Scaffold-Mediated Gene Delivery for Osteochondral Repair
Abstract
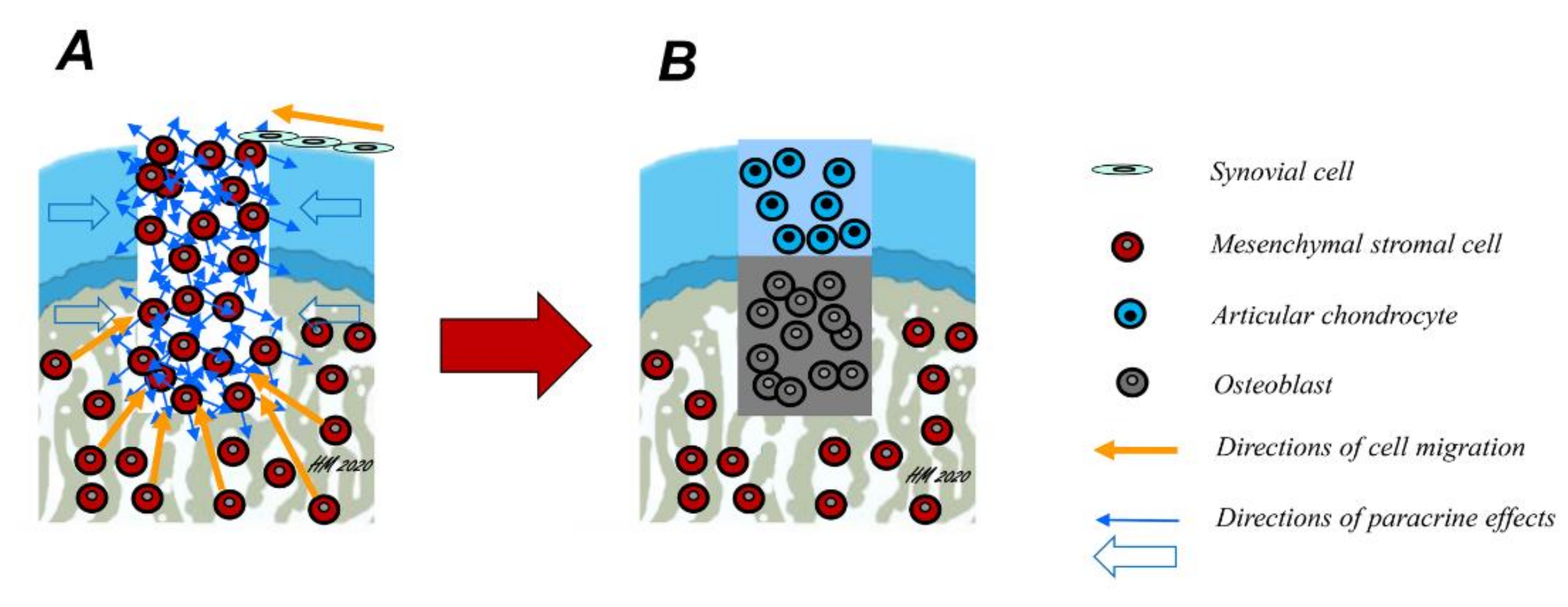
1. Introduction
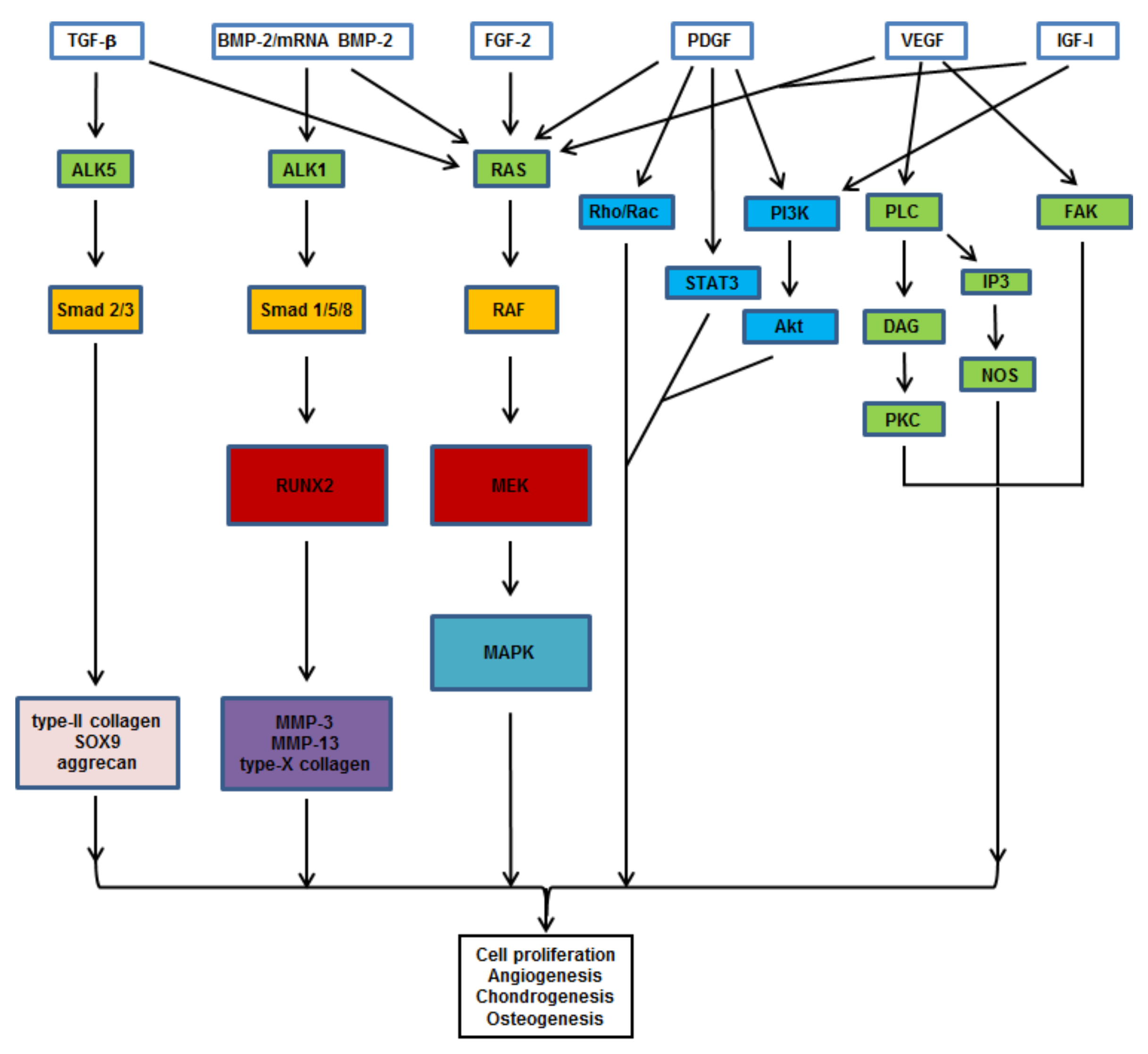
2. Candidate Genes for Osteochondral Repair
3. Nonviral Gene Delivery Systems
4. Viral Vectors
4.1. Adenoviral Vectors
4.2. Retroviral Vectors
4.3. Baculoviral Vectors
4.4. Recombinant Adeno-Associated Viral (rAAV) Vectors
5. Scaffolds for Osteochondral Repair
6. Scaffold-Mediated Nonviral In Vitro Gene Delivery
7. Scaffold-Mediated Nonviral In Vivo Gene Delivery for Osteochondral Repair
8. Scaffold-Mediated Viral In Vitro Gene Delivery for Osteochondral Repair
9. Scaffold-Mediated Viral In Vivo Gene Delivery for Osteochondral Repair
10. Clinical Scaffolds for Osteochondral Repair
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buckwalter, J.A. Articular cartilage: Injuries and potential for healing. J. Orthop. Sports Phys. Ther. 1998, 28, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Brown, W.E.; Lee, C.A.; Wang, D.; Paschos, N.; Hu, J.C.; Athanasiou, K.A. Surgical and tissue engineering strategies for articular cartilage and meniscus repair. Nat. Rev. Rheumatol. 2019, 15, 550–570. [Google Scholar] [CrossRef] [PubMed]
- Schinhan, M.; Gruber, M.; Vavken, P.; Dorotka, R.; Samouh, L.; Chiari, C.; Gruebl-Barabas, R.; Nehrer, S. Critical-size defect induces unicompartmental osteoarthritis in a stable ovine knee. J. Orthop. Res. 2012, 30, 214–220. [Google Scholar] [CrossRef]
- Orth, P.; Eldracher, M.; Cucchiarini, M.; Madry, H. Small-diameter subchondral drilling improves DNA and proteoglycan content of the cartilaginous repair tissue in a large animal model of a full-thickness chondral defect. J. Clin. Med. 2020, 9, 1903. [Google Scholar] [CrossRef] [PubMed]
- Madry, H.; Kon, E.; Condello, V.; Peretti, G.M.; Steinwachs, M.; Seil, R.; Berruto, M.; Engebretsen, L.; Filardo, G.; Angele, P. Early osteoarthritis of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 1753–1762. [Google Scholar] [CrossRef]
- Perelli, S.; Molina Romoli, A.R.; Costa-Paz, M.; Erquicia, J.I.; Gelber, P.E.; Monllau, J.C. Internal fixation of osteochondritis dissecans of the knee leads to good long-term outcomes and high degree of healing without differences between fixation devices. J. Clin. Med. 2019, 8, 1934. [Google Scholar] [CrossRef]
- Sanders, T.L.; Pareek, A.; Obey, M.R.; Johnson, N.R.; Carey, J.L.; Stuart, M.J.; Krych, A.J. High rate of osteoarthritis after osteochondritis dissecans fragment excision compared with surgical restoration at a mean 16-year follow-up. Am. J. Sports Med. 2017, 45, 1799–1805. [Google Scholar] [CrossRef]
- Saris, D.B.; Vanlauwe, J.; Victor, J.; Haspl, M.; Bohnsack, M.; Fortems, Y.; Vandekerckhove, B.; Almqvist, K.F.; Claes, T.; Handelberg, F.; et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am. J. Sports Med. 2008, 36, 235–246. [Google Scholar] [CrossRef]
- Barry, F.; Murphy, M. Mesenchymal stem cells in joint disease and repair. Nat. Rev. Rheumatol. 2013, 9, 584–594. [Google Scholar] [CrossRef]
- Van den Berg, W.B.; van der Kraan, P.M.; Scharstuhl, A.; van Beuningen, H.M. Growth factors and cartilage repair. Clin. Orthop. Relat Res. 2001, S244–S250. [Google Scholar] [CrossRef]
- Devescovi, V.; Leonardi, E.; Ciapetti, G.; Cenni, E. Growth factors in bone repair. Chir. Organi. Mov. 2008, 92, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Fortier, L.A.; Barker, J.U.; Strauss, E.J.; McCarrel, T.M.; Cole, B.J. The role of growth factors in cartilage repair. Clin. Orthop. Relat Res. 2011, 469, 2706–2715. [Google Scholar] [CrossRef] [PubMed]
- Cucchiarini, M.; Madry, H. The potential of gene transfer for the treatment of osteoarthritis. Regen. Med. 2014, 9, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.J.; Kim, T.H.; Choi, S.J.; Park, J.H.; Wall, I.B.; Kim, H.W. Gene delivery techniques for adult stem cell-based regenerative therapy. Nanomedicine 2013, 8, 1875–1891. [Google Scholar] [CrossRef]
- Hanada, K.; Solchaga, L.A.; Caplan, A.I.; Hering, T.M.; Goldberg, V.M.; Yoo, J.U.; Johnstone, B. BMP-2 induction and TGF-beta 1 modulation of rat periosteal cell chondrogenesis. J. Cell Biochem. 2001, 81, 284–294. [Google Scholar] [CrossRef]
- Lee, K.H.; Song, S.U.; Hwang, T.S.; Yi, Y.; Oh, I.S.; Lee, J.Y.; Choi, K.B.; Choi, M.S.; Kim, S.J. Regeneration of hyaline cartilage by cell-mediated gene therapy using transforming growth factor beta 1-producing fibroblasts. Hum. Gene Ther. 2001, 12, 1805–1813. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Deng, C.; Li, Y.P. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef]
- Nixon, A.J.; Fortier, L.A.; Williams, J.; Mohammed, H. Enhanced repair of extensive articular defects by insulin-like growth factor-I-laden fibrin composites. J. Orthop. Res. 1999, 17, 475–487. [Google Scholar] [CrossRef]
- Schmidmaier, G.; Wildemann, B.; Heeger, J.; Gabelein, T.; Flyvbjerg, A.; Bail, H.J.; Raschke, M. Improvement of fracture healing by systemic administration of growth hormone and local application of insulin-like growth factor-1 and transforming growth factor-beta1. Bone 2002, 31, 165–172. [Google Scholar] [CrossRef]
- Jentzsch, K.D.; Wellmitz, G.; Heder, G.; Petzold, E.; Buntrock, P.; Oehme, P. A bovine brain fraction with fibroblast growth factor activity inducing articular cartilage regeneration in vivo. Acta Biol. Med. Ger. 1980, 39, 967–971. [Google Scholar]
- Cucchiarini, M.; Madry, H.; Ma, C.; Thurn, T.; Zurakowski, D.; Menger, M.D.; Kohn, D.; Trippel, S.B.; Terwilliger, E.F. Improved tissue repair in articular cartilage defects in vivo by rAAV-mediated overexpression of human fibroblast growth factor 2. Mol. Ther. 2005, 12, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhao, M.; Mundy, G.R. Bone morphogenetic proteins. Growth Factors 2004, 22, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Klein-Nulend, J.; Louwerse, R.T.; Heyligers, I.C.; Wuisman, P.I.; Semeins, C.M.; Goei, S.W.; Burger, E.H. Osteogenic protein (OP-1, BMP-7) stimulates cartilage differentiation of human and goat perichondrium tissue in vitro. J. Biomed. Mater. Res. 1998, 40, 614–620. [Google Scholar] [CrossRef]
- Street, J.; Bao, M.; deGuzman, L.; Bunting, S.; Peale, F.V., Jr.; Ferrara, N.; Steinmetz, H.; Hoeffel, J.; Cleland, J.L.; Daugherty, A.; et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc. Natl. Acad. Sci. USA 2002, 99, 9656–9661. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.S.; Sable, R.B.; Kothari, R.M. Occurrence, biochemical profile of vascular endothelial growth factor (VEGF) isoforms and their functions in endochondral ossification. J Cell Physiol. 2012, 227, 1298–1308. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.B.; Chen, E.H.; Lynch, S.E. A review of the effects of insulin-like growth factor and platelet derived growth factor on in vivo cartilage healing and repair. Osteoarthr. Cartil. 2006, 14, 403–412. [Google Scholar] [CrossRef]
- Caplan, A.I.; Correa, D. PDGF in bone formation and regeneration: New insights into a novel mechanism involving MSCs. J. Orthop. Res. 2011, 29, 1795–1803. [Google Scholar] [CrossRef]
- Lefebvre, V.; Behringer, R.R.; de Crombrugghe, B. L-Sox5, Sox6 and Sox9 control essential steps of the chondrocyte differentiation pathway. Osteoarthr. Cartil. 2001, 9 (Suppl. A), S69–S75. [Google Scholar] [CrossRef]
- Inada, M.; Yasui, T.; Nomura, S.; Miyake, S.; Deguchi, K.; Himeno, M.; Sato, M.; Yamagiwa, H.; Kimura, T.; Yasui, N.; et al. Maturational disturbance of chondrocytes in Cbfa1-deficient mice. Dev. Dyn. 1999, 214, 279–290. [Google Scholar] [CrossRef]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef]
- Raisin, S.; Belamie, E.; Morille, M. Non-viral gene activated matrices for mesenchymal stem cells based tissue engineering of bone and cartilage. Biomaterials 2016, 104, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Athirasala, A.; Menezes, P.P.; Ashwanikumar, N.; Zou, T.; Sahay, G.; Bertassoni, L.E. Messenger RNA delivery for tissue engineering and regenerative medicine applications. Tissue Eng. Part A 2019, 25, 91–112. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.C.; Raftery, R.M.; Curtin, C.M.; O’Driscoll, C.M.; O’Brien, F.J. Scaffold-based delivery of nucleic acid therapeutics for enhanced bone and cartilage repair. J. Orthop. Res. 2019, 37, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Carballo-Pedrares, N.; Fuentes-Boquete, I.; Díaz-Prado, S.; Rey-Rico, A. Hydrogel-based localized nonviral gene delivery in regenerative medicine approaches-an overview. Pharmaceutics 2020, 12, 752. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, M.; Oskuee, R.K.; Nassirli, H.; Malaekeh-Nikouei, B. Progress in the development of lipopolyplexes as efficient non-viral gene delivery systems. J. Control Release 2016, 236, 1–14. [Google Scholar] [CrossRef]
- Bono, N.; Ponti, F.; Mantovani, D.; Candiani, G. Non-viral in vitro gene delivery: It is now time to set the bar! Pharmaceutics 2020, 12, 183. [Google Scholar] [CrossRef]
- Al Qtaish, N.; Gallego, I.; Villate-Beitia, I.; Sainz-Ramos, M.; Lopez-Mendez, T.B.; Grijalvo, S.; Eritja, R.; Soto-Sanchez, C.; Martinez-Navarrete, G.; Fernandez, E.; et al. Niosome-based approach for in situ gene delivery to retina and brain cortex as immune-privileged tissues. Pharmaceutics 2020, 12, 198. [Google Scholar] [CrossRef]
- Rai, R.; Alwani, S.; Badea, I. Polymeric nanoparticles in gene therapy: New avenues of design and optimization for delivery applications. Polymers 2019, 11, 745. [Google Scholar] [CrossRef]
- Sum, C.H.; Shortall, S.M.; Wong, S.; Wettig, S.D. Non-viral gene delivery. Exp. Suppl. 2018, 110, 3–68. [Google Scholar] [CrossRef]
- Thomas, C.E.; Ehrhardt, A.; Kay, M.A. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet 2003, 4, 346–358. [Google Scholar] [CrossRef]
- Tokushige, K.; Moradpour, D.; Wakita, T.; Geissler, M.; Hayashi, N.; Wands, J.R. Comparison between cytomegalovirus promoter and elongation factor-1 alpha promoter-driven constructs in the establishment of cell lines expressing hepatitis C virus core protein. J. Virol. Methods 1997, 64, 73–80. [Google Scholar] [CrossRef]
- Chung, S.; Andersson, T.; Sonntag, K.C.; Bjorklund, L.; Isacson, O.; Kim, K.S. Analysis of different promoter systems for efficient transgene expression in mouse embryonic stem cell lines. Stem Cells 2002, 20, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Raup, A.; Jerome, V.; Freitag, R.; Synatschke, C.V.; Muller, A.H. Promoter, transgene, and cell line effects in the transfection of mammalian cells using PDMAEMA-based nano-stars. Biotechnol. Rep. 2016, 11, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Sum, C.H.; Wettig, S.; Slavcev, R.A. Impact of DNA vector topology on non-viral gene therapeutic safety and efficacy. Curr. Gene Ther. 2014, 14, 309–329. [Google Scholar] [CrossRef]
- Nafissi, N.; Alqawlaq, S.; Lee, E.A.; Foldvari, M.; Spagnuolo, P.A.; Slavcev, R.A. DNA ministrings: Highly safe and effective gene delivery vectors. Mol. Ther. Nucleic Acids 2014, 3, e165. [Google Scholar] [CrossRef]
- Aronovich, E.L.; McIvor, R.S.; Hackett, P.B. The Sleeping Beauty transposon system: A non-viral vector for gene therapy. Hum. Mol. Genet 2011, 20, R14–R20. [Google Scholar] [CrossRef]
- Qian, Q.; Che, J.; Ye, L.; Zhong, B. [The improvement and application of piggyBac transposon system in mammals]. Yi Chuan 2014, 36, 965–973. [Google Scholar] [CrossRef]
- Vargas, J.E.; Chicaybam, L.; Stein, R.T.; Tanuri, A.; Delgado-Canedo, A.; Bonamino, M.H. Retroviral vectors and transposons for stable gene therapy: Advances, current challenges and perspectives. J. Transl. Med. 2016, 14, 288–303. [Google Scholar] [CrossRef]
- Cucchiarini, M. Human gene therapy: Novel approaches to improve the current gene delivery systems. Discov. Med. 2016, 21, 495–506. [Google Scholar]
- Shi, S.; Chan, A.G.; Mercer, S.; Eckert, G.J.; Trippel, S.B. Endogenous versus exogenous growth factor regulation of articular chondrocytes. J. Orthop. Res. 2014, 32, 54–60. [Google Scholar] [CrossRef]
- Shi, S.; Mercer, S.; Eckert, G.J.; Trippel, S.B. Regulation of articular chondrocyte aggrecan and collagen gene expression by multiple growth factor gene transfer. J. Orthop. Res. 2012, 30, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Cheng, Y.; Yuan, Q.; Li, J. IGF-1 and BMP-2 induces differentiation of adipose-derived mesenchymal stem cells into chondrocytes-like cells. Ann. Biomed. Eng. 2010, 38, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Pagnotto, M.R.; Wang, Z.; Karpie, J.C.; Ferretti, M.; Xiao, X.; Chu, C.R. Adeno-associated viral gene transfer of transforming growth factor-beta1 to human mesenchymal stem cells improves cartilage repair. Gene. Ther. 2007, 14, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Jin, Y.Q.; Kretlow, J.D.; Liu, W.; Ding, W.; Sun, H.; Zhou, G.; Zhang, W.; Cao, Y. Adenoviral transduction of hTGF-beta1 enhances the chondrogenesis of bone marrow derived stromal cells. Biotechnol. Lett. 2009, 31, 639–646. [Google Scholar] [CrossRef]
- Garza-Veloz, I.; Romero-Diaz, V.J.; Martinez-Fierro, M.L.; Marino-Martinez, I.A.; Gonzalez-Rodriguez, M.; Martinez-Rodriguez, H.G.; Espinoza-Juarez, M.A.; Bernal-Garza, D.A.; Ortiz-Lopez, R.; Rojas-Martinez, A. Analyses of chondrogenic induction of adipose mesenchymal stem cells by combined co-stimulation mediated by adenoviral gene transfer. Arthr. Res. Ther. 2013, 15, R80–R82. [Google Scholar] [CrossRef]
- Neumann, A.J.; Gardner, O.F.; Williams, R.; Alini, M.; Archer, C.W.; Stoddart, M.J. Human articular cartilage progenitor cells are responsive to mechanical stimulation and adenoviral-mediated overexpression of bone-morphogenetic protein 2. PLoS ONE 2015, 10, e0136229. [Google Scholar] [CrossRef]
- Chopra, I.; Hodgson, J.; Metcalf, B.; Poste, G. New approaches to the control of infections caused by antibiotic-resistant bacteria. An industry perspective. JAMA 1996, 275, 401–403. [Google Scholar] [CrossRef]
- Evans, C.H.; Liu, F.J.; Glatt, V.; Hoyland, J.A.; Kirker-Head, C.; Walsh, A.; Betz, O.; Wells, J.W.; Betz, V.; Porter, R.M.; et al. Use of genetically modified muscle and fat grafts to repair defects in bone and cartilage. Eur. Cell Mater. 2009, 18, 96–111. [Google Scholar] [CrossRef]
- Goodrich, L.R.; Hidaka, C.; Robbins, P.D.; Evans, C.H.; Nixon, A.J. Genetic modification of chondrocytes with insulin-like growth factor-1 enhances cartilage healing in an equine model. J. Bone Joint Surg. Br. 2007, 89, 672–685. [Google Scholar] [CrossRef]
- Goodrich, L.R.; Brower-Toland, B.D.; Warnick, L.; Robbins, P.D.; Evans, C.H.; Nixon, A.J. Direct adenovirus-mediated IGF-I gene transduction of synovium induces persisting synovial fluid IGF-I ligand elevations. Gene Ther. 2006, 13, 1253–1262. [Google Scholar] [CrossRef]
- Menendez, M.I.; Clark, D.J.; Carlton, M.; Flanigan, D.C.; Jia, G.; Sammet, S.; Weisbrode, S.E.; Knopp, M.V.; Bertone, A.L. Direct delayed human adenoviral BMP-2 or BMP-6 gene therapy for bone and cartilage regeneration in a pony osteochondral model. Osteoarthr. Cartil. 2011, 19, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zheng, Z.; Liu, P.; Ma, Y.; Lin, L.; Lang, N.; Fu, X.; Zhang, J.; Ma, K.; Chen, P.; et al. The synergistic effects of microfracture, perforated decalcified cortical bone matrix and adenovirus-bone morphogenetic protein-4 in cartilage defect repair. Biomaterials 2008, 29, 4616–4629. [Google Scholar] [CrossRef] [PubMed]
- Marshall, E. Gene therapy death prompts review of adenovirus vector. Science 1999, 286, 2244–2245. [Google Scholar] [CrossRef] [PubMed]
- Ueblacker, P.; Wagner, B.; Vogt, S.; Salzmann, G.; Wexel, G.; Kruger, A.; Plank, C.; Brill, T.; Specht, K.; Hennig, T.; et al. In vivo analysis of retroviral gene transfer to chondrocytes within collagen scaffolds for the treatment of osteochondral defects. Biomaterials 2007, 28, 4480–4487. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, R.; Usas, A.; Kubo, S.; Corsi, K.; Peng, H.; Rose, T.; Cummins, J.; Fu, F.H.; Huard, J. Cartilage repair using bone morphogenetic protein 4 and muscle-derived stem cells. Arthr. Rheum. 2006, 54, 433–442. [Google Scholar] [CrossRef]
- Kubo, S.; Cooper, G.M.; Matsumoto, T.; Phillippi, J.A.; Corsi, K.A.; Usas, A.; Li, G.; Fu, F.H.; Huard, J. Blocking vascular endothelial growth factor with soluble Flt-1 improves the chondrogenic potential of mouse skeletal muscle-derived stem cells. Arthr. Rheum. 2009, 60, 155–165. [Google Scholar] [CrossRef]
- Lee, J.M.; Im, G.I. SOX trio-co-transduced adipose stem cells in fibrin gel to enhance cartilage repair and delay the progression of osteoarthritis in the rat. Biomaterials 2012, 33, 2016–2024. [Google Scholar] [CrossRef]
- Yoon, H.J.; Kim, S.B.; Somaiya, D.; Noh, M.J.; Choi, K.B.; Lim, C.L.; Lee, H.Y.; Lee, Y.J.; Yi, Y.; Lee, K.H. Type II collagen and glycosaminoglycan expression induction in primary human chondrocyte by TGF-beta1. BMC Musculoskelet Disord. 2015, 16, 141–152. [Google Scholar] [CrossRef]
- Lu, C.H.; Yeh, T.S.; Yeh, C.L.; Fang, Y.H.; Sung, L.Y.; Lin, S.Y.; Yen, T.C.; Chang, Y.H.; Hu, Y.C. Regenerating cartilages by engineered ASCs: Prolonged TGF-beta3/BMP-6 expression improved articular cartilage formation and restored zonal structure. Mol. Ther. 2014, 22, 186–195. [Google Scholar] [CrossRef]
- Chen, H.C.; Chang, Y.H.; Chuang, C.K.; Lin, C.Y.; Sung, L.Y.; Wang, Y.H.; Hu, Y.C. The repair of osteochondral defects using baculovirus-mediated gene transfer with de-differentiated chondrocytes in bioreactor culture. Biomaterials 2009, 30, 674–681. [Google Scholar] [CrossRef]
- Cucchiarini, M.; Thurn, T.; Weimer, A.; Kohn, D.; Terwilliger, E.F.; Madry, H. Restoration of the extracellular matrix in human osteoarthritic articular cartilage by overexpression of the transcription factor SOX9. Arthr. Rheum. 2007, 56, 158–167. [Google Scholar] [CrossRef]
- Watanabe, S.; Imagawa, T.; Boivin, G.P.; Gao, G.; Wilson, J.M.; Hirsch, R. Adeno-associated virus mediates long-term gene transfer and delivery of chondroprotective IL-4 to murine synovium. Mol. Ther. 2000, 2, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.G.; Xie, J.; Yang, P.; Wang, Y.; Xu, L.; Liu, D.; Hsu, H.C.; Zhou, T.; Edwards, C.K., 3rd; Mountz, J.D. Adeno-associated virus production of soluble tumor necrosis factor receptor neutralizes tumor necrosis factor alpha and reduces arthritis. Hum. Gene Ther. 2000, 11, 2431–2442. [Google Scholar] [CrossRef] [PubMed]
- Nooeaid, P.; Salih, V.; Beier, J.P.; Boccaccini, A.R. Osteochondral tissue engineering: Scaffolds, stem cells and applications. J. Cell Mol. Med. 2012, 16, 2247–2270. [Google Scholar] [CrossRef] [PubMed]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- Keeney, M.; Pandit, A. The osteochondral junction and ist repair via bi-phasic tissue engineering scaffolds. Tissue Eng Part B Rev. 2009, 15, 55–73. [Google Scholar] [CrossRef]
- Lopa, S.; Madry, H. Bioinspired scaffolds for osteochondral regeneration. Tissue Eng. Part A 2014, 20, 2052–2076. [Google Scholar] [CrossRef]
- Shpichka, A.; Koroleva, A.; Kuznetsova, D.; Dmitriev, R.I.; Timashev, P. Fabrication and handling of 3D scaffolds based on polymers and decellularized tissues. Adv. Exp. Med. Biol. 2017, 1035, 71–81. [Google Scholar] [CrossRef]
- Jun, I.; Han, H.S.; Edwards, J.R.; Jeon, H. Electrospun fibrous scaffolds for tissue engineering: Viewpoints on architecture and fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef]
- Wubneh, A.; Tsekoura, E.K.; Ayranci, C.; Uludag, H. Current state of fabrication technologies and materials for bone tissue engineering. Acta Biomater. 2018, 80, 1–30. [Google Scholar] [CrossRef]
- De Mori, A.; Pena Fernandez, M.; Blunn, G.; Tozzi, G.; Roldo, M. 3D printing and electrospinning of composite hydrogels for cartilage and bone tissue engineering. Polymers 2018, 10, 285. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Gao, L.; Venkatesan, J.K.; Wang, G.; Madry, H.; Cucchiarini, M. Translational applications of photopolymerizable hydrogels for cartilage repair. J. Exp. Orthop. 2019, 6, 47–58. [Google Scholar] [CrossRef]
- Critchley, S.; Sheehy, E.J.; Cunniffe, G.; Diaz-Payno, P.; Carroll, S.F.; Jeon, O.; Alsberg, E.; Brama, P.A.J.; Kelly, D.J. 3D printing of fibre-reinforced cartilaginous templates for the regeneration of osteochondral defects. Acta Biomater. 2020, 113, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Camarero-Espinosa, S.; Cooper-White, J. Tailoring biomaterial scaffolds for osteochondral repair. Int. J. Pharm. 2017, 523, 476–489. [Google Scholar] [CrossRef]
- Cassaro, A. Fracture resistance of prosthetic abutments reconstructed with different methods. Stomatol. Mediterr. 1988, 8, 133–138. [Google Scholar] [PubMed]
- Zhu, C.; Wu, Q.; Wang, F.; Zhang, X.; Chen, F.; Liu, X.; Yang, Q.; Zhu, L. Animal models used for testing hydrogels in cartilage regeneration. Curr. Stem Cell Res. Ther. 2018, 13, 517–525. [Google Scholar] [CrossRef]
- Verrier, S.; Alini, M.; Alsberg, E.; Buchman, S.R.; Kelly, D.; Laschke, M.W.; Menger, M.D.; Murphy, W.L.; Stegemann, J.P.; Schutz, M.; et al. Tissue engineering and regenerative approaches to improving the healing of large bone defects. Eur. Cell. Mater. 2016, 32, 87–110. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Rothrauff, B.B.; Tuan, R.S. Tendon and ligament regeneration and repair: Clinical relevance and developmental paradigm. Birth Defects Res. C Embryo Today 2013, 99, 203–222. [Google Scholar] [CrossRef]
- Chajra, H.; Rousseau, C.F.; Cortial, D.; Ronziere, M.C.; Herbage, D.; Mallein-Gerin, F.; Freyria, A.M. Collagen-based biomaterials and cartilage engineering. Application to osteochondral defects. Biomed. Mater. Eng. 2008, 18, S33–S45. [Google Scholar]
- Hishimura, R.; Onodera, T.; Hontani, K.; Baba, R.; Homan, K.; Matsubara, S.; Joutoku, Z.; Kim, W.; Nonoyama, T.; Kurokawa, T.; et al. Osteochondral autograft transplantation technique augmented by an ultrapurified alginate gel enhances osteochondral repair in a rabbit model. Am. J. Sports Med. 2019, 47, 468–478. [Google Scholar] [CrossRef]
- Costa, L.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Gellan gum-based hydrogels for osteochondral repair. Adv. Exp. Med. Biol. 2018, 1058, 281–304. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, V.P.; Pina, S.; Oliveira, J.M.; Reis, R.L. Silk fibroin-based hydrogels and scaffolds for osteochondral repair and regeneration. Adv. Exp. Med. Biol. 2018, 1058, 305–325. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, J.; Ma, L.; Li, F.; Tu, Z.; Gao, C. Fabrication of poly(lactide-co-glycolide) scaffold filled with fibrin gel, mesenchymal stem cells, and poly(ethylene oxide)-b-poly(L-lysine)/TGF-beta1 plasmid DNA complexes for cartilage restoration in vivo. J. Biomed. Mater. Res. A 2013, 101, 3097–3108. [Google Scholar] [CrossRef] [PubMed]
- Iulian, A.; Dan, L.; Camelia, T.; Claudia, M.; Sebastian, G. Synthetic materials for osteochondral tissue engineering. Adv. Exp. Med. Biol. 2018, 1058, 31–52. [Google Scholar] [CrossRef]
- Xie, H.; Ji, Y.; Tian, Q.; Wang, X.; Zhang, N.; Zhang, Y.; Xu, J.; Wang, N.; Yan, J. Autogenous bone particle/titanium fiber composites for bone regeneration in a rabbit radius critical-size defect model. Connect Tissue Res. 2017, 58, 553–561. [Google Scholar] [CrossRef]
- Freitas, G.P.; Lopes, H.B.; Almeida, A.L.G.; Abuna, R.P.F.; Gimenes, R.; Souza, L.E.B.; Covas, D.T.; Beloti, M.M.; Rosa, A.L. Potential of osteoblastic cells derived from bone marrow and adipose tissue associated with a polymer/ceramic composite to repair bone tissue. Calcif. Tissue Int. 2017, 101, 312–320. [Google Scholar] [CrossRef]
- Kawai, T.; Matsui, K.; Ezoe, Y.; Kajii, F.; Suzuki, O.; Takahashi, T.; Kamakura, S. Efficacy of octacalcium phosphate collagen composite for titanium dental implants in dogs. Materials 2018, 11, 229. [Google Scholar] [CrossRef]
- Julier, Z.; Park, A.J.; Briquez, P.S.; Martino, M.M. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017, 53, 13–28. [Google Scholar] [CrossRef]
- Akagi, D.; Oba, M.; Koyama, H.; Nishiyama, N.; Fukushima, S.; Miyata, T.; Nagawa, H.; Kataoka, K. Biocompatible micellar nanovectors achieve efficient gene transfer to vascular lesions without cytotoxicity and thrombus formation. Gene Ther. 2007, 14, 1029–1038. [Google Scholar] [CrossRef]
- Wang, H.; Ding, S.; Zhang, Z.; Wang, L.; You, Y. Cationic micelle: A promising nanocarrier for gene delivery with high transfection efficiency. J. Gene Med. 2019, 21, e3101. [Google Scholar] [CrossRef]
- Rey-Rico, A.; Venkatesan, J.K.; Frisch, J.; Rial-Hermida, I.; Schmitt, G.; Concheiro, A.; Madry, H.; Alvarez-Lorenzo, C.; Cucchiarini, M. PEO-PPO-PEO micelles as effective rAAV-mediated gene delivery systems to target human mesenchymal stem cells without altering their differentiation potency. Acta Biomater. 2015, 27, 42–52. [Google Scholar] [CrossRef]
- Venkatesan, J.K.; Gardner, O.; Rey-Rico, A.; Eglin, D.; Alini, M.; Stoddart, M.J.; Cucchiarini, M.; Madry, H. Improved chondrogenic differentiation of rAAV SOX9-modified human MSCs seeded in fibrin-polyurethane scaffolds in a hydrodynamic environment. Int. J. Mol. Sci. 2018, 19, 2635. [Google Scholar] [CrossRef]
- Frisch, J.; Rey-Rico, A.; Venkatesan, J.K.; Schmitt, G.; Madry, H.; Cucchiarini, M. rAAV-mediated overexpression of sox9, TGF-beta and IGF-I in minipig bone marrow aspirates to enhance the chondrogenic processes for cartilage repair. Gene Ther. 2016, 23, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Cucchiarini, M.; Madry, H. Biomaterial-guided delivery of gene vectors for targeted articular cartilage repair. Nat. Rev. Rheumatol. 2019, 15, 18–29. [Google Scholar] [CrossRef] [PubMed]
- He, C.X.; Zhang, T.Y.; Miao, P.H.; Hu, Z.J.; Han, M.; Tabata, Y.; Hu, Y.L.; Gao, J.Q. TGF-beta1 gene-engineered mesenchymal stem cells induce rat cartilage regeneration using nonviral gene vector. Biotechnol. Appl. Biochem. 2012, 59, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Lv, L.; Dai, Y.; Wu, G.; Zhao, H.; Zhang, F. Porous chitosan scaffolds with embedded hyaluronic acid/chitosan/plasmid-DNA nanoparticles encoding TGF-beta1 induce DNA controlled release, transfected chondrocytes, and promoted cell proliferation. PLoS ONE 2013, 8, e69950. [Google Scholar] [CrossRef]
- Elangovan, S.; D’Mello, S.R.; Hong, L.; Ross, R.D.; Allamargot, C.; Dawson, D.V.; Stanford, C.M.; Johnson, G.K.; Sumner, D.R.; Salem, A.K. The enhancement of bone regeneration by gene activated matrix encoding for platelet derived growth factor. Biomaterials 2014, 35, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Curtin, C.M.; Tierney, E.G.; McSorley, K.; Cryan, S.A.; Duffy, G.P.; O’Brien, F.J. Combinatorial gene therapy accelerates bone regeneration: Non-viral dual delivery of VEGF and BMP2 in a collagen-nanohydroxyapatite scaffold. Adv. Healthc. Mater. 2015, 4, 223–227. [Google Scholar] [CrossRef]
- D’Mello, S.R.; Elangovan, S.; Hong, L.; Ross, R.D.; Sumner, D.R.; Salem, A.K. A pilot study evaluating combinatorial and simultaneous delivery of polyethylenimine-plasmid DNA complexes encoding for VEGF and PDGF for bone regeneration in calvarial bone defects. Curr. Pharm. Biotechnol. 2015, 16, 655–660. [Google Scholar] [CrossRef]
- Keeney, M.; Chung, M.T.; Zielins, E.R.; Paik, K.J.; McArdle, A.; Morrison, S.D.; Ransom, R.C.; Barbhaiya, N.; Atashroo, D.; Jacobson, G.; et al. Scaffold-mediated BMP-2 minicircle DNA delivery accelerated bone repair in a mouse critical-size calvarial defect model. J. Biomed. Mater. Res. A 2016, 104, 2099–2107. [Google Scholar] [CrossRef]
- Plonka, A.B.; Khorsand, B.; Yu, N.; Sugai, J.V.; Salem, A.K.; Giannobile, W.V.; Elangovan, S. Effect of sustained PDGF nonviral gene delivery on repair of tooth-supporting bone defects. Gene Ther. 2017, 24, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Khorsand, B.; Nicholson, N.; Do, A.V.; Femino, J.E.; Martin, J.A.; Petersen, E.; Guetschow, B.; Fredericks, D.C.; Salem, A.K. Regeneration of bone using nanoplex delivery of FGF-2 and BMP-2 genes in diaphyseal long bone radial defects in a diabetic rabbit model. J. Control Release 2017, 248, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Raftery, R.M.; Mencia Castano, I.; Chen, G.; Cavanagh, B.; Quinn, B.; Curtin, C.M.; Cryan, S.A.; O’Brien, F.J. Translating the role of osteogenic-angiogenic coupling in bone formation: Highly efficient chitosan-pDNA activated scaffolds can accelerate bone regeneration in critical-sized bone defects. Biomaterials 2017, 149, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Raftery, R.M.; Mencia-Castano, I.; Sperger, S.; Chen, G.; Cavanagh, B.; Feichtinger, G.A.; Redl, H.; Hacobian, A.; O’Brien, F.J. Delivery of the improved BMP-2-advanced plasmid DNA within a gene-activated scaffold accelerates mesenchymal stem cell osteogenesis and critical size defect repair. J. Control Release 2018, 283, 20–31. [Google Scholar] [CrossRef]
- Tierney, E.G.; Duffy, G.P.; Hibbitts, A.J.; Cryan, S.A.; O’Brien, F.J. The development of non-viral gene-activated matrices for bone regeneration using polyethyleneimine (PEI) and collagen-based scaffolds. J. Control Release 2012, 158, 304–311. [Google Scholar] [CrossRef]
- Mano, J.F.; Silva, G.A.; Azevedo, H.S.; Malafaya, P.B.; Sousa, R.A.; Silva, S.S.; Boesel, L.F.; Oliveira, J.M.; Santos, T.C.; Marques, A.P.; et al. Natural origin biodegradable systems in tissue engineering and regenerative medicine: Present status and some moving trends. J. R. Soc. Interface 2007, 4, 999–1030. [Google Scholar] [CrossRef]
- Saravanan, S.; Leena, R.S.; Selvamurugan, N. Chitosan based biocomposite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1354–1365. [Google Scholar] [CrossRef]
- Wang, W.; Li, B.; Li, Y.; Jiang, Y.; Ouyang, H.; Gao, C. In vivo restoration of full-thickness cartilage defects by poly(lactide-co-glycolide) sponges filled with fibrin gel, bone marrow mesenchymal stem cells and DNA complexes. Biomaterials 2010, 31, 5953–5965. [Google Scholar] [CrossRef]
- Chen, J.; Chen, H.; Li, P.; Diao, H.; Zhu, S.; Dong, L.; Wang, R.; Guo, T.; Zhao, J.; Zhang, J. Simultaneous regeneration of articular cartilage and subchondral bone in vivo using MSCs induced by a spatially controlled gene delivery system in bilayered integrated scaffolds. Biomaterials 2011, 32, 4793–4805. [Google Scholar] [CrossRef]
- Li, B.; Li, F.; Ma, L.; Yang, J.; Wang, C.; Wang, D.; Gao, C. Poly(lactide-co-glycolide)/fibrin gel construct as a 3D model to evaluate gene therapy of cartilage in vivo. Mol. Pharm. 2014, 11, 2062–2070. [Google Scholar] [CrossRef]
- Lee, Y.H.; Wu, H.C.; Yeh, C.W.; Kuan, C.H.; Liao, H.T.; Hsu, H.C.; Tsai, J.C.; Sun, J.S.; Wang, T.W. Enzyme-crosslinked gene-activated matrix for the induction of mesenchymal stem cells in osteochondral tissue regeneration. Acta. Biomater 2017, 63, 210–226. [Google Scholar] [CrossRef] [PubMed]
- Ledo, A.M.; Senra, A.; Rilo-Alvarez, H.; Borrajo, E.; Vidal, A.; Alonso, M.J.; Garcia-Fuentes, M. mRNA-activated matrices encoding transcription factors as primers of cell differentiation in tissue engineering. Biomaterials 2020, 247, 120016–120027. [Google Scholar] [CrossRef] [PubMed]
- Balmayor, E.R.; Geiger, J.P.; Koch, C.; Aneja, M.K.; van Griensven, M.; Rudolph, C.; Plank, C. Modified mRNA for BMP-2 in combination with biomaterials serves as a transcript-activated matrix for effectively inducing osteogenic pathways in stem cells. Stem Cells Dev. 2017, 26, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.D.; Salter, E.; Chen, E.; Sutter, K.A.; Alsberg, E. Calcium phosphate-DNA nanoparticle gene delivery from alginate hydrogels induces in vivo osteogenesis. J. Biomed. Mater. Res. A 2010, 92, 1131–1138. [Google Scholar] [CrossRef]
- Wegman, F.; Bijenhof, A.; Schuijff, L.; Oner, F.C.; Dhert, W.J.; Alblas, J. Osteogenic differentiation as a result of BMP-2 plasmid DNA based gene therapy in vitro and in vivo. Eur. Cell Mater. 2011, 21, 230–242. [Google Scholar] [CrossRef]
- Leng, P.; Ding, C.R.; Zhang, H.N.; Wang, Y.Z. Reconstruct large osteochondral defects of the knee with hIGF-1 gene enhanced Mosaicplasty. Knee 2012, 19, 804–811. [Google Scholar] [CrossRef]
- Wegman, F.; Geuze, R.E.; van der Helm, Y.J.; Cumhur Oner, F.; Dhert, W.J.; Alblas, J. Gene delivery of bone morphogenetic protein-2 plasmid DNA promotes bone formation in a large animal model. J. Tissue Eng. Regen Med. 2014, 8, 763–770. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, T.; Tierney, E.G.; Cunniffe, G.M.; O’Brien, F.J.; Kelly, D.J. Gene delivery of TGF-beta3 and BMP2 in an MSC-laden alginate hydrogel for articular cartilage and endochondral bone tissue engineering. Tissue Eng. Part A 2016, 22, 776–787. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, T.; Rathan, S.; Hobbs, C.; Pitacco, P.; Freeman, F.E.; Cunniffe, G.M.; Dunne, N.J.; McCarthy, H.O.; Nicolosi, V.; O’Brien, F.J.; et al. Pore-forming bioinks to enable spatio-temporally defined gene delivery in bioprinted tissues. J. Control Release 2019, 301, 13–27. [Google Scholar] [CrossRef]
- Loozen, L.D.; Wegman, F.; Oner, F.C.; Dhert, W.J.A.; Alblas, J. Porous bioprinted constructs in BMP-2 non-viral gene therapy for bone tissue engineering. J. Mater. Chem. B 2013, 1, 6619–6626. [Google Scholar] [CrossRef]
- Yang, H.N.; Park, J.S.; Jeon, S.Y.; Park, K.H. Carboxymethylcellulose (CMC) formed nanogels with branched poly(ethyleneimine) (bPEI) for inhibition of cytotoxicity in human MSCs as a gene delivery vehicles. Carbohydr. Polym. 2015, 122, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Huang, Z.; Cai, X.; Xiang, J.; Zhu, Y.A.; Wang, R.; Chen, J.; Zhang, J. Localized delivery of antisense oligonucleotides by cationic hydrogel suppresses TNF-alpha expression and endotoxin-induced osteolysis. Pharm. Res. 2011, 28, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Li, D.D.; Pan, J.F.; Ji, Q.X.; Yu, X.B.; Liu, L.S.; Li, H.; Jiao, X.J.; Wang, L. Characterization and cytocompatibility of thermosensitive hydrogel embedded with chitosan nanoparticles for delivery of bone morphogenetic protein-2 plasmid DNA. J. Mater. Sci. Mater. Med. 2016, 27, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ji, Q.; Chen, X.; Sun, Y.; Xu, Q.; Deng, P.; Hu, F.; Yang, J. Accelerated bony defect healing based on chitosan thermosensitive hydrogel scaffolds embedded with chitosan nanoparticles for the delivery of BMP2 plasmid DNA. J. Biomed. Mater. Res. A 2017, 105, 265–273. [Google Scholar] [CrossRef]
- Komatsu, K.; Shibata, T.; Shimada, A.; Ideno, H.; Nakashima, K.; Tabata, Y.; Nifuji, A. Cationized gelatin hydrogels mixed with plasmid DNA induce stronger and more sustained gene expression than atelocollagen at calvarial bone defects in vivo. J. Biomater. Sci. Polym. Ed. 2016, 27, 419–430. [Google Scholar] [CrossRef]
- Needham, C.J.; Shah, S.R.; Dahlin, R.L.; Kinard, L.A.; Lam, J.; Watson, B.M.; Lu, S.; Kasper, F.K.; Mikos, A.G. Osteochondral tissue regeneration through polymeric delivery of DNA encoding for the SOX trio and RUNX2. Acta Biomater. 2014, 10, 4103–4112. [Google Scholar] [CrossRef]
- Wehrhan, F.; Amann, K.; Molenberg, A.; Lutz, R.; Neukam, F.W.; Schlegel, K.A. Critical size defect regeneration using PEG-mediated BMP-2 gene delivery and the use of cell occlusive barrier membranes—The osteopromotive principle revisited. Clin. Oral Implants Res. 2013, 24, 910–920. [Google Scholar] [CrossRef]
- Dash, T.K.; Konkimalla, V.B. Poly-ε-caprolactone based formulations for drug delivery and tissue engineering: A review. J. Control Release 2012, 158, 15–33. [Google Scholar] [CrossRef]
- Li, Z.; Tan, B.H. Towards the development of polycaprolactone based amphiphilic block copolymers: Molecular design, self-assembly and biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 45, 620–634. [Google Scholar] [CrossRef]
- Brunger, J.M.; Huynh, N.P.; Guenther, C.M.; Perez-Pinera, P.; Moutos, F.T.; Sanchez-Adams, J.; Gersbach, C.A.; Guilak, F. Scaffold-mediated lentiviral transduction for functional tissue engineering of cartilage. Proc. Natl. Acad. Sci. USA 2014, 111, E798–E806. [Google Scholar] [CrossRef]
- Moutos, F.T.; Glass, K.A.; Compton, S.A.; Ross, A.K.; Gersbach, C.A.; Guilak, F.; Estes, B.T. Anatomically shaped tissue-engineered cartilage with tunable and inducible anticytokine delivery for biological joint resurfacing. Proc. Natl. Acad. Sci. USA 2016, 113, E4513–E4522. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.K.; Moutos, F.T.; Rey-Rico, A.; Estes, B.T.; Frisch, J.; Schmitt, G.; Madry, H.; Guilak, F.; Cucchiarini, M. Chondrogenic differentiation processes in human bone-marrow aspirates seeded in three-dimensional-woven poly(ε-caprolactone) scaffolds enhanced by recombinant adeno-associated virus-mediated SOX9 gene transfer. Hum. Gene Ther. 2018, 29, 1277–1286. [Google Scholar] [CrossRef]
- Johnstone, B.; Alini, M.; Cucchiarini, M.; Dodge, G.R.; Eglin, D.; Guilak, F.; Madry, H.; Mata, A.; Mauck, R.L.; Semino, C.E.; et al. Tissue engineering for articular cartilage repair—The state of the art. Eur. Cell Mater. 2013, 25, 248–267. [Google Scholar] [CrossRef] [PubMed]
- Rohman, G.; Huot, S.; Vilas-Boas, M.; Radu-Bostan, G.; Castner, D.G.; Migonney, V. The grafting of a thin layer of poly(sodium styrene sulfonate) onto poly(ε-caprolactone) surface can enhance fibroblast behavior. J. Mater. Sci. Mater. Med. 2015, 26, 206–215. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Venkatesan, J.K.; Meng, W.; Rey-Rico, A.; Schmitt, G.; Speicher-Mentges, S.; Falentin-Daudre, C.; Leroux, A.; Madry, H.; Migonney, V.; Cucchiarini, M. Enhanced chondrogenic differentiation activities in human bone marrow aspirates via sox9 overexpression mediated by pNaSS-grafted PCL film-guided rAAV gene transfer. Pharmaceutics 2020, 12, 280. [Google Scholar] [CrossRef]
- Neumann, A.J.; Alini, M.; Archer, C.W.; Stoddart, M.J. Chondrogenesis of human bone marrow-derived mesenchymal stem cells is modulated by complex mechanical stimulation and adenoviral-mediated overexpression of bone morphogenetic protein 2. Tissue Eng. Part A 2013, 19, 1285–1294. [Google Scholar] [CrossRef]
- Rowland, C.R.; Glass, K.A.; Ettyreddy, A.R.; Gloss, C.C.; Matthews, J.R.L.; Huynh, N.P.T.; Guilak, F. Regulation of decellularized tissue remodeling via scaffold-mediated lentiviral delivery in anatomically-shaped osteochondral constructs. Biomaterials 2018, 177, 161–175. [Google Scholar] [CrossRef]
- Rey-Rico, A.; Venkatesan, J.K.; Schmitt, G.; Concheiro, A.; Madry, H.; Alvarez-Lorenzo, C.; Cucchiarini, M. rAAV-mediated overexpression of TGF-beta via vector delivery in polymeric micelles stimulates the biological and reparative activities of human articular chondrocytes in vitro and in a human osteochondral defect model. Int. J. Nanomed. 2017, 12, 6985–6996. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Han, R.; He, C.; Wang, G.; Wang, J.; Zheng, J.; Pei, M.; Wei, L. Demineralized bone matrix combined bone marrow mesenchymal stem cells, bone morphogenetic protein-2 and transforming growth factor-beta3 gene promoted pig cartilage defect repair. PLoS ONE 2014, 9, e116061. [Google Scholar] [CrossRef]
- Cao, L.; Yang, F.; Liu, G.; Yu, D.; Li, H.; Fan, Q.; Gan, Y.; Tang, T.; Dai, K. The promotion of cartilage defect repair using adenovirus mediated Sox9 gene transfer of rabbit bone marrow mesenchymal stem cells. Biomaterials 2011, 32, 3910–3920. [Google Scholar] [CrossRef]
- Madry, H.; Gao, L.; Rey-Rico, A.; Venkatesan, J.K.; Muller-Brandt, K.; Cai, X.; Goebel, L.; Schmitt, G.; Speicher-Mentges, S.; Zurakowski, D.; et al. Thermosensitive hydrogel based on PEO-PPO-PEO poloxamers for a controlled in situ release of recombinant adeno-associated viral vectors for effective gene therapy of cartilage defects. Adv. Mater. 2020, 32, e1906508. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Filardo, G.; Perdisa, F.; Venieri, G.; Marcacci, M. Clinical results of multilayered biomaterials for osteochondral regeneration. J. Exp. Orthop. 2014, 1, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Glass, K.A.; Link, J.M.; Brunger, J.M.; Moutos, F.T.; Gersbach, C.A.; Guilak, F. Tissue-engineered cartilage with inducible and tunable immunomodulatory properties. Biomaterials 2014, 35, 5921–5931. [Google Scholar] [CrossRef] [PubMed]
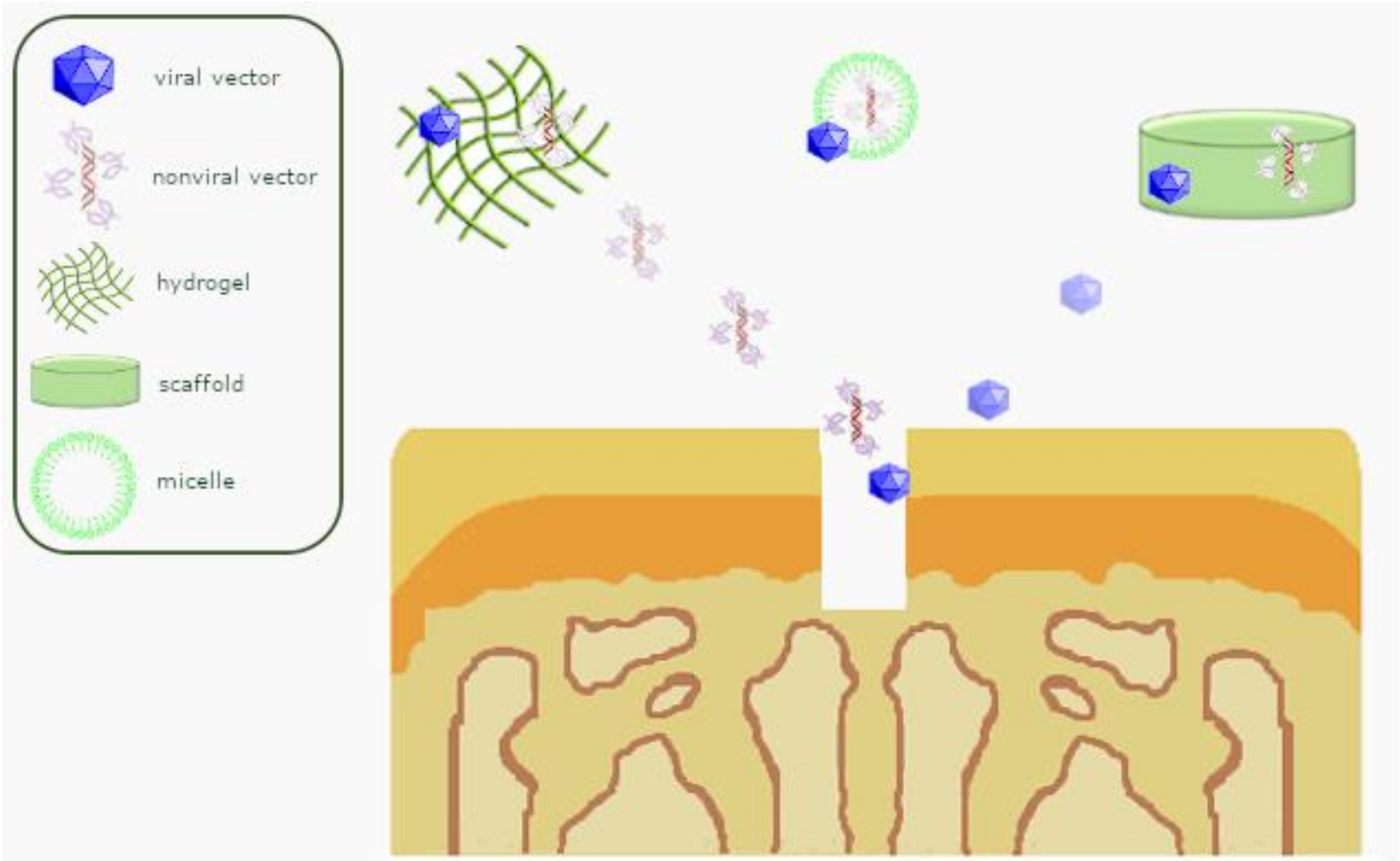
| Systems | Vectors | Efficacy | Integration | Features |
|---|---|---|---|---|
| Nonviral | naked pDNA | very low | no | very short-term expression, very low efficiency |
| lipoplexes | low | no | short-term expression, low immunogenicity, cytotoxicity at high concentrations | |
| polyplexes | low | no | short-term expression, low immunogenicity, cytotoxicity at high concentrations | |
| lipopolyplexes | medium | no | short-term expression, low immunogenicity, low cytotoxicity | |
| nanoparticles | medium | no | short-term expression, costly, quality control difficulties | |
| transposons | medium | yes | long-term expression, low immunogenicity, low cytotoxicity | |
| Viral | adenoviral | very high | no | short-term expression, strong immunogenicity |
| retroviral | high | yes | long-term expression, strong immunogenicity | |
| baculoviral | high | no | short-term expression | |
| rAAV | very high | no | long-term expression, low immunogenicity |
| Systems | Biocompatibility | Biodegradation | Mechanical/ Physico-Chemical Properties | Biological Properties |
|---|---|---|---|---|
| hydrogels (alginate, chitosan, collagen, gelatin, etc.) | high | high | poor mechanical strength, high porosity and swelling ratio | ECM-like properties |
| solid scaffolds (PCL, PLGA, etc.) | low | low | high mechanical strength, tuneable properties | controlled release of biomolecule cargos |
| hybrid scaffolds (fibrin/PLGA, gelatin/collagen, etc.) | moderate-high | moderate | combination of hydrogels and solid scaffolds properties | high cell adhesion and sustained release profiles |
| Vectors | Genes | Scaffolds | In Vitro Target Cells | In Vivo Models | Applications | Ref. |
|---|---|---|---|---|---|---|
| PEI complexes | PDGF | collagen | BMSCs | rat | bone repair (cell proliferation, osteogenesis) | [107] |
| VEGF, BMP-2 | collagen-nHA | rMSCs | rat | bone repair (cell proliferation, osteogenesis, angiogenesis) | [108] | |
| VEGF, PDGF | collagen | BMSCs | rat | bone repair (cell proliferation, osteogenesis, angiogenesis) | [109] | |
| PDGF | collagen | hPLFs hGFs | rat | bone repair (cell proliferation, osteogenesis) | [111] | |
| FGF-2 BMP-2 | collagen | BMSCs | rabbit | bone repair (cell proliferation, osteogenesis, angiogenesis) | [112] | |
| GFP, luc | collagen-nHA | rMSCs | - | transgene expression | [115] | |
| OSX | CMC nanogel | hMSCs | - | bone repair (osteogenesis) | [131] | |
| bPEI-HA complexes | SOX trio, RUNX2 | OPF hydrogel | - | rat | osteochondral repair (osteo-/chondrogenesis) | [136] |
| CaP/PEI nanoparticles | TGF-β3, BMP-2 | collagen-nHA | hMSCs | - | osteochondral repair (osteo- /chondrogenesis) | [121] |
| CaP nanoparticles | BMP-2 | 3D-printed alginate hydrogel | gMSCs | mouse | bone repair (osteogenesis) | [130] |
| BMP-2 | alginate hydrogels | MC3T3-E1 | mouse | bone repair (osteogenesis) | [124] | |
| nHA particles | TGF-β3, BMP-2, SOX9 | 3D-printed alginate-MC hydrogel | hMSCs | mouse | osteochondral repair (osteo-/chondrogenesis) | [129] |
| TGF-β3, BMP-2 | alginate hydrogels | MSCs | - | bone repair (osteogenesis) | [128] | |
| chitosan nanoparticles | VEGF, BMP-2 | collagen-nHA | rMSCs | rat | bone repair (cell proliferation, osteogenesis, angiogenesis) | [113] |
| BMP-2, BMP-7 | collagen-nHA | rMSCs | rat | bone repair (osteogenesis) | [114] | |
| ASO, TNF-α | gelatin-chitosan hydrogel | RAW 264.7 | mouse | bone repair (suppression of osteoclastogenesis) | [132] | |
| BMP-2 | chitosan hydrogel | - | rat, beagle dog | bone repair (osteogenesis) | [134] | |
| BMP-2 | chitosan hydrogel | hPDLCs | - | bone repair (osteogenesis) | [133] | |
| hyaluronic acid-chitosan nanoparticles | TGF-β1 | porous chitosan | chondrocytes | - | cartilage repair (chondrogenesis) | [106] |
| PEO-b-PLL complexes | TGF-β1 | PLGA | rbMSCs | rabbit | osteochondral repair (chondrogenesis) | [93] |
| pullulan-spermine complexes | TGF-β1 | gelatin sponge | rMSCs | rat | cartilage repair (chondrogenesis) | [105] |
| TMC complexes | TGF-β1 | PLGA sponge | BMSCs | rabbit | cartilage repair (chondrogenesis) | [119] |
| superFect complexes | BMP-2 | PLGA | skull-derived osteoblasts | mouse | bone repair (osteogenesis) | [110] |
| BMP-2 | PEG hydrogel | hFOB | pig | bone repair (osteogenesis) | [137] | |
| lipofectamine complexes | TGF-β1 | PLGA/fibrin hydrogel | rMSCs | rabbit | cartilage repair (chondrogenesis) | [120] |
| FuGene6 complexes | hIGF-I | calcium alginate hydrogel | BMSCs | goat | osteochondral repair (osteo-/chondrogenesis) | [126] |
| naked pDNA | TGF-β1, BMP-2 | CG/HCG | rMSCs | rabbit | osteochondral repair (osteo-/chondrogenesis) | [119] |
| BMP-2 | alginate hydrogel | hMSCs, MG-63 | mouse | bone repair (osteogenesis) | [125] | |
| BMP-2 | alginate hydrogel | gMSCs | goat | bone repair (osteogenesis) | [127] | |
| BMP-2 | collagen and gelatin hydrogels | - | mouse | bone repair (osteogenesis) | [135] | |
| mRNA 3DfectIN® complexes | SOX9 | fibrin hydrogel | hMSCs | - | cartilage repair (chondrogenesis) | [122] |
| mRNA DreamFect Gold complexes | fibrin gel or MBCP granules | rMSCs | - | bone repair (osteogenesis) | [123] |
| Vectors | Genes | Scaffolds | In Vitro Target Cells | In Vivo Models | Applications | Ref. |
|---|---|---|---|---|---|---|
| lentiviral | IL-1Ra | PCL | ASCs | - | cartilage repair (reduction of MMP activity) | [141] |
| eGFP, TGF-β3, BMP-2, IL-1Ra | CDM | hMSCS | - | cartilage repair (protection against tissue degradation) | [147] | |
| rAAV | SOX9 | PU | hMSCs | - | cartilage repair (cell proliferation, ECM deposition, reduced hypertrophy) | [102] |
| SOX9 | PCL | hBMA | - | cartilage repair (cell proliferation, ECM deposition, reduced hypertrophy) | [142] | |
| SOX9 | pNaSS-grafted PCL | hBMA | cartilage repair (cell proliferation, ECM deposition) | [145] | ||
| TGF-β1 | PEO-PPO-PEO micelles | chondrocytes | - | cartilage repair (cell proliferation, ECM deposition) | [148] | |
| SOX9 | PEO-PPO-PEO hydrogel | - | minipig | osteochondral repair (ECM deposition) | [151] | |
| adenoviral | BMP-2 | PU | hMSCS | cartilage repair (ECM deposition) | [146] | |
| BMP-2, TGF-β3 | DBM | BMSCs | pig | cartilage repair (ECM deposition) | [149] | |
| TGF-β1 | PGA | BMSCs | mice | cartilage repair (ECM deposition) | [54] | |
| SOX9 | PGA | BMSCs | rabbit | cartilage repair (ECM deposition) | [150] | |
| baculoviral | TGF-β1, BMP-6 | PLGA | rASCs | rabbit | cartilage repair (neocartilage formation) | [69] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madry, H.; Venkatesan, J.K.; Carballo-Pedrares, N.; Rey-Rico, A.; Cucchiarini, M. Scaffold-Mediated Gene Delivery for Osteochondral Repair. Pharmaceutics 2020, 12, 930. https://doi.org/10.3390/pharmaceutics12100930
Madry H, Venkatesan JK, Carballo-Pedrares N, Rey-Rico A, Cucchiarini M. Scaffold-Mediated Gene Delivery for Osteochondral Repair. Pharmaceutics. 2020; 12(10):930. https://doi.org/10.3390/pharmaceutics12100930
Chicago/Turabian StyleMadry, Henning, Jagadeesh Kumar Venkatesan, Natalia Carballo-Pedrares, Ana Rey-Rico, and Magali Cucchiarini. 2020. "Scaffold-Mediated Gene Delivery for Osteochondral Repair" Pharmaceutics 12, no. 10: 930. https://doi.org/10.3390/pharmaceutics12100930
APA StyleMadry, H., Venkatesan, J. K., Carballo-Pedrares, N., Rey-Rico, A., & Cucchiarini, M. (2020). Scaffold-Mediated Gene Delivery for Osteochondral Repair. Pharmaceutics, 12(10), 930. https://doi.org/10.3390/pharmaceutics12100930







