Effect of Hyaluronic Acid and Pluronic-F68 on the Surface Properties of Foam as a Delivery System for Polidocanol in Sclerotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
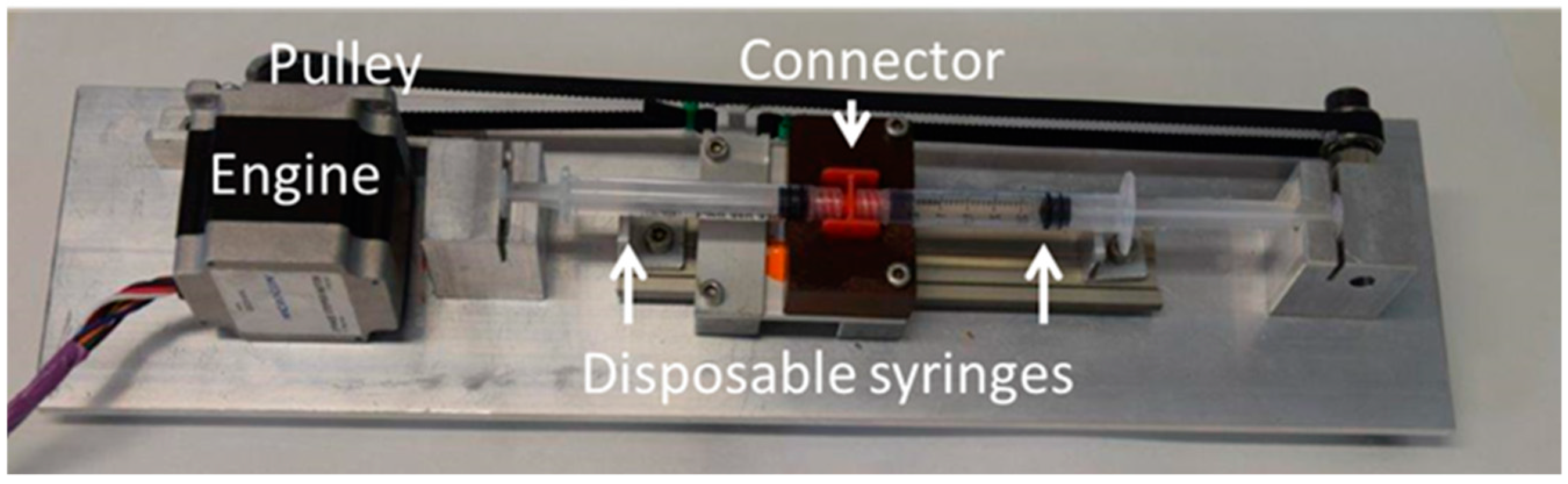
2.2.1. Double Syringe Method
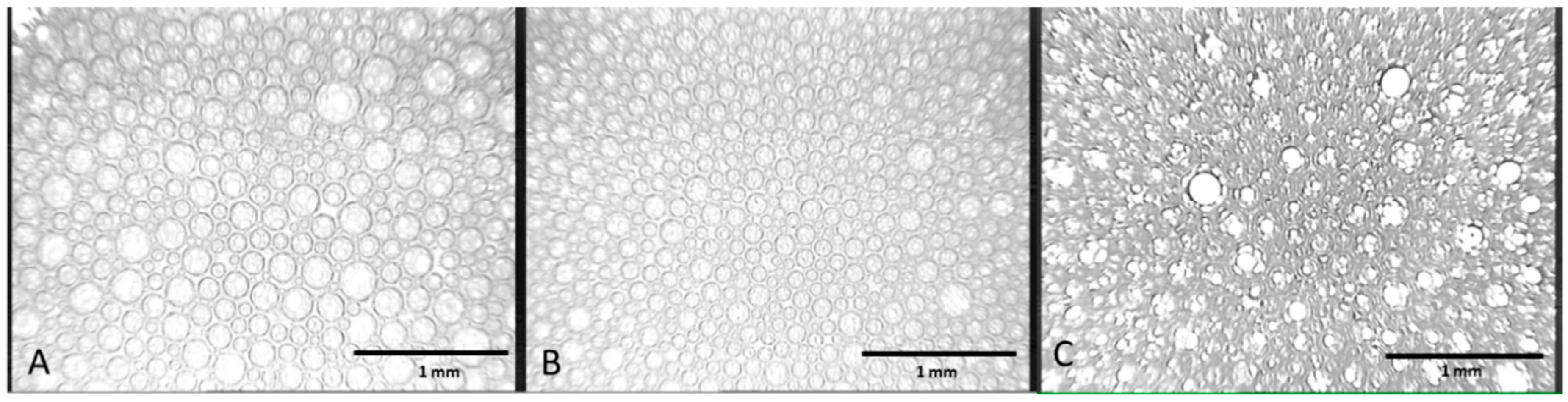
2.2.2. Microscope Images
2.2.3. Pendant Drop Surface Film Balance (DINATEN)
2.2.4. Langmuir Film Balance
2.2.5. Brewster Angle Microscopy
2.2.6. Statistical Analysis
3. Results
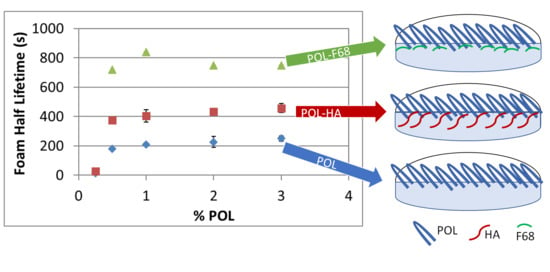
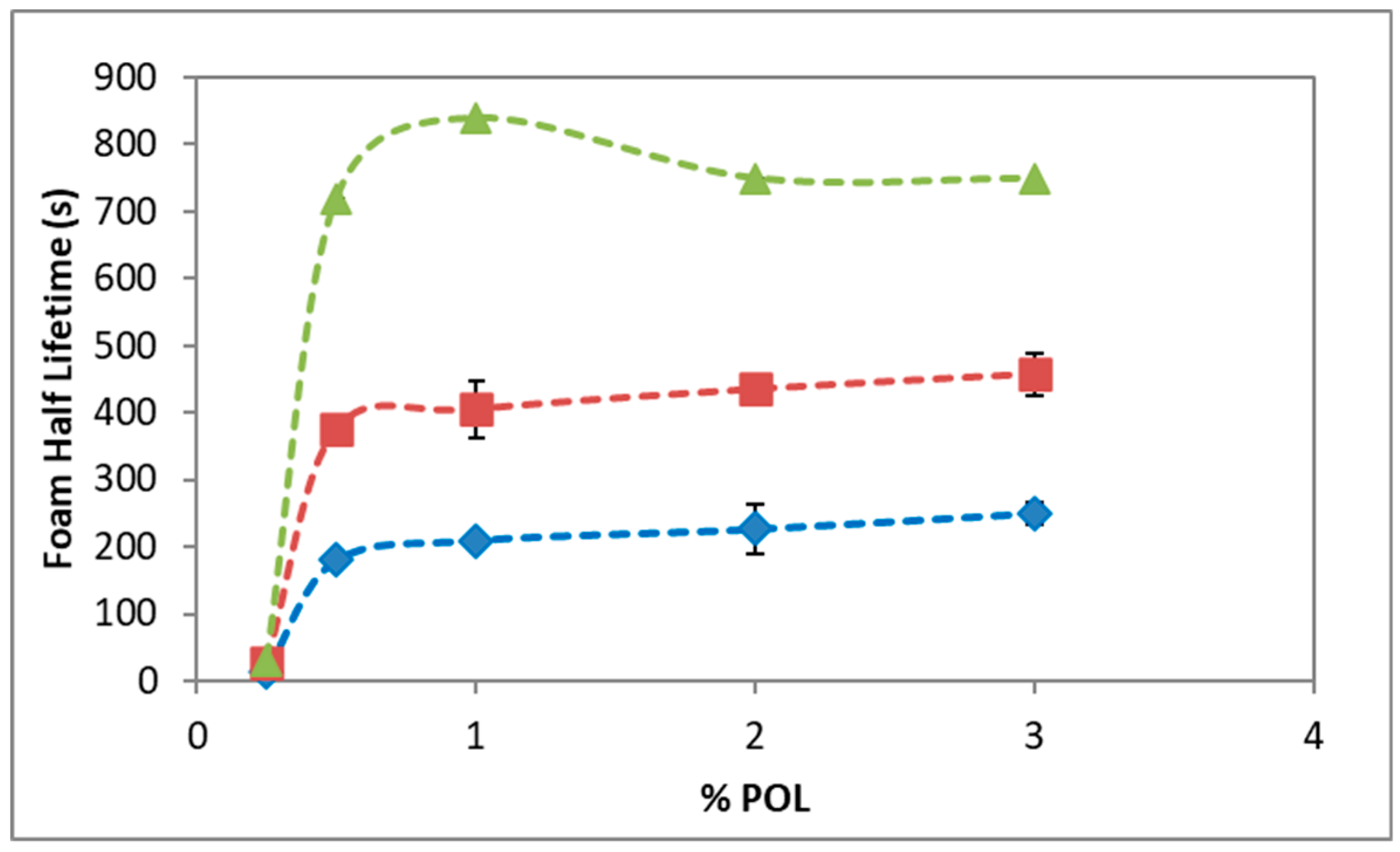
3.1. Foam Stability and Bubble Structure
3.2. Surface Tension
3.3. Surface Dilatational Elasticity and Viscosity
3.4. Compression Isotherm and microBAM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Su, S.; Kang, P.M. Recent Advances in Nanocarrier-Assisted Therapeutics Delivery Systems. Pharmaceutics 2020, 12, 837. [Google Scholar] [CrossRef]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G.; Lee, W.; Park, H.H. Protein-based nanoparticles as drug delivery systems. Pharmaceutics 2020, 12, 604. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jones, S.A.; Brown, M.B. Dynamic foams in topical drug delivery. J. Pharm. Pharmacol. 2010, 62, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Shinde, N.G.; Aloorkar, N.H.; Bangar, B.N.; Deshmukh, S.M.; Shirke, M.V.; Kale, B.B. Pharmaceutical Foam Drug Delivery System: General Considerations. Indo Am. J. Pharm. Res. 2013, 3, 1322–1327. [Google Scholar]
- Star, P.; Connor, D.E.; Parsi, K. Novel developments in foam sclerotherapy: Focus on Varithena® (polidocanol endovenous microfoam) in the management of varicose veins. Phlebology 2017, 33, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Eckmann, D.M. Polidocanol for endovenous microfoam sclerosant therapy. Expert Opin. Investig. Drugs 2009, 18, 1919–1927. [Google Scholar] [CrossRef]
- Frullini, A.; Cavezzi, A. Sclerosing foam in the treatment of varicose veins and telangiectases: History and analysis of safety and complications. Dermatol. Surg. 2002, 28, 11–15. [Google Scholar] [CrossRef]
- Nastasa, V.; Samaras, K.; Ampatzidis, C.; Karapantsios, T.D.; Trelles, M.A.; Moreno-Moraga, J.; Smarandache, A.; Pascu, M.L. Properties of polidocanol foam in view of its use in sclerotherapy. Int. J. Pharm. 2015, 478, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Wollmann, J.C. Sclerosant foams: Stabilities, physical properties and rheological behaviour. Phlebologie 2010, 39, 208–217. [Google Scholar] [CrossRef]
- Duffy, D.M. Sclerosants: A comparative review. Dermatol. Surg. 2010, 36, 1010–1025. [Google Scholar] [CrossRef] [PubMed]
- Parsi, K. Interaction of detergent sclerosants with cell membranes. Phlebology 2015, 30, 306–315. [Google Scholar] [CrossRef]
- Bai, T.; Jiang, W.; Chen, Y.; Yan, F.; Xu, Z.; Fan, Y. Effect of Multiple Factors on Foam Stability in Foam Sclerotherapy. Sci. Rep. 2018, 8, 15683. [Google Scholar] [CrossRef]
- Maldonado-Valderrama, J.; Martín-Rodriguez, A.; Gálvez-Ruiz, M.J.; Miller, R.; Langevin, D.; Cabrerizo-Vílchez, M.A. Foams and emulsions of β-casein examined by interfacial rheology. Colloids Surf. A Physicochem. Eng. Asp. 2008, 323, 116–122. [Google Scholar] [CrossRef]
- Wilde, P.J. Interfaces: Their role in foam and emulsion behaviour. Curr. Opin. Colloid Interface Sci. 2000, 5, 176–181. [Google Scholar] [CrossRef]
- Rio, E.; Drenckhan, W.; Salonen, A.; Langevin, D. Unusually stable liquid foams. Adv. Colloid Interface Sci. 2014, 205, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Langevin, D. Aqueous foams: A field of investigation at the Frontier between chemistry and physics. ChemPhysChem 2008, 9, 510–522. [Google Scholar] [CrossRef]
- Maldonado-valderrama, J.; Martín-Molina, A.; Martín-Rodríguez, A.; Cabrerizo-Vílchez, M.A.; Gálvez-Ruiz, M.J.; Langevin, D. Surface Properties and Foam Stability of Protein/Surfactant Mixtures: Theory and Experiment. J. Phys. Chem. C 2007, 2715–2723. [Google Scholar] [CrossRef]
- Tessari, L.; Cavezzi, A.; Frullini, A. Preliminary experience with a new sclerosing foam in the treatment of varicose veins. Dermatol. Surg. 2001, 27, 58–60. [Google Scholar] [CrossRef]
- Gaillard, T.; Roché, M.; Honorez, C.; Jumeau, M.; Balan, A.; Jedrzejczyk, C.; Drenckhan, W. Controlled foam generation using cyclic diphasic flows through a constriction. Int. J. Multiph. Flow 2017, 96, 173–187. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Chen, A.; Wang, T.; Liu, S. A modified Tessari method for producing more foam. Springerplus 2016, 5, 129. [Google Scholar] [CrossRef]
- Cabrera, J.; Cabrera, J.; Garcia-Olmedo, M.A.; Redondo, P. Treatment of Venous Malformations with Sclerosant in Microfoam Form. Arch. Dermatol. 2003, 139, 1409–1416. [Google Scholar] [CrossRef]
- Rial, R.; Hervas, L.S.; Monux, G.; Galindo, A.; Martin, A.; Hernando, M.; Martinez, I.; Hernando, A.; Serrano, F.J. Polidocanol foam stability in terms of its association with glycerin. Phlebology 2014, 29, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Nastasa, V.; Samaras, K.; Pascu, M.L.; Karapantsios, T.D. Moderately stable emulsions produced by a double syringe method. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 460, 321–326. [Google Scholar] [CrossRef]
- Rao, N.V.; Rho, J.G.; Um, W.; Ek, P.K.; Nguyen, V.Q.; Oh, B.H.; Kim, W.; Park, J.H. Hyaluronic acid nanoparticles as nanomedicine for treatment of inflammatory diseases. Pharmaceutics 2020, 12, 931. [Google Scholar] [CrossRef]
- Fakhari, A.; Berkland, C. Applications and emerging trends of hyaluronic acid in tissue engineering, as a dermal filler and in osteoarthritis treatment. Acta Biomater. 2013, 9, 7081–7092. [Google Scholar] [CrossRef]
- Dodero, A.; Williams, R.; Gagliardi, S.; Vicini, S.; Alloisio, M.; Castellano, M. A micro-rheological and rheological study of biopolymers solutions: Hyaluronic acid. Carbohydr. Polym. 2019, 203, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Torcello-Gómez, A.; Wulff-Pérez, M.; Gálvez-Ruiz, M.J.; Martín-Rodríguez, A.; Cabrerizo-Vílchez, M.; Maldonado-Valderrama, J. Block copolymers at interfaces: Interactions with physiological media. Adv. Colloid Interface Sci. 2014, 206, 414–427. [Google Scholar] [CrossRef]
- Cao, J.; Su, M.; Hasan, N.; Lee, J.; Kwak, D.; Kim, D.Y.; Kim, K.; Lee, E.H.; Jung, J.H.; Yoo, J. Nitric Oxide-Releasing Thermoresponsive Pluronic F127/Alginate Hydrogel for Enhanced Antibacterial Activity and Accelerated Healing of Infected Wounds. Pharmaceutics 2020, 12, 926. [Google Scholar] [CrossRef]
- Patel, H.R.; Patel, R.P.; Patel, M.M. Poloxamers: A pharmaceutical excipients with therapeutic behaviours. Int. J. PharmTech Res. 2009, 1, 299–303. [Google Scholar]
- Maldonado-Valderrama, J.; Torcello-Gómez, A.; del Castillo-Santaella, T.; Holgado-Terriza, J.A.; Cabrerizo-Vílchez, M.A. Subphase exchange experiments with the pendant drop technique. Adv. Colloid Interface Sci. 2015, 222, 488–501. [Google Scholar] [CrossRef]
- Maldonado-Valderrama, J.; Holgado-Terriza, J.A.; Torcello-Gómez, A.; Cabrerizo-Vílchez, M.A. In vitro digestion of interfacial protein structures. Soft Matter 2013, 1043–1053. [Google Scholar] [CrossRef]
- Hoenig, D.; Moebius, D. Direct visualization of monolayers at the air-water interface by Brewster angle microscopy. J. Phys. Chem. 1991, 95, 4590–4592. [Google Scholar] [CrossRef]
- Wang, R.; Guo, Y.; Liu, H.; Chen, Y.; Shang, Y.; Liu, H. The effect of chitin nanoparticles on surface behaviour of DPPC/DPPG Langmuir monolayers. J. Colloid Interface Sci. 2018, 519, 186–193. [Google Scholar] [CrossRef]
- Wong, K.; Chen, T.; Connor, D.E.; Behnia, M.; Parsi, K. Basic physiochemical and rheological properties of detergent sclerosants. Phlebology 2015, 30, 339–349. [Google Scholar] [CrossRef]
- Dinache, A.; Smarandache, A.; Andrei, I.R.; Urzica, I.; Nichita, C.; Boni, M.; Nastasa, V.; Pascu, M.L. Laser assisted generation of micro/nanosize emulsions. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 577, 265–273. [Google Scholar] [CrossRef]
- Smarandache, A.; Moreno, J.; Staicu, A.; Trelles, M.; Pascu, M.-L. Applications of Polidocanol in Varicose Vein Treatment Assisted by Exposure to Nd:YAG Laser Radiation. Nd YAG Laser 2012. [Google Scholar] [CrossRef]
- López-Cervantes, J.L.; Gracia-Fadrique, J.; Calvo, E.; Amigo, A. Surface tensions, densities, and speeds of sound for aqueous solutions of lauryl ether ethoxylates. Fluid Phase Equilib. 2013, 356, 193–200. [Google Scholar] [CrossRef]
- Rodriguez Patino, J.M.; Pilosof, A.M.R. Protein-polysaccharide interactions at fluid interfaces. Food Hydrocoll. 2011, 25, 1925–1937. [Google Scholar] [CrossRef]
- Maldonado-Valderrama, J.; Fainerman, V.B.; Gálvez-Ruiz, M.J.; Martín-Rodriguez, A.; Cabrerizo-Vílchez, M.A.; Miller, R. Dilatational Rheology of β-Casein Adsorbed Layers at Liquid−Fluid Interfaces. J. Phys. Chem. B 2005, 109, 17608–17616. [Google Scholar] [CrossRef]
- Pérez-Mosqueda, L.M.; Maldonado-Valderrama, J.; Ramírez, P.; Cabrerizo-Vílchez, M.A.; Muñoz, J. Interfacial characterization of Pluronic PE9400 at biocompatible (air-water and limonene-water) interfaces. Colloids Surf. B Biointerfaces 2013, 111, 171–178. [Google Scholar] [CrossRef]
- Maldonado-Valderrama, J.; Muros-Cobos, J.L.; Holgado-Terriza, J.A.; Cabrerizo-Vílchez, M.A. Bile salts at the air-water interface: Adsorption and desorption. Colloids Surfaces B Biointerfaces 2014, 120, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, G.A.; Yokoi, N.; Ivanova, S.; Dimitrov, T.; Andreev, K.; Krastev, R.; Lalchev, Z. Surface chemistry study of the interactions of hyaluronic acid and benzalkonium chloride with meibomian and corneal cell lipids. Soft Matter 2013, 9, 10841–10856. [Google Scholar] [CrossRef]
- Bringezu, F.; Ding, J.; Brezesinski, G.; Waring, A.J.; Zasadzinski, J.A. Influence of pulmonary surfactant protein B on model lung surfactant monolayers. Langmuir 2002, 18. [Google Scholar] [CrossRef]
- Yang, Y.; Maldonado-Valderrama, J.; Martín-Molina, A. Temperature and electrostatics effects on charged poly(N-isopropylacrylamide) microgels at the interface. J. Mol. Liq. 2020, 303, 112678. [Google Scholar] [CrossRef]
- Miller, R.; Aksenenko, E.V.; Alahverdjieva, V.S.; Fainerman, V.B.; Kotsmar, C.S.; Krägel, J.; Leser, M.E.; Maldonado-Valderrama, J.; Pradines, V.; Stefaniu, C.; et al. Proteins in Solution and at Interfaces. In Methods and Applications in Biotechnology and Materials Science; Ruso, J.M., Piñeiro, A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 389–427. [Google Scholar]
- Del Castillo-Santaella, T.; Cebrián, R.; Maqueda, M.; Gálvez-Ruiz, M.J.; Maldonado-Valderrama, J. Assessing in vitro digestibility of food biopreservative AS-48. Food Chem. 2018, 246, 249–257. [Google Scholar] [CrossRef]
- Martin, A.H.; Grolle, K.; Bos, M.A.; Cohen Stuart, M.A.; Van Vliet, T. Network forming properties of various proteins adsorbed at the air/water interface in relation to foam stability. J. Colloid Interface Sci. 2002, 254, 175–183. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
del Castillo-Santaella, T.; Yang, Y.; Martínez-González, I.; Gálvez-Ruiz, M.J.; Cabrerizo-Vílchez, M.Á.; Holgado-Terriza, J.A.; Selles-Galiana, F.; Maldonado-Valderrama, J. Effect of Hyaluronic Acid and Pluronic-F68 on the Surface Properties of Foam as a Delivery System for Polidocanol in Sclerotherapy. Pharmaceutics 2020, 12, 1039. https://doi.org/10.3390/pharmaceutics12111039
del Castillo-Santaella T, Yang Y, Martínez-González I, Gálvez-Ruiz MJ, Cabrerizo-Vílchez MÁ, Holgado-Terriza JA, Selles-Galiana F, Maldonado-Valderrama J. Effect of Hyaluronic Acid and Pluronic-F68 on the Surface Properties of Foam as a Delivery System for Polidocanol in Sclerotherapy. Pharmaceutics. 2020; 12(11):1039. https://doi.org/10.3390/pharmaceutics12111039
Chicago/Turabian Styledel Castillo-Santaella, Teresa, Yan Yang, Inmaculada Martínez-González, María José Gálvez-Ruiz, Miguel Ángel Cabrerizo-Vílchez, Juan Antonio Holgado-Terriza, Fernando Selles-Galiana, and Julia Maldonado-Valderrama. 2020. "Effect of Hyaluronic Acid and Pluronic-F68 on the Surface Properties of Foam as a Delivery System for Polidocanol in Sclerotherapy" Pharmaceutics 12, no. 11: 1039. https://doi.org/10.3390/pharmaceutics12111039
APA Styledel Castillo-Santaella, T., Yang, Y., Martínez-González, I., Gálvez-Ruiz, M. J., Cabrerizo-Vílchez, M. Á., Holgado-Terriza, J. A., Selles-Galiana, F., & Maldonado-Valderrama, J. (2020). Effect of Hyaluronic Acid and Pluronic-F68 on the Surface Properties of Foam as a Delivery System for Polidocanol in Sclerotherapy. Pharmaceutics, 12(11), 1039. https://doi.org/10.3390/pharmaceutics12111039