniger, F.; van Hoogevest, P.; Luciani, P. Study on the in situ aggregation of liposomes with negatively charged phospholipids for use as injectable depot formulation. Colloids Surf. B 2018, 168, 10–17. [Google Scholar] [CrossRef]
- Rahnfeld, L.; Luciani, P. Injectable Lipid-Based Depot Formulations: Where Do We Stand? Pharmaceutics 2020, 12, 567. [Google Scholar] [CrossRef]
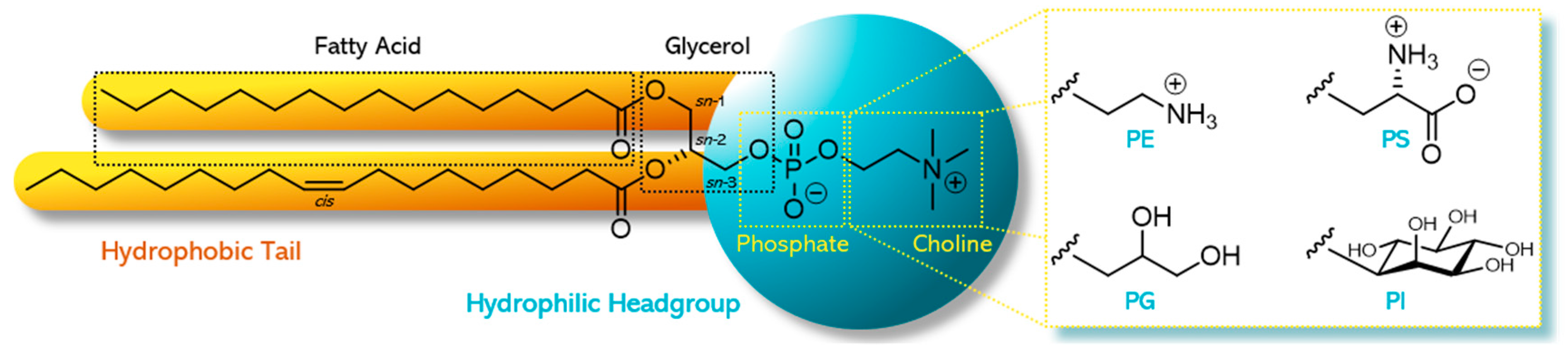

| Phospholipid | Lecithin and Phospholipids (% w/w) | |||||
|---|---|---|---|---|---|---|
| Soybean | Sunflower Seed | Rapeseed | Egg (64–79% PC) | Egg (80–85% PC) | Egg (≥98% PC) | |
| PC | 20–22 | 20–26 | 23–31 | 72 | 81 | 99 |
| PE | 16–22 | 4–10 | 9–15 | 17 | 8.5 | 0.0 |
| PI | 13–16 | 15–19 | 15–18 | - | - | - |
| PA | 5–10 | 2–5 | 5–10 | - | - | - |
| SM | - | - | - | 2.0 | 2.0 | 0.4 |
| LPC | <3 | <3 | <3 | 2.0 | 2.0 | 0.0 |
| LPE | - | - | - | 1.0 | 0.3 | 0.0 |
| Fatty Acid | Lecithin and Phospholipids (% w/w) | |||||
|---|---|---|---|---|---|---|
| Soybean | Sunflower Seed | Rapeseed | Egg (64–79% PC) | Egg (80–85% PC) | Egg (≥98% PC) | |
| C14:0 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.2 |
| C16:0 | 21 | 16 | 10 | 31 | 31 | 34 |
| C18:0 | 4.7 | 5.3 | 0.8 | 15 | 14 | 12 |
| C18:1 | 9.9 | 21 | 49 | 24 | 28 | 28 |
| C18:2 | 57 | 54 | 31 | 16 | 15 | 16 |
| C18:3 | 5.0 | 0.2 | 4.4 | - | - | - |
| C20:0 | 0.1 | 0.3 | 0.1 | - | - | - |
| C20:4 | - | - | - | 5.6 | 4.8 | 3.6 |
| C22:0 | 0.4 | 1.5 | 0.1 | - | - | - |
| C22:4 | - | - | - | 0.3 | 0.3 | 0.2 |
| C22:5 | - | - | - | 0.2 | 0.2 | 0.1 |
| C22:6 | - | - | - | 2.2 | 1.8 | 1.8 |
| Principal Investigator | Host | Start/ Status | Title of Project |
|---|---|---|---|
| Stimuli-Responsive Liposomes | |||
| Robbert Jan Kok | University Utrecht, the Netherlands | 2017, finished | Encapsulation of plant-derived toxins in stimuli-sensitive liposomes |
| Tatu Lajunen | University of Helsinki, Finland | 2019, ongoing | Light activated liposomes for cancer therapy |
| Mans Broekgaarden | University Grenoble, France | 2020, ongoing | Radiation-responsive liposomes for controlled release and tumor permeation of radiotherapy dose enhancers |
| Heijan Xiong | University of Texas at Dallas, USA | 2020, ongoing | Highly photosensitive phospholipid nanovesicles for near infrared light-triggered local anesthesia |
| Targeted Liposomes | |||
| Luisa Corvo | University Lisbon, Portugal | 2017, finished | Targeted liposomal antioxidant and anti- inflammatory therapy for liver ischemic reperfusion injury |
| Jai Prakash | University Twente, the Netherlands | 2018, finished | Modulating tumor-associated macrophages using cell-specific targeted liposomes |
| Avi Schroeder | Israel Institute of Technology | 2018, ongoing | Phospholipids as metastases-targeting molecules using barcoding as a new research tool in liposome discovery |
| Michele Bernasconi | Bern University Hospital, Switzerland | 2020, ongoing | Targeted liposomal drug delivery to pediatric sarcomas: beyond the EPR effect |
| Joke den Haan | Amsterdam UMC, the Netherlands | 2020, ongoing | Virus-like liposomes targeting CD169+ dendritic cells as a novel carrier for cancer immunotherapy |
| Ulrike Müller | University Heidelberg, Germany | 2020, ongoing | Liposome mediated delivery of biologicals to the brain as a novel therapeutic strategy for Alzheimer’s disease |
| Exosomes | |||
| Paola Luciani, Gregor Fuhrmann | University Bern, Switzerland and HIPS, Germany | 2017, ongoing | Lipid-based therapeutics for liver fibrosis and their impact on extracellular vesicles |
| Raymond Schiffelers | University Medical Center Utrecht, the Netherlands | 2018, ongoing | Liposome–extracellular vesicle hybrids for therapeutic RNA delivery |
| Jean-Christophe Leroux | ETH Zurich, Switzerland | 2018, ongoing | Research on the drug loading of exosomes |
| Other Liposomal Approaches | |||
| Federico Bordi, Simona Sennato | La Sapienza University and CNR-ISC Rome, Italy | 2017, ongoing | Antitubercular drug-loaded multi-liposomes vectors |
| Hermann Nirschl | KIT, Germany | 2019, ongoing | Encapsulation of active pharmaceutical ingredients into liposomes via centrifugation of water-in-oil nano-emulsions |
| Ruchi Bansal | University Twente, the Netherlands | 2019, ongoing | Bioactive liposomes for the treatment of non-alcoholic steatohepatitis (NASH) |
| Enrico Mastrobattista | University Utrecht, the Netherlands | 2020, ongoing | Liposome-based coatings for immune stimulation and bone regeneration |
| Principal Investigator | Host | Start/ Status | Title of Project |
|---|---|---|---|
| Giuseppe De Rosa | University Federico II, Naples, Italy | 2017, ongoing | Lipid nanovectors to use non-coding RNA oligonucleotides in glioblastoma in combination with standard therapy |
| Andreas Koeberle | University Innsbruck, Austria | 2019, ongoing | Potential of algal phosphatidylcholines containing Ω3 fatty acids in the supportive therapy of leukemia and lymphoma |
| Roland Bodmeier, Marina Kolbina | Freie Universität Berlin, Germany | 2019, ongoing | Twin-screw extruded phospholipid implants for controlled parenteral delivery |
| Karsten Mäder, Annette Meister | University Halle, Germany | 2020, ongoing | PS and PG enriched extrudates and nanofibers for local anti-inflammatory therapies |
| Klazina Kooiman | Erasmus MC University Medical Center Rotterdam, the Netherlands | 2017, ongoing | Theranostic phospholipid-coated ultrasound contrast agents: response on demand |
| Katarina Edwards | Uppsala University, Sweden | 2019, ongoing | Lipodisks for dual delivery of chemo- therapeutic drugs and anticancer peptides |
| Principal Investigator | Host | Start/ Status | Title of Project |
|---|---|---|---|
| Simon Drescher | University Halle, Germany | 2017, finished | Liposomal oral drug delivery: the use of bipolar amphiphiles to stabilize liposomes |
| Alexander Treusch | University of Southern Denmark | 2019, ongoing | Improving nano-particulate carriers for oral drug delivery using archaeal tetraether lipids from novel sources |
| Sandra Klein | University Greifswald, Germany | 2019, ongoing | Oral mixed micelle formulations—a novel phospholipid-based platform for safe and effective pediatric drug delivery |
| Anthony Attama | University of Nigeria | 2018, ongoing | Development of phospholipid-based depot antimalaria tablets of Azadirachta indica leaf extract and artemether/lumefantrine for oral delivery |
| Thomas Rades | University of Copenhagen, Denmark | 2019, ongoing | Co-amorphous drug–lecithin systems— bridging the gap between amorphous solid dispersions and lipid-based drug delivery |
| Anette Müllertz | University of Copenhagen, Denmark | 2019, ongoing | Enabling oral delivery of peptides by designing phospholipid complexes for self-emulsifying drug delivery systems |
| Paulina Skupin-Mrugalska | Poznan University of Medical Sciences, Poland | 2020, ongoing | Phospholipids as excipients in amorphous solid dispersions: an attempt to establish hot-melt-extrusion for oral formulations of poorly soluble drugs |
| Principal Investigator | Host | Start/ Status | Title of Project |
|---|---|---|---|
| Željka Vanić | University Zagreb, Croatia | 2017, finished | Synergy-based delivery system for combating sexually-transmitted bacterial infections: liposomal azithromycin-in-chitosan hydrogel |
| Rolf Daniels | University Tübingen, Germany | 2017, finished | Electrospun bioactive wound dressing containing phospholipid stabilized nanodispersions of a birch bark dry extract |
| Claudia Valenta | University Vienna, Austria | 2018, ongoing | Development and analysis of different phospholipid formulations for dermal application and their effect on human dermal cell viability |
| Reinhard Neubert, Gerald Brezesinski | Institute of Applied Dermatopharmacy, Halle/Saale, Germany | 2019, ongoing | Characterization of plant-based hydrogenated phospholipids for cosmetic and dermal application |
| Principal Investigator | Host | Start/ Status | Title of Project |
|---|---|---|---|
| Thomas Gutsmann, Christian Nehls | Research Center Borstel, Germany | 2018, ongoing | Bottom-up designed synthetic bacteria—a tool to develop new antibiotic strategies |
| Sylvio May | NDSU, USA | 2018, ongoing | Theoretical model to describe formation and stability of liposome–drug complexes |
| Heiko Heerklotz, Leonie Naßwetter | University Freiburg, Germany | 2020, ongoing | Establishing a fundamental understanding of the fate of mixed micellar formulations after intravenous administration |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drescher, S.; van Hoogevest, P. The Phospholipid Research Center: Current Research in Phospholipids and Their Use in Drug Delivery. Pharmaceutics 2020, 12, 1235. https://doi.org/10.3390/pharmaceutics12121235
Drescher S, van Hoogevest P. The Phospholipid Research Center: Current Research in Phospholipids and Their Use in Drug Delivery. Pharmaceutics. 2020; 12(12):1235. https://doi.org/10.3390/pharmaceutics12121235
Chicago/Turabian StyleDrescher, Simon, and Peter van Hoogevest. 2020. "The Phospholipid Research Center: Current Research in Phospholipids and Their Use in Drug Delivery" Pharmaceutics 12, no. 12: 1235. https://doi.org/10.3390/pharmaceutics12121235
APA StyleDrescher, S., & van Hoogevest, P. (2020). The Phospholipid Research Center: Current Research in Phospholipids and Their Use in Drug Delivery. Pharmaceutics, 12(12), 1235. https://doi.org/10.3390/pharmaceutics12121235





