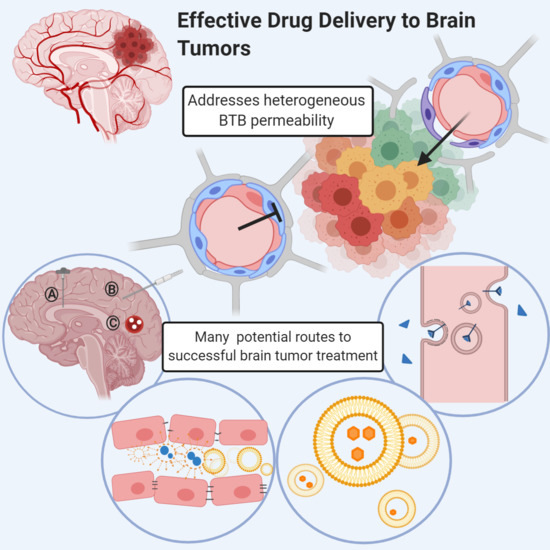
Addressing BBB Heterogeneity: A New Paradigm for Drug Delivery to Brain Tumors
Abstract
:1. Introduction
2. Barriers and Boundaries in the Brain
2.1. CNS Blood–Tissue Barriers
2.2. Neurovascular Unit
2.3. Blood to Brain Permeability and Transport
3. Heterogeneous Blood–Tumor Barrier Permeability
4. Invasive Technologies
4.1. Intrathecal and Intraventricular Injections
4.2. Convection-Enhanced Delivery
| Title | Purpose | NCT Number | Phase | Status/Outcome | Ref |
|---|---|---|---|---|---|
| MTX110 by Convection-Enhanced Delivery in Treating Participants With Newly-Diagnosed Diffuse Intrinsic Pontine Glioma (PNOC015) | To study the side effects of panobinostat nanoparticles formulation MTX110 in participants with newly diagnosed DIPG | NCT03566199 | I/II | Active, not recruiting | [96] |
| Chronic Convection Enhanced Delivery of Topotecan | Primarily to establish the safety of prolonged intracerebral CED of chemotherapy in patients with recurrent HGG. Secondly to determine topotecan distribution and radiographic tumor response under the given CED conditions | i. NCT03154996 ii. NCT03927274 iii. NCT02278510 iv. NCT00308165 | i. I ii., iii. Early phase I iv. I/II | i.Active, not recruiting ii. Recruiting iii. Completed: Safety of CMC catheters has been reported iv. Recruiting | [97,98,99,100,101] |
| CED With Irinotecan Liposome Injection Using Real-Time Imaging in Children With Diffuse Intrinsic Pontine Glioma (DIPG; PNOC 009) | Phase I and Early Efficacy Study of CED of irinotecan liposome injection (nal-IRI) using real-time imaging with gadolinium in children with DIPG who have completed focal radiotherapy | NCT03086616 | I | Recruiting | [102] |
| CED of 124I-Omburtamab for Patients With Non-Progressive Diffuse Pontine Gliomas Previously Treated With External Beam Radiation Therapy | To studythe safety of 124I-omburtamab given by CED at different dose levels for DIPG | NCT01502917 | I | Recruiting | [103] |
| CED of MTX110 Newly Diagnosed Diffuse Midline Gliomas | To find the maximum tolerated dose of MTX110 (a water-soluble Panobinostat nanoparticle formulation) and Gadolinium that can be given safely in children with newly DIPG | NCT04264143 | I | Recruiting | [104] |
| Carboplatin in Treating Patients With Recurrent High-Grade Gliomas | To evaluate the safety and toxicity of carboplatin administered by CED in HGG. It is a dose-escalating study. | NCT01644955 | I | Completed | [105] |
| Convection-Enhanced Delivery (CED) of MDNA55 in Adults With Recurrent or Progressive Glioblastoma | Single-arm study with the primary endpoint of median overall survival (mOS) and a secondary endpoint of objective response rate (ORR) following a single intra-tumoral infusion of MDNA55 in adult recurrent GBM subjects | NCT02858895 | II | Completed: mOS 12.4 months for all patients vs. 7.2 months in synthetic control arm (SCA); 13.2 months in patients with high IL4R expression vs. 6.1 months in SCA | [106,107,108] |
| An Open-Label Dose Escalation Safety Study of Convection-Enhanced Delivery of IL13-PE38QQR in Patients With Progressive Pediatric Diffuse Infiltrating Brainstem Glioma and Supratentorial High-Grade Glioma | Test the safety and feasibility of giving IL13-PE38QQR directly into regions of the brain in pediatric patients with DIPG or HGG, using CED | NCT00880061 | I | Terminated: did not reach the entire MRI-defined tumor volume in any patient, short-term radiographic effects were observed in 2 of the 5 patients treated. | [109,110] |
| Study of Convection-Enhanced, Image-Assisted Delivery of Liposomal-Irinotecan In Recurrent High-Grade Glioma | Dose toleration study to determine MTD of nanoliposomal irinotecan in adults with recurrent HGG by CED | NCT02022644 | I | Recruiting | [111] |
| IL13-PE38QQR Infusion After Tumor Resection, Followed by Radiation Therapy With or Without Temozolomide in Patients With Newly Diagnosed Malignant Glioma | Determine the highest dose of IL13-PE38QQR that can be safely administered by CED to the area around the tumor site after surgical resection and concurrent radiation or TMZ | NCT00089427 | I | Completed: Positive results, overall survival linked to catheter placement | [112,113] |
| Safety and Efficacy Study to Treat Recurrent Grade 4 Malignant Brain Tumors | To study the safety and efficacy of TP-38 at 100 ng/mL | NCT00104091 | II | Completed: Results pending | [114] |
| Maximum Tolerated Dose, Safety, and Efficacy of Rhenium Nanoliposomes in Recurrent Glioma (ReSPECT) | A multicenter, sequential cohort, open-label, volume, and dose-escalation study of the safety, tolerability, and distribution of 186RNL given by CED to patients with recurrent or progressive malignant glioma after standard surgical, radiation, and/or chemotherapy treatment | NCT01906385 | I/II | Recruiting | [115] |
| Safety Study of Replication-competent Adenovirus (Delta-24-RGD) in Patients With Recurrent Glioblastoma | To determine the safety and tolerability of Delta-24-RGD administered by CED to the tumor and the surrounding infiltrated brain in patients with recurrent GBM | NCT01582516 | I/II | Completed: Safe and robust replication of the AAV, killing of rHGG cells. ≥95% reduction in tumor size in some patients, 5 patients survived >3 years | [116,117] |
| A Dose-Escalation Phase I Study Of Human-Recombinant Bone Morphogenetic Protein 4 Administered Via CED In GBM Patients | To evaluate the feasibility and safety of intratumor and interstitial therapy with hBMP4 in increasing doses in patients with progressive and/or multiple recurrent GBM | NCT02869243 | I | Recruiting | [118] |
| The PRECISE Trial: Study of IL13-PE38QQR Compared to GLIADEL Wafer in Patients With Recurrent Glioblastoma Multiforme | To determine whether overall survival duration, safety, and quality of life are improved for patients treated with IL13-PE38QQR compared to patients treated with GLIADEL® Wafer following surgical tumor removal in treatment of first recurrence GBM | NCT00076986 | III | Completed: There was no survival difference between CB administered via CED and Gliadel® Wafer | [119,120] |
| Phase 1 Trial of D2C7-IT in Combination With i. 2141-V11 for Recurrent Malignant Glioma ii. Atezolimab for recurrent gliomas | Phase 1 study of D2C7-IT in combination with monoclonal antibodies | i. NCT04547777 ii. NCT04160494 iii. NCT02303678 | I | i. Not yet recruiting ii, iii. Recruiting | [121,122,123] |
| Phase 1b Study PVSRIPO for Recurrent Malignant Glioma in Children | Confirm the safety of the selected dose and potential toxicity of oncolytic poliovirus (PV) immunotherapy with PVSRIPO for pediatric patients with recurrent WHO grade III or IV malignant glioma, to determine MTD for phase 2 | i. NCT03043391, ii. NCT01491893, iii. NCT04479241 | I | i. Recruiting ii. Active, not recruiting | [124,125,126] |
| Phase IIb Clinical Trial With TGF-β2 Antisense Compound AP 12009 for Recurrent or Refractory High-Grade Glioma | Multinational dose-finding Phase IIb study of the efficacy and safety of two doses of AP 12009 (OT-101/trabedersen) compared to standard chemotherapy (TMZ or PCV) in adult patients with confirmed recurrent high-grade glioma | NCT00431561 | IIb | Completed: OT-101 is an effective agent against recurrent gliomas without the myelosuppression effects of chemotherapy, which is unavailable | [127,128,129] |
4.3. Biodegradable Wafers
5. Blood–Brain Barrier Disrupting Strategies
5.1. Osmotic Blood-Brain Barrier Disruption
5.2. Microbubble-Mediated Focused Ultrasound
6. Nanoparticles
6.1. Biological Vectors
6.1.1. Viral Vectors
6.1.2. Exosomes
6.1.3. Cell Delivery
6.2. Synthetic Vehicles
| Ref | [207,208,209] | [210,211,213] | [214,215,216,218] | [221,222] | [227,228] | [230,233,234] [231,235] |
|---|---|---|---|---|---|---|
| Examples | AAV9-hIFNβ, retroviral herpes simplex virus-thymidine kinase (HSV-tk), Toca 511 delivers suicide gene, cytosine deaminase (CD), and in combination with oral prodrug, adenoviral vector carrying the wild-type p53 gene (Ad-p53) | Paclitaxel with bEND.3 cell-derived exosome, doxorubicin with U-87 MG cell-derived exosome, miRNA-486-5p transferred exosomes, siKrasG12D iExosomes, tumor-cell-derived exosomes and α-GalCer on a DC-based vaccine | Neutrophil-mediated paclitaxel cationic liposomes, carboxylesterase-expressing allogeneic neural stem cells, bone morphogenetic protein 4 (BMP4) expressing adipose-derived mesenchymal stem cells, neural stem cells engineered to express membrane-bound TRAIL (NSCs-mTRAIL) | Iron oxide nanoparticles, gold nanoclusters, mesoporous silica nanoparticles, lanthanide upconversion particles | Cationized bovine serum albumin modified NPs, polysorbate 80 or poloxamer 188 overcoated NPs, apolipoprotein bound nanoparticles, | Transferrin receptor-targeted (OX26) immunoliposomes, LDLR-DHA nanoparticles, insulin-mAb-modified HSA NPs; Glutathione-modified liposomes, choline-derivate-modified NPs |
| Disadvantages | i. Limited brain tumor penetration ii. Highly invasive administration method iii. Prevailing risk of oncogenesis and lethality | i. Lacking standardized isolation and purification procedure, ii. Donor cells choice iii. Potential tumor induction risk of tumor cell-derived exosomes | i. Potentially toxic effects of the cargo on the cell carrier itself ii. Spatial and temporal release of the therapeutic agent iii. Limited loading efficiency | i. Neurotoxicity ii. Unspecific distribution | i. Poor selectivity ii. Protein adsorption and corona formation | i. Protein adsorption and corona formation ii. Potential neurotoxicity iii. Difficulty of manufacturing |
| Advantages | i. High efficiency for gene delivery, ii. Innate ability to infect cells | i. Nonimmunogenic ii. Stable and long circulation iii. Cross BBB iv. Target the tissue via their natural surface proteins | i. Cross BBB ii. Naturally recruited to sites of brain tumors | i. Ultrasmall size ii. Easily modified iii. Contrast imaging iv. Phototherapeutics | i. Electrostatic adsorption ii. Improve cellular uptake iii. Improve penetrating efficiency | i. High selectivity ii. Enhanced brain accumulation iii. Cross BBB iv. Decrease systemic toxicity |
| Strategy | AMT | RMT and TMT | ||||
| Viral vectors | Exosomes | Cell carriers | Passive diffusion | Actively targeted delivery | ||
| Biological vectors | Synthetic vehicles | |||||
7. Receptor-Mediated Transcytosis
7.1. BBB Shuttle Peptides
7.2. Antibody-Based Delivery Systems
8. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAV | Adeno-associated virus |
| ABC | ATP-binding cassette protein |
| AMT | Adsorptive-mediated transcytosis |
| BBB | Blood–brain barrier |
| BBBD | Blood–brain barrier disruption |
| BCRP | Breast cancer resistance protein |
| BTB | Blood–tumor barrier |
| CED | Convection-enhanced delivery |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| CVO | Circumventricular organs |
| DIPG | Diffuse intrinsic pontine glioma |
| EC | Endothelial cells |
| EGFR | Epidermal growth factor receptor |
| FDA | Food and Drug Administration |
| FUS | Focused ultrasound |
| GBM | Glioblastoma miltiforme |
| GLUT1 | Glucose transporter 1 |
| HDAC | Histone deacetylase |
| IGFR | Insulin-like growth factor receptor |
| IR | Insulin receptor |
| IT | Intrathecal |
| LDLR | Low-density lipoprotein receptor |
| LRP1 | Low-density lipoprotein-related protein 1 |
| MCT1 | Monocarboxylate transporter 1 |
| MNP | Magnetic nanoparticles |
| MSC | Mesenchymal stem cells |
| MRI | Magnetic resonance imaging |
| MRP | Multidrug resistance protein |
| NSC | Neural stem cell |
| NVU | Neurovascular unit |
| NVU/BBB | Neurovascular unit/blood–brain barrier |
| PDGFR-β | Platelet-derived growth factor-β |
| P-gp | P-glycoprotein |
| PLGA | poly(lactic-co-glycolic acid) |
| RMT | Receptor-mediated transcytosis |
| TfR1 | Transferrin receptor 1 |
| TMT | Transporter-mediated transcytosis |
| VEGF | Vascular endothelial growth factor |
References
- Sarkaria, J.N.; Hu, L.S.; Parney, I.F.; Pafundi, D.H.; Brinkmann, D.H.; Laack, N.N.; Giannini, C.; Burns, T.C.; Kizilbash, S.H.; Laramy, J.K.; et al. Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro-Oncology 2018, 20, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Pitz, M.W.; Desai, A.; Grossman, S.A.; Blakeley, J.O. Tissue concentration of systemically administered antineoplastic agents in human brain tumors. J. Neuro-Oncol. 2011, 104, 629–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arvold, N.D.; Lee, E.Q.; Mehta, M.P.; Margolin, K.; Alexander, B.M.; Lin, N.U.; Anders, C.K.; Soffietti, R.; Camidge, D.R.; Vogelbaum, M.A.; et al. Updates in the management of brain metastases. Neuro-Oncology 2016, 18, 1043–1065. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, T.; Ohtsuki, S. Brain-to-blood transporters for endogenous substrates and xenobiotics at the blood-brain barrier: An overview of biology and methodology. Neurotherapeutics 2005, 2, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol. Biomarkers Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, L.; Lee, E.Q.; Wen, P.Y. Epidemiology of brain metastases. Curr. Oncol. Rep. 2012. [Google Scholar] [CrossRef]
- Neuwelt, E.A.; Bauer, B.; Fahlke, C.; Fricker, G.; Iadecola, C.; Janigro, D.; Leybaert, L.; Molnár, Z.; O’Donnell, M.E.; Povlishock, J.T.; et al. Engaging neuroscience to advance translational research in brain barrier biology. Nat. Rev. Neurosci. 2011, 12, 169–182. [Google Scholar] [CrossRef] [Green Version]
- Mastorakos, P.; McGAVERN, D.B. The anatomy and immunology of vasculature in the central nervous system. Sci. Immunol. 2019, 4, eaav0492. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef]
- Daneman, R.; Zhou, L.; Kebede, A.A.; Barres, B.A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 2010, 468, 562–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Lin, W.J.; Tang, Y. New Insights into the Dysfunctions of Pericytes and Neurovascular Units in Neurodegenerative Diseases. Neurosci. Bull. 2020. [Google Scholar] [CrossRef] [PubMed]
- Pandit, R.; Chen, L.; Götz, J. The blood-brain barrier: Physiology and strategies for drug delivery. Adv. Drug Deliv. Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Lockman, P.R.; Mittapalli, R.K.; Taskar, K.S.; Rudraraju, V.; Gril, B.; Bohn, K.A.; Adkins, C.E.; Roberts, A.; Thorsheim, H.R.; Gaasch, J.A.; et al. Heterogeneous Blood-Tumor Barrier Permeability Determines Drug Efficacy in Experimental Brain Metastases of Breast Cancer. Clin. Cancer Res. 2010, 16, 5664–5678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argaw, A.T.; Gurfein, B.T.; Zhang, Y.; Zameer, A.; John, G.R. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc. Natl. Acad. Sci. USA 2009, 106, 1977–1982. [Google Scholar] [CrossRef] [Green Version]
- Watkins, S.; Robel, S.; Kimbrough, I.F.; Robert, S.M.; Ellis-Davies, G.; Sontheimer, H. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat. Commun. 2014, 5, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef]
- Lyle, L.T.; Lockman, P.R.; Adkins, C.E.; Mohammad, A.S.; Sechrest, E.; Hua, E.; Palmieri, D.; Liewehr, D.J.; Steinberg, S.M.; Kloc, W.; et al. Alterations in Pericyte Subpopulations Are Associated with Elevated Blood-Tumor Barrier Permeability in Experimental Brain Metastasis of Breast Cancer. Clin. Cancer Res. 2016, 22, 5287–5299. [Google Scholar] [CrossRef] [Green Version]
- Adkins, C.E.; Mohammad, A.S.; Terrell-Hall, T.B.; Dolan, E.L.; Shah, N.; Sechrest, E.; Griffith, J.; Lockman, P.R. Characterization of passive permeability at the blood-tumor barrier in five preclinical models of brain metastases of breast cancer. Clin. Exp. Metastasis 2016, 33, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Gampa, G.; Kenchappa, R.S.; Mohammad, A.S.; Parrish, K.E.; Kim, M.; Crish, J.F.; Luu, A.; West, R.; Hinojosa, A.Q.; Sarkaria, J.N.; et al. Enhancing Brain Retention of a KIF11 Inhibitor Significantly Improves its Efficacy in a Mouse Model of Glioblastoma. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.D.; Price, J.E.; Fujimaki, T.; Bucana, C.D.; Fidler, I.J. Differential permeability of the blood-brain barrier in experimental brain metastases produced by human neoplasms implanted into nude mice. Am. J. Pathol. 1992, 141, 1115–1124. [Google Scholar]
- Terrell-Hall, T.B.; Nounou, M.I.; El-Amrawy, F.; Griffith, J.I.G.; Lockman, P.R. Trastuzumab distribution in an in-vivo and in-vitro model of brain metastases of breast cancer. Oncotarget 2017, 8, 83734–83744. [Google Scholar] [CrossRef]
- Taskar, K.S.; Rudraraju, V.; Mittapalli, R.K.; Samala, R.; Thorsheim, H.R.; Lockman, J.; Gril, B.; Hua, E.; Palmieri, D.; Polli, J.W.; et al. Lapatinib Distribution in HER2 Overexpressing Experimental Brain Metastases of Breast Cancer. Pharm. Res. 2012, 29, 770–781. [Google Scholar] [CrossRef] [Green Version]
- Gril, B.; Wei, D.; Zimmer, A.S.; Robinson, C.; Khan, I.; Difilippantonio, S.; Overstreet, M.G.; Steeg, P.S. HER2 antibody-drug conjugate controls growth of breast cancer brain metastases in hematogenous xenograft models, with heterogeneous blood–tumor barrier penetration unlinked to a passive marker. Neuro-Oncology 2020, 22, 1625–1636. [Google Scholar] [CrossRef]
- Askoxylakis, V.; Ferraro, G.B.; Kodack, D.P.; Badeaux, M.; Shankaraiah, R.C.; Seano, G.; Kloepper, J.; Vardam, T.; Martin, J.D.; Naxerova, K.; et al. Preclinical Efficacy of Ado-trastuzumab Emtansine in the Brain Microenvironment. J. Natl. Cancer Inst. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- On, N.H.; Mitchell, R.; Savant, S.D.; Bachmeier, C.J.; Hatch, G.M.; Miller, D.W. Examination of blood-brain barrier (BBB) integrity in a mouse brain tumor model. J. Neuro-Oncol. 2013, 111, 133–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, S.; Elmquist, W.F. Insight into the Cooperation of P-glycoprotein (ABCB1) and Breast Cancer Resistance Protein (ABCG2) at the Blood–Brain Barrier: A Case Study Examining Sorafenib Efflux Clearance. Mol. Pharm. 2012, 9, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Parrish, K.E.; Pokorny, J.; Mittapalli, R.K.; Bakken, K.; Sarkaria, J.N.; Elmquist, W.F. Efflux Transporters at the Blood-Brain Barrier Limit Delivery and Efficacy of Cyclin-Dependent Kinase 4/6 Inhibitor Palbociclib (PD-0332991) in an Orthotopic Brain Tumor Model. J. Pharmacol. Exp. Ther. 2015, 355, 264–271. [Google Scholar] [CrossRef]
- Gampa, G.; Kim, M.; Mohammad, A.S.; Parrish, K.E.; Mladek, A.C.; Sarkaria, J.N.; Elmquist, W.F. Brain Distribution and Active Efflux of Three panRAF Inhibitors: Considerations in the Treatment of Melanoma Brain Metastases. J. Pharmacol. Exp. Ther. 2019, 368, 446–461. [Google Scholar] [CrossRef] [Green Version]
- Mittapalli, R.K.; Chung, A.H.; Parrish, K.E.; Crabtree, D.; Halvorson, K.G.; Hu, G.; Elmquist, W.F.; Becher, O.J. ABCG2 and ABCB1 Limit the Efficacy of Dasatinib in a PDGF-B-Driven Brainstem Glioma Model. Mol. Cancer Ther. 2016, 15, 819–829. [Google Scholar] [CrossRef] [Green Version]
- Pokorny, J.L.; Calligaris, D.; Gupta, S.K.; Iyekegbe, D.O.; Mueller, D.; Bakken, K.K.; Carlson, B.L.; Schroeder, M.A.; Evans, D.L.; Lou, Z.; et al. The efficacy of the wee1 inhibitor MK-1775 combined with temozolomide is limited by heterogeneous distribution across the blood-brain barrier in glioblastoma. Clin. Cancer Res. 2015, 21, 1916–1924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakoma, A.; Barbieri, E.; Agarwal, S.; Jackson, J.; Chen, Z.; Kim, Y.; McVay, M.; Shohet, J.M.; Kim, E.S. The MDM2 small-molecule inhibitor RG7388 leads to potent tumor inhibition in p53 wild-type neuroblastoma. Cell Death Discov. 2015, 1, 15026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.; Ma, D.J.; Calligaris, D.; Zhang, S.; Feathers, R.W.; Vaubel, R.A.; Meaux, I.; Mladek, A.C.; Parrish, K.E.; Jin, F.; et al. Efficacy of the MDM2 Inhibitor SAR405838 in Glioblastoma Is Limited by Poor Distribution Across the Blood–Brain Barrier. Mol. Cancer Ther. 2018, 17, 1893–1901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breckwoldt, M.O.; Bode, J.; Sahm, F.; Krüwel, T.; Solecki, G.; Hahn, A.; Wirthschaft, P.; Berghoff, A.S.; Haas, M.; Venkataramani, V.; et al. Correlated MRI and ultramicroscopy (MR-UM) of brain tumors reveals vast heterogeneity of tumor infiltration and neoangiogenesis in preclinical models and human disease. Front. Neurosci. 2019, 12, 1004. [Google Scholar] [CrossRef] [Green Version]
- Fink, J.R.; Muzi, M.; Peck, M.; Krohn, K.A. Multimodality brain tumor imaging: MR imaging, PET, and PET/MR imaging. J. Nucl. Med. 2015, 56, 1554–1561. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, M.; Tanaka, R.; Takeda, N. Magnetic resonance imaging and histopathology of cerebral gliomas. Neuroradiology 1992, 34, 463–469. [Google Scholar] [CrossRef]
- Kelly, P.J.; Daumas-Duport, C.; Kispert, D.B.; Kall, B.A.; Scheithauer, B.W.; Illig, J.J. Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J. Neurosurg. 1987, 66, 865–874. [Google Scholar] [CrossRef]
- Pessina, F.; Navarria, P.; Cozzi, L.; Ascolese, A.M.; Simonelli, M.; Santoro, A.; Clerici, E.; Rossi, M.; Scorsetti, M.; Bello, L. Maximize surgical resection beyond contrast-enhancing boundaries in newly diagnosed glioblastoma multiforme: Is it useful and safe? A single institution retrospective experience. J. Neuro-Oncol. 2017, 135, 129–139. [Google Scholar] [CrossRef]
- Sanai, N.; Berger, M.S. Glioma extent of resection and its impact on patient outcome. Neurosurgery 2008, 62, 753–766. [Google Scholar] [CrossRef] [Green Version]
- Brown, T.J.; Brennan, M.C.; Li, M.; Church, E.W.; Brandmeir, N.J.; Rakszawski, K.L.; Patel, A.S.; Rizk, E.B.; Suki, D.; Sawaya, R.; et al. Association of the Extent of Resection With Survival in Glioblastoma. JAMA Oncol. 2016, 2, 1460–1469. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, S.; Mittapalli, R.K.; Zellmer, D.M.; Gallardo, J.L.; Donelson, R.; Seiler, C.; Decker, S.A.; SantaCruz, K.S.; Pokorny, J.L.; Sarkaria, J.N.; et al. Active efflux of dasatinib from the brain limits efficacy against murine glioblastoma: Broad implications for the clinical use of molecularly targeted agents. Mol. Cancer Ther. 2012, 11, 2183–2192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanai, N.; Li, J.; Boerner, J.; Stark, K.; Wu, J.; Kim, S.; Derogatis, A.; Mehta, S.; Dhruv, H.D.; Heilbrun, L.K.; et al. Phase 0 trial of azd1775 in first-recurrence glioblastoma patients. Clin. Cancer Res. 2018, 24, 3820–3828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milano, M.T.; Okunieff, P.; Donatello, R.S.; Mohile, N.A.; Sul, J.; Walter, K.A.; Korones, D.N. Patterns and timing of recurrence after temozolomide-based chemoradiation for glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1147–1155. [Google Scholar] [CrossRef]
- Osswald, M.; Blaes, J.; Liao, Y.; Solecki, G.; Gömmel, M.; Berghoff, A.S.; Salphati, L.; Wallin, J.J.; Phillips, H.S.; Wick, W.; et al. Impact of blood-brain barrier integrity on tumor growth and therapy response in brain metastases. Clin. Cancer Res. 2016, 22, 6078–6087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salphati, L.; Heffron, T.P.; Alicke, B.; Nishimura, M.; Barck, K.; Carano, R.A.; Cheong, J.; Edgar, K.A.; Greve, J.; Kharbanda, S.; et al. Targeting the PI3K pathway in the brain—Efficacy of a PI3K inhibitor optimized to cross the blood-brain barrier. Clin. Cancer Res. 2012, 18, 6239–6248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballard, P.; Yates, J.W.T.; Yang, Z.; Kim, D.W.; Yang, J.C.H.; Cantarini, M.; Pickup, K.; Jordan, A.; Hickey, M.; Grist, M.; et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin. Cancer Res. 2016, 22, 5130–5140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reungwetwattana, T.; Nakagawa, K.; Cho, B.C.; Cobo, M.; Cho, E.K.; Bertolini, A.; Bohnet, S.; Zhou, C.; Lee, K.H.; Nogami, N.; et al. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 3290–3297. [Google Scholar] [CrossRef]
- Fowler, M.J.; Cotter, J.D.; Knight, B.E.; Sevick-Muraca, E.M.; Sandberg, D.I.; Sirianni, R.W. Intrathecal drug delivery in the era of nanomedicine. Adv. Drug Deliv. Rev. 2020. [Google Scholar] [CrossRef]
- Costa, R.; Kumthekar, P. Management of central nervous system metastases in breast cancer. In The Breast: Comprehensive Management of Benign and Malignant Diseases; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 942–960.e7. ISBN 9780323359559. [Google Scholar]
- Ammaya, A.K. Subcutaneous Reservoir and Pump For Sterile Access to Ventricular Cerebrospinal Fluid. Lancet 1963, 186, 983–984. [Google Scholar] [CrossRef]
- Witorsch, P.; Williams, T.W.; Ommaya, A.K.; Utz, J.P. Intraventricular Administration of Amphotericin B: Use of Subcutaneous Reservoir in Four Patients With Mycotic Meningitis. JAMA J. Am. Med. Assoc. 1965, 194, 699–702. [Google Scholar] [CrossRef]
- Ozerov, S.; Thomale, U.W.; Schulz, M.; Schaumann, A.; Samarin, A.; Kumirova, E. The use of a smartphone-assisted ventricle catheter guide for Ommaya reservoir placement—Experience of a retrospective bi-center study. Child’s Nerv. Syst. 2018, 34, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Malani, R.; Fleisher, M.; Kumthekar, P.; Lin, X.; Omuro, A.; Groves, M.D.; Lin, N.U.; Melisko, M.; Lassman, A.B.; Jeyapalan, S.; et al. Cerebrospinal fluid circulating tumor cells as a quantifiable measurement of leptomeningeal metastases in patients with HER2 positive cancer. J. Neuro-Oncol. 2020, 148, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Pluchart, H.; Jacquet, E.; Charlety, D.; Allenet, B.; Bedouch, P.; Mousseau, M. Long-Term Survivor with Intrathecal and Intravenous Trastuzumab Treatment in Metastatic Breast Cancer. Target. Oncol. 2016, 11, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.T.; Raizer, J.; Gabor, E.P.; Liu, N.M.; Vu, J.Q.; Slamon, D.J.; Barstis, J.L. Intrathecal trastuzumab: Immunotherapy improves the prognosis of leptomeningeal metastases in HER-2+ breast cancer patient. J. Immunother. Cancer 2015, 3, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulia, S.; Gupta, S.; Singh, A. Intrathecal trastuzumab for leptomeningeal carcinomatosis in patients with human epidermal growth factor receptor 2 positive breast cancer. Indian J. Med. Paediatr. Oncol. 2016, 37, 196–198. [Google Scholar] [CrossRef] [Green Version]
- Figura, N.B.; Long, W.; Yu, M.; Robinson, T.J.; Mokhtari, S.; Etame, A.B.; Tran, N.D.; Diaz, R.; Soliman, H.; Han, H.S.; et al. Intrathecal trastuzumab in the management of HER2+ breast leptomeningeal disease: A single institution experience. Breast Cancer Res. Treat. 2018, 169, 391–396. [Google Scholar] [CrossRef]
- García, F.J.V.; Carrión, N.P.; de la Cruz-Merino, L. Long-term complete response to intrathecal trastuzumab in a patient with leptomeningeal carcinomatosis due to her2- overexpressing breast cancer. Medicine 2020, 99, e18298. [Google Scholar] [CrossRef]
- Zagouri, F.; Zoumpourlis, P.; Le Rhun, E.; Bartsch, R.; Zografos, E.; Apostolidou, K.; Dimopoulos, M.-A.; Preusser, M. Intrathecal administration of anti-HER2 treatment for the treatment of meningeal carcinomatosis in breast cancer: A metanalysis with meta-regression. Cancer Treat. Rev. 2020, 88, 102046. [Google Scholar] [CrossRef]
- Mrugala, M.M.; Kim, B.; Sharma, A.; Johnson, N.; Graham, C.; Kurland, B.F.; Gralow, J. Phase II Study of Systemic High-dose Methotrexate and Intrathecal Liposomal Cytarabine for Treatment of Leptomeningeal Carcinomatosis From Breast Cancer. Clin. Breast Cancer 2019, 19, 311–316. [Google Scholar] [CrossRef]
- Scott, B.J.; Oberheim-Bush, N.A.; Kesari, S. Leptomeningeal metastasis in breast cancer-a systematic review. Oncotarget 2016, 7, 3740–3747. [Google Scholar] [CrossRef] [Green Version]
- Taillibert, S.; Chamberlain, M.C. Leptomeningeal Metastasis. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 149, pp. 169–204. [Google Scholar]
- Niwińska, A.; Rudnicka, H.; Murawska, M. Breast cancer leptomeningeal metastasis: The results of combined treatment and the comparison of methotrexate and liposomal cytarabine as intra-cerebrospinal fluid chemotherapy. Clin. Breast Cancer 2015, 15, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Yang, G.; He, H.; Zhao, G.; Yuan, T.; Li, Y.; Shi, W.; Gao, P.; Dong, L.; Li, Y. Concurrent radiotherapy and intrathecal methotrexate for treating leptomeningeal metastasis from solid tumors with adverse prognostic factors: A prospective and single-arm study. Int. J. Cancer 2016, 139, 1864–1872. [Google Scholar] [CrossRef] [PubMed]
- Assi, H.I.; Mahmoud, T.; Saadeh, F.S.; El Darsa, H. Management of leptomeningeal metastasis in breast cancer. Clin. Neurol. Neurosurg. 2018, 172, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.J.; van Vugt, V.A.; Rush, T.; Brown, T.; Chen, C.C.; Carter, B.S.; Schwab, R.; Fanta, P.; Helsten, T.; Bazhenova, L.; et al. Concurrent intrathecal methotrexate and liposomal cytarabine for leptomeningeal metastasis from solid tumors: A retrospective cohort study. J. Neuro-Oncol. 2014, 119, 361–368. [Google Scholar] [CrossRef]
- Rothwell, W.T.; Bell, P.; Richman, L.K.; Limberis, M.P.; Tretiakova, A.P.; Li, M.; Wilson, J.M. Intrathecal viral vector delivery of trastuzumab prevents or inhibits tumor growth of human HER2-positive xenografts in mice. Cancer Res. 2018, 78, 6171–6182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutova, M.; Flores, L.; Adhikarla, V.; Tsaturyan, L.; Tirughana, R.; Aramburo, S.; Metz, M.; Gonzaga, J.; Annala, A.; Synold, T.W.; et al. Quantitative Evaluation of Intraventricular Delivery of Therapeutic Neural Stem Cells to Orthotopic Glioma. Front. Oncol. 2019, 9, 68. [Google Scholar] [CrossRef] [Green Version]
- Householder, K.T.; Dharmaraj, S.; Sandberg, D.I.; Wechsler-Reya, R.J.; Sirianni, R.W. Fate of nanoparticles in the central nervous system after intrathecal injection in healthy mice. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.M.; Chen, M.C.; Tsai, C.M.; Perng, R.P. Intrathecal gemcitabine chemotherapy for non-small cell lung cancer patients with meningeal carcinomatosis—A case report. Lung Cancer 2003, 40, 99–101. [Google Scholar] [CrossRef]
- Jabbour, E.; O’Brien, S.; Kantarjian, H.; Garcia-Manero, G.; Ferrajoli, A.; Ravandi, F.; Cabanillas, M.; Thomas, D.A. Neurologic complications associated with intrathecal liposomal cytarabine given prophylactically in combination with high-dose methotrexate and cytarabine to patients with acute lymphocytic leukemia. Blood 2007, 109, 3214–3218. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, C.; Wang, H.; Tao, Q.; Xiong, S.; Zhai, Z. Transverse myelopathy occurring with intrathecal administration of methotrexate and cytarabine chemotherapy: A case report. Oncol. Lett. 2016, 11, 4066–4068. [Google Scholar] [CrossRef] [Green Version]
- Chotsampancharoen, T.; Sripornsawan, P.; Wongchanchailert, M. Two Fatal Cases of Accidental Intrathecal Vincristine Administration: Learning from Death Events. Chemotherapy 2016, 61, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Nair, A. Implications of Intrathecal Chemotherapy for Anaesthesiologists: A Brief Review. Scientifica 2016, 2016, 3759845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Partap, S.; Murphy, P.A.; Vogel, H.; Barnes, P.D.; Edwards, M.S.B.; Fisher, P.G. Liposomal cytarabine for central nervous system embryonal tumors in children and young adults. J. Neuro-Oncol. 2011, 103, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, R.K.; Woodbury, D.M. Penetration of 14C-inulin and 14C-sucrose into brain, cerebrospinal fluid, and skeletal muscle of developing rats. Exp. Brain Res. 1969, 7, 181–194. [Google Scholar] [CrossRef]
- Bergman, I.; Burckart, G.; Pohi, C.R.; Venkataramanan, R.; Barmada, M.A.; Griffin, J.A.; Cheung, N.K. Pharmacokinetics of IgG and IgM anti-ganglioside antibodies in rats and monkeys after intrathecal administration. ASPET 1998, 284, 111–115. [Google Scholar]
- Collins, J.M. Pharmacokinetics of intraventricular administration. J. Neuro-Oncol. 1983, 1, 283–291. [Google Scholar] [CrossRef]
- Pardridge, W.M. CSF, blood-brain barrier, and brain drug delivery. Expert Opin. Drug Deliv. 2016, 13, 963–975. [Google Scholar] [CrossRef]
- Hunt Bobo, R.; Laske, D.W.; Akbasak, A.; Morrison, P.F.; Dedrick, R.L.; Oldfield, E.H. Convection-enhanced delivery of macromolecules in the brain. Proc. Natl. Acad. Sci. USA 1994, 91, 2076–2080. [Google Scholar] [CrossRef] [Green Version]
- Mehta, A.M.; Sonabend, A.M.; Bruce, J.N. Convection-Enhanced Delivery. Neurotherapeutics 2017, 14, 358–371. [Google Scholar] [CrossRef] [Green Version]
- Saka, R.; Sathe, P.; Khan, W. Brain local delivery strategy. In Brain Targeted Drug Delivery System; Elsevier: Amsterdam, The Netherlands, 2019; pp. 241–286. [Google Scholar]
- Tosi, U.; Kommidi, H.; Adeuyan, O.; Guo, H.; Maachani, U.B.; Chen, N.; Su, T.; Zhang, G.; Pisapia, D.J.; Dahmane, N.; et al. PET, image-guided HDAC inhibition of pediatric diffuse midline glioma improves survival in murine models. Sci. Adv. 2020, 6, eabb4105. [Google Scholar] [CrossRef]
- Pang, H.H.; Chen, P.Y.; Wei, K.C.; Huang, C.W.; Shiue, Y.L.; Huang, C.Y.; Yang, H.W. Convection-enhanced delivery of a virus-like nanotherapeutic agent with dual-modal imaging for besiegement and eradication of brain tumors. Theranostics 2019, 9, 1752–1763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Nance, E.A.; Mastorakos, P.; Chisholm, J.; Berry, S.; Eberhart, C.; Tyler, B.; Brem, H.; Suk, J.S.; Hanes, J. Convection enhanced delivery of cisplatin-loaded brain penetrating nanoparticles cures malignant glioma in rats. J. Control. Release 2017, 263, 112–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahangiri, A.; Chin, A.T.; Flanigan, P.M.; Chen, R.; Bankiewicz, K.; Aghi, M.K. Convection-enhanced delivery in glioblastoma: A review of preclinical and clinical studies. J. Neurosurg. 2017, 126, 191–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokosawa, M.; Sonoda, Y.; Sugiyama, S.; Saito, R.; Yamashita, Y.; Nishihara, M.; Satoh, T.; Kumabe, T.; Yokoyama, M.; Tominaga, T. Convection-enhanced delivery of a synthetic retinoid Am80, loaded into polymeric micelles, prolongs the survival of rats bearing intracranial glioblastoma xenografts. Tohoku J. Exp. Med. 2010, 221, 257–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grahn, A.Y.; Bankiewicz, K.S.; Dugich-Djordjevic, M.; Bringas, J.R.; Hadaczek, P.; Johnson, G.A.; Eastman, S.; Luz, M. Non-PEGylated liposomes for convection-enhanced delivery of topotecan and gadodiamide in malignant glioma: Initial experience. J. Neuro-Oncol. 2009, 95, 185–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.Y.; Li, R.J.; Huang, C.Y.; Wei, K.C.; Chen, P.Y. Controlled release of liposome-encapsulated temozolomide for brain tumour treatment by convection-enhanced delivery. J. Drug Target. 2018, 26, 325–332. [Google Scholar] [CrossRef]
- Nordling-David, M.M.; Yaffe, R.; Guez, D.; Meirow, H.; Last, D.; Grad, E.; Salomon, S.; Sharabi, S.; Levi-Kalisman, Y.; Golomb, G.; et al. Liposomal temozolomide drug delivery using convection enhanced delivery. J. Control. Release 2017, 261, 138–146. [Google Scholar] [CrossRef]
- Zhang, R.; Saito, R.; Mano, Y.; Sumiyoshi, A.; Kanamori, M.; Sonoda, Y.; Kawashima, R.; Tominaga, T. Convection-enhanced delivery of SN-38-loaded polymeric micelles (NK012) enables consistent distribution of SN-38 and is effective against rodent intracranial brain tumor models. Drug Deliv. 2016, 23, 2780–2786. [Google Scholar] [CrossRef] [Green Version]
- Rechberger, J.S.; Power, E.A.; Lu, V.M.; Zhang, L.; Sarkaria, J.N.; Daniels, D.J. Evaluating infusate parameters for direct drug delivery to the brainstem: A comparative study of convection-enhanced delivery versus osmotic pump delivery. Neurosurg. Focus 2020, 48. [Google Scholar] [CrossRef] [Green Version]
- Zhan, W.; Wang, C.H. Convection enhanced delivery of liposome encapsulated doxorubicin for brain tumour therapy. J. Control. Release 2018, 285, 212–229. [Google Scholar] [CrossRef]
- Zhan, W.; Arifin, D.Y.; Lee, T.K.; Wang, C.H. Mathematical Modelling of Convection Enhanced Delivery of Carmustine and Paclitaxel for Brain Tumour Therapy. Pharm. Res. 2017, 34, 860–873. [Google Scholar] [CrossRef] [PubMed]
- Singleton, W.G.B.; Bieneman, A.S.; Woolley, M.; Johnson, D.; Lewis, O.; Wyatt, M.J.; Damment, S.J.P.; Boulter, L.J.; Killick-Cole, C.L.; Asby, D.J.; et al. The distribution, clearance, and brainstem toxicity of panobinostat administered by convection-enhanced delivery. Proc. J. Neurosurg. Pediatrics Am. Assoc. Neurol. Surg. 2018, 22, 288–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MTX110 by Convection-Enhanced Delivery in Treating Participants with Newly-Diagnosed Diffuse Intrinsic Pontine Glioma-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03566199?term=convection+enhanced+delivery&draw=2&rank=1 (accessed on 21 September 2020).
- Chronic Convection Enhanced Delivery of Topotecan-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03154996?term=convection+enhanced+delivery&draw=2&rank=2 (accessed on 21 September 2020).
- Intratumorally-Administered Topotecan Using Convection-Enhanced Delivery in Patients With Grade III/IV Glioma-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03927274?term=convection+enhanced+delivery&draw=2&rank=4 (accessed on 21 September 2020).
- Topotecan Using Convection-Enhanced Delivery (CED) in High Grade Glioma-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02278510 (accessed on 3 October 2020).
- Vogelbaum, M.A.; Brewer, C.; Barnett, G.H.; Mohammadi, A.M.; Peereboom, D.M.; Ahluwalia, M.S.; Gao, S. First-in-human evaluation of the Cleveland Multiport Catheter for convection-enhanced delivery of topotecan in recurrent high-grade glioma: Results of pilot trial 1. J. Neurosurg. 2018, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safety Study of Intracerebral Topotecan for Recurrent Brain Tumors-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT00308165?term=convection+enhanced+delivery&draw=3&rank=17 (accessed on 21 September 2020).
- CED With Irinotecan Liposome Injection Using Real Time Imaging in Children with Diffuse Intrinsic Pontine Glioma (DIPG) (PNOC 009)-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03086616?term=convection+enhanced+delivery&draw=2&rank=3 (accessed on 21 September 2020).
- Convection-Enhanced Delivery of 124I-Omburtamab for Patients With Non-Progressive Diffuse Pontine Gliomas Previously Treated With External Beam Radiation Therapy-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01502917?term=convection+enhanced+delivery&draw=2&rank=5 (accessed on 21 September 2020).
- CED of MTX110 Newly Diagnosed Diffuse Midline Gliomas-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04264143?term=convection+enhanced+delivery&draw=2&rank=6 (accessed on 21 September 2020).
- Carboplatin in Treating Patients With Recurrent High-Grade Gliomas-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01644955?term=convection+enhanced+delivery&draw=2&rank=7 (accessed on 21 September 2020).
- Zacks Small Cap Research-MDNA.TO: New Data for MDNA55 and MDNA11 Presented at ASCO 2020. Available online: https://scr.zacks.com/News/Press-Releases/Press-Release-Details/2020/MDNATO-New-Data-for-MDNA55-and-MDNA11-Presented-at-ASCO-2020/default.aspx (accessed on 21 September 2020).
- Randazzo, D.; Achrol, A.; Aghi, M.K.; Bexon, M.; Brem, S.; Brenner, A.J.; Butowski, N.A.; Chandhasin, C.; Chowdhary, S.A.; Coello, M.; et al. MDNA55: A locally administered IL4 guided toxin as a targeted treatment for recurrent glioblastoma. J. Clin. Oncol. 2019, 37, 2039. [Google Scholar] [CrossRef]
- Convection-Enhanced Delivery (CED) of MDNA55 in Adults with Recurrent or Progressive Glioblastoma-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02858895?term=convection+enhanced+delivery&draw=2&rank=10 (accessed on 21 September 2020).
- Chittiboina, P.; Heiss, J.D.; Warren, K.E.; Lonser, R.R. Magnetic resonance imaging properties of convective delivery in diffuse intrinsic pontine gliomas: Clinical article. J. Neurosurg. Pediatr. 2014, 13, 273–275. [Google Scholar] [CrossRef]
- An Open Label Dose Escalation Safety Study of Convection-Enhanced Delivery of IL13-PE38QQR in Patients with Progressive Pediatric Diffuse Infiltrating Brainstem Glioma and Supratentorial High-Grade Glioma-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT00880061?term=convection+enhanced+delivery&draw=3&rank=11 (accessed on 21 September 2020).
- Study of Convection-Enhanced, Image-Assisted Delivery of Liposomal-Irinotecan In Recurrent High Grade Glioma-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02022644?term=convection+enhanced+delivery&draw=3&rank=18 (accessed on 21 September 2020).
- Kunwar, S.; Prados, M.D.; Chang, S.M.; Berger, M.S.; Lang, F.F.; Piepmeier, J.M.; Sampson, J.H.; Ram, Z.; Gutin, P.H.; Gibbons, R.D.; et al. Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: A report by the cintredekin besudotox intraparenchymal study group. J. Clin. Oncol. 2007, 25, 837–844. [Google Scholar] [CrossRef]
- IL13-PE38QQR Infusion After Tumor Resection, Followed by Radiation Therapy With or Without Temozolomide in Patients With Newly Diagnosed Malignant Glioma-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT00089427 (accessed on 3 October 2020).
- Safety and Efficacy Study to Treat Recurrent Grade 4 Malignant Brain Tumors-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT00104091?term=convection+enhanced+delivery&draw=5&rank=34 (accessed on 21 September 2020).
- Maximum Tolerated Dose, Safety, and Efficacy of Rhenium Nanoliposomes in Recurrent Glioma (ReSPECT)-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01906385?term=convection+enhanced+delivery&draw=5&rank=31 (accessed on 21 September 2020).
- Lang, F.F.; Conrad, C.; Gomez-Manzano, C.; Alfred Yung, W.K.; Sawaya, R.; Weinberg, J.S.; Prabhu, S.S.; Rao, G.; Fuller, G.N.; Aldape, K.D.; et al. Phase I study of DNX-2401 (delta-24-RGD) oncolytic adenovirus: Replication and immunotherapeutic effects in recurrent malignant glioma. J. Clin. Oncol. 2018, 36, 1419–1427. [Google Scholar] [CrossRef]
- Safety Study of Replication-Competent Adenovirus (Delta-24-Rgd) in Patients with Recurrent Glioblastoma-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01582516?term=convection+enhanced+delivery&draw=4&rank=22 (accessed on 21 September 2020).
- A Dose Escalation Phase I Study Of Human-Recombinant Bone Morphogenetic Protein 4 Administrated Via CED In GBM Patients-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02869243?term=convection+enhanced+delivery&draw=4&rank=23 (accessed on 21 September 2020).
- Kunwar, S.; Chang, S.; Westphal, M.; Vogelbaum, M.; Sampson, J.; Barnett, G.; Shaffrey, M.; Ram, Z.; Piepmeier, J.; Prados, M.; et al. Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro-Oncology 2010, 12, 871–881. [Google Scholar] [CrossRef] [Green Version]
- The PRECISE Trial: Study of IL13-PE38QQR Compared to GLIADEL Wafer in Patients With Recurrent Glioblastoma Multiforme-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT00076986?term=convection+enhanced+delivery&draw=4&rank=25 (accessed on 21 September 2020).
- Phase 1 Trial of D2C7-IT in Combination With 2141-V11 for Recurrent Malignant Glioma-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04547777?term=convection+enhanced+delivery&draw=4&rank=26 (accessed on 21 September 2020).
- D2C7-IT With Atezolizumab for Recurrent Gliomas-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04160494?term=convection+enhanced+delivery&draw=4&rank=29 (accessed on 21 September 2020).
- D2C7 for Adult Patients With Recurrent Malignant Glioma-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02303678?term=convection+enhanced+delivery&draw=4&rank=21 (accessed on 21 September 2020).
- Phase 1b Study PVSRIPO for Recurrent Malignant Glioma in Children-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03043391?term=convection+enhanced+delivery&draw=4&rank=28 (accessed on 21 September 2020).
- PVSRIPO for Recurrent Glioblastoma (GBM)-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01491893?term=convection+enhanced+delivery&draw=4&rank=30 (accessed on 21 September 2020).
- PVSRIPO and Pembrolizumab in Patients With Recurrent Glioblastoma-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04479241?term=convection+enhanced+delivery&draw=5&rank=36 (accessed on 21 September 2020).
- Available online: http://oncotelic.com/wp-content/uploads/AACR-2019-GBM-Final-For-Web.pdf (accessed on 21 September 2020).
- Phase IIb Clinical Trial With TGF-β2 Antisense Compound AP 12009 for Recurrent or Refractory High-Grade Glioma-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT00431561?term=convection+enhanced+delivery&draw=5&rank=38 (accessed on 21 September 2020).
- Uckun, F.M.; Qazi, S.; Hwang, L.; Trieu, V.N. Recurrent or refractory high-grade gliomas treated by convection-enhanced delivery of a TGFΒ2-targeting RNA therapeutic: A post-hoc analysis with long-term follow-up. Cancers 2019, 11, 1892. [Google Scholar] [CrossRef] [Green Version]
- FDA. GLIADEL® WAFER (Carmustine Implant), for Intracranial Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/020637s029lbl.pdf (accessed on 21 September 2020).
- Brem, H.; Mahaley, M.S.; Vick, N.A.; Black, K.L.; Schold, S.C.; Burger, P.C.; Friedman, A.H.; Ciric, I.S.; Eller, T.W.; Cozzens, J.W.; et al. Interstitial chemotherapy with drug polymer implants for the treatment of recurrent gliomas. J. Neurosurg. 1991, 74, 441–446. [Google Scholar] [CrossRef]
- Ashby, L.S.; Smith, K.A.; Stea, B. Gliadel wafer implantation combined with standard radiotherapy and concurrent followed by adjuvant temozolomide for treatment of newly diagnosed high-grade glioma: A systematic literature review. World J. Surg. Oncol. 2016, 14, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, T.; Fukai, J.; Kodama, Y.; Hirose, T.; Okita, Y.; Moriuchi, S.; Nonaka, M.; Tsuyuguchi, N.; Terakawa, Y.; Uda, T.; et al. Characteristics and outcomes of elderly patients with diffuse gliomas: A multi-institutional cohort study by Kansai Molecular Diagnosis Network for CNS Tumors. J. Neuro-Oncol. 2018, 140, 329–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wait, S.D.; Prabhu, R.S.; Burri, S.H.; Atkins, T.G.; Asher, A.L. Polymeric drug delivery for the treatment of glioblastoma. Neuro. Oncol. 2015, 17, ii9–ii23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lechapt-Zalcman, E.; Levallet, G.; Dugué, A.E.; Vital, A.; Diebold, M.D.; Menei, P.; Colin, P.; Peruzzy, P.; Emery, E.; Bernaudin, M.; et al. O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation and low MGMT-encoded protein expression as prognostic markers in glioblastoma patients treated with biodegradable carmustine wafer implants after initial surgery followed by radiotherapy with concomitant and adjuvant temozolomide. Cancer 2012, 118, 4545–4554. [Google Scholar] [CrossRef] [PubMed]
- Metellus, P.; Coulibaly, B.; Nanni, I.; Fina, F.; Eudes, N.; Giorgi, R.; Barrie, M.; Chinot, O.; Fuentes, S.; Dufour, H.; et al. Prognostic impact of O6-methylguanine-DNA methyltransferase silencing in patients with recurrent glioblastoma multiforme who undergo surgery and carmustine wafer implantation: A prospective patient cohort. Cancer 2009, 115, 4783–4794. [Google Scholar] [CrossRef]
- Gutenberg, A.; Bock, H.C.; Brück, W.; Doerner, L.; Mehdorn, H.M.; Roggendorf, W.; Westphal, M.; Felsberg, J.; Reifenberger, G.; Giese, A. MGMT promoter methylation status and prognosis of patients with primary or recurrent glioblastoma treated with carmustine wafers. Br. J. Neurosurg. 2013, 27, 772–778. [Google Scholar] [CrossRef]
- Ducray, F.; Honnorat, J. New adjuvant chemotherapy for glioblastoma. Press. Medicale 2007, 36, 1249–1254. [Google Scholar] [CrossRef]
- Fukai, J.; Nishibayashi, H.; Uematsu, Y.; Kanemura, Y.; Fujita, K.; Nakao, N. Rapid regression of glioblastoma following carmustine wafer implantation: A case report. Mol. Clin. Oncol. 2016, 5, 153–157. [Google Scholar] [CrossRef] [Green Version]
- Sippl, C.; Ketter, R.; Braun, L.; Teping, F.; Schoeneberger, L.; Kim, Y.J.; List, M.; Nakhoda, A.; Wemmert, S.; Oertel, J.; et al. miRNA-26a expression influences the therapy response to carmustine wafer implantation in patients with glioblastoma multiforme. Acta Neurochir. 2019, 161, 2299–2309. [Google Scholar] [CrossRef]
- Shapira-Furman, T.; Serra, R.; Gorelick, N.; Doglioli, M.; Tagliaferri, V.; Cecia, A.; Peters, M.; Kumar, A.; Rottenberg, Y.; Langer, R.; et al. Biodegradable wafers releasing Temozolomide and Carmustine for the treatment of brain cancer. J. Control. Release 2019, 295, 93–101. [Google Scholar] [CrossRef]
- Lee, J.; Cho, H.R.; Cha, G.D.; Seo, H.; Lee, S.; Park, C.K.; Kim, J.W.; Qiao, S.; Wang, L.; Kang, D.; et al. Flexible, sticky, and biodegradable wireless device for drug delivery to brain tumors. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Santini, J.T.; Cima, M.J.; Langer, R. A controlled-release microchip. Nature 1999, 397, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Frazier, J.L.; Wang, P.P.; Case, D.; Tyler, B.M.; Pradilla, G.; Weingart, J.D.; Brem, H. Local delivery of minocycline and systemic BCNU have synergistic activity in the treatment of intracranial glioma. J. Neuro-Oncol. 2003, 64, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Bow, H.; Hwang, L.S.; Schildhaus, N.; Xing, J.; Murray, L.; Salditch, Q.; Ye, X.; Zhang, Y.; Weingart, J.; Brem, H.; et al. Local delivery of angiogenesis-inhibitor minocycline combined with radiotherapy and oral temozolomide chemotherapy in 9L glioma: Laboratory investigation. J. Neurosurg. 2014, 120, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Weingart, J.D.; Sipos, E.P.; Brem, H. The role of minocycline in the treatment of intracranial 9L glioma. J. Neurosurg. 1995, 82, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Li, K.W.; Dang, W.; Tyler, B.M.; Troiano, G.; Tihan, T.; Brem, H.; Walter, K.A. Polilactofate microspheres for paclitaxel delivery to central nervous system malignancies. Clin. Cancer Res. 2003, 9, 3441–3447. [Google Scholar]
- Graham-Gurysh, E.; Moore, K.M.; Satterlee, A.B.; Sheets, K.T.; Lin, F.C.; Bachelder, E.M.; Miller, C.R.; Hingtgen, S.D.; Ainslie, K.M. Sustained Delivery of Doxorubicin via Acetalated Dextran Scaffold Prevents Glioblastoma Recurrence after Surgical Resection. Mol. Pharm. 2018, 15, 1309–1318. [Google Scholar] [CrossRef]
- Yuan, X.; Dillehay, L.E.; Williams, J.R.; Williams, J.A. Synthetic, implantable polymers for IUdR radiosensitization of experimental human malignant glioma. Cancer Biother. Radiopharm. 1999, 14, 187–202. [Google Scholar] [CrossRef]
- Yuan, X.; Tabassi, K.; Williams, J.A. Implantable polymers for tirapazamine treatments of experimental intracranial malignant glioma. Radiat. Oncol. Investig. 1999, 7, 218–230. [Google Scholar] [CrossRef]
- Ranganath, S.H.; Wang, C.H. Biodegradable microfiber implants delivering paclitaxel for post-surgical chemotherapy against malignant glioma. Biomaterials 2008, 29, 2996–3003. [Google Scholar] [CrossRef]
- Fleming, A.B.; Saltzman, W.M. Pharmacokinetics of the carmustine implant. Clin. Pharmacokinet. 2002, 41, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Graham-Gurysh, E.G.; Murthy, A.B.; Moore, K.M.; Hingtgen, S.D.; Bachelder, E.M.; Ainslie, K.M. Synergistic drug combinations for a precision medicine approach to interstitial glioblastoma therapy. J. Control. Release 2020, 323, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Storm, P.B.; Moriarity, J.L.; Tyler, B.; Burger, P.C.; Brem, H.; Weingart, J. Polymer delivery of camptothecin against 9L gliosarcoma: Release, distribution, and efficacy. J. Neuro-Oncol. 2002, 56, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Sampath, P.; Amundson, E.; Wall, M.E.; Tyler, B.M.; Wani, M.C.; Alderson, L.M.; Colvin, M.; Brem, H.; Weingart, J.D. Camptothecin analogs in malignant gliomas: Comparative analysis and characterization. J. Neurosurg. 2003, 98, 570–577. [Google Scholar] [CrossRef]
- Sampath, P.; Rhines, L.D.; DiMeco, F.; Tyler, B.M.; Park, M.C.; Brem, H. Interstitial docetaxel (Taxotere), ccarmustine and ccombined interstitial ttherapy: A novel treatment for experimental malignant glioma. J. Neuro-Oncol. 2006, 80, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Shibahara, I.; Hanihara, M.; Watanabe, T.; Dan, M.; Sato, S.; Kuroda, H.; Inamura, A.; Inukai, M.; Hara, A.; Yasui, Y.; et al. Tumor microenvironment after biodegradable BCNU wafer implantation: Special consideration of immune system. J. Neuro-Oncol. 2018, 137, 417–427. [Google Scholar] [CrossRef]
- Wolinsky, J.B.; Colson, Y.L.; Grinstaff, M.W. Local drug delivery strategies for cancer treatment: Gels, nanoparticles, polymeric films, rods, and wafers. J. Control. Release 2012, 159, 14–26. [Google Scholar] [CrossRef] [Green Version]
- Thiagarajah, J.R.; Kim, J.K.; Magzoub, M.; Verkman, A.S. Slowed diffusion in tumors revealed by microfiberoptic epifluorescence photobleaching. Nat. Methods 2006, 3, 275–280. [Google Scholar] [CrossRef]
- Rapoport, S.I.; Hori, M.; Klatzo, I. Testing of a hypothesis for osmotic opening of the blood-brain barrier. Am. J. Physiol. 1972, 223, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Neuwelt, E.A. Reversible osmotic blood-brain barrier disruption in humans: Implications for the chemotherapy of malignant brain tumors. Neurosurgery 1980, 7, 204. [Google Scholar] [CrossRef]
- Kroll, R.A.; Neuwelt, E.A. Outwitting the blood-brain barrier for therapeutic purposes: Osmotic opening and other means. Neurosurgery 1998, 42, 1083–1100. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.B.; Duff, T.A.; Javid, M.J. Treatment of increased intracranial pressure: A comparison of different hyperosmotic agents and the use of thiopental. Neurosurgery 1979, 5, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, S.I. Osmotic opening of the blood-brain barrier: Principles, mechanism, and therapeutic applications. Cell. Mol. Neurobiol. 2000, 20, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Kenta, A. Sex differences in Drosophila behavior: Qualitative and quantitative dimorphism. Curr. Opin. Physiol. 2018, 6, 35–45. [Google Scholar] [CrossRef]
- Neuwelt, E.A.; Goldman, D.L.; Dahlborg, S.A.; Crossen, J.; Ramsey, F.; Roman-Goldstein, S.; Braziel, R.; Dana, B. Primary CNS lymphoma treated with osmotic blood-brain barrier disruption: Prolonged survival and preservation of cognitive function. J. Clin. Oncol. 1991, 9, 1580–1590. [Google Scholar] [CrossRef]
- Angelov, L.; Doolittle, N.D.; Kraemer, D.F.; Siegal, T.; Barnett, G.H.; Peereboom, D.M.; Stevens, G.; McGregor, J.; Jahnke, K.; Lacy, C.A.; et al. Blood-brain barrier disruption and intra-arterial methotrexate-based therapy for newly diagnosed primary CNS lymphoma: A multi-institutional experience. J. Clin. Oncol. 2009, 27, 3503–3509. [Google Scholar] [CrossRef] [Green Version]
- Doolittle, N.D.; Korfel, A.; Lubow, M.A.; Schorb, E.; Schlegel, U.; Rogowski, S.; Fu, R.; Dósa, E.; Illerhaus, G.; Kraemer, D.F.; et al. Long-term cognitive function, neuroimaging, and quality of life in primary CNS lymphoma. Neurology 2013, 81, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Tyson, R.M.; Siegal, T.; Doolittle, N.D.; Lacy, C.; Kraemer, D.F.; Neuwelt, E.A. Current status and future of relapsed primary central nervous system lymphoma (PCNSL). Leuk. Lymphoma 2003, 44, 627–633. [Google Scholar] [CrossRef]
- Siegal, T.; Rubinstein, R.; Bokstein, F.; Schwartz, A.; Lossos, A.; Shalom, E.; Chisin, R.; Gomori, J.M. In vivo assessment of the window of barrier opening after osmotic blood- brain barrier disruption in humans. J. Neurosurg. 2000, 92, 599–605. [Google Scholar] [CrossRef] [Green Version]
- Rapoport, S.I.; Fredericks, W.R.; Ohno, K.; Pettigrew, K.D. Quantitative aspects of reversible osmotic opening of the blood-brain barrier. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1980, 7, 421–431. [Google Scholar] [CrossRef]
- Bellavance, M.A.; Blanchette, M.; Fortin, D. Recent advances in blood-brain barrier disruption as a CNS delivery strategy. AAPS J. 2008, 10, 166–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemper, E.M.; Boogerd, W.; Thuis, I.; Beijnen, J.H.; van Tellingen, O. Modulation of the blood-brain barrier in oncology: Therapeutic opportunities for the treatment of brain tumours? Cancer Treat. Rev. 2004, 30, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Doolittle, N.D.; Miner, M.E.; Hall, W.A.; Siegal, T.; Hanson, E.J.; Osztie, E.; McAllister, L.D.; Bubalo, J.S.; Kraemer, D.F.; Fortin, D.; et al. Safety and efficacy of a multicenter study using intraarterial chemotherapy in conjunction with osmotic opening of the blood-brain barrier for the treatment of patients with malignant brain tumors. Cancer 2000, 88, 637–647. [Google Scholar] [CrossRef]
- Sheikov, N.; McDannold, N.; Sharma, S.; Hynynen, K. Effect of Focused Ultrasound Applied With an Ultrasound Contrast Agent on the Tight Junctional Integrity of the Brain Microvascular Endothelium. Ultrasound Med. Biol. 2008, 34, 1093–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hynynen, K.; Jolesz, F.A. Demonstration of potential noninvasive ultrasound brain therapy through an intact skull. Ultrasound Med. Biol. 1998, 24, 275–283. [Google Scholar] [CrossRef]
- Lipsman, N.; Meng, Y.; Bethune, A.J.; Huang, Y.; Lam, B.; Masellis, M.; Herrmann, N.; Heyn, C.; Aubert, I.; Boutet, A.; et al. Blood–brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat. Commun. 2018, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Treat, L.H.; McDannold, N.; Vykhodtseva, N.; Zhang, Y.; Tam, K.; Hynynen, K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int. J. Cancer 2007, 121, 901–907. [Google Scholar] [CrossRef]
- Choi, J.J.; Pernot, M.; Small, S.A.; Konofagou, E.E. Noninvasive, transcranial and localized opening of the blood-brain barrier using focused ultrasound in mice. Ultrasound Med. Biol. 2007, 33, 95–104. [Google Scholar] [CrossRef]
- Himuro, S. Physicochemical characteristics of microbubbles. Kagaku Koguku 2007, 71, 165–169. [Google Scholar] [CrossRef]
- Timbie, K.F.; Mead, B.P.; Price, R.J. Drug and gene delivery across the blood-brain barrier with focused ultrasound. J. Control. Release 2015, 219, 61–75. [Google Scholar] [CrossRef] [Green Version]
- Tung, Y.-S.; Vlachos, F.; Feshitan, J.A.; Borden, M.A.; Konofagou, E.E. The mechanism of interaction between focused ultrasound and microbubbles in blood-brain barrier opening in mice. J. Acoust. Soc. Am. 2011, 130, 3059–3067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, H.C.; Tsai, C.H.; Chen, W.S.; Inserra, C.; Wei, K.C.; Liu, H.L. Safety evaluation of frequent application of microbubble-enhanced focused ultrasound blood-brain-barrier opening. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Idbaih, A.; Canney, M.; Belin, L.; Desseaux, C.; Vignot, A.; Bouchoux, G.; Asquier, N.; Law-Ye, B.; Leclercq, D.; Bissery, A.; et al. Safety and feasibility of repeated and transient blood-brain barrier disruption by pulsed ultrasound in patients with recurrent glioblastoma. Clin. Cancer Res. 2019, 25, 3793–3801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpentier, A.; Canney, M.; Vignot, A.; Reina, V.; Beccaria, K.; Horodyckid, C.; Karachi, C.; Leclercq, D.; Lafon, C.; Chapelon, J.Y.; et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci. Transl. Med. 2016, 8. [Google Scholar] [CrossRef]
- Mainprize, T.; Lipsman, N.; Huang, Y.; Meng, Y.; Bethune, A.; Ironside, S.; Heyn, C.; Alkins, R.; Trudeau, M.; Sahgal, A.; et al. Blood-Brain Barrier Opening in Primary Brain Tumors with Non-invasive MR-Guided Focused Ultrasound: A Clinical Safety and Feasibility Study. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Aryal, M.; Vykhodtseva, N.; Zhang, Y.Z.; McDannold, N. Evaluation of permeability, doxorubicin delivery, and drug retention in a rat brain tumor model after ultrasound-induced blood-tumor barrier disruption. J. Control. Release 2017, 250, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Kovacs, Z.; Werner, B.; Rassi, A.; Sass, J.O.; Martin-Fiori, E.; Bernasconi, M. Prolonged survival upon ultrasound-enhanced doxorubicin delivery in two syngenic glioblastoma mouse models. J. Control. Release 2014, 187, 74–82. [Google Scholar] [CrossRef]
- Park, E.J.; Zhang, Y.Z.; Vykhodtseva, N.; McDannold, N. Ultrasound-mediated blood-brain/blood-tumor barrier disruption improves outcomes with trastuzumab in a breast cancer brain metastasis model. J. Control. Release 2012, 163, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Kobus, T.; Zervantonakis, I.K.; Zhang, Y.; McDannold, N.J. Growth inhibition in a brain metastasis model by antibody delivery using focused ultrasound-mediated blood-brain barrier disruption. J. Control. Release 2016, 238, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.L.; Hsu, P.H.; Lin, C.Y.; Huang, C.W.; Chai, W.Y.; Chu, P.C.; Huang, C.Y.; Chen, P.Y.; Yang, L.Y.; Kuo, J.S.; et al. Focused ultrasound enhances central nervous system delivery of Bevacizumab for malignant glioma treatment. Radiology 2016, 281, 99–108. [Google Scholar] [CrossRef]
- Yang, F.Y.; Chang, W.Y.; Lin, W.T.; Hwang, J.J.; Chien, Y.C.; Wang, H.E.; Tsai, M.L. Focused ultrasound enhanced molecular imaging and gene therapy for multifusion reporter gene in glioma-bearing rat model. Oncotarget 2015, 6, 36260–36268. [Google Scholar] [CrossRef] [Green Version]
- Chang, E.L.; Ting, C.Y.; Hsu, P.H.; Lin, Y.C.; Liao, E.C.; Huang, C.Y.; Chang, Y.C.; Chan, H.L.; Chiang, C.S.; Liu, H.L.; et al. Angiogenesis-targeting microbubbles combined with ultrasound-mediated gene therapy in brain tumors. J. Control. Release 2017, 255, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Treat, L.H.; McDannold, N.; Zhang, Y.; Vykhodtseva, N.; Hynynen, K. Improved Anti-Tumor Effect of Liposomal Doxorubicin After Targeted Blood-Brain Barrier Disruption by MRI-Guided Focused Ultrasound in Rat Glioma. Ultrasound Med. Biol. 2012, 38, 1716–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.Y.; Wang, H.E.; Liu, R.S.; Teng, M.C.; Li, J.J.; Lu, M.; Wei, M.C.; Wong, T.T. Pharmacokinetic Analysis of 111In-Labeled Liposomal Doxorubicin in Murine Glioblastoma after Blood-Brain Barrier Disruption by Focused Ultrasound. PLoS ONE 2012, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ting, C.Y.; Fan, C.H.; Liu, H.L.; Huang, C.Y.; Hsieh, H.Y.; Yen, T.C.; Wei, K.C.; Yeh, C.K. Concurrent blood-brain barrier opening and local drug delivery using drug-carrying microbubbles and focused ultrasound for brain glioma treatment. Biomaterials 2012, 33, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.H.; Ting, C.Y.; Chang, Y.C.; Wei, K.C.; Liu, H.L.; Yeh, C.K. Drug-loaded bubbles with matched focused ultrasound excitation for concurrent blood-brain barrier opening and brain-tumor drug delivery. Acta Biomater. 2015, 15, 89–101. [Google Scholar] [CrossRef]
- Chen, P.Y.; Liu, H.L.; Hua, M.Y.; Yang, H.W.; Huang, C.Y.; Chu, P.C.; Lyu, L.A.; Tseng, I.C.; Feng, L.Y.; Tsai, H.C.; et al. Novel magnetic/ultrasound focusing system enhances nanoparticle drug delivery for glioma treatment. Neuro. Oncol. 2010, 12, 1050–1060. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.L.; Hua, M.Y.; Yang, H.W.; Huang, C.Y.; Chu, P.N.; Wu, J.S.; Tseng, I.C.; Wang, J.J.; Yen, T.C.; Chen, P.Y.; et al. Magnetic resonance monitoring of focused ultrasound/magnetic nanoparticle targeting delivery of therapeutic agents to the brain. Proc. Natl. Acad. Sci. USA 2010, 107, 15205–15210. [Google Scholar] [CrossRef] [Green Version]
- Burgess, A.; Hynynen, K. Noninvasive and targeted drug delivery to the brain using focused ultrasound. ACS Chem. Neurosci. 2013, 4, 519–526. [Google Scholar] [CrossRef] [Green Version]
- Arvanitis, C.D.; Askoxylakis, V.; Guo, Y.; Datta, M.; Kloepper, J.; Ferraro, G.B.; Bernabeu, M.O.; Fukumura, D.; McDannold, N.; Jain, R.K. Mechanisms of enhanced drug delivery in brain metastases with focused ultrasound-induced blood–tumor barrier disruption. Proc. Natl. Acad. Sci. USA 2018, 115, E8717–E8726. [Google Scholar] [CrossRef] [Green Version]
- Burgess, A.; Shah, K.; Hough, O.; Hynynen, K. Endothelial cell metabolism in normal and diseased vasculature. Circ. Res. 2016, 116, 1231–1244. [Google Scholar] [CrossRef]
- Miller, D.B.; O’Callaghan, J.P. New horizons for focused ultrasound (FUS)—Therapeutic applications in neurodegenerative diseases. Metabolism 2017, 69, S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Lockman, P.R.; Mumper, R.J.; Khan, M.A.; Allen, D.D. Nanoparticle Technology for Drug Delivery Across the Blood-Brain Barrier. Drug Dev. Ind. Pharm. 2002, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Caffery, B.; Lee, J.S.; Alexander-Bryant, A.A. Vectors for Glioblastoma Gene Therapy: Viral & Non-Viral Delivery Strategies. Nanomaterials 2019, 9, 105. [Google Scholar] [CrossRef] [Green Version]
- Dong, X. Current Strategies for Brain Drug Delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.T.; Hlavaty, J.; Ostertag, D.; Espinoza, F.L.; Martin, B.; Petznek, H.; Rodriguez-Aguirre, M.; Ibañez, C.E.; Kasahara, N.; Gunzburg, W.; et al. Toca 511 gene transfer and 5-fluorocytosine in combination with temozolomide demonstrates synergistic therapeutic efficacy in a temozolomide-sensitive glioblastoma model. Cancer Gene Ther. 2013, 20, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Guhasarkar, D.; Su, Q.; Gao, G.; Sena-Esteves, M. Systemic AAV9-IFNβ gene delivery treats highly invasive glioblastoma. Neuro-Oncology 2016, 18, 1508–1518. [Google Scholar] [CrossRef] [Green Version]
- Lang, F.F.; Bruner, J.M.; Fuller, G.N.; Aldape, K.; Prados, M.D.; Chang, S.; Berger, M.S.; McDermoff, M.W.; Kunwar, S.M.; Junck, L.R.; et al. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: Biological and clinical results. J. Clin. Oncol. 2003, 21, 2508–2518. [Google Scholar] [CrossRef]
- Liao, W.; Du, Y.; Zhang, C.; Pan, F.; Yao, Y.; Zhang, T.; Peng, Q. Exosomes: The next generation of endogenous nanomaterials for advanced drug delivery and therapy. Acta Biomater. 2019, 86, 1–14. [Google Scholar] [CrossRef]
- Yang, T.; Martin, P.; Fogarty, B.; Brown, A.; Schurman, K.; Phipps, R.; Yin, V.P.; Lockman, P.; Bai, S. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio Rerio. Pharm. Res. 2015, 32, 2003–2014. [Google Scholar] [CrossRef]
- Ha, D.; Yang, N.; Nadithe, V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharm. Sin. B 2016, 6, 287–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Chen, L.; Liu, J.; Meng, H.; Zhang, R.; Ma, L.; Wu, L.; Yu, S.; Shi, F.; Li, Y.; et al. Co-delivery of tumor-derived exosomes with alpha-galactosylceramide on dendritic cell-based immunotherapy for glioblastoma. Cancer Lett. 2017, 411, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Aboody, K.S.; Najbauer, J.; Danks, M.K. Stem and progenitor cell-mediated tumor selective gene therapy. Gene Ther. 2008, 15, 739–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheets, K.T.; Bagó, J.R.; Hingtgen, S.D. Delivery of cytotoxic mesenchymal stem cells with biodegradable scaffolds for treatment of postoperative brain cancer. In Targeted Drug Delivery; Sirianni, R.W., Behkam, B., Eds.; Springer International Publishing: Cham, Switzerland, 2018; Volume 1831, pp. 49–58. [Google Scholar]
- Xue, J.; Zhao, Z.; Zhang, L.; Xue, L.; Shen, S.; Wen, Y.; Wei, Z.; Wang, L.; Kong, L.; Sun, H.; et al. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat. Nanotechnol. 2017, 12, 692–700. [Google Scholar] [CrossRef]
- Hersh, D.S.; Wadajkar, A.S.; Roberts, N.B.; Perez, J.G.; Connolly, N.P.; Frenkel, V.; Winkles, J.A.; Woodworth, G.F.; Kim, A.J. Evolving Drug Delivery Strategies to Overcome the Blood Brain Barrier. Curr. Pharm. Des. 2016, 22, 1177–1193. [Google Scholar] [CrossRef] [Green Version]
- Balyasnikova, I.V.; Ferguson, S.D.; Han, Y.; Liu, F.; Lesniak, M.S. Therapeutic effect of neural stem cells expressing TRAIL and bortezomib in mice with glioma xenografts. Cancer Lett. 2011, 310, 148–159. [Google Scholar] [CrossRef] [Green Version]
- Parodi, A.; Rudzinska, M.; Deviatkin, A.A.; Soond, S.M.; Baldin, A.V.; Zamyatnin, J.A.A. Established and Emerging Strategies for Drug Delivery Across the Blood-Brain Barrier in Brain Cancer. Pharmaceutics 2019, 11, 245. [Google Scholar] [CrossRef] [Green Version]
- Batrakova, E.V.; Gendelman, H.E.; Kabanov, A.V. Cell-mediated drug delivery. Expert Opin. Drug Deliv. 2011, 8, 415–433. [Google Scholar] [CrossRef] [Green Version]
- Luther, D.C.; Huang, R.; Jeon, T.; Zhang, X.; Lee, Y.-W.; Nagaraj, H.; Rotello, V.M. Delivery of drugs, proteins, and nucleic acids using inorganic nanoparticles. Adv. Drug Deliv. Rev. 2020. [Google Scholar] [CrossRef]
- Sawicki, K.; Czajka, M.; Matysiak-Kucharek, M.; Fal, B.; Drop, B.; Mȩczyńska-Wielgosz, S.; Sikorska, K.; Kruszewski, M.; Kapka-Skrzypczak, L. Toxicity of metallic nanoparticles in the central nervous system. Nanotechnol. Rev. 2019, 8, 175–200. [Google Scholar] [CrossRef] [Green Version]
- Azcona, P.; Zysler, R.; Lassalle, V. Simple and novel strategies to achieve shape and size control of magnetite nanoparticles intended for biomedical applications. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 504, 320–330. [Google Scholar] [CrossRef] [Green Version]
- Prades, R.; Guerrero, S.; Araya, E.; Molina, C.; Salas, E.; Zurita, E.; Selva, J.; Egea, G.; López-Iglesias, C.; Teixidó, M.; et al. Delivery of gold nanoparticles to the brain by conjugation with a peptide that recognizes the transferrin receptor. Biomaterials 2012, 33, 7194–7205. [Google Scholar] [CrossRef] [PubMed]
- Múzquiz-Ramos, E.M.; Guerrero-Chávez, V.; Macías-Martínez, B.I.; López-Badillo, C.M.; García-Cerda, L.A. Synthesis and characterization of maghemite nanoparticles for hyperthermia applications. Ceram. Int. 2015, 41, 397–402. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J. Mechanism of polymeric nanoparticle-based drug transport across the blood-brain barrier (BBB). J. Microencapsul. 2013. [Google Scholar] [CrossRef] [PubMed]
- Jallouli, Y.; Paillard, A.; Chang, J.; Sevin, E.; Betbeder, D. Influence of surface charge and inner composition of porous nanoparticles to cross blood-brain barrier in vitro. Int. J. Pharm. 2007, 344, 103–109. [Google Scholar] [CrossRef]
- Lu, W.; Wan, J.; She, Z.; Jiang, X. Brain delivery property and accelerated blood clearance of cationic albumin conjugated pegylated nanoparticle. J. Control. Release 2007, 118, 38–53. [Google Scholar] [CrossRef]
- Mendes, M.; Sousa, J.; Pais, A.A.; Vitorino, C. Targeted Theranostic Nanoparticles for Brain Tumor Treatment. Pharmaceutics 2018, 10, 181. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhou, L.; Ye, D.; Huang, S.; Shao, K.; Huang, R.; Han, L.; Liu, Y.; Liu, S.; Ye, L.; et al. Choline-derivate-modified nanoparticles for brain-targeting gene delivery. Adv. Mater. 2011, 23, 4516–4520. [Google Scholar] [CrossRef]
- Rip, J.; Chen, L.; Hartman, R.; Van Den Heuvel, A.; Reijerkerk, A.; Van Kregten, J.; Van Der Boom, B.; Appeldoorn, C.; De Boer, M.; Maussang, D.; et al. Glutathione PEGylated liposomes: Pharmacokinetics and delivery of cargo across the blood-brain barrier in rats. J. Drug Target. 2014, 22, 460–467. [Google Scholar] [CrossRef] [Green Version]
- Johnsen, K.B.; Burkhart, A.; Melander, F.; Kempen, P.J.; Vejlebo, J.B.; Siupka, P.; Nielsen, M.S.; Andresen, T.L.; Moos, T. Targeting transferrin receptors at the blood-brain barrier improves the uptake of immunoliposomes and subsequent cargo transport into the brain parenchyma. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Knobloch, T.; Kreuter, J. Targeting the insulin receptor: Nanoparticles for drug delivery across the blood-brain barrier (BBB). J. Drug Target. 2011. [Google Scholar] [CrossRef] [PubMed]
- Mulik, R.S.; Bing, C.; Ladouceur-Wodzak, M.; Munaweera, I.; Chopra, R.; Corbin, I.R. Localized delivery of low-density lipoprotein docosahexaenoic acid nanoparticles to the rat brain using focused ultrasound. Biomaterials 2016, 83, 257–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, S.; Sane, R.; Oberoi, R.; Ohlfest, J.R.; Elmquist, W.F. Delivery of molecularly targeted therapy to malignant glioma, a disease of the whole brain. Expert Rev. Mol. Med. 2011, 13, e17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.J.; Zhang, Y.; Kenrick, M.; Hoyte, K.; Luk, W.; Lu, Y.; Atwal, J.; Elliott, J.M.; Prabhu, S.; Watts, R.J.; et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci. Transl. Med. 2011, 3, 84ra44. [Google Scholar] [CrossRef] [PubMed]
- Oller-Salvia, B.; Sánchez-Navarro, M.; Giralt, E.; Teixidó, M. Blood-brain barrier shuttle peptides: An emerging paradigm for brain delivery. Chem. Soc. Rev. 2016, 45, 4690–4707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haqqani, A.S.; Thom, G.; Burrell, M.; Delaney, C.E.; Brunette, E.; Baumann, E.; Sodja, C.; Jezierski, A.; Webster, C.; Stanimirovic, D.B. Intracellular sorting and transcytosis of the rat transferrin receptor antibody OX26 across the blood–brain barrier in vitro is dependent on its binding affinity. J. Neurochem. 2018, 146, 735–752. [Google Scholar] [CrossRef] [Green Version]
- Uchida, Y.; Yagi, Y.; Takao, M.; Takao, M.; Tano, M.; Umetsu, M.; Hirano, S.; Usui, T.; Tachikawa, M.; Terasaki, T. Comparison of Absolute Protein Abundances of Transporters and Receptors among Blood-Brain Barriers at Different Cerebral Regions and the Blood-Spinal Cord Barrier in Humans and Rats. Mol. Pharm. 2020, 17, 2006–2020. [Google Scholar] [CrossRef]
- Uchida, Y.; Ohtsuki, S.; Katsukura, Y.; Ikeda, C.; Suzuki, T.; Kamiie, J.; Terasaki, T. Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors. J. Neurochem. 2011, 117, 333–345. [Google Scholar] [CrossRef]
- Aasen, S.N.; Espedal, H.; Holte, C.F.; Keunen, O.; Karlsen, T.V.; Tenstad, O.; Maherally, Z.; Miletic, H.; Hoang, T.; Eikeland, A.V.; et al. Improved drug delivery to brain metastases by peptide-mediated permeabilization of the blood–brain barrier. Mol. Cancer Ther. 2019, 18, 2171–2181. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, G.; Curran, G.L.; Sarkaria, J.N.; Lowe, V.J.; Jenkins, R.B. Peptide carrier-mediated non-covalent delivery of unmodified cisplatin, methotrexate and other agents via intravenous route to the brain. PLoS ONE 2014, 9, e97655. [Google Scholar] [CrossRef] [PubMed]
- Bird, G.H.; Madani, N.; Perry, A.F.; Princiotto, A.M.; Supko, J.G.; He, X.; Gavathiotis, E.; Sodroski, J.G.; Walensky, L.D. Hydrocarbon double-stapling remedies the proteolytic instability of a lengthy peptide therapeutic. Proc. Natl. Acad. Sci. USA 2010, 107, 14093–14098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardridge, W.M. Blood-Brain Barrier and Delivery of Protein and Gene Therapeutics to Brain. Front. Aging Neurosci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.R.; Morgan, E.H. The transferrin homologue, melanotransferrin (p97), is rapidly catabolized by the liver of the rat and does not effectively donate iron to the brain. Biochim. Biophys. Acta-Mol. Basis Dis. 2004, 1690, 124–133. [Google Scholar] [CrossRef] [Green Version]
- Gobinda, S.; Geoffry, C.; Robert, J. Crossing the blood-brain barrier: Brain delivery of unmodified cancer therapeutics via intravenous route mediated by a peptide transporter. Neuro. Oncol. 2013, 15, iii37–iii61. [Google Scholar] [CrossRef] [Green Version]
- Demeule, M.; Regina, A.; Ché, C.; Poirier, J.; Nguyen, T.; Gabathuler, R.; Castaigne, J.P.; Béliveau, R. Identification and design of peptides as a new drug delivery system for the brain. J. Pharmacol. Exp. Ther. 2008, 324, 1064–1072. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Jin, K.; Pang, Q.; Shen, S.; Yan, Z.; Jiang, T.; Zhu, X.; Yu, L.; Pang, Z.; Jiang, X. On-Demand Drug Release from Dual-Targeting Small Nanoparticles Triggered by High-Intensity Focused Ultrasound Enhanced Glioblastoma-Targeting Therapy. ACS Appl. Mater. Interfaces 2017, 9, 31612–31625. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiang, Y.; Meng, F.; Deng, C.; Cheng, R.; Zhang, J.; Feijen, J.; Zhong, Z. Highly efficacious and specific anti-glioma chemotherapy by tandem nanomicelles co-functionalized with brain tumor-targeting and cell-penetrating peptides. J. Control. Release 2018. [Google Scholar] [CrossRef]
- Shi, Y.; Jiang, Y.; Cao, J.; Yang, W.; Zhang, J.; Meng, F.; Zhong, Z. Boosting RNAi therapy for orthotopic glioblastoma with nontoxic brain-targeting chimaeric polymersomes. J. Control. Release 2018, 292, 163–171. [Google Scholar] [CrossRef]
- Regina, A.; Demeule, M.; Tripathy, S.; Lord-Dufour, S.; Currie, J.C.; Iddir, M.; Annabi, B.; Castaigne, J.P.; Lachowicz, J.E. ANG4043, a novel brain-penetrant peptide-mAb conjugate, is efficacious against HER2-positive intracranial tumors in mice. Mol. Cancer Ther. 2015. [Google Scholar] [CrossRef] [Green Version]
- Hoyos-Ceballos, G.P.; Ruozi, B.; Ottonelli, I.; Da Ros, F.; Vandelli, M.A.; Forni, F.; Daini, E.; Vilella, A.; Zoli, M.; Tosi, G.; et al. PLGA-PEG-Ang–2 nanoparticles for blood–brain barrier crossing: Proof-of-concept study. Pharmaceutics 2020, 12, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Xiong, Z.; Liu, Z.; Huang, X.; Jiang, X. Angiopep-2/IP10-EGFRvIIIscFv modified nanoparticles and CTL synergistically inhibit malignant glioblastoma. Sci. Rep. 2018, 8, 12827. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Nyberg, S.; Sharp, P.S.; Madsen, J.; Daneshpour, N.; Armes, S.P.; Berwick, J.; Azzouz, M.; Shaw, P.; Abbott, N.J.; et al. LRP-1-mediated intracellular antibody delivery to the Central Nervous System. Sci. Rep. 2015, 5, srep11990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumthekar, P.; Tang, S.; Brenner, A.J.; Kesari, S.; Anders, C.K.; Carrillo, J.A.; Chalasani, P.; Kabos, P.; Ahluwalia, M.S.; Ibrahim, N.K. OS7.2 A Phase II Study of ANG1005, a novel BBB/BCB Penetratant Taxane in Patients with Recurrent Brain Metastases and Leptomeningeal Carcinomatosis from Breast Cancer. Neuro-Oncology 2016, 18, iv16. [Google Scholar] [CrossRef] [Green Version]
- Kumthekar, P.; Tang, S.C.; Brenner, A.J.; Kesari, S.; Piccioni, D.E.; Anders, C.; Carrillo, J.; Chalasani, P.; Kabos, P.; Puhalla, S.; et al. ANG1005, a Brain-Penetrating Peptide–Drug Conjugate, Shows Activity in Patients with Breast Cancer with Leptomeningeal Carcinomatosis and Recurrent Brain Metastases. Clin. Cancer Res. 2020, 26, 2789–2799. [Google Scholar] [CrossRef] [Green Version]
- Thomas, F.C.; Taskar, K.; Rudraraju, V.; Goda, S.; Thorsheim, H.R.; Gaasch, J.A.; Mittapalli, R.K.; Palmieri, D.; Steeg, P.S.; Lockman, P.R.; et al. Uptake of ANG1005, a novel paclitaxel derivative, through the blood-brain barrier into brain and experimental brain metastases of breast cancer. Pharm. Res. 2009, 26, 2486–2494. [Google Scholar] [CrossRef] [Green Version]
- Gaillard, P.J.; Appeldoorn, C.C.M.; Dorland, R.; Van Kregten, J.; Manca, F.; Vugts, D.J.; Windhorst, B.; Van Dongen, G.A.M.S.; De Vries, H.E.; Maussang, D.; et al. Pharmacokinetics, brain delivery, and efficacy in brain tumor-bearing mice of glutathione pegylated liposomal doxorubicin (2B3-101). PLoS ONE 2014, 9, e82331. [Google Scholar] [CrossRef] [Green Version]
- Brandsma, D.; Dieras, V.; Altintas, S.; Anders, C.; Arnedos, M.; Gelderblom, H.; Soetekouw, P.; Jager, A.; van Linde, M.; Aftimos, P. p08.03 * 2b3-101, glutathione pegylated liposomal doxorubicin, in patients with recurrent high grade gliomas and breast cancer brain metastases. Neuro. Oncol. 2014, 16, ii50–ii51. [Google Scholar] [CrossRef] [Green Version]
- Recht, L.; Torres, C.O.; Smith, T.W.; Raso, V.; Griffin, T.W. Transferrin receptor in normal and neoplastic brain tissue: Implications for brain-tumor immunotherapy. J. Neurosurg. 1990, 72, 941–945. [Google Scholar] [CrossRef]
- Kim, G.; Kim, M.; Lee, Y.; Byun, J.W.; Hwang, D.W.; Lee, M. Systemic delivery of microRNA-21 antisense oligonucleotides to the brain using T7-peptide decorated exosomes. J. Control. Release 2020, 317, 273–281. [Google Scholar] [CrossRef]
- Kuang, Y.; An, S.; Guo, Y.; Huang, S.; Shao, K.; Liu, Y.; Li, J.; Ma, H.; Jiang, C. T7 peptide-functionalized nanoparticles utilizing RNA interference for glioma dual targeting. Int. J. Pharm. 2013, 454, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Novak, T.; Miller, K.; Zhu, Y.; Kenney, M.E.; Broome, A.M. Transferrin receptor-targeted theranostic gold nanoparticles for photosensitizer delivery in brain tumors. Nanoscale 2015, 7, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, T.; Chai, Z.; Samulski, R.J.; Li, C. Blood-brain barrier shuttle peptides enhance AAV transduction in the brain after systemic administration. Biomaterials 2018, 176, 71–83. [Google Scholar] [CrossRef]
- Abuqayyas, L.; Balthasar, J.P. Investigation of the role of FcγR and FcRn in mAb distribution to the brain. Mol. Pharm. 2013, 10, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pardridge, W.M. Mediated efflux of IgG molecules from brain to blood across the blood-brain barrier. J. Neuroimmunol. 2001, 114, 168–172. [Google Scholar] [CrossRef]
- Schlachetzki, F.; Zhu, C.; Pardridge, W.M. Expression of the neonatal Fc receptor (FcRn) at the blood-brain barrier. J. Neurochem. 2002, 81, 203–206. [Google Scholar] [CrossRef]
- St-Amour, I.; Paré, I.; Alata, W.; Coulombe, K.; Ringuette-Goulet, C.; Drouin-Ouellet, J.; Vandal, M.; Soulet, D.; Bazin, R.; Calon, F. Brain bioavailability of human intravenous immunoglobulin and its transport through the murine blood-brain barrier. J. Cereb. Blood Flow Metab. 2013, 33, 1983–1992. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.N.; Pizzo, M.E.; Nehra, G.; Wilken-Resman, B.; Boroumand, S.; Thorne, R.G. Passive Immunotherapies for Central Nervous System Disorders: Current Delivery Challenges and New Approaches. Bioconjug. Chem. 2018, 29, 3937–3966. [Google Scholar] [CrossRef]
- Gan, H.K.; van den Bent, M.; Lassman, A.B.; Reardon, D.A.; Scott, A.M. Antibody–drug conjugates in glioblastoma therapy: The right drugs to the right cells. Nat. Rev. Clin. Oncol. 2017, 14, 695–707. [Google Scholar] [CrossRef]
- Cavaco, M.; Gaspar, D.; Castanho, M.; Neves, V. Antibodies for the Treatment of Brain Metastases, a Dream or a Reality? Pharmaceutics 2020, 12, 62. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhu, C.; Pardridge, W.M. Antisense gene therapy of brain cancer with an artificial virus gene delivery system. Mol. Ther. 2002, 6, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Boado, R.J.; Hui, E.K.W.; Lu, J.Z.; Pardridge, W.M. Very high plasma concentrations of a monoclonal antibody against the human insulin receptor are produced by subcutaneous injection in the rhesus monkey. Mol. Pharm. 2016, 13, 3241–3246. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Huang, C.C.; Huang, W.; Tang, W.H.; Rait, A.; Yin, Y.Z.; Cruz, I.; Xiang, L.M.; Pirollo, K.F.; Chang, E.H. Systemic tumor-targeted gene delivery by anti-transferrin receptor scFv-immunoliposomes. Mol. Cancer Ther. 2002, 1, 5. [Google Scholar]
- Kim, S.S.; Harford, J.B.; Moghe, M.; Slaughter, T.; Doherty, C.; Chang, E.H. A tumor-targeting nanomedicine carrying the p53 gene crosses the blood–brain barrier and enhances anti-PD-1 immunotherapy in mouse models of glioblastoma. Int. J. Cancer 2019, 145, 2535–2546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senzer, N.; Nemunaitis, J.; Nemunaitis, D.; Bedell, C.; Edelman, G.; Barve, M.; Nunan, R.; Pirollo, K.F.; Rait, A.; Chang, E.H. Phase I Study of a Systemically Delivered p53 Nanoparticle in Advanced Solid Tumors. Mol. Ther. 2013, 21, 1096–1103. [Google Scholar] [CrossRef] [Green Version]
- Stanimirovic, D.B.; Kemmerich, K.; Haqqani, A.S.; Farrington, G.K. Engineering and Pharmacology of Blood–Brain Barrier-Permeable Bispecific Antibodies. In Advances in Pharmacology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 71, pp. 301–335. [Google Scholar]
- VVerdino, P.; Atwell, S.; Demarest, S.J. Emerging trends in bispecific antibody and scaffold protein therapeutics. Curr. Opin. Chem. Eng. 2018, 19, 107–123. [Google Scholar] [CrossRef]
- Karaoglu Hanzatian, D.; Schwartz, A.; Gizatullin, F.; Erickson, J.; Deng, K.; Villanueva, R.; Stedman, C.; Harris, C.; Ghayur, T.; Goodearl, A. Brain uptake of multivalent and multi-specific DVD-Ig proteins after systemic administration. mAbs 2018, 10, 765–777. [Google Scholar] [CrossRef] [Green Version]
- Kariolis, M.S.; Wells, R.C.; Getz, J.A.; Kwan, W.; Mahon, C.S.; Tong, R.; Kim, D.J.; Srivastava, A.; Bedard, C.; Henne, K.R.; et al. Brain delivery of therapeutic proteins using an Fc fragment blood-brain barrier transport vehicle in mice and monkeys. Sci. Transl. Med. 2020, 12, eaay1359. [Google Scholar] [CrossRef]
- Ullman, J.C.; Arguello, A.; Getz, J.A.; Bhalla, A.; Mahon, C.S.; Wang, J.; Giese, T.; Bedard, C.; Kim, D.J.; Blumenfeld, J.R.; et al. Brain delivery and activity of a lysosomal enzyme using a blood-brain barrier transport vehicle in mice. Sci. Transl. Med. 2020, 12, eaay1163. [Google Scholar] [CrossRef]
- Tzeng, S.Y.; Green, J.J. Therapeutic nanomedicine for brain cancer. Ther. Deliv. 2013, 4, 687–704. [Google Scholar] [CrossRef] [Green Version]
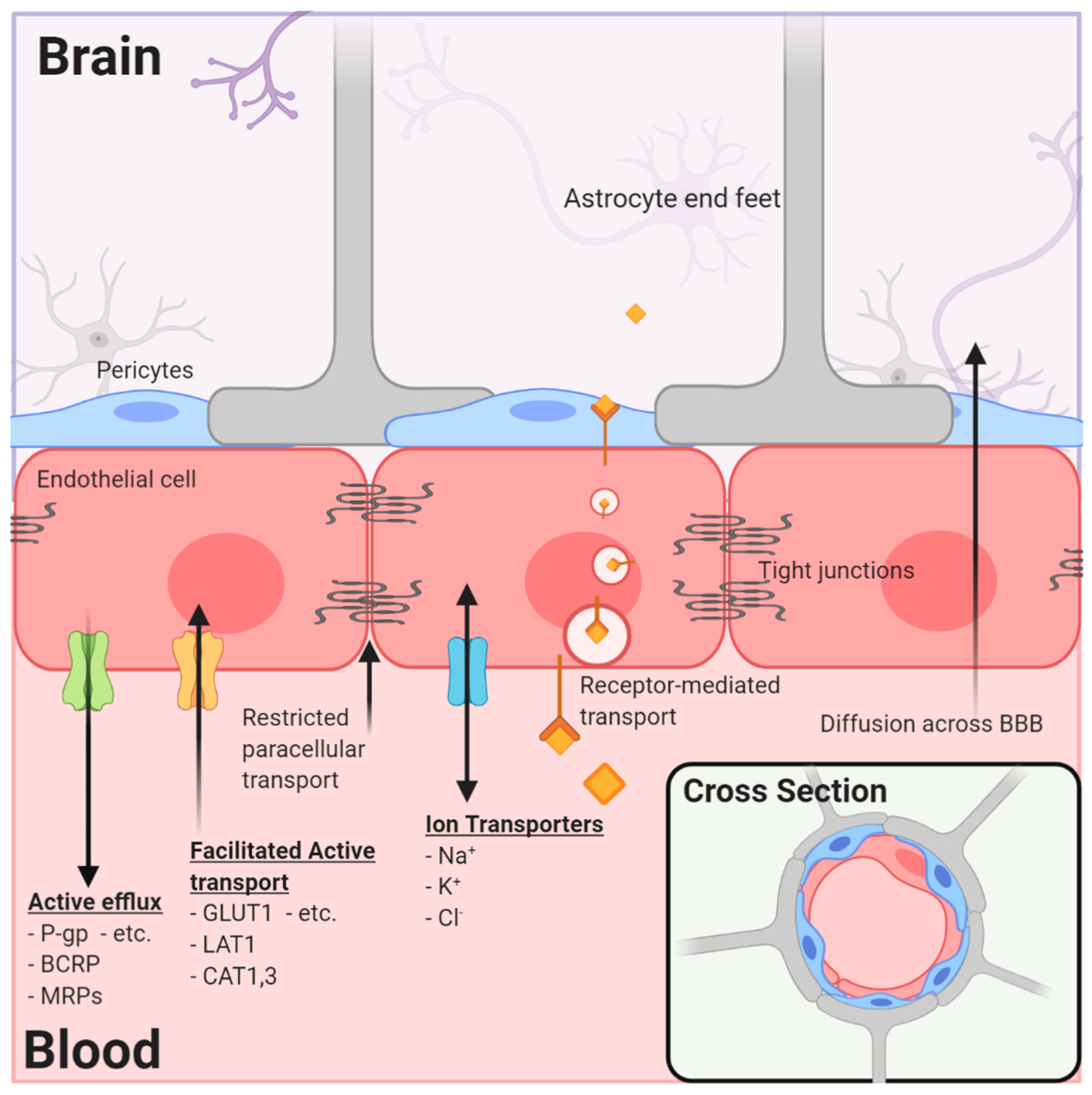
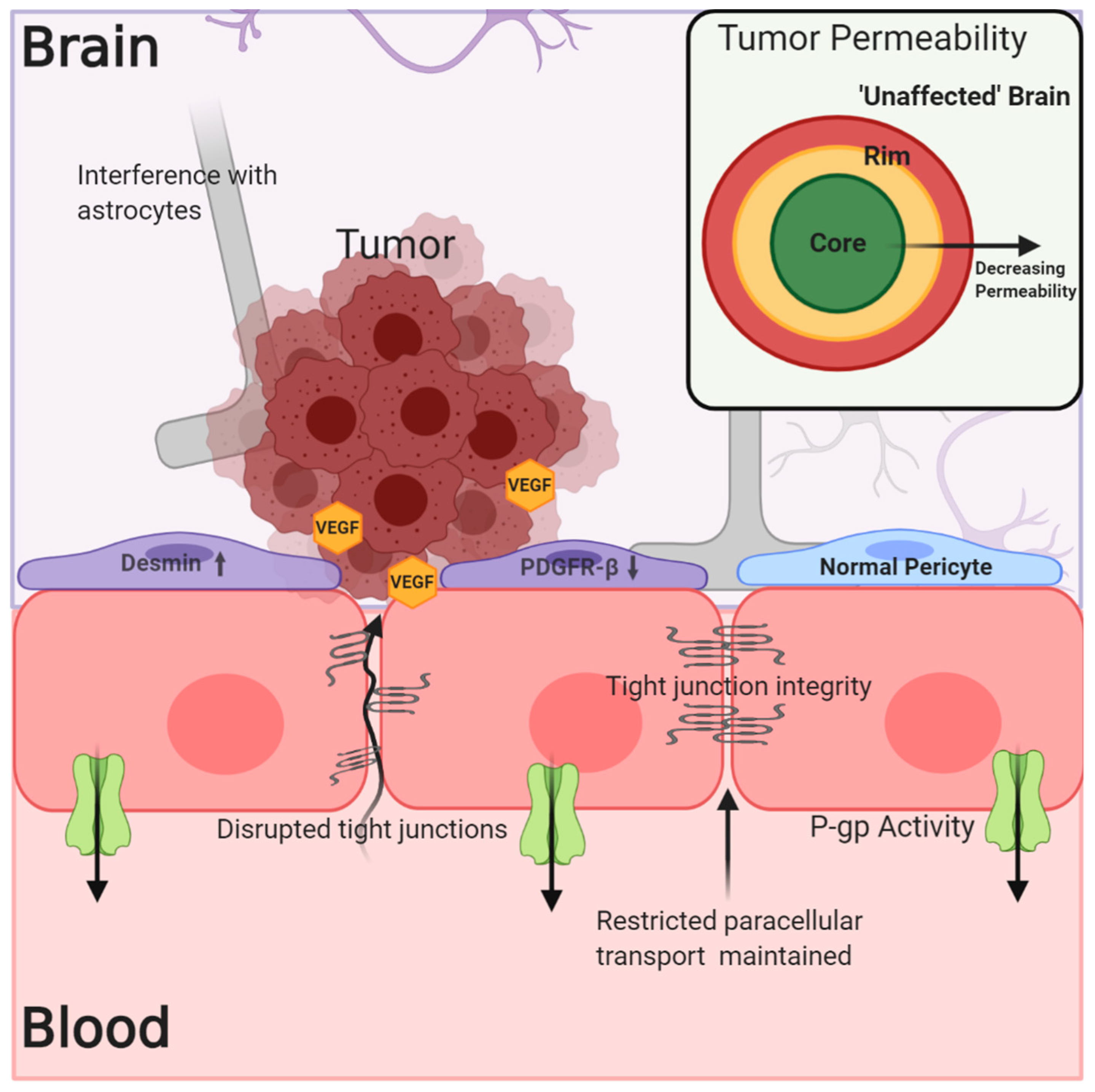
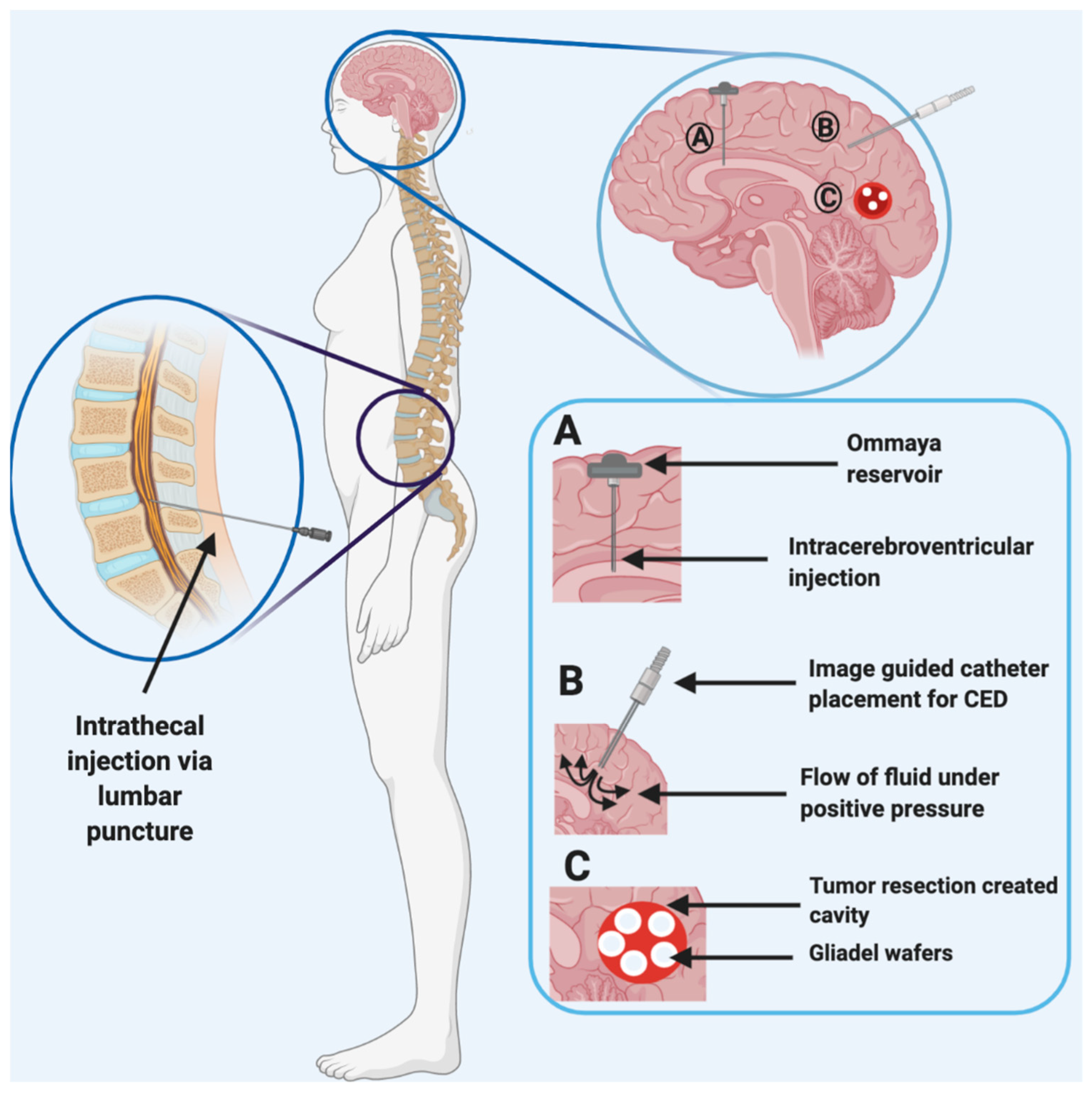
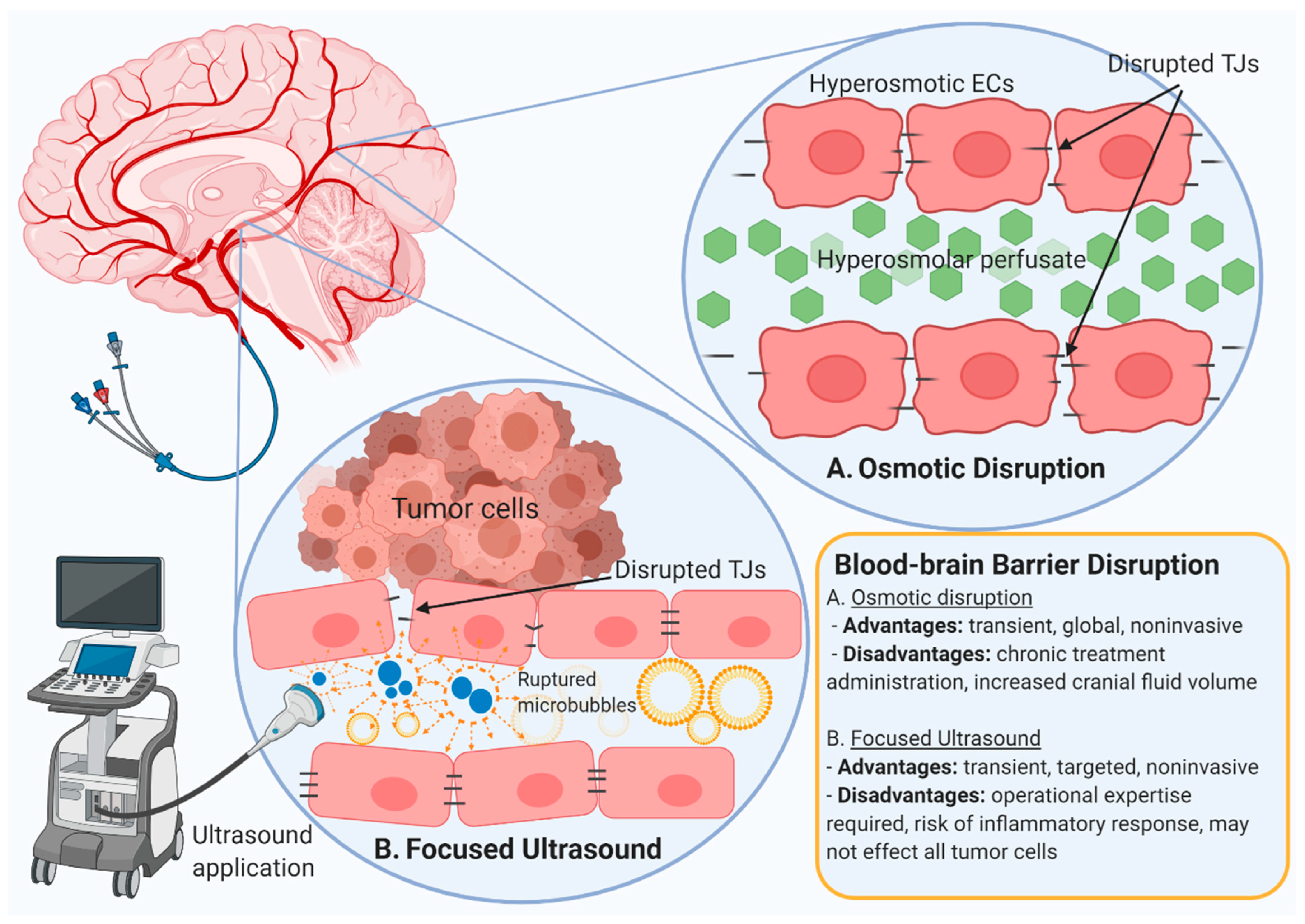
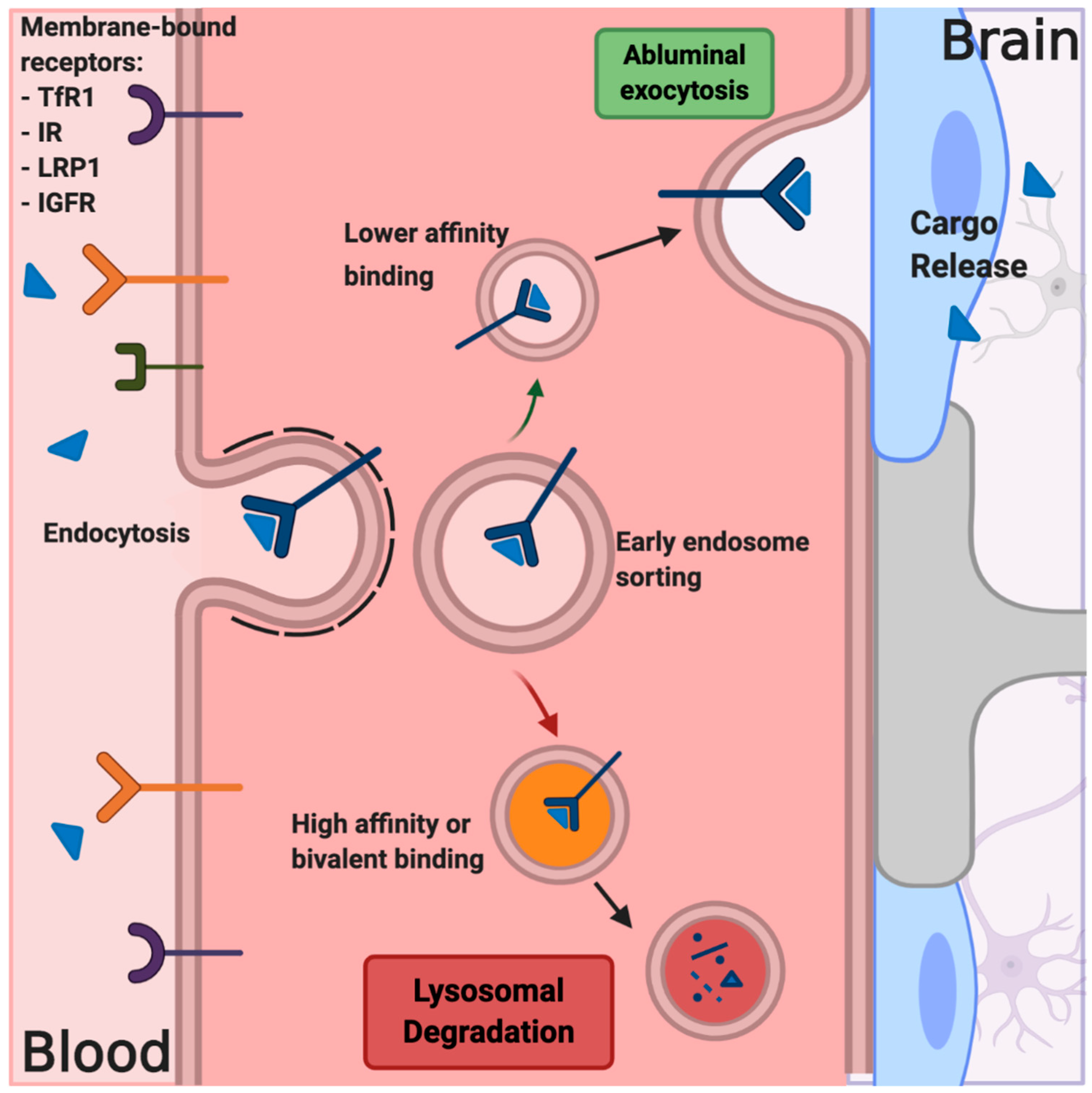
| Transport System | Typical Substrate | SLC Family | Common Name |
|---|---|---|---|
| Carbohydrates | |||
| Hexose | Glucose | SLC2A1 | Glut1 |
| Sodium Myo-inositol | Myo-inositol | SLC5A3 | SMIT |
| Monocarboxylates | |||
| Monocarboxylic acid | Lactic acid ketones | SLC16A1 | MCT1 |
| Amino Acids | |||
| Large neutral amino acid | Phenylalanine | SLC7A5 | LAT1 |
| Small neutral amino acid | Alanine | SLC38A2 | SNAT2, -3, -5 |
| Cationic amino acid | Lysine | SLC7A1 | Cat1, CAT3 |
| Beta amino acid | Taurine | SLC6A6 | TauT |
| Ala-Ser-Cys | Ala, ser, cys | SLC1A4 | ASCT1, -2 |
| Excitatory amino acid | Glutamic acid | SLC1A2 | EAAT-1, -2, -3 |
| Glycine | Glycine | SLC6A9, A5 | GT-1 |
| Others | |||
| Fatty acids | Essential FA LPC-PC (DHA) | SLC44A1/2 Mfsd2A | FATP-1, -4 Mfsd2A |
| Nucleoside | Adenosine | SLC29A1 SLC28A1 | ENT-1, -2; CNT1–3 |
| Hormones | Thyroid T3 Thyroid T4 | SLC16A2 OATP1C1 | MCT8 OATP1C1 |
| Biotin, pantothenic acid | biotin | SLC5A6 | SMVT |
| Folic acid | Folinic acid | SLC46A1 | PCFT |
| Copper | Cu+ | SLC31A1 | CTR1 |
| Transport System | Common Name | Typical Substrate |
|---|---|---|
| ATP Binding Cassette Transporter (ABC) | ||
| ABCB1 | P-gp | Broad-spectrum, xenobiotics |
| ABCG2 | BCRP | mitoxantrone anthracycline xenobiotics |
| ABCC1 | MRP1 | GSSG, leukotrienes |
| ABCC5 | MRP5 | Thiopurines, cyclic nucleotides |
| ABCC4 | MRP4 | Organic anions |
| Non-ABC Transporters | ||
| SLC22A7 | OAT2-3 | Organic ions |
| SLC22A8 | OATP1A4 | |
| SLC20A2 | OATP2B1 | |
| SLCO1A4 | OCTN2 | |
| SLCO2B1 | OCT1-3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Griffith, J.I.; Rathi, S.; Zhang, W.; Zhang, W.; Drewes, L.R.; Sarkaria, J.N.; Elmquist, W.F. Addressing BBB Heterogeneity: A New Paradigm for Drug Delivery to Brain Tumors. Pharmaceutics 2020, 12, 1205. https://doi.org/10.3390/pharmaceutics12121205
Griffith JI, Rathi S, Zhang W, Zhang W, Drewes LR, Sarkaria JN, Elmquist WF. Addressing BBB Heterogeneity: A New Paradigm for Drug Delivery to Brain Tumors. Pharmaceutics. 2020; 12(12):1205. https://doi.org/10.3390/pharmaceutics12121205
Chicago/Turabian StyleGriffith, Jessica I., Sneha Rathi, Wenqiu Zhang, Wenjuan Zhang, Lester R. Drewes, Jann N. Sarkaria, and William F. Elmquist. 2020. "Addressing BBB Heterogeneity: A New Paradigm for Drug Delivery to Brain Tumors" Pharmaceutics 12, no. 12: 1205. https://doi.org/10.3390/pharmaceutics12121205
APA StyleGriffith, J. I., Rathi, S., Zhang, W., Zhang, W., Drewes, L. R., Sarkaria, J. N., & Elmquist, W. F. (2020). Addressing BBB Heterogeneity: A New Paradigm for Drug Delivery to Brain Tumors. Pharmaceutics, 12(12), 1205. https://doi.org/10.3390/pharmaceutics12121205