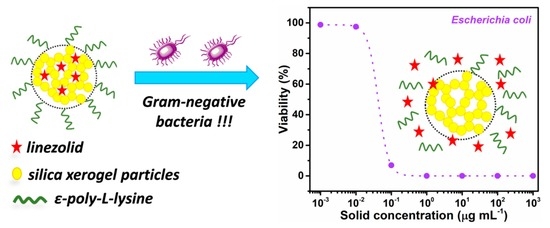
Antibacterial Activity of Linezolid against Gram-Negative Bacteria: Utilization of ε-Poly-l-Lysine Capped Silica Xerogel as an Activating Carrier
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Culture Media
2.2. Preparation of Silica Xerogel Using Volcanic Tuff (Solid S0)
2.3. Synthesis of Linezolid Loaded Silica Xerogel (Solid S1) and ε-Poly-l-Lysine Capped Linezolid Loaded Silica Xerogel (Solid S2)
2.4. Material Characterization
2.5. Antibacterial Activity
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hammad, A.; Abutaleb, N.S.; Elsebaei, M.M.; Norvil, A.B.; Alswah, M.; Ali, A.O.; Abdel-Aleem, J.A.; Alattar, A.; Bayoumi, S.A.; Gowher, H.; et al. From Phenylthiazoles to Phenylpyrazoles: Broadening the Antibacterial Spectrum toward Carbapenem-Resistant Bacteria. J. Med. Chem. 2019, 62, 7998–8010. [Google Scholar] [CrossRef] [PubMed]
- Yarlagadda, V.; Manjunath, G.B.; Sarkar, P.; Akkapeddi, P.; Paramanandham, K.; Shome, B.R.; Ravikumar, R.; Haldar, J. Glycopeptide Antibiotic to Overcome the Intrinsic Resistance of Gram-Negative Bacteria. ACS Infect. Dis. 2016, 2, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Serri, A.; Mahboubi, A.; Zarghi, A.; Moghimi, H.R. PAMAM-dendrimer Enhanced Antibacterial Effect of Vancomycin Hydrochloride Against Gram-Negative Bacteria. J. Pharm. Pharm. Sci. 2019, 22, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Bernardos, A.; Piacenza, E.; Sancenon, F.; Hamidi, M.; Maleki, A.; Turner, R.J.; Martínez-Máñez, R. Mesoporous Silica-Based Materials with Bactericidal Properties. Small 2019, 15, e1900669. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Ingle, A.P.; Pandit, R.; Paralikar, P.; Gupta, I.; Chaud, M.V.; Dos Santos, C.A. Broadening the spectrum of small-molecule antibacterials by metallic nanoparticles to overcome microbial resistance. Int. J. Pharm. 2017, 532, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Nicolosi, D.; Scalia, M.; Nicolosi, V.M.; Pignatello, R. Encapsulation in fusogenic liposomes broadens the spectrum of action of vancomycin against Gram-negative bacteria. Int. J. Antimicrob. Agents 2010, 35, 553–558. [Google Scholar] [CrossRef]
- Cottarel, G.; Wierzbowski, J. Combination drugs, an emerging option for antibacterial therapy. Trends Biotechnol. 2007, 25, 547–555. [Google Scholar] [CrossRef]
- Ulubayram, K.; Calamak, S.; Shahbazi, R.; Eroglu, I. Nanofibers Based Antibacterial Drug Design, Delivery and Applications. Curr. Pharm. Des. 2015, 21, 1930–1943. [Google Scholar] [CrossRef]
- Chen, C.; Yang, K. Ebselen bearing polar functionality: Identification of potent antibacterial agents against multidrug-resistant Gram-negative bacteria. Bioorg. Chem. 2019, 93, 103286. [Google Scholar] [CrossRef]
- Mas, N.; Galiana, I.; Mondragon, L.; Aznar, E.; Climent, E.; Cabedo, N.; Sancenon, F.; Murguia, J.R.; Martínez-Máñez, R.; Marcos, M.D.; et al. Enhanced efficacy and broadening of antibacterial action of drugs via the use of capped mesoporous nanoparticles. Chemistry 2013, 19, 11167–11171. [Google Scholar] [CrossRef]
- Sperandio, F.F.; Huang, Y.-Y.; Hamblin, M.R. Antimicrobial Photodynamic Therapy to Kill Gram-negative Bacteria. Recent Pat. Anti-Infect. Drug Discov. 2013, 8, 108–120. [Google Scholar] [CrossRef]
- Velikova, N.; Mas, N.; Miguel-Romero, L.; Polo, L.; Stolte, E.; Zaccaria, E.; Cao, R.; Taverne, N.; Murguia, J.R.; Martínez-Máñez, R.; et al. Broadening the antibacterial spectrum of histidine kinase autophosphorylation inhibitors via the use of epsilon-poly-L-lysine capped mesoporous silica-based nanoparticles. Nanomedicine 2017, 13, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Hernández Montoto, A.; Montes, R.; Samadi, A.; Gorbe, M.; Terrés, J.M.; Cao-Milán, R.; Aznar, E.; Ibañez, J.; Masot, R.; Marcos, M.D.; et al. Gold Nanostars Coated with Mesoporous Silica Are Effective and Nontoxic Photothermal Agents Capable of Gate Keeping and Laser-Induced Drug Release. ACS Appl. Mater. Interface 2018, 10, 27644–27656. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, A.; Aznar, E.; Martínez-Máñez, R.; Sancenón, F. New Advances in In Vivo Applications of Gated Mesoporous Silica as Drug Delivery Nanocarriers. Small 2020, 16, 1902242. [Google Scholar] [CrossRef] [PubMed]
- Giménez, C.; de la Torre, C.; Gorbe, M.; Aznar, E.; Sancenón, F.; Murguía, J.R.; Martínez-Máñez, R.; Marcos, M.D.; Amorós, P. Gated Mesoporous Silica Nanoparticles for the Controlled Delivery of Drugs in Cancer Cells. Langmuir 2015, 31, 3753–3762. [Google Scholar] [CrossRef] [PubMed]
- Moreno, V.M.; Álvarez, E.; Izquierdo-Barba, I.; Baeza, A.; Serrano-López, J.; Vallet-Regí, M. Bacteria as nanoparticles carrier for enhancing penetration in a tumoral matrix model. Adv. Mater. Interfaces 2020, 7, 1901942. [Google Scholar] [CrossRef] [PubMed]
- Shadjou, N.; Hasanzadeh, M. Bone tissue engineering using silica-based mesoporous nanobiomaterials: Recent progress. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 55, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Mas, N.; Arcos, D.; Polo, L.; Aznar, E.; Sánchez-Salcedo, S.; Sancenón, F.; García, A.; Marcos, M.D.; Baeza, A.; Vallet-Regí, M.; et al. Towards the Development of Smart 3D “Gated Scaffolds” for On-Command Delivery. Small 2014, 10, 4859–4864. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomedicine 2015, 11, 313–327. [Google Scholar] [CrossRef]
- Llopis-Lorente, A.; Diez, P.; Sanchez, A.; Marcos, M.D.; Sancenon, F.; Martinez-Ruiz, P.; Villalonga, R.; Martínez-Máñez, R. Interactive models of communication at the nanoscale using nanoparticles that talk to one another. Nat. Commun. 2017, 8, 15511. [Google Scholar] [CrossRef]
- Giménez, C.; Climent, E.; Aznar, E.; Martínez-Máñez, R.; Sancenón, F.; Marcos, M.D.; Amorós, P.; Rurack, K. Towards chemical communication between gated nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 12629–12633. [Google Scholar] [CrossRef] [PubMed]
- de Luis, B.; Llopis-Lorente, A.; Rincón, P.; Gadea, J.; Sancenón, F.; Aznar, E.; Villalonga, R.; Murguía, J.R.; Martínez-Máñez, R. An interactive model of communication between abiotic nanodevices and microorganisms. Angew. Chem. Int. Ed. 2019, 58, 14986–14990. [Google Scholar] [CrossRef] [PubMed]
- Hasanzadeh, M.; Shadjou, N.; de la Guardia, M.; Eskandani, M.; Sheikhzadeh, P. Mesoporous silica-based materials for use in biosensors. TrAC Trends Anal. Chem. 2012, 33, 117–129. [Google Scholar] [CrossRef]
- El-Safty, S.A.; Shenashen, M.A. Nanoscale dynamic chemical, biological sensor material designs for control monitoring and early detection of advanced diseases. Mater. Today Bio 2020, 5, 100044. [Google Scholar] [CrossRef]
- Pla, L.; Santiago-Felipe, S.; Tormo-Mas, M.A.; Pemán, J.; Sancenón, F.; Aznar, E.; Martínez-Máñez, R. Aptamer-capped nanoporous anodic alumina for Staphylococcus aureus detection. Sens. Actuators B Chem. 2020, 320, 128281. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Pandarus, V.; Beland, F.; Ilharco, L.M.; Pagliaro, M. The sol-gel route to advanced silica-based materials and recent applications. Chem. Rev. 2013, 113, 6592–6620. [Google Scholar] [CrossRef]
- Ulker, Z.; Erkey, C. An emerging platform for drug delivery: Aerogel based systems. J. Control. Release 2014, 177, 51–63. [Google Scholar] [CrossRef]
- Guzel Kaya, G.; Yilmaz, E.; Deveci, H. Sustainable nanocomposites of epoxy and silica xerogel synthesized from corn stalk ash: Enhanced thermal and acoustic insulation performance. Compos. Part. B Eng. 2018, 150, 1–6. [Google Scholar] [CrossRef]
- Maleki, H.; Duraes, L.; Garcia-Gonzalez, C.A.; Del Gaudio, P.; Portugal, A.; Mahmoudi, M. Synthesis and biomedical applications of aerogels: Possibilities and challenges. Adv. Colloid Interface Sci. 2016, 236, 1–27. [Google Scholar] [CrossRef]
- Quintanar-Guerrero, D.; Ganem-Quintanar, A.; Nava-Arzaluz, M.G.; Pinon-Seundo, E. Silica xerogels as pharmaceutical drug carriers. Expert Opin. 2009, 6, 485–498. [Google Scholar] [CrossRef]
- Pérez, N.A.; Lima, E.; Bosch, P.; Méndez-Vivar, J. Consolidating materials for the volcanic tuff in western Mexico. J. Cult. Herit. 2014, 15, 352–358. [Google Scholar] [CrossRef]
- Lemougna, P.N.; Wang, K.-T.; Tang, Q.; Nzeukou, A.N.; Billong, N.; Melo, U.C.; Cui, X.-M. Review on the use of volcanic ashes for engineering applications. Resour. Conserv. Recycl. 2018, 137, 177–190. [Google Scholar] [CrossRef]
- Maranon, E.; Ulmanu, M.; Fernandez, Y.; Anger, I.; Castrillon, L. Removal of ammonium from aqueous solutions with volcanic tuff. J. Hazard. Mater. 2006, 137, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Brum, L.F.W.; dos Santos, C.; Gnoatto, J.A.; Moura, D.J.; Santos, J.H.Z.; Brandelli, A. Silica xerogels as novel streptomycin delivery platforms. J. Drug Deliv. Sci. Technol. 2019, 53, 101210. [Google Scholar] [CrossRef]
- Deon, M.; Morawski, F.M.; Passaia, C.; Dalmás, M.; Laranja, D.C.; Malheiros, P.S.; Nicolodi, S.; Arenas, L.T.; Costa, T.M.H.; de Menezes, E.W.; et al. Chitosan-stabilized gold nanoparticles supported on silica/titania magnetic xerogel applied as antibacterial system. J. Sol-Gel Sci. Technol. 2018, 89, 333–342. [Google Scholar] [CrossRef]
- Storm, W.L.; Youn, J.; Reighard, K.P.; Worley, B.V.; Lodaya, H.M.; Shin, J.H.; Schoenfisch, M.H. Superhydrophobic nitric oxide-releasing xerogels. Acta Biomater. 2014, 10, 3442–3448. [Google Scholar] [CrossRef]
- Guzel Kaya, G.; Yilmaz, E.; Deveci, H. A novel silica xerogel synthesized from volcanic tuff as an adsorbent for high-efficient removal of methylene blue: Parameter optimization using Taguchi experimental design. J. Chem. Technol. Biotechnol. 2019, 94, 2729–2737. [Google Scholar] [CrossRef]
- Mohammadian, M.; Jafarzadeh Kashi, T.S.; Erfan, M.; Soorbaghi, F.P. Synthesis and characterization of silica aerogel as a promising drug carrier system. J. Drug Deliv. Sci. Technol. 2018, 44, 205–212. [Google Scholar] [CrossRef]
- Hu, W.; Li, M.; Chen, W.; Zhang, N.; Li, B.; Wang, M.; Zhao, Z. Preparation of hydrophobic silica aerogel with kaolin dried at ambient pressure. Colloid Surf. A Physicochem. Eng. Asp. 2016, 501, 83–91. [Google Scholar] [CrossRef]
- Zhu, H.; Jia, S.; Yang, H.; Tang, W.; Jia, Y.; Tan, Z. Characterization of bacteriostatic sausage casing: A composite of bacterial cellulose embedded with ɛ-polylysine. Food Sci. Biotechnol. 2010, 19, 1479–1484. [Google Scholar] [CrossRef]
- Lei, Y.; Hu, Z.; Cao, B.; Chen, X.; Song, H. Enhancements of thermal insulation and mechanical property of silica aerogel monoliths by mixing graphene oxide. Mater. Chem. Phys. 2017, 187, 183–190. [Google Scholar] [CrossRef]
- Huang, D.; Guo, C.; Zhang, M.; Shi, L. Characteristics of nanoporous silica aerogel under high temperature from 950 °C to 1200 °C. Mater. Des. 2017, 129, 82–90. [Google Scholar] [CrossRef]
- Kashyap Khatri, S.; Bathnanand, M.; Nikhila, R. Formulation and Evaluation of Wound Healing Activity of Linezolid Topical Preparations on Diabetic Rats. Int. J. Appl. Pharm. 2016, 8, 30–36. [Google Scholar]
- Shi, K.; Liu, Y.; Ke, L.; Fang, Y.; Yang, R.; Cui, F. Epsilon-poly-L-lysine guided improving pulmonary delivery of supramolecular self-assembled insulin nanospheres. Int. J. Biol. Macromol. 2015, 72, 1441–1450. [Google Scholar] [CrossRef]
- Lin, L.; Xue, L.; Duraiarasan, S.; Haiying, C. Preparation of ε-polylysine/chitosan nanofibers for food packaging against Salmonella on chicken. Food Pack Shelf Life 2018, 17, 134–141. [Google Scholar] [CrossRef]
- Lv, J.M.; Meng, Y.C.; Shi, Y.G.; Li, Y.H.; Chen, J.; Sheng, F. Properties of epsilon-polylysine·HCl/high-methoxyl pectin polyelectrolyte complexes and their commercial application. J. Food Process. Preserv. 2020, 44, e14320. [Google Scholar] [CrossRef]
- Huber, L.; Zhao, S.; Malfait, W.J.; Vares, S.; Koebel, M.M. Fast and Minimal-Solvent Production of Superinsulating Silica Aerogel Granulate. Angew. Chem. Int. Ed. 2017, 56, 4753–4756. [Google Scholar] [CrossRef]
- Shi, F.; Liu, J.-X.; Song, K.; Wang, Z.-Y. Cost-effective synthesis of silica aerogels from fly ash via ambient pressure drying. J. Non-Cryst. Solids 2010, 356, 2241–2246. [Google Scholar] [CrossRef]
- Sarawade, P.B.; Kim, J.-K.; Hilonga, A.; Kim, H.T. Production of low-density sodium silicate-based hydrophobic silica aerogel beads by a novel fast gelation process and ambient pressure drying process. Solid State Sci. 2010, 12, 911–918. [Google Scholar] [CrossRef]
- Liu, G.; Yang, R.; Li, M. Liquid adsorption of basic dye using silica aerogels with different textural properties. J. Non-Cryst. Solids 2010, 356, 250–257. [Google Scholar] [CrossRef]
- Guzel Kaya, G.; Deveci, H. Effect of Aging Solvents on Physicochemical and Thermal Properties of Silica Xerogels Derived from Steel Slag. ChemistrySelect 2020, 5, 1586–1591. [Google Scholar] [CrossRef]
- Rao, A.P.; Rao, A.V.; Gurav, J.L. Effect of protic solvents on the physical properties of the ambient pressure dried hydrophobic silica aerogels using sodium silicate precursor. J. Porous Mater. 2007, 15, 507–512. [Google Scholar] [CrossRef]
- Sperling, R.A.; Parak, W.J. Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Philos. Trans. A Math. Phys. Eng. Sci. 2010, 368, 1333–1383. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-k.; Lee, N.-H.; Oh, H.-J.; Kim, J.-W.; Rhee, C.-K.; Park, K.-S.; Kim, S.-J. Surface modification and characterization of highly dispersed silica nanoparticles by a cationic surfactant. Colloid Surf. A Physicochem. Eng. Asp. 2010, 358, 172–176. [Google Scholar] [CrossRef]
- Tan, L.; Tan, X.; Fang, M.; Yu, Z.; Wang, X. Effects of humic acid and Mg2+ on morphology and aggregation behavior of silica aerogels. J. Mol. Liq. 2018, 264, 261–268. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Hasan, J.; Crawford, R.J.; Ivanova, E.P. Antibacterial surfaces: The quest for a new generation of biomaterials. Trends Biotechnol. 2013, 31, 295–304. [Google Scholar] [CrossRef]
- Katsikogianni, M.; Missirlis, Y.F. Concise Review of Mechanisms of Bacterial Adhesion to Biomaterials and of Techniques Used in Estimating Bacteria-Material Interactions. Eur. Cell Mater. 2004, 8, 37–57. [Google Scholar] [CrossRef]
- Ruiz-Rico, M.; Fuentes, C.; Pérez-Esteve, É.; Jiménez-Belenguer, A.I.; Quiles, A.; Marcos, M.D.; Martínez-Máñez, R.; Barat, J.M. Bactericidal activity of caprylic acid entrapped in mesoporous silica nanoparticles. Food Control 2015, 56, 77–85. [Google Scholar] [CrossRef]
- Zahedi Bialvaei, A.; Rahbar, M.; Yousefi, M.; Asgharzadeh, M.; Samadi Kafil, H. Linezolid: A promising option in the treatment of Gram-positives. J. Antimicrob. Chemother. 2017, 72, 354–364. [Google Scholar] [CrossRef]
- Chang, Y.; McLandsborough, L.; McClements, D.J. Cationic Antimicrobial (ε-Polylysine)–Anionic Polysaccharide (Pectin) Interactions: Influence of Polymer Charge on Physical Stability and Antimicrobial Efficacy. J. Agric. Food Chem. 2012, 60, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.C.; Singh, A.; Pandey, A.K.; Mishra, A. Review on production and medical applications of ε-polylysine. Biochem. Eng. J. 2012, 65, 70–81. [Google Scholar] [CrossRef]
- Liu, J.-N.; Chang, S.-L.; Xu, P.-W.; Tan, M.-H.; Zhao, B.; Wang, X.-D.; Zhao, Q.-S. Structural Changes and Antibacterial Activity of Epsilon-poly-L-lysine in Response to pH and Phase Transition and Their Mechanisms. J. Agric. Food Chem. 2020, 68, 1101–1109. [Google Scholar] [CrossRef] [PubMed]







| Material | Composition |
|---|---|
| Solid S0 | Silica xerogel |
| Solid S1 | Silica xerogel loaded with linezolid |
| Solid S2 | Silica xerogel loaded with linezolid and capped with ε-poly-l-lysine |
| Material | SBET a (m2 g−1) | Pore Size (nm) | Pore Volume (cm3 g−1) | Bulk Density (g cm−3) |
|---|---|---|---|---|
| solid S0 | 195 | 10 | 0.50 | 0.037 |
| Material | Zeta Potential (mV) | Electrophoretic Mobility (μm cm V−1 S−1) | Conductivity (mS cm−1) |
|---|---|---|---|
| solid S0 | −46.1 ± 0.1 | −3.62 ± 0.01 | 0.0327 |
| solid S1 | −42.0 ± 1.1 | −3.29 ± 0.09 | 0.0314 |
| solid S2 | 16.9 ± 0.6 | 1.33 ± 0.05 | 0.0271 |
| Material | Linezolid (mmol g−1) | ε-poly-l-lysine (mmol g−1) |
|---|---|---|
| solid S1 | 0.188 | - |
| solid S2 | 0.187 | 0.022 |
| Active Compound | Escherichia coli | Pseudomonas aeruginosa | Staphylococcus aureus |
|---|---|---|---|
| linezolid | --- | --- | 1.00 |
| ε-poly-l-lysine | 0.13 | 0.21 | 0.08 |
| Material | Escherichia coli | Pseudomonas aeruginosa | Staphylococcus aureus |
|---|---|---|---|
| solid S1 | --- | --- | 1.37 |
| solid S2 | 0.04 | 0.02 | 0.07 |
| solid S3 | 0.29 | 0.21 | 0.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzel Kaya, G.; Medaglia, S.; Candela-Noguera, V.; Tormo-Mas, M.Á.; Marcos, M.D.; Aznar, E.; Deveci, H.; Martínez-Máñez, R. Antibacterial Activity of Linezolid against Gram-Negative Bacteria: Utilization of ε-Poly-l-Lysine Capped Silica Xerogel as an Activating Carrier. Pharmaceutics 2020, 12, 1126. https://doi.org/10.3390/pharmaceutics12111126
Guzel Kaya G, Medaglia S, Candela-Noguera V, Tormo-Mas MÁ, Marcos MD, Aznar E, Deveci H, Martínez-Máñez R. Antibacterial Activity of Linezolid against Gram-Negative Bacteria: Utilization of ε-Poly-l-Lysine Capped Silica Xerogel as an Activating Carrier. Pharmaceutics. 2020; 12(11):1126. https://doi.org/10.3390/pharmaceutics12111126
Chicago/Turabian StyleGuzel Kaya, Gulcihan, Serena Medaglia, Vicente Candela-Noguera, María Ángeles Tormo-Mas, María Dolores Marcos, Elena Aznar, Huseyin Deveci, and Ramón Martínez-Máñez. 2020. "Antibacterial Activity of Linezolid against Gram-Negative Bacteria: Utilization of ε-Poly-l-Lysine Capped Silica Xerogel as an Activating Carrier" Pharmaceutics 12, no. 11: 1126. https://doi.org/10.3390/pharmaceutics12111126
APA StyleGuzel Kaya, G., Medaglia, S., Candela-Noguera, V., Tormo-Mas, M. Á., Marcos, M. D., Aznar, E., Deveci, H., & Martínez-Máñez, R. (2020). Antibacterial Activity of Linezolid against Gram-Negative Bacteria: Utilization of ε-Poly-l-Lysine Capped Silica Xerogel as an Activating Carrier. Pharmaceutics, 12(11), 1126. https://doi.org/10.3390/pharmaceutics12111126