Drug Delivery Systems on Leprosy Therapy: Moving Towards Eradication?
Abstract
:1. Introduction
1.1. Ethiologic Agent, the Mycobacterium leprae
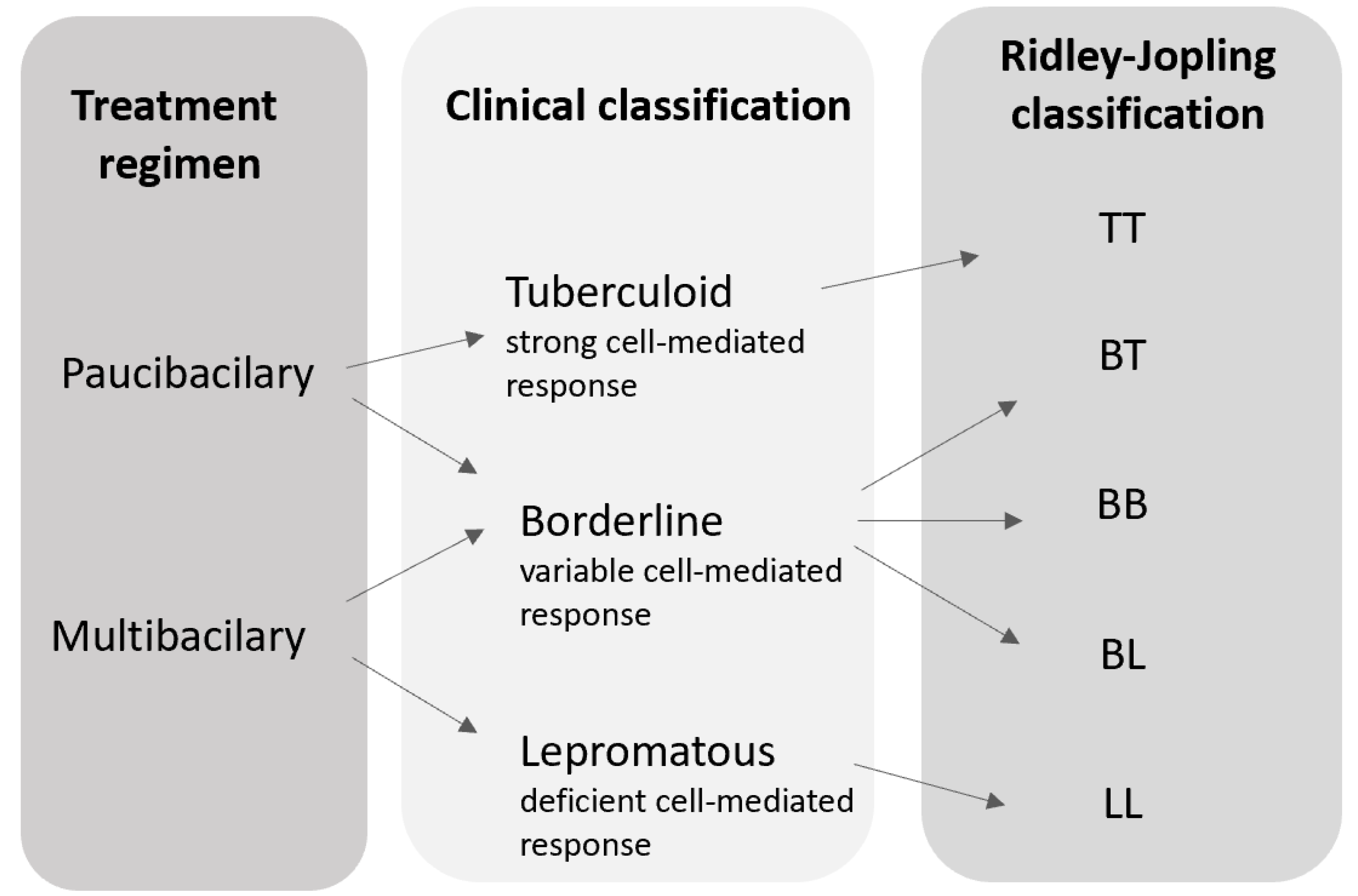
1.2. Classification of Leprosy
1.3. Diagnosis of Leprosy
2. Leprosy Treatment Approaches
2.1. Conventional Treatment
2.2. Issues and Challenges of Leprosy Treatment
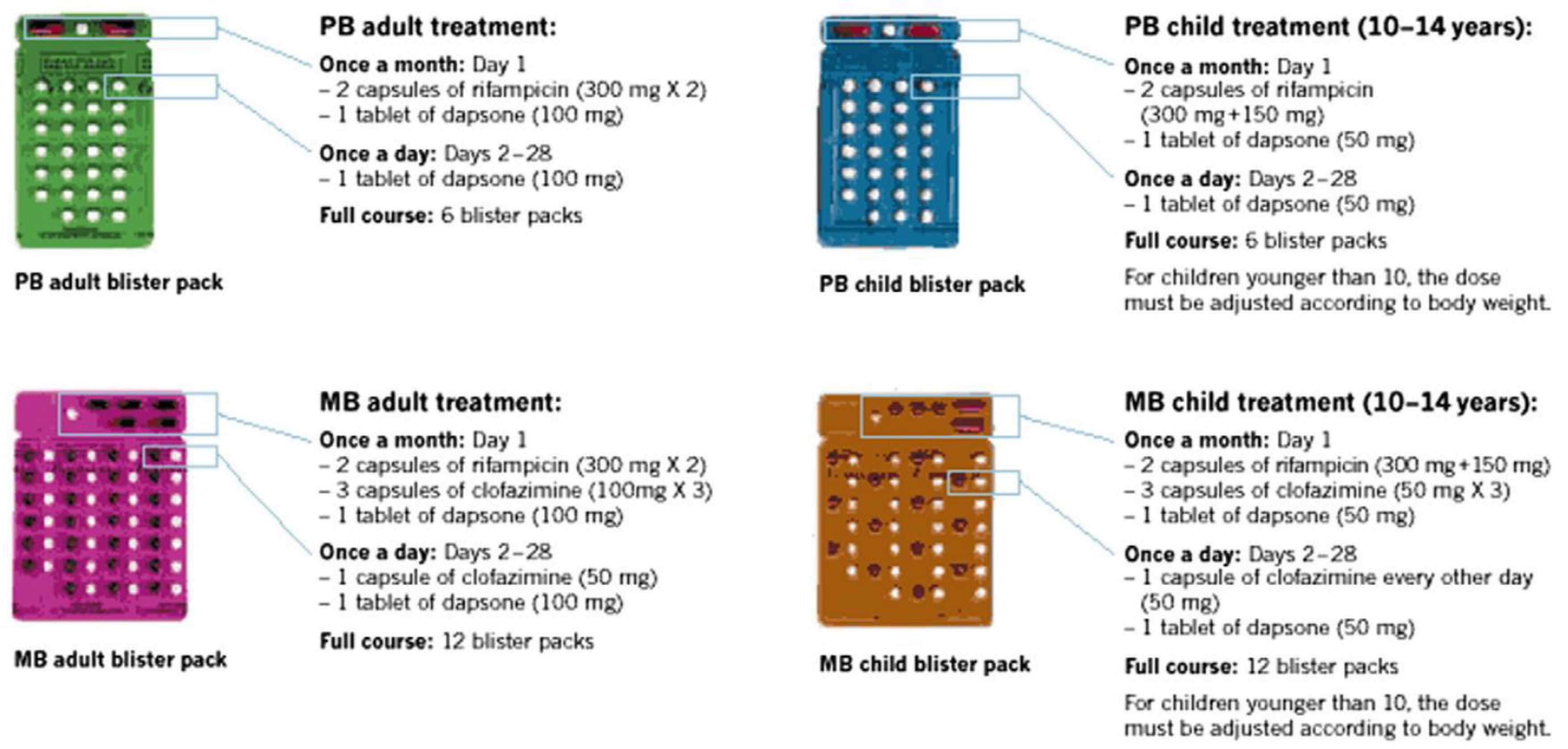
2.3. Uniform-Multidrug Therapy Regimen
2.4. The role of Drug Delivery Strategies in Leprosy Treatment
| Type of Delivery System | Composition | Major Outcome | Ref |
|---|---|---|---|
| Oral Administration | |||
| Solid lipid nanoparticles | Precirol ATO 5 Tween 80 | gastric and intestinal cells tolerate more CLZ when loaded in the nanoparticles than free in solution | [84] |
| Polymeric nanoparticles | PLGA | nanoparticles decreased drug intrinsic toxicity, with improved intestinal permeation | [85] |
| Transdermal administration | |||
| Liposomes | Phosphatidylcholine Cholesterol HPMC (matrix to deliver liposomes) | liposomal gels were found to be stable at room temperature for up to 3 months | [86] |
| Other applications | |||
| Solid dispersions | Hypromellose phthalate Hypromellose vinylpyrrolidone-vinyl acetate | elucidation of a possible structural model of the drug–polymer complex | [87] |
| Solid dispersions | Hypromellose phthalate Hypromellose vinylpyrrolidone-vinyl acetate | contribution to a rational selection of appropriate polymeric carriers | [88] |
2.5. Targeting Drug Delivery—A Future Perspective
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- White, C.; Franco-Paredes, C. Leprosy in the 21st Century. Clin. Microbiol. Rev. 2015, 28, 80–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virmond, M.D.C.L.; Grzybowski, A.; Virmond, L. Leprosy: A glossary. Clin. Dermatol. 2015, 33, 8–18. [Google Scholar] [CrossRef]
- Bhat, R.M.; Prakash, C. Leprosy: An Overview of Pathophysiology. Interdiscip. Perspect. Infect. Dis. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Organization WH. Global Leprosy Strategy 2016–2020: Accelerating Towards a Leprosy-Free World: WHO Library Cataloguing-in-Publication Data. 2016. Available online: https://apps.who.int/neglected_diseases/ntddata/leprosy/leprosy.html (accessed on 26 November 2020).
- Eichelmann, K.; González, S.G.; Salas-Alanis, J.; Ocampo-Candiani, J. Leprosy. An Update: Definition, Pathogenesis, Classification, Diagnosis, and Treatment. Actas Dermo Sifiliográficas 2013, 104, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Akpolat, N.D.; Akkus, A.; Kaynak, E. An Update on the Epidemiology, Diagnosis and Treatment of Leprosy. Hansen Dis. Forgot. Negl. Dis. 2019, 45, 383–393. [Google Scholar] [CrossRef] [Green Version]
- Gillis, T.P. Chapter 93–Mycobacterium leprae A2. In Molecular Medical Microbiology, 2nd ed.; Tang, Y.-W., Sussman, M., Liu, D., Poxton, I., Schwartzman, J., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 1655–1668. [Google Scholar]
- Rambukkana, A. How does Mycobacterium leprae target the peripheral nervous system? Trends Microbiol. 2000, 8, 23–28. [Google Scholar] [CrossRef]
- Abed, N.; Couvreur, P. Nanocarriers for antibiotics: A promising solution to treat intracellular bacterial infections. Int. J. Antimicrob. Agents 2014, 43, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Takeshita, F.; Nakata, N.; Ishii, N.; Makino, M. Localization of CORO1A in the Macrophages Containing Mycobacterium leprae. Acta Histochem. ET Cytochem. 2006, 39, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Mattos, K.A.; Lara, F.A.; Oliveira, V.G.C.; Rodrigues, L.S.; D’Avila, H.; Melo, R.C.N.; Manso, P.P.A.; Sarno, E.N.; Bozza, P.T.; Pessolani, M.C.V. Modulation of lipid droplets by Mycobacterium leprae in Schwann cells: A putative mechanism for host lipid acquisition and bacterial survival in phagosomes. Cell. Microbiol. 2011, 13, 259–273. [Google Scholar] [CrossRef]
- Mattos, K.A.; Oliveira, V.C.; Berrêdo-Pinho, M.; Amaral, J.J.; Antunes, L.C.M.; Melo, R.C.; Rosa, P.S. Mycobacterium leprae intracellular survival relies on cholesterol accumulation in infected macrophages: A potential target for new drugs for leprosy treatment. Cell. Microbiol. 2014, 16, 797–815. [Google Scholar] [CrossRef] [Green Version]
- Cruz, D.; Watson, A.D.; Miller, C.S.; Montoya, D.; Ochoa, M.-T.; Sieling, P.A.; Gutierrez, M.A.; Navab, M.; Reddy, S.T.; Witztum, J.L.; et al. Host-derived oxidized phospholipids and HDL regulate innate immunity in human leprosy. J. Clin. Investig. 2008, 118, 2917–2928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaschignard, J.; Grant, A.V.; Thu, H.N.; Orlova, M.; Cobat, A.; Huong, N.T.; Ba, N.N.; Thai, V.H.; Abel, L.; Schurr, E.; et al. Pauci and Multibacillary Leprosy: Two Distinct, Genetically Neglected Diseases. PLoS Negl. Trop. Dis. 2016, 10, e0004345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Organization WH. Global Leprosy Programme. 2017. Available online: https://apps.who.int/iris/bitstream/handle/10665/274289/WER9335.pdf?ua=1 (accessed on 16 July 2019).
- WHO. International Classification of Functioning, Disability And Health (ICF); WHO: Geneva, Switzerland, 2001. [Google Scholar]
- de Souza, V.; da Silva-Junior, W.; de Jesus, A.M.; Oliveira, D.; Raptis, H.; Freitas, P.H.; Schneiberg, S. Is the WHO disability grading system for leprosy related to the level of functional activity and social participation? Lepr. Rev. 2016, 87, 191–200. [Google Scholar] [CrossRef]
- Freitas, A.A.; Oliveira, R.M.; Hungria, E.M.; Cardoso, L.P.V.; Sousa, A.L.O.M.; Costa, M.B.; Reed, S.G.; Duthie, M.S.; Stefani, M.M.D.A. Alterations to antigen-specific immune responses before and after multidrug therapy of leprosy. Diagn. Microbiol. Infect. Dis. 2015, 83, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Moura, R.S.; Penna, G.D.O.; Fujiwara, T.; Pontes, M.A.D.A.; Cruz, R.; Gonçalves, H.D.S.; Penna, M.P.; Cardoso, L.P.V.; Stefani, M.M.A.; Bührer-Sékula, S. Evaluation of a rapid serological test for leprosy classification using human serum albumin as the antigen carrier. J. Immunol. Methods 2014, 412, 35–41. [Google Scholar] [CrossRef]
- Legendre, D.P.; Muzny, C.A.; Swiatlo, E. Hansen’s Disease (Leprosy): Current and Future Pharmacotherapy and Treatment of Disease-Related Immunologic Reactions. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2012, 32, 27–37. [Google Scholar] [CrossRef]
- Fischer, M. Leprosy—An overview of clinical features, diagnosis, and treatment. J. Dtsch. Dermatol. Ges. 2017, 15, 801–827. [Google Scholar] [CrossRef] [Green Version]
- Akash, M.S.H.; Rehman, K.; Chen, S.-Q. Polymeric-based particulate systems for delivery of therapeutic proteins. Pharm. Dev. Technol. 2015, 21, 367–378. [Google Scholar] [CrossRef]
- Hungria, E.M.; Freitas, A.A.; Pontes, M.A.A.; Gonçalves, H.S.; Sousa, A.L.O.M.; Costa, M.B.; Castilho, M.L.O.R.; Duthie, M.S.; Stefani, M.M.A. Antigen-specific secretion of IFNγ and CXCL10 in whole blood assay detects Mycobacterium leprae infection but does not discriminate asymptomatic infection from symptomatic leprosy. Diagn. Microbiol. Infect. Dis. 2017, 87, 328–334. [Google Scholar] [CrossRef]
- Hunter, S.W.; Brennan, P.J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J. Bacteriol. 1981, 147, 728–735. [Google Scholar] [CrossRef] [Green Version]
- Duthie, M.S.; Goto, W.; Ireton, G.C.; Reece, S.T.; Cardoso, L.P.V.; Martelli, C.M.T.; Stefani, M.M.A.; Nakatani, M.; De Jesus, R.C.; Martins, E.; et al. Use of Protein Antigens for Early Serological Diagnosis of Leprosy. Clin. Vaccine Immunol. 2007, 14, 1400–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duthie, M.S.; Raychaudhuri, R.; Tutterrow, Y.L.; Misquith, A.; Bowman, J.; Casey, A.; Balagon, M.F.; Maghanoy, A.; Beltran-Alzate, J.C.; Romero-Alzate, M.; et al. A rapid ELISA for the diagnosis of MB leprosy based on complementary detection of antibodies against a novel protein-glycolipid conjugate. Diagn. Microbiol. Infect. Dis. 2014, 79, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.S.; Kim, H.J.; Wheat, W.H.; Chatterjee, D.; Balagon, M.V.; Cellona, R.V.; Tan, E.V.; Gelber, R.; Saunderson, P.; Duthie, M.S.; et al. Analysis of Antibody Responses to Mycobacterium leprae Phenolic Glycolipid I, Lipoarabinomannan, and Recombinant Proteins To Define Disease Subtype-Specific Antigenic Profiles in Leprosy. Clin. Vaccine Immunol. 2010, 18, 260–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Z.; Wang, X.; Li, Z.-J.; Ren, S.-Q.; Chen, G.; Ying, X.-T.; Lin, J.-M. Development of a sensitive, rapid, biotin–streptavidin based chemiluminescent enzyme immunoassay for human thyroid stimulating hormone. Talanta 2008, 75, 965–972. [Google Scholar] [CrossRef]
- Casey, J.L.; Coley, A.M.; Street, G.; Parisi, K.; Devine, P.L.; Foley, M. Peptide Mimotopes Selected from a Random Peptide Library for Diagnosis of Epstein-Barr Virus Infection. J. Clin. Microbiol. 2006, 44, 764–771. [Google Scholar] [CrossRef] [Green Version]
- Duthie, M.S.; Balagon, M.F. Combination chemoprophylaxis and immunoprophylaxis in reducing the incidence of leprosy. Risk Manag. Heal. Policy 2016, 9, 43–53. [Google Scholar] [CrossRef] [Green Version]
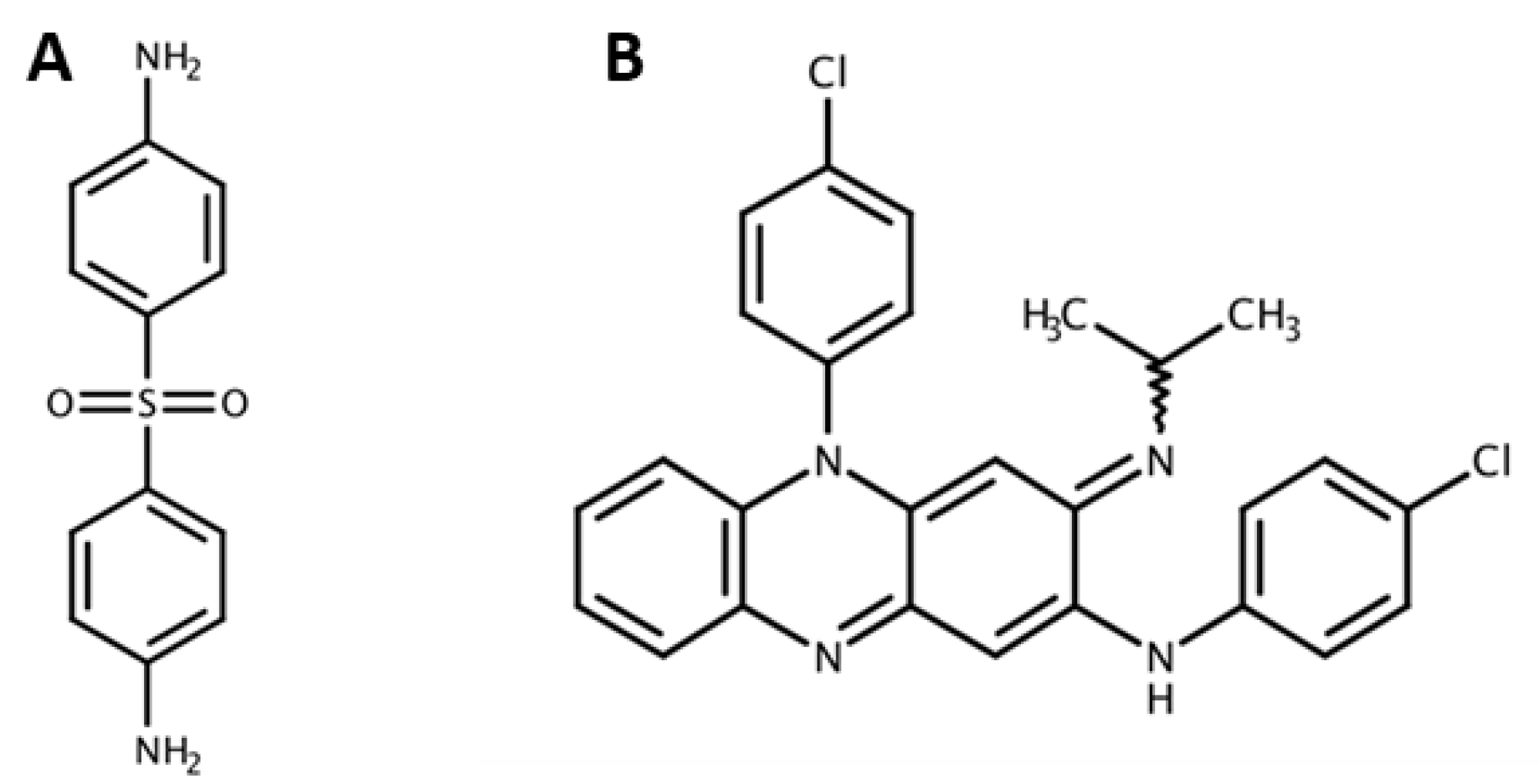
- Zhu, Y.; Stiller, M.J. Dapsone and sulfones in dermatology: Overview and update. J. Am. Acad. Dermatol. 2001, 45, 420–434. [Google Scholar] [CrossRef]
- Kar, H.K.; Gupta, R. Treatment of leprosy. Clin. Dermatol. 2015, 33, 55–65. [Google Scholar] [CrossRef]
- Anusuya, S.; Natarajan, J. The eradication of leprosy: Molecular modeling techniques for novel drug discovery. Expert Opin. Drug Discov. 2013, 8, 1239–1251. [Google Scholar] [CrossRef]
- Lazo-Porras, M.; Prutski, G.J.; Barrionuevo, P.; Tapia, J.C.; Ugarte-Gil, C.; Ponce, O.J.; Málaga, G. World Health Organization (WHO) Antibiotic Regimen Against Other Regimens for the Treatment of Leprosy: A Systematic Review and Meta-Analysis. BMC Infect. Dis. 2020, 20, 62. [Google Scholar] [CrossRef]
- Organization WH. WHO Expert Committee on Leprosy: Seventh Report: World Health Organization. 1998. Available online: https://apps.who.int/iris/bitstream/handle/10665/42060/WHO_TRS_874.pdf (accessed on 12 February 2017).
- Rodrigues, L.C.; Lockwood, D.N. Leprosy now: Epidemiology, progress, challenges, and research gaps. Lancet Infect. Dis. 2011, 11, 464–470. [Google Scholar] [CrossRef]
- Jacobson, R.; Hastings, R. Rifampicin-resistant leprosy. Lancet 1976. [Google Scholar] [CrossRef]
- Ji, B.; Perani, E.G.; Petinom, C.; Grosset, J.H. Bactericidal activities of combinations of new drugs against Mycobacterium leprae in nude mice. Antimicrob. Agents Chemother. 1996, 40, 393–399. [Google Scholar] [CrossRef] [Green Version]
- González, A.; Maestre, J.; Hernández, O.; Columbié, Y.; Atrio, N.; Martín, M.; Rodríguez, J. Survey for secondary dapsone and rifampicin resistance in Cuba. Leprosy Rev. 1993, 64, 128. [Google Scholar] [CrossRef]
- Wang, H.; Yu, M.; Wu, K.; Pei, B.; Yang, D.; Wang, Q.; Shen, J.; Yan, L.; Zhang, G. Rifampicin-resistant Mycobacterium leprae in an elderly leprosy patient in the People’s Republic of China. Clin. Interv. Aging 2013, 8, 1097–1099. [Google Scholar] [CrossRef] [Green Version]
- Matsuoka, M. Global surveillance system to monitor the development of drug resistance in Mycobacterium leprae. Res. Rep. Trop. Med. 2015, 6, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Prasad, P.V.S.; Kaviarasan, P.K. Leprosy therapy, past and present: Can we hope to eliminate it? Indian J. Dermatol. 2010, 55, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Saunderson, P.R. Drug-resistant M leprae. Clin. Dermatol. 2016, 34, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.T.; Eiglmeier, K.; Parkhill, J.; James, K.D.; Thomson, N.R.; Wheeler, P.R.; Honoré, N.; Garnier, T.; Churcher, C.; Harris, D.; et al. Massive gene decay in the leprosy bacillus. Nat. Cell Biol. 2001, 409, 1007–1011. [Google Scholar] [CrossRef]
- Organization WH. Guidelines for Global Surveillance of Drug Resistance in Leprosy. India, 2009. Available online: https://apps.who.int/iris/handle/10665/205158 (accessed on 12 February 2017).
- Cambau, E.; Saunderson, P.; Matsuoka, M.; Cole, S.T.; Kai, M.; Suffys, P.; Rosa, P.S.; Williams, D.; Gupta, U.D.; Lavania, M.; et al. Antimicrobial resistance in leprosy: Results of the first prospective open survey conducted by a WHO surveillance network for the period 2009–15. Clin. Microbiol. Infect. 2018, 24, 1305–1310. [Google Scholar] [CrossRef] [Green Version]
- Organization WH. A Guide for Surveillance of Antimicrobial Resistance in Leprosy. Delhi, 2017. Available online: https://www.who.int/lep/resources/9789290226192/en/ (accessed on 16 July 2019).
- Penna, M.L.F.; Bührer-Sékula, S.; Pontes, M.A.D.A.; Cruz, R.; Gonçalves, H.D.S.; Penna, G.O. Primary results of clinical trial for uniform multidrug therapy for leprosy patients in Brazil (U-MDT/CT-BR): Reactions frequency in multibacillary patients. Lepr. Rev. 2012, 83, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Sener, O.; Doganci, L.; Safali, M.; Besirbellioglu, B.; Bulucu, F.; Pahsa, A. Severe dapsone hypersensitivity syndrome. J. Investig. Allergol. Clin. Immunol. 2006, 16, 268–270. [Google Scholar] [PubMed]
- Oliveira, F.R.; Pessoa, M.C.; Albuquerque, R.F.V.; Schalcher, T.R.; Monteiro, M.C. Clinical Applications and Methemoglobinemia Induced by Dapsone. J. Braz. Chem. Soc. 2014, 25, 1770–1779. [Google Scholar] [CrossRef]
- Wozel, G.; Blasum, C. Dapsone in dermatology and beyond. Arch. Dermatol. Res. 2014, 306, 103–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuoka, M. Drug Resistance in Leprosy. IAL Textb. Lepr. 2017, 63, 573. [Google Scholar] [CrossRef]
- Chaves, L.L.; Vieira, A.C.C.; Ferreira, D.; Sarmento, B.; Reis, S. Rational and precise development of amorphous polymeric systems with dapsone by response surface methodology. Int. J. Biol. Macromol. 2015, 81, 662–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, D.L.; Spring, L.; Harris, E.; Roche, P.; Gillis, T.P. Dihydropteroate Synthase of Mycobacterium leprae and Dapsone Resistance. Antimicrob. Agents Chemother. 2000, 44, 1530–1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wozel, V.G. Innovative Use of Dapsone. Dermatol. Clin. 2010, 28, 599–610. [Google Scholar] [CrossRef]
- Coleman, M.D. Dapsone-mediated agranulocytosis: Risks, possible mechanisms and prevention. Toxicology 2001, 162, 53–60. [Google Scholar] [CrossRef]
- Gopal, M.; Padayatchi, N.; Metcalfe, J.Z.; O’Donnell, M.R. Systematic review of clofazimine for the treatment of drug-resistant tuberculosis [Review article]. Int. J. Tuberc. Lung Dis. 2013, 17, 1001–1007. [Google Scholar] [CrossRef] [Green Version]
- Mafukidze, A.; Harausz, E.; Furin, J. An update on repurposed medications for the treatment of drug-resistant tuberculosis. Expert Rev. Clin. Pharmacol. 2016, 9, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Cholo, M.C.; Steel, H.C.; Fourie, P.B.; Germishuizen, W.A.; Anderson, R. Clofazimine: Current status and future prospects. J. Antimicrob. Chemother. 2012, 67, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Szeto, W.; Garcia-Buitrago, M.T.; Abbo, L.; Rosenblatt, J.D.; Moshiree, B.; Morris, M.I. Clofazimine Enteropathy: A Rare and Underrecognized Complication of Mycobacterial Therapy. Open Forum Infect. Dis. 2016, 3, ofw004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, R.; Silva, C.D.C.; Chaves, L. Tissue-based in vitro and ex vivo models for intestinal permeability studies. In Concepts and Models for Drug Permeability Studies; Elsevier BV: Cambridge, MA, USA, 2016; pp. 203–236. [Google Scholar]
- Baik, J.; Rosania, G.R. Macrophages Sequester Clofazimine in an Intracellular Liquid Crystal-Like Supramolecular Organization. PLoS ONE 2012, 7, e47494. [Google Scholar] [CrossRef] [PubMed]
- Yoon, G.S.; Keswani, R.K.; Sud, S.; Rzeczycki, P.M.; Murashov, M.D.; Koehn, T.A.; Standiford, T.J.; Stringer, K.A.; Rosania, G.R. Clofazimine Biocrystal Accumulation in Macrophages Upregulates Interleukin 1 Receptor Antagonist Production To Induce a Systemic Anti-Inflammatory State. Antimicrob. Agents Chemother. 2016, 60, 3470–3479. [Google Scholar] [CrossRef] [Green Version]
- Arbiser, J.L.; Moschella, S.L. Clofazimine: A review of its medical uses and mechanisms of action. J. Am. Acad. Dermatol. 1995, 32, 241–247. [Google Scholar] [CrossRef]
- Baik, J.; Stringer, K.A.; Mane, G.; Rosania, G.R. Multiscale Distribution and Bioaccumulation Analysis of Clofazimine Reveals a Massive Immune System-Mediated Xenobiotic Sequestration Response. Antimicrob. Agents Chemother. 2012, 57, 1218–1230. [Google Scholar] [CrossRef] [Green Version]
- Yoon, G.S.; Sud, S.; Keswani, R.K.; Baik, J.; Standiford, T.J.; Stringer, K.A.; Rosania, G.R. Phagocytosed clofazimine biocrystals can modulate innate immune signaling by inhibiting TNFalpha and boosting IL-1RA secretion. Mol. Pharm. 2015, 12, 2517–2527. [Google Scholar] [CrossRef] [Green Version]
- Rocha, A.D.S.; Cunha, M.D.G.; Diniz, L.M.; Salgado, C.G.; Aires, M.A.P.; Nery, J.A.; Gallo, E.N.; Miranda, A.; Magnanini, M.M.F.; Matsuoka, M.; et al. Drug and Multidrug Resistance among Mycobacterium leprae Isolates from Brazilian Relapsed Leprosy Patients. J. Clin. Microbiol. 2012, 50, 1912–1917. [Google Scholar] [CrossRef] [Green Version]
- Islan, G.; Durán, M.; Cacicedo, M.L.; Nakazato, G.; Kobayashi, R.K.; Martinez, D.S.; Castro, G.R.; Durán, N. Nanopharmaceuticals as a solution to neglected diseases: Is it possible? Acta Trop. 2017, 170, 16–42. [Google Scholar] [CrossRef] [Green Version]
- Khan, J.; Rades, T.; Boyd, B.J. The Precipitation Behavior of Poorly Water-Soluble Drugs with an Emphasis on the Digestion of Lipid Based Formulations. Pharm. Res. 2016, 33, 548–562. [Google Scholar] [CrossRef]
- Chaves, L.L.; Vieira, A.C.C.; Reis, S.; Sarmento, B.; Ferreira, D. Quality by Design: Discussing and Assessing the Solid Dispersions Risk. Curr. Drug Deliv. 2014, 11, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, M.; Couto, A.; Chaves, L.L.; Ferreira, D.; Sarmento, B.; Reis, S.; Vieira, A.C.C. Design and statistical modeling of mannose-decorated dapsone-containing nanoparticles as a strategy of targeting intestinal M-cells. Int. J. Nanomed. 2016, 11, 2601–2617. [Google Scholar] [CrossRef] [Green Version]
- Chaves, L.L.; Costa Lima, S.A.; Vieira, A.C.; Barreiros, L.; Segundo, M.A.; Ferreira, D.; Sarmento, B.; Reis, S. pH-sensitive nanoparticles for improved oral delivery of dapsone: Risk assessment, design, optimization and characterization. Nanomedicine 2017, 12, 1975–1990. [Google Scholar] [CrossRef]
- Chaves, L.L.; Silveri, A.; Vieira, A.C.C.; Ferreira, D.; Cristiano, M.C.; Paolino, D.; Celia, C. pH-responsive chitosan based hydrogels affect the release of dapsone: Design, set-up, and physicochemical characterization. Int. J. Biol. Macromol. 2019, 133, 1268–1279. [Google Scholar] [CrossRef]
- De Sousa, V.P.; Monteiro, L.M.; Lione, V.F.; Carmo, F.A.D.; Amaral, L.H.D.; Da Silva, J.H.; Nasciutti, E.L.; Castro, H.C.; Rodrigues, C.R.; Cabral, L.M. Development and characterization of a new oral dapsone nanoemulsion system: Permeability and in silico bioavailability studies. Int. J. Nanomed. 2012, 7, 5175–5182. [Google Scholar] [CrossRef] [Green Version]
- Grebogi, I.H.; Tibola, A.P.O.V.; Barison, A.; Grandizoli, C.W.P.S.; Ferraz, H.G.; Rodrigues, L.N.C. Binary and ternary inclusion complexes of dapsone in cyclodextrins and polymers: Preparation, characterization and evaluation. J. Incl. Phenom. Macrocycl. Chem. 2011, 73, 467–474. [Google Scholar] [CrossRef]
- Tiwari, R.; Tiwari, G.; Wal, P.; Wal, A.; Maurya, P. Development, Characterization and Transdermal Delivery of Dapsone and an Antibiotic Entrapped in Ethanolic Liposomal Gel for the Treatment of Lapromatous Leprosy. Open Nanomed. J. 2018, 5, 1–15. [Google Scholar] [CrossRef]
- Kanwar, R.; Gradzielski, M.; Mehta, S.K. Biomimetic Solid Lipid Nanoparticles of Sophorolipids Designed for Antileprosy Drugs. J. Phys. Chem. B 2018, 122, 6837–6845. [Google Scholar] [CrossRef]
- De Sousa, V.P.; Borges, V.R.D.A.; Simon, A.; Cabral, L.M..; Sena, A.R.C. Nanoemulsion containing dapsone for topical administration: A study of in vitro release and epidermal permeation. Int. J. Nanomed. 2013, 8, 535–544. [Google Scholar] [CrossRef] [Green Version]
- Ordaz, J.C.; Chigo-Anota, E.; Villanueva, M.S.; Castro, M. Possibility of a magnetic [BN fullerene:B6 cluster]− nanocomposite as a vehicle for the delivery of dapsone. New J. Chem. 2017, 41, 8045–8052. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, S.; Zhang, M.; Sun, J. Nanocarriers for oral drug delivery. J. Drug Target. 2013, 21, 515–527. [Google Scholar] [CrossRef]
- Chime, F.K.A.A.A.S.; Kenechukwu, F.C.; Attama, A.A. Nanoemulsions—Advances in Formulation, Characterization and Applications in Drug Delivery. In Application of Nanotechnology in Drug Delivery; IntechOpen: London, UK, 2014; p. 03. [Google Scholar]
- Cyphert, E.L.; Von Recum, H.A. Emerging technologies for long-term antimicrobial device coatings: Advantages and limitations. Exp. Biol. Med. 2017, 242, 788–798. [Google Scholar] [CrossRef]
- Chaves, L.L.; Lima, S.A.C.; Vieira, A.C.C.; Ferreira, D.; Sarmento, B.; Reis, S. Overcoming clofazimine intrinsic toxicity: Statistical modelling and characterization of solid lipid nanoparticles. J. R. Soc. Interface 2018, 15, 20170932. [Google Scholar] [CrossRef]
- Chaves, L.L.; Lima, S.A.C.; Vieira, A.C.C.; Barreiros, L.; Segundo, M.A.; Ferreira, D.; Sarmento, B.; Reis, S. Development of PLGA nanoparticles loaded with clofazimine for oral delivery: Assessment of formulation variables and intestinal permeability. Eur. J. Pharm. Sci. 2018, 112, 28–37. [Google Scholar] [CrossRef]
- Patel, A.N.M.V.B. Encapsulation and stability of clofazimine liposomes. J. Microencapsul. 1999, 16, 357–367. [Google Scholar] [CrossRef]
- Nie, H.; Su, Y.; Zhang, M.; Song, Y.; Leone, A.; Taylor, L.S.; Marsac, P.J.; Li, T.; Byrn, S.R. Solid-State Spectroscopic Investigation of Molecular Interactions between Clofazimine and Hypromellose Phthalate in Amorphous Solid Dispersions. Mol. Pharm. 2016, 13, 3964–3975. [Google Scholar] [CrossRef]
- Nie, H.; Mo, H.; Zhang, M.; Song, Y.; Fang, K.; Taylor, L.S.; Li, T.; Byrn, S.R. Investigating the Interaction Pattern and Structural Elements of a Drug–Polymer Complex at the Molecular Level. Mol. Pharm. 2015, 12, 2459–2468. [Google Scholar] [CrossRef]
- Chen, W.; Cheng, C.A.; Lee, B.Y.; Clemens, D.L.; Huang, W.Y.; Horwitz, M.A.; Zink, J.I. Facile strategy enabling both high joading and high release amounts of the water-insoluble drug clofazimine using mesoporous silica nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 31870–31881. [Google Scholar] [CrossRef]
- Maitra, A.; Bates, S.; Kolvekar, T.; Devarajan, P.V.; Guzman, J.D.; Bhakta, S. Repurposing–A ray of hope in tackling extensively drug resistance in tuberculosis. Int. J. Infect. Dis. 2015, 32, 50–55. [Google Scholar] [CrossRef] [Green Version]
- De Castro, R.R.; Todaro, V.; Da Silva, L.C.R.P.; Simon, A.; Carmo, F.A.D.; De Sousa, V.P.; Rodrigues, C.R.; Sarmento, B.; Healy, A.M.; Cabral, L.M. Development of inhaled formulation of modified clofazimine as an alternative to treatment of tuberculosis. J. Drug Deliv. Sci. Technol. 2020, 58, 101805. [Google Scholar] [CrossRef]
- Verma, R.K.; Germishuizen, W.A.; Motheo, M.P.; Agrawal, A.K.; Singh, A.K.; Mohan, M.; Gupta, P.; Gupta, U.D.; Cholo, M.C.; Anderson, R.; et al. Inhaled Microparticles Containing Clofazimine Are Efficacious in Treatment of Experimental Tuberculosis in Mice. Antimicrob. Agents Chemother. 2012, 57, 1050–1052. [Google Scholar] [CrossRef] [Green Version]
- Brunaugh, A.D.; Jan, S.U.; Ferrati, S.; Smyth, H.D.C. Excipient-Free Pulmonary Delivery and Macrophage Targeting of Clofazimine via Air Jet Micronization. Mol. Pharm. 2017, 14, 4019–4031. [Google Scholar] [CrossRef]
- Sabnis, A.A. Inhaled Clofazimine Delivery for the Treatment of Pulmonary Tuberculosis. Ph.D. Thesis, Creighton University, Omaha, NE, USA, 2015. [Google Scholar]
- Burger, C.; Aucamp, M.; Du Preez, J.L.; Haynes, R.K.; Ngwane, A.H.; Du Plessis, J.; Gerber, M. Formulation of Natural Oil Nano-Emulsions for the Topical Delivery of Clofazimine, Artemisone and Decoquinate. Pharm. Res. 2018, 35, 186. [Google Scholar] [CrossRef]
- Van Zyl, L.; Viljoen, J.; Haynes, R.K.; Aucamp, M.; Ngwane, A.H.; Du Plessis, J. Topical Delivery of Artemisone, Clofazimine and Decoquinate Encapsulated in Vesicles and Their In vitro Efficacy Against Mycobacterium tuberculosis. AAPS PharmSciTech 2019, 20, 33. [Google Scholar] [CrossRef]
- Valetti, S.; Xia, X.; Gouveia, J.C.; Brodin, P.; Bernet-Camard, M.-F.; Andersson, M.; Feiler, A. Clofazimine encapsulation in nanoporous silica particles for the oral treatment of antibiotic-resistant Mycobacterium tuberculosis infections. Nanomed. 2017, 12, 831–844. [Google Scholar] [CrossRef]
- Walvekar, P.; Gannimani, R.; Govender, T. Combination drug therapy via nanocarriers against infectious diseases. Eur. J. Pharm. Sci. 2019, 127, 121–141. [Google Scholar] [CrossRef]
- Li, H.; Ma, S.; Zhang, H.; Liu, J.; Wu, Y.; Cao, P.; Gao, X. Nano carrier mediated co-delivery of dapsone and clofazimine for improved therapeutic efficacy against tuberculosis in rats. Biomed. Res. 2017, 28, 1284–1289. [Google Scholar]
- Chaves, L.L.; Lima, S.A.C.; Vieira, A.C.; Barreiros, L.; Segundo, M.A.; Ferreira, D.; Sarmento, B.; Reis, S. Nanosystems as modulators of intestinal dapsone and clofazimine delivery. Biomed. Pharmacother. 2018, 103, 1392–1396. [Google Scholar] [CrossRef]
- Fitzgerald, L.E.; Abendaño, N.; Juste, R.A.; Alonso-Hearn, M. Three-Dimensional In Vitro Models of Granuloma to Study Bacteria-Host Interactions, Drug-Susceptibility, and Resuscitation of Dormant Mycobacteria. BioMed Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Maeda, Y.; Fukutomi, Y.; Makino, M. An in vitro model of Mycobacterium leprae induced granuloma formation. BMC Infect Dis. 2013, 13, 279. [Google Scholar] [CrossRef] [Green Version]
- Balamayooran, G.; Pena, M.; Sharma, R.; Truman, R.W. The armadillo as an animal model and reservoir host for Mycobacterium leprae. Clin. Dermatol. 2015, 33, 108–115. [Google Scholar] [CrossRef]
- Sharma, R.; Lahiri, R.; Scollard, D.M.; Pena, M.; Williams, D.L.; Adams, L.B.; Figarola, J.; Truman, R.W. The armadillo: A model for the neuropathy of leprosy and potentially other neurodegenerative diseases. Dis. Model. Mech. 2012, 6, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Madigan, A.C.; Cameron, J.; Ramakrishnan, L. A Zebrafish Model of Mycobacterium leprae Granulomatous Infection. J. Infect. Dis. 2017, 216, 776–779. [Google Scholar] [CrossRef] [Green Version]
- Tapinos, N.; Ohnishi, M.; Rambukkana, A. ErbB2 receptor tyrosine kinase signaling mediates early demyelination induced by leprosy bacilli. Nat. Med. 2006, 12, 961–966. [Google Scholar] [CrossRef]
- Mattos, K.A.; D’Avila, H.; Rodrigues, L.S.; Oliveira, V.G.C.; Sarno, E.N.; Atella, G.C.; Pereira, G.M.; Bozza, P.T.; Pessolani, M.C.V. Lipid droplet formation in leprosy: Toll-like receptor-regulated organelles involved in eicosanoid formation and Mycobacterium leprae pathogenesis. J. Leukoc. Biol. 2009, 87, 371–384. [Google Scholar] [CrossRef] [Green Version]
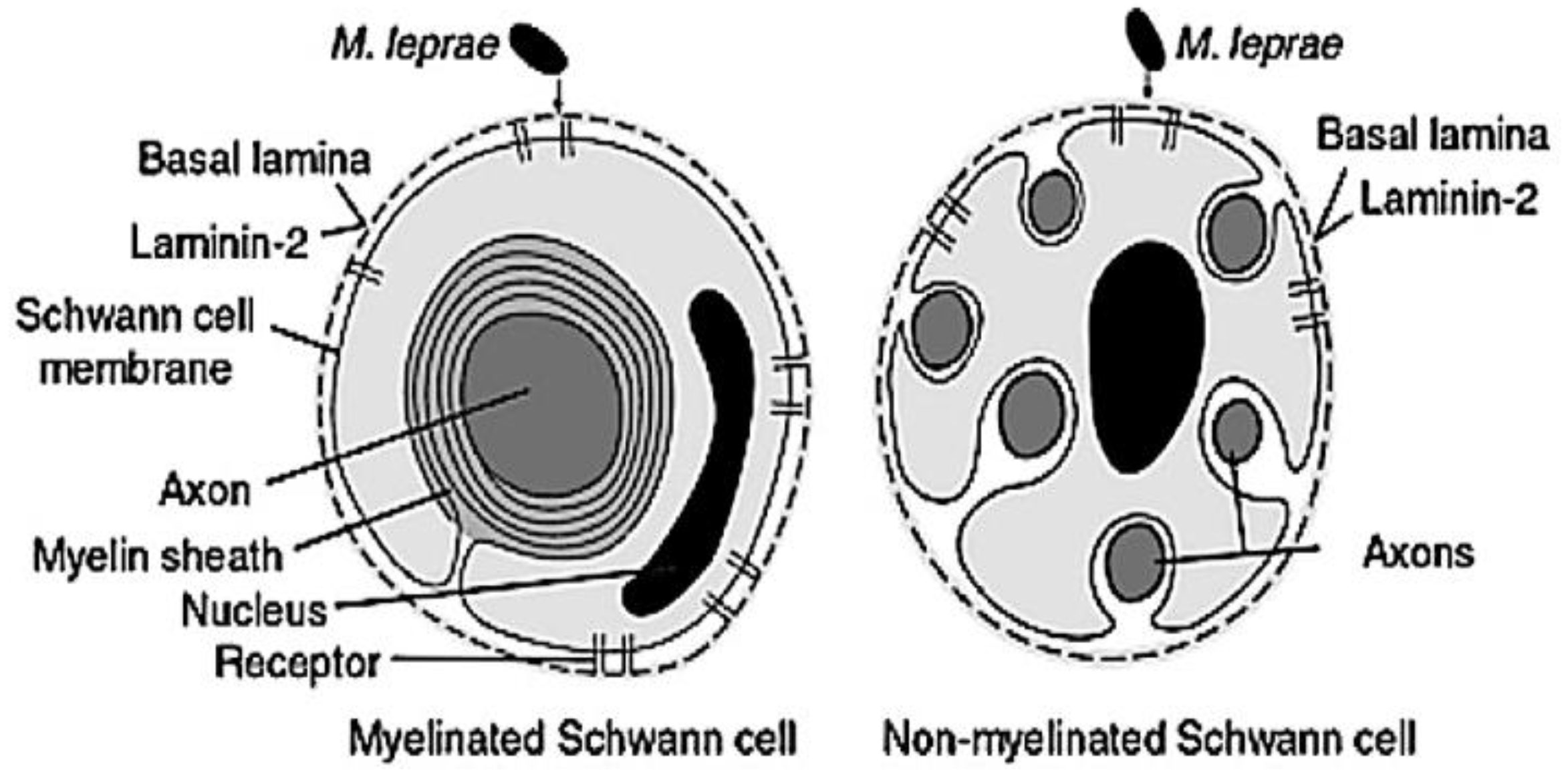
- Rambukkana, A.; Yamada, H.; Zanazzi, G.; Mathus, T.; Salzer, J.L.; Yurchenco, P.D.; Fischetti, V.A. Role of α-dystroglycan as a Schwann cell receptor for Mycobacterium leprae. Science 1998, 282, 2076–2079. [Google Scholar] [CrossRef]
- Van Der Meer-Janssen, Y.P.; Van Galen, J.; Batenburg, J.J.; Helms, J.B. Lipids in host-pathogen interactions: Pathogens exploit the complexity of the host cell lipidome. Prog. Lipid Res. 2010, 49, 1–26. [Google Scholar] [CrossRef]
| Type of Delivery System | Composition | Major Outcome | Ref |
|---|---|---|---|
| Oral Administration | |||
| Solid lipid nanoparticles | Cetyl palmitate Stearylamine Tween 80 Mannose | exhibits a pH-sensitive DAP release profile, with a faster drug release at acidic pH than at a neutral pH | [72] |
| Polymeric nanoparticles | Eudragit® L100 Pluronic® F-68 Polyvinyl alcohol | DAP in vitro release assay from the nanoparticles confirmed the nanoparticles’ pH sensitivity; nanoparticles showed increased DAP permeation in intestinal cell models compared to the drug solution | [73] |
| Hydrogel | Chitosan Glutaraldehyde Hydroxypropyl methylcellulose | loading of DAP within the pH-responsive interpenetrating polymer networks led to in vitro drug controlled release under gastrointestinal pH conditions | [74] |
| Nanoemulsions | Isopropyl myristate Span 80 or 20 Tween 80, 40 or 20 Propylene glycol Ethanol | DAP release profiles of the nanoemulsions showed a higher dissolution than free DAP | [75] |
| Polymeric dispersions | Polyvinylpyrrolidone (PVP K30) Pluronic F68 | in vivo dissolution rate of PDs was significantly faster compared to free DAP | [53] |
| Polymeric dispersions | 2-hydroxypropyl-β-cyclodextrin (HP β CD) β -cyclodextrin (CD) PVP K30 | complex DAP/HP β CD provided a great increase in DAP solubility | [76] |
| Transdermal administration | |||
| Ethosomes | Cholesterol Soy lecitin Ethanol Carbopol 934/PEG 400 with Cloxacillin Sodium | ethosomes in gel matrix exhibited a stable in vitro permeation study with uniform and sustained permeation of drugs | [77] |
| Solid lipid nanoparticles | Lactonic sophorolipid Pluronics with Rifampicin | released DAP remained in the therapeutic concentration window | [78] |
| Nanoemulsions | Isopropyl myristate n-methyl pyrrolidone Tween 80 and Span 20 | isopropyl myristate promoted an increase in DAP in vitro epidermal permeation | [79] |
| Other applications | |||
| Nanocomposites | Fullerene Boron nitride fullerene | improves the solubility and achieves drug dispersion for biological applications | [80] |
| Receptors of SC | Expressing Cells | Role | Ref |
|---|---|---|---|
| Laminin-α2 | Schwann cells | Cause early nerve degeneration | [20] |
| Tyrosine kinase receptor (ErB2 and ErK ½) | Schwann cells | Result in demyelination | [105] |
| Adipose differentiation-related protein (ADRP) | Macrophages | Lipid accumulation | [106] |
| LDL-R | Macrophages | Native cholesterol uptake | [107] |
| SRA-1, SRB-2, LRP-1 | Macrophages | Modified cholesterol uptake | [107] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaves, L.L.; Patriota, Y.; Soares-Sobrinho, J.L.; Vieira, A.C.C.; Lima, S.A.C.; Reis, S. Drug Delivery Systems on Leprosy Therapy: Moving Towards Eradication? Pharmaceutics 2020, 12, 1202. https://doi.org/10.3390/pharmaceutics12121202
Chaves LL, Patriota Y, Soares-Sobrinho JL, Vieira ACC, Lima SAC, Reis S. Drug Delivery Systems on Leprosy Therapy: Moving Towards Eradication? Pharmaceutics. 2020; 12(12):1202. https://doi.org/10.3390/pharmaceutics12121202
Chicago/Turabian StyleChaves, Luíse L., Yuri Patriota, José L. Soares-Sobrinho, Alexandre C. C. Vieira, Sofia A. Costa Lima, and Salette Reis. 2020. "Drug Delivery Systems on Leprosy Therapy: Moving Towards Eradication?" Pharmaceutics 12, no. 12: 1202. https://doi.org/10.3390/pharmaceutics12121202
APA StyleChaves, L. L., Patriota, Y., Soares-Sobrinho, J. L., Vieira, A. C. C., Lima, S. A. C., & Reis, S. (2020). Drug Delivery Systems on Leprosy Therapy: Moving Towards Eradication? Pharmaceutics, 12(12), 1202. https://doi.org/10.3390/pharmaceutics12121202








