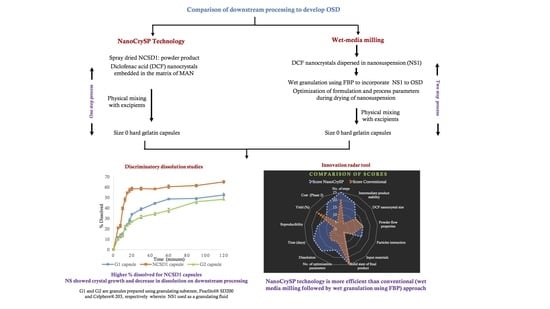
Comparison of Downstream Processing of Nanocrystalline Solid Dispersion and Nanosuspension of Diclofenac Acid to Develop Solid Oral Dosage Form
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Analytical Method Development of DCF Using HPLC
2.2.2. Generation of NCSD of DCF Using NanoCrySP Technology
2.2.3. Characterization of NCSD
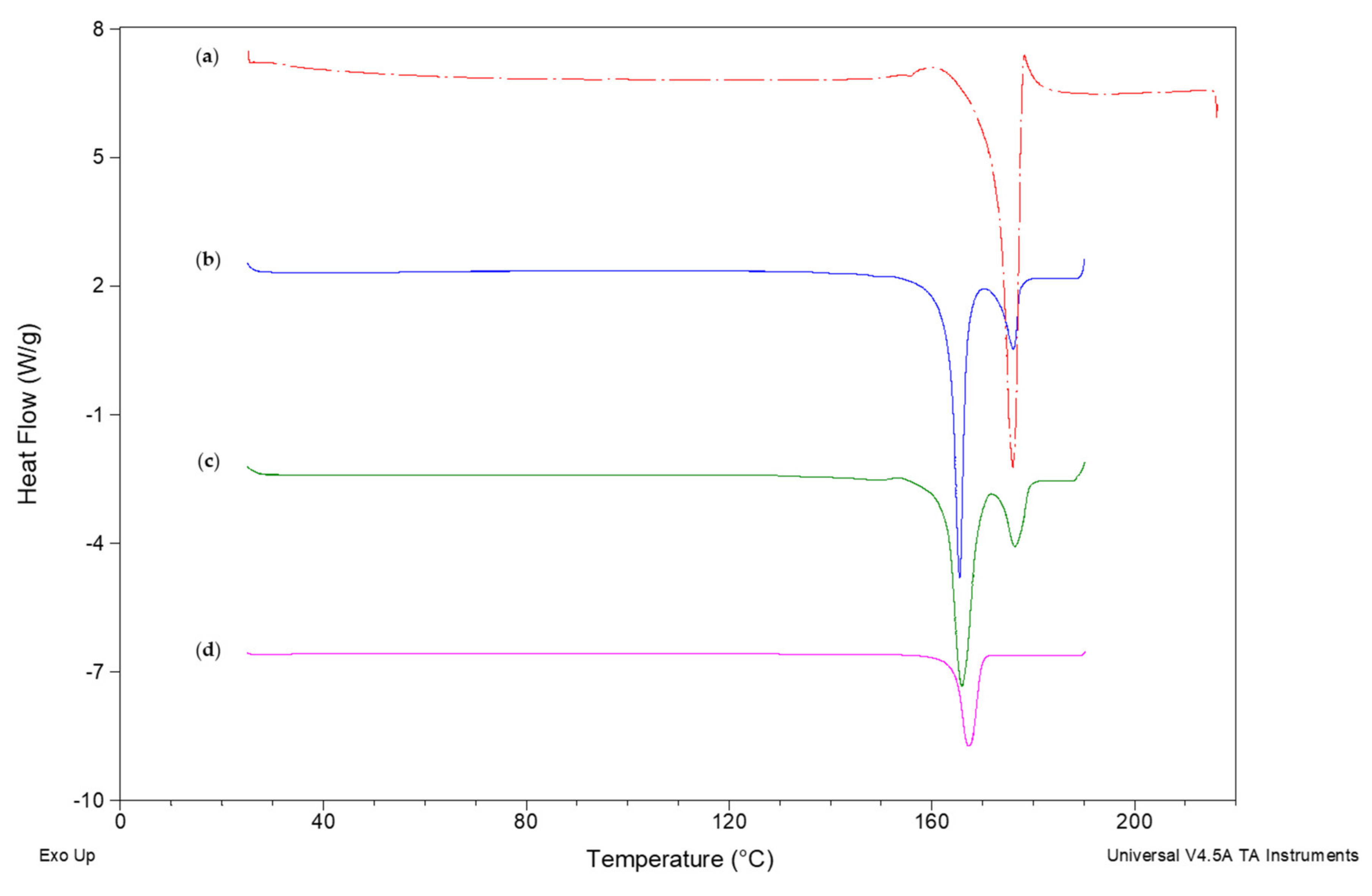
Differential Scanning Calorimetry (DSC)
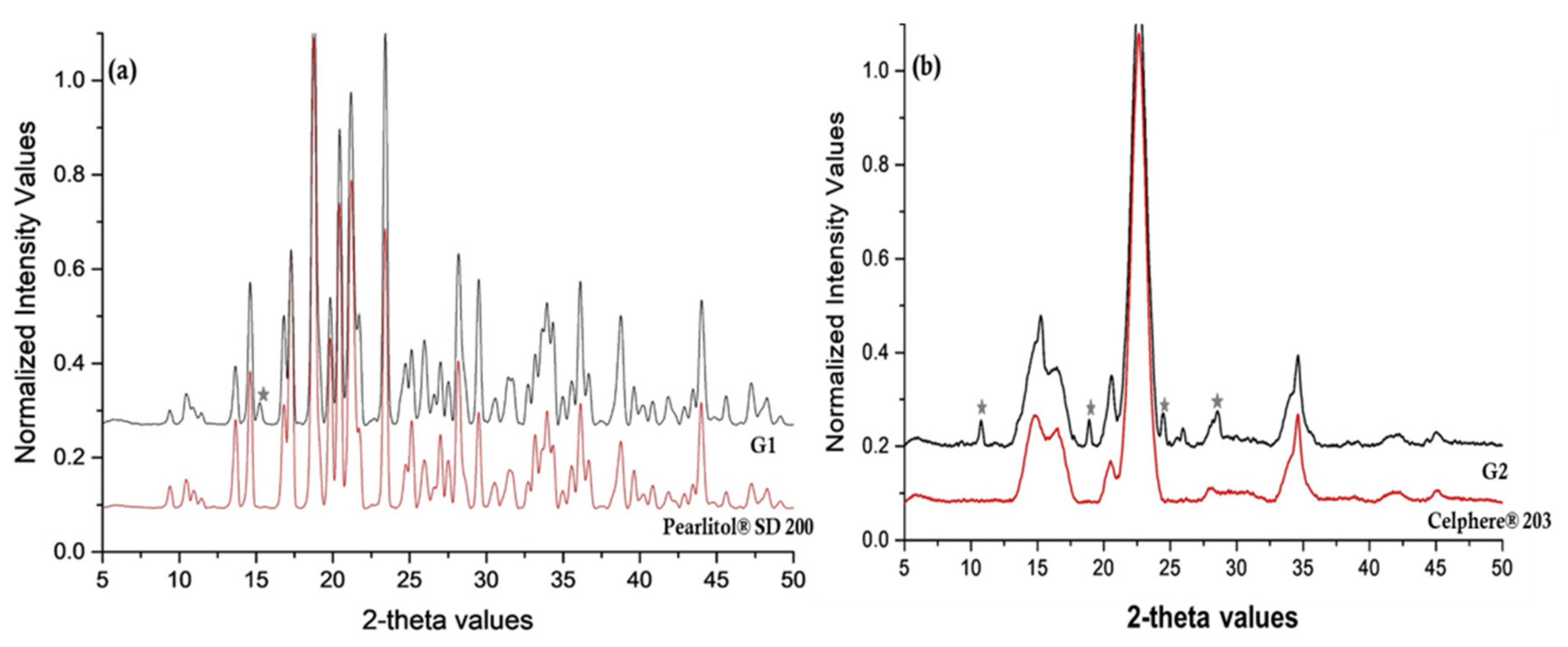
Powder X-ray Diffraction (PXRD)
Size Determination Using Zetasizer®
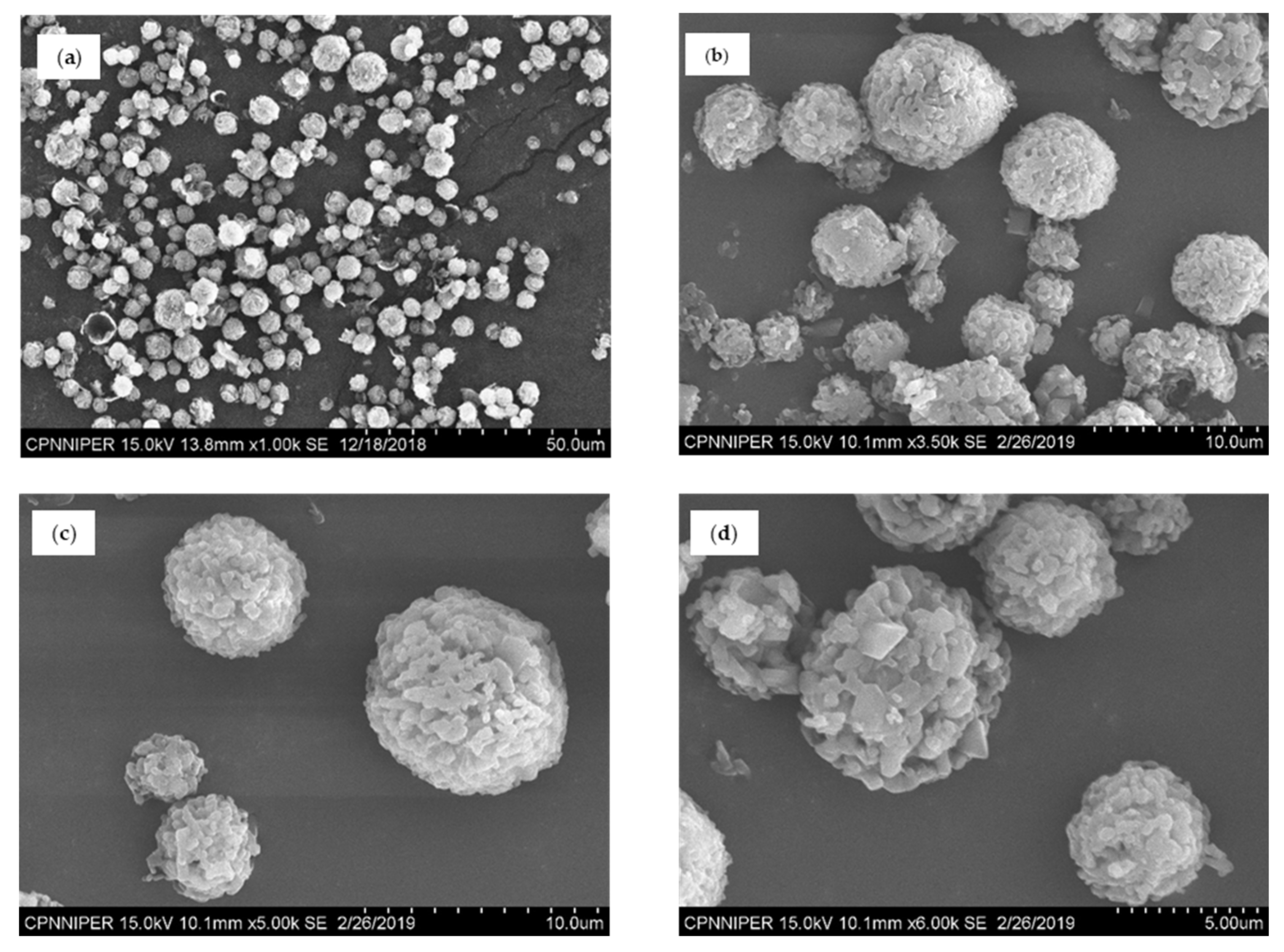
Scanning Electron Microscopy (SEM)
Assay
Determination of % Yield
Moisture Content Determination
Flow Properties
2.2.4. Generation and Characterization of DCF Nanocrystals Prepared by Wet Media Milling
2.2.5. Wet Granulation Using Nanosuspension
2.2.6. Characterization of Granules
2.2.7. Incorporation of Nanocrystals to Solid Oral Dosage Form
2.2.8. Discriminatory Drug Dissolution Studies
2.2.9. Stability of Intermediate Products
2.2.10. Comparison of Process Efficiency of NanoCrySP Technology and Conventional (Wet Media Milling Followed by Wet Granulation Using FBP) Approach
Costing of Both the Approaches
Comparison of Various Quantitative and Qualitative Aspects of Both the Processes
3. Results
3.1. Generation of NCSDs
3.2. Characterization of NCSD
3.3. Generation and Characterization of DCF Nanocrystals Prepared by Wet Media Milling
3.4. Wet Granulation Using Nanosuspension
3.4.1. Impact of Wet Granulation Method
3.4.2. Influence of Process Parameters
3.4.3. Effect of Granulating Substrate
3.4.4. Impact of Drug Loading of Nanosuspension on Wet Granulation
3.5. Characterization of FBP Processed Wet Granules
3.6. Formulation of Solid Oral Dosage Form: Effect of Dosage Form
3.7. Discriminatory Drug Dissolution Studies
3.7.1. Development of Discriminatory Dissolution Medium
3.7.2. Comparison of Dissolution Profiles
3.8. Stability of Intermediate Products
3.9. Comparison of Process Efficiency
3.9.1. Costing
3.9.2. Comparison of Various Attributes
3.9.3. Innovation Radar
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gao, L.; Liu, G.; Ma, J.; Wang, X.; Zhou, L.; Li, X.; Wang, F. Application of drug nanocrystal technologies on oral drug delivery of poorly soluble drugs. Pharm. Res. 2013, 30, 307–324. [Google Scholar] [CrossRef] [PubMed]
- Möschwitzer, J.P. Drug nanocrystals in the commercial pharmaceutical development process. Int. J. Pharm. 2013, 453, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Nancollas, G.H. A new understanding of the relationship between solubility and particle size. J. Solut. Chem. 1998, 27, 521–531. [Google Scholar] [CrossRef]
- Mohana Raghava Srivalli, K.; Mishra, B. Drug nanocrystals: Four basic prerequisites for formulation development and scale-up. Curr. Drug Targets 2015, 16, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.K.C.; Yang, X.; Chang, X.; Gao, J. Nanonization strategies for poorly water-soluble drugs. Drug Discov. Today 2011, 16, 354–360. [Google Scholar] [CrossRef]
- Sinha, B.; Müller, R.H.; Möschwitzer, J.P. Bottom-up approaches for preparing drug nanocrystals: Formulations and factors affecting particle size. Int. J. Pharm. 2013, 453, 126–141. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, D.; Chen, M. Drug nanocrystals for the formulation of poorly soluble drugs and its application as a potential drug delivery system. J. Nanoparticle Res. 2008, 10, 845–862. [Google Scholar] [CrossRef]
- Shegokar, R.; Müller, R.H. Nanocrystals: Industrially feasible multifunctional formulation technology for poorly soluble actives. Int. J. Pharm. 2010, 399, 129–139. [Google Scholar] [CrossRef]
- Verma, S.; Gokhale, R.; Burgess, D.J. A comparative study of top-down and bottom-up approaches for the preparation of micro/nanosuspensions. Int. J. Pharm. 2009, 380, 216–222. [Google Scholar] [CrossRef]
- De Waard, H.; Hinrichs, W.; Frijlink, H. A novel bottom–up process to produce drug nanocrystals: Controlled crystallization during freeze-drying. J. Control. Release 2008, 128, 179–183. [Google Scholar] [CrossRef]
- Chan, H.-K.; Kwok, P.C.L. Production methods for nanodrug particles using the bottom-up approach. Adv. Drug Deliv. Rev. 2011, 63, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Jacobs, C.; Kayser, O. Nanosuspensions as particulate drug formulations in therapy: Rationale for development and what we can expect for the future. Adv. Drug Deliv. Rev. 2001, 47, 3–19. [Google Scholar] [CrossRef]
- Shegokar, R.; Singh, K.K.; Müller, R.H. Nevirapine nanosuspension: Comparative investigation of production methods. Nanotechnol. Dev. 2011, 1:e4, 16–22. [Google Scholar] [CrossRef]
- Chin, W.W.L.; Parmentier, J.; Widzinski, M.; Tan, E.H.; Gokhale, R. A brief literature and patent review of nanosuspensions to a final drug product. J. Pharm. Sci. 2014, 103, 2980–2999. [Google Scholar] [CrossRef] [PubMed]
- Malamatari, M.; Somavarapu, S.; Taylor, K.M.; Buckton, G. Solidification of nanosuspensions for the production of solid oral dosage forms and inhalable dry powders. Expert Opin. Drug Deliv. 2016, 13, 435–450. [Google Scholar] [CrossRef]
- Patel, M.; Shah, A.; Patel, N.; Patel, M.; Patel, K. Nanosuspension: A novel approach for drug delivery system. J. Pharm. Sci. Bio-Sci. Res. 2011, 1, 1–10. [Google Scholar]
- Goel, S.; Sachdeva, M.; Agarwal, V. Nanosuspension technology: Recent patents on drug delivery and their characterizations. Recent Pat Drug Deliv Formul 2019, 13, 91–104. [Google Scholar] [CrossRef]
- Ali, H.S.; Hanafy, A.F.; Alqurshi, A. Engineering of solidified glyburide nanocrystals for tablet formulation via loading of carriers: Downstream processing, characterization, and bioavailability. Int. J. Nanomed. 2019, 14, 1893–1906. [Google Scholar] [CrossRef]
- Chaubal, M.V.; Popescu, C. Conversion of nanosuspensions into dry powders by spray drying: A case study. Pharm. Res. 2008, 25, 2302–2308. [Google Scholar] [CrossRef]
- Salazar, J.; Ghanem, A.; Müller, R.H.; Möschwitzer, J.P. Nanocrystals: Comparison of the size reduction effectiveness of a novel combinative method with conventional top-down approaches. Eur. J. Pharm. Biopharm. 2012, 81, 82–90. [Google Scholar] [CrossRef]
- Van Eerdenbrugh, B.; Froyen, L.; Van Humbeeck, J.; Martens, J.A.; Augustijns, P.; Van Den Mooter, G. Alternative matrix formers for nanosuspension solidification: Dissolution performance and X-ray microanalysis as an evaluation tool for powder dispersion. Eur. J. Pharm. Sci. 2008, 35, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Dolenc, A.; Kristl, J.; Baumgartner, S.; Planinšek, O. Advantages of celecoxib nanosuspension formulation and transformation into tablets. Int. J. Pharm. 2009, 376, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Lee, J. Drug nano-and microparticles processed into solid dosage forms: Physical properties. J. Pharm. Sci. 2003, 92, 2057–2068. [Google Scholar] [CrossRef] [PubMed]
- Gulsun, T.; Gursoy, R.N.; Oner, L. Design and characterization of nanocrystal formulations containing ezetimibe. Chem. Pharm. Bull. 2011, 59, 41–45. [Google Scholar] [CrossRef]
- Van Eerdenbrugh, B.; Van den Mooter, G.; Augustijns, P. Top-down production of drug nanocrystals: Nanosuspension stabilization, miniaturization and transformation into solid products. Int. J. Pharm. 2008, 364, 64–75. [Google Scholar] [CrossRef]
- van der Aalst, W.M.; De Medeiros, A.A.; Weijters, A. Process Equivalence: Comparing Two Process Models Based on Observed Behavior BPM Heidelberg, Germany; Dustdar, S., Fiadeiro, J.L., Sheth, A.P., Eds.; Springer: Heidelberg, Germany, 2006. [Google Scholar]
- Statistical Analysis in Method Comparison Studies—Part One. Available online: https://acutecaretesting.org/en/articles/statistical-analysis-in-method-comparison-studies-part-one (accessed on 6 September 2020).
- Nekkanti, V.; Pillai, R.; Venkateshwarlu, V.; Harisudhan, T. Development and characterization of solid oral dosage form incorporating candesartan nanoparticles. Pharm. Dev. Technol. 2009, 14, 290–298. [Google Scholar] [CrossRef]
- Shete, G.; Bansal, A.K. NanoCrySP technology for generation of drug nanocrystals: Translational aspects and business potential. Drug Deliv. Transl. Res. 2016, 6, 392–398. [Google Scholar] [CrossRef]
- Bansal, A.K.; Dantuluri, A.K.; Shete, G.; Pawar, Y. Nanocrystalline Solid Dispersion Compositions. 2018. Patent EP2822539B1, 21 November 2018. [Google Scholar]
- Conaghan, P.G. A turbulent decade for NSAIDs: Update on current concepts of classification, epidemiology, comparative efficacy, and toxicity. Rheumatol. Int. 2012, 32, 1491–1502. [Google Scholar] [CrossRef]
- Bhala, N.; Emberson, J.; Merhi, A.; Abramson, S.; Arber, N.; Baron, J.; Bombardier, C.; Cannon, C.; Farkouh, M.; FitzGerald, G. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: Meta-analyses of individual participant data from randomised trials. Lancet 2013, 382, 769–779. [Google Scholar]
- Schneider, V.; Lévesque, L.E.; Zhang, B.; Hutchinson, T.; Brophy, J.M. Association of selective and conventional nonsteroidal antiinflammatory drugs with acute renal failure: A population-based, nested case-control analysis. Am. J. Epidemiol 2006, 164, 881–889. [Google Scholar] [CrossRef]
- Desjardins, P.J.; Olugemo, K.; Solorio, D.; Young, C.L. Pharmacokinetic properties and tolerability of low-dose SoluMatrix diclofenac. Clin. Ther. 2015, 37, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Llinas, A.; Burley, J.C.; Box, K.J.; Glen, R.C.; Goodman, J.M. Diclofenac solubility: Independent determination of the intrinsic solubility of three crystal forms. J. Med. Chem. 2007, 50, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, V.; Shete, G.; Bansal, A.K. Mechanism of generation of drug nanocrystals in celecoxib: Mannitol nanocrystalline solid dispersion. Int. J. Pharm. 2015, 495, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Sinico, C.; Ennas, G.; Marongiu, F.; Marongiu, G.; Fadda, A.M. Diclofenac nanosuspensions: Influence of preparation procedure and crystal form on drug dissolution behaviour. Int. J. Pharm. 2009, 373, 124–132. [Google Scholar] [CrossRef]
- Pireddu, R.; Sinico, C.; Ennas, G.; Marongiu, F.; Muzzalupo, R.; Lai, F.; Fadda, A.M. Novel nanosized formulations of two diclofenac acid polymorphs to improve topical bioavailability. Eur. J. Pharm. Sci. 2015, 77, 208–215. [Google Scholar] [CrossRef]
- Food Drug Administration. Q3C-Tables and List Guidance for Industry; ICH (Revision 3); U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research (CDER); Center for Biologics Evaluation and Research (CBER): Maryland, MD, USA, 2017. [Google Scholar]
- Kaur, A.; Parmar, P.K.; Bansal, A.K. Evaluation of different techniques for size determination of drug nanocrystals: A case study of celecoxib nanocrystalline solid dispersion. Pharmaceutics 2019, 11, 516. [Google Scholar] [CrossRef]
- Carr, R.L. Evaluating flow properties of solids. Chem. Eng. 1965, 18, 163–168. [Google Scholar]
- Loehnert, S. About statistical analysis of qualitative survey data. Qual. Reliab. Eng. Int. 2010, 2010. [Google Scholar] [CrossRef]
- Sandelowski, M.; Voils, C.I.; Knafl, G. On quantitizing. J. Mix. Methods Res. 2009, 3, 208–222. [Google Scholar] [CrossRef]
- Srnka, K.J.; Koeszegi, S.T. From words to numbers: How to transform qualitative data into meaningful quantitative results. Schmalenbach Bus Rev. 2007, 59, 29–57. [Google Scholar] [CrossRef]
- Morris, L. Innovation metrics: The innovation process and how to measure it. In An InnovationLabs White Paper; InnovationLab. LLC: New York, NY, USA, 2008. [Google Scholar]
- Becher, R.-D.; Schlünder, E.-U. Fluidized bed granulation—the importance of a drying zone for the particle growth mechanism. Chem. Eng. Process. 1998, 37, 1–6. [Google Scholar] [CrossRef]
- Gao, J.Z.; Jain, A.; Motheram, R.; Gray, D.; Hussain, M. Fluid bed granulation of a poorly water soluble, low density, micronized drug: Comparison with high shear granulation. Int. J. Pharm. 2002, 237, 1–14. [Google Scholar] [CrossRef]
- Kleinebudde, P. The crystallite-gel-model for microcrystalline cellulose in wet-granulation, extrusion, and spheronization. Pharm. Res. 1997, 14, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Murdande, S.B.; Shah, D.A.; Dave, R.H. Impact of nanosizing on solubility and dissolution rate of poorly soluble pharmaceuticals. J. Pharm. Sci. 2015, 104, 2094–2102. [Google Scholar] [CrossRef]
- Braig, V.; Konnerth, C.; Peukert, W.; Lee, G. Enhanced dissolution of naproxen from pure-drug, crystalline nanoparticles: A case study formulated into spray-dried granules and compressed tablets. Int. J. Pharm. 2019, 554, 54–60. [Google Scholar] [CrossRef]
- Sun, D.D.; Wen, H.; Taylor, L.S. Non-sink dissolution conditions for predicting product quality and in vivo performance of supersaturating drug delivery systems. J. Pharm. Sci. 2016, 105, 2477–2488. [Google Scholar] [CrossRef]
- Guidance for Industry: Dissolution Testing of Immediate Release Solid Oral Dosage Forms; U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research (CDER): Maryland, MD, USA, 1997.
- Shah, V.P.; Tsong, Y.; Sathe, P.; Liu, J.-P. In vitro dissolution profile comparison—Statistics and analysis of the similarity factor, f2. Pharm. Res. 1998, 15, 889–896. [Google Scholar] [CrossRef]
- Singh, S.K.; Srinivasan, K.; Gowthamarajan, K.; Narayan, G. Development and validation of discriminatory dissolution procedure for poorly soluble glyburide. Asian J. Pharm. 2014, 4. [Google Scholar] [CrossRef]
- Ashokraj, Y.; Daroi, A.; Gupta, R.; Khanolkar, A.; Kulkarni, A.; Laud, S.; Pokale, M.; Shedge, S.; Date, P. Discriminatory dissolution method development and validation of etoricoxib tablets. Dissolut. Technol. 2016, 23, 30–34. [Google Scholar] [CrossRef]
- Pawar, H.A.; Joshi, P.R. Development and validation of a discriminating in vitro dissolution method for oral formulations containing satranidazole. Int. J. Spectrosc. 2014, 2014, 624635. [Google Scholar] [CrossRef]
- Bhakay, A.; Davé, R.; Bilgili, E. Recovery of BCS Class II drugs during aqueous redispersion of core–shell type nanocomposite particles produced via fluidized bed coating. Powder Technol. 2013, 236, 221–234. [Google Scholar] [CrossRef]
- Kasi, V.; Tang, X. (Eds.) Design attributes and performance outcomes: A framework for comparing business processes. In SAIS 2005 Proceedings; AIS eLibrary: Georgia, GA, USA, 2005. [Google Scholar]
- Arroniz, I.; Wolcott, R.C.; Sawhney, M. The 12 different ways for companies to innovate. Engineering Management Review, IEEE 2006, 47, 75–81. [Google Scholar]
- Bose, S.; Schenck, D.; Ghosh, I.; Hollywood, A.; Maulit, E.; Ruegger, C. Application of spray granulation for conversion of a nanosuspension into a dry powder form. Eur. J. Pharm. Sci. 2012, 47, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Beirowski, J.; Inghelbrecht, S.; Arien, A.; Gieseler, H. Freeze-drying of nanosuspensions, 1: Freezing rate versus formulation design as critical factors to preserve the original particle size distribution. J. Pharm. Sci. 2011, 100, 1958–1968. [Google Scholar] [CrossRef]
- Figueroa, C.E.; Bose, S. Spray granulation: Importance of process parameters on in vitro and in vivo behavior of dried nanosuspensions. Eur. J. Pharm. Biopharm. 2013, 85, 1046–1055. [Google Scholar] [CrossRef]
- Berggren, J.; Alderborn, G. Drying behaviour of two sets of microcrystalline cellulose pellets. Int. J. Pharm. 2001, 219, 113–126. [Google Scholar] [CrossRef]
- Kállai, N.; Luhn, O.; Dredán, J.; Kovács, K.; Lengyel, M.; Antal, I. Evaluation of drug release from coated pellets based on isomalt, sugar, and microcrystalline cellulose inert cores. AAPS PharmSciTech 2010, 11, 383–391. [Google Scholar] [CrossRef]
- Johannesson, M.; O’Brien, B.J. Economics, pharmaceuticals, and pharmacoeconomics. Med. Decis. Mak. 1998, 18, S1–S3. [Google Scholar] [CrossRef]






| Ingredients | Role | Quantity (%) | Quantity per Capsule (mg) |
|---|---|---|---|
| NCSD1 † | API | 25.14 | 91.75 |
| Pearlitol® SD 200/Celphere® 203 * | Diluent | 72.31 | 263.94 |
| Aerosil® 200 | Glidant | 0.30 | 1.08 |
| croscarmellose sodium (CCS) | Superdisintegrant | 1.76 | 6.42 |
| sodium stearyl fumarate (SSF) | Lubricant | 0.49 | 1.80 |
| Total fill weight | 100 | 365 |
| Ingredients | Role | Quantity (%) | Quantity per Capsule for G1 Granules (mg) | Quantity per Capsule for G2 Granules (mg) |
|---|---|---|---|---|
| G1 granules † | API | 97.15 | 355.70 | - |
| G2 granules # | 97.15 | - | 355.70 | |
| Aerosil® 200 | Glidant | 0.30 | 1.08 | 1.08 |
| croscarmellose sodium | Superdisintegrant | 1.76 | 6.42 | 6.42 |
| sodium stearyl fumarate | Lubricant | 0.49 | 1.8 | 1.80 |
| Total fill weight | 100 | 365 | 365 |
| Time (Hours) | D90 (nm) | PDI |
|---|---|---|
| 2 | 810.07 ± 88.11 | 0.79 ± 0.09 |
| 4 | 596.29 ± 151.08 | 0.76 ± 0.07 |
| 6 | 361.61 ± 11.78 | 0.52 ± 0.14 |
| 8 | 351.32 ± 7.53 | 0.53 ± 0.16 |
| Ingredients | Quantity | Rate/kg or Liter (INR) | Cost (INR) |
|---|---|---|---|
| Phase 1 | |||
| DCF | 7.2 kg | 1100 | 7920 |
| MAN | 10.35 kg | 473 | 4896 |
| SLS USPNF | 0.45 kg | 950 | 428 |
| Purified Water | 800 L | 10 | 8000 |
| Isopropyl alcohol | 2800 L | 275 | 792,000 |
| Total ingredient | 813,244 | ||
| Spray dryer | 600,000 | ||
| Manpower | INR 320 × 60 days × 2 persons | 38,400 | |
| Electricity | INR 300 × 60 days | 18,000 | |
| Phase 1 NCSD1 | 1,469,644 | ||
| Phase 2 | |||
| Capsule filling machine | 80,000 | ||
| 100,000 capsules | 0.18/capsule | 18,000 | |
| Aerosil® 200 | 0.11 kg | 625 | 69 |
| Croscarmellose sodium | 0.642 kg | 1450 | 931 |
| Sodium stearyl fumarate | 0.18 kg | 13,750 | 2475 |
| Inert excipient (Pearlitol® SD 200) | 26.39 kg | 1150 | 30,348 |
| or Celphere® 203 | 26.39 kg | 3023 | 79,780 |
| Manpower | INR 320 ×3 days × 1 person | 960 | |
| Electricity | INR 300 × 3 days | 900 | |
| Analytical | 50,000 | ||
| Phase 2 NCSD1 (Celphere® 203) | 233,115 | ||
| Phase 2 NCSD1 (Pearlitol® SD 200) | 183,683 | ||
| Ingredients | Quantity | Rate/kg or Liter (INR) | Cost (INR) |
|---|---|---|---|
| Phase 1 | |||
| DCF | 4.4 kg | 1100 | 4840 |
| HPC EP | 0.88 kg | 3300 | 2904 |
| SLS USPNF | 0.088 kg | 950 | 84 |
| Purified Water | 88 L | 10 | 880 |
| Total ingredient | 8708 | ||
| Wet media mill (dyno mill) | 500,000 | ||
| Manpower | INR 320 × 2 days × 2 persons | 1280 | |
| Electricity | INR 300 × 2 days | 600 | |
| Phase 1 NS1 | 510,588 | ||
| Phase 2 | |||
| Wet granulation using NS | |||
| FBP | 750,000 | ||
| Inert excipient Pearlitol® SD 200 or | 37.7 kg | 1150 | 43,355 |
| Celphere® 203 | 37.7 kg | 3023 | 113,967 |
| Manpower | INR 320 × 8 days × 2 persons | 5120 | |
| Electricity | INR 300 × 8 days | 2400 | |
| Capsule manufacturing | |||
| Capsule filling machine | 80,000 | ||
| Aerosil 200 | 0.11 kg | 625 | 68 |
| Croscarmellose sodium | 0.642 kg | 1450 | 931 |
| Sodium stearyl fumarate | 0.18 kg | 13,750 | 2475 |
| 100,000 capsules | 0.18/capsule | 18,000 | |
| Manpower | INR 320 × 3 days × 1 person | 960 | |
| Electricity | INR 300 × 3 days | 900 | |
| Analytical | 50,000 | ||
| Phase 2 NS1 (Celphere® 203) | 1,024,821 | ||
| Phase 2 NS1 (Pearlitol® SD 200) | 954,209 | ||
| Parameters | (a) Weight | (b) NanoCrySP Rating | (c) Conventional Rating | (a × b) Score NanoCrySP | (a × c) Score Conventional |
|---|---|---|---|---|---|
| No. of steps | 5 | 5 | 4 | 25 | 20 |
| Intermediate product stability | 4 | 5 | 1 | 20 | 4 |
| DCF nanocrystal size of intermediate product | 3 | 4 | 4 | 12 | 12 |
| Solid state of final product | 5 | 5 | 5 | 25 | 25 |
| Yield (%) | 4 | 3 | 5 | 12 | 20 |
| Time (days) for phase 2 | 4 | 5 | 2 | 20 | 8 |
| Powder flow properties | 3 | 5 | 5 | 15 | 15 |
| Particles interaction | 3 | 5 | 3 | 15 | 9 |
| Input materials | 3 | 1 | 1 | 3 | 3 |
| No. of optimization parameters in phase 2 | 3 | 5 | 3 | 15 | 9 |
| Dissolution | 5 | 4 | 2 | 20 | 10 |
| Reproducibility | 4 | 4 | 1 | 16 | 4 |
| Phase 2 cost | 5 | 4 | 1 | 20 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jadhav, S.; Kaur, A.; Bansal, A.K. Comparison of Downstream Processing of Nanocrystalline Solid Dispersion and Nanosuspension of Diclofenac Acid to Develop Solid Oral Dosage Form. Pharmaceutics 2020, 12, 1015. https://doi.org/10.3390/pharmaceutics12111015
Jadhav S, Kaur A, Bansal AK. Comparison of Downstream Processing of Nanocrystalline Solid Dispersion and Nanosuspension of Diclofenac Acid to Develop Solid Oral Dosage Form. Pharmaceutics. 2020; 12(11):1015. https://doi.org/10.3390/pharmaceutics12111015
Chicago/Turabian StyleJadhav, Sanika, Amanpreet Kaur, and Arvind Kumar Bansal. 2020. "Comparison of Downstream Processing of Nanocrystalline Solid Dispersion and Nanosuspension of Diclofenac Acid to Develop Solid Oral Dosage Form" Pharmaceutics 12, no. 11: 1015. https://doi.org/10.3390/pharmaceutics12111015
APA StyleJadhav, S., Kaur, A., & Bansal, A. K. (2020). Comparison of Downstream Processing of Nanocrystalline Solid Dispersion and Nanosuspension of Diclofenac Acid to Develop Solid Oral Dosage Form. Pharmaceutics, 12(11), 1015. https://doi.org/10.3390/pharmaceutics12111015