A Novel Co-Crystal of Bexarotene and Ligustrazine Improves Pharmacokinetics and Tissue Distribution of Bexarotene in SD Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Compounds and Agents
2.2. Experimental Animals
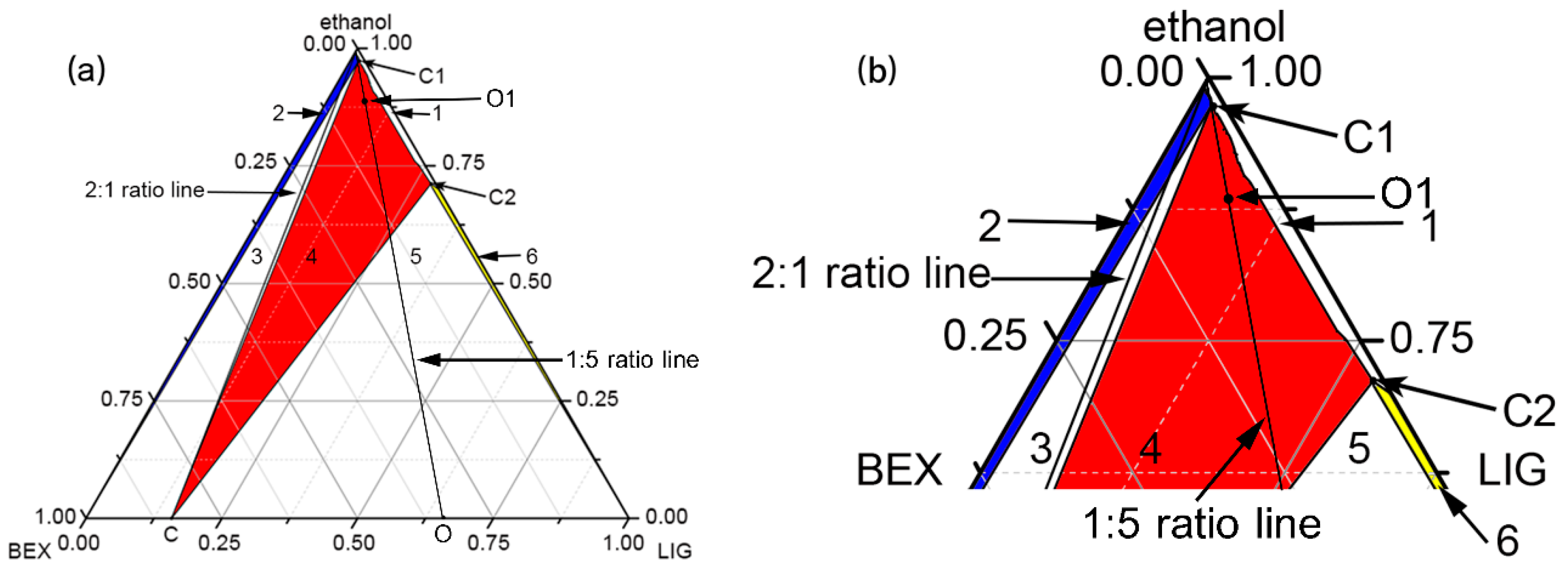
2.3. Construction of Ternary Phase Diagram
2.4. Preparation of 2BEX-LIG and the Elimination of the Excessive LIG from the Mixture
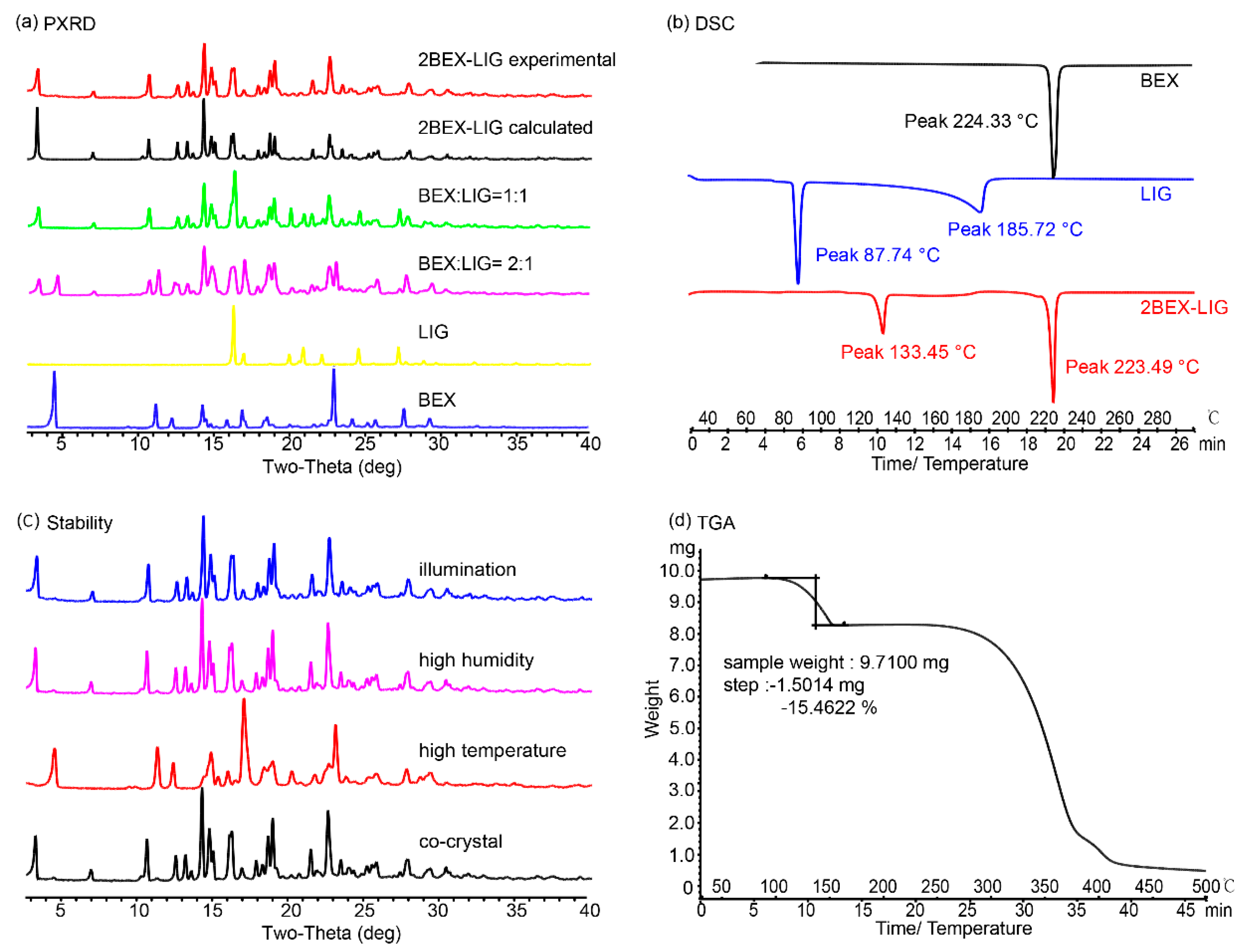
2.5. Characterization of 2BEX-LIG
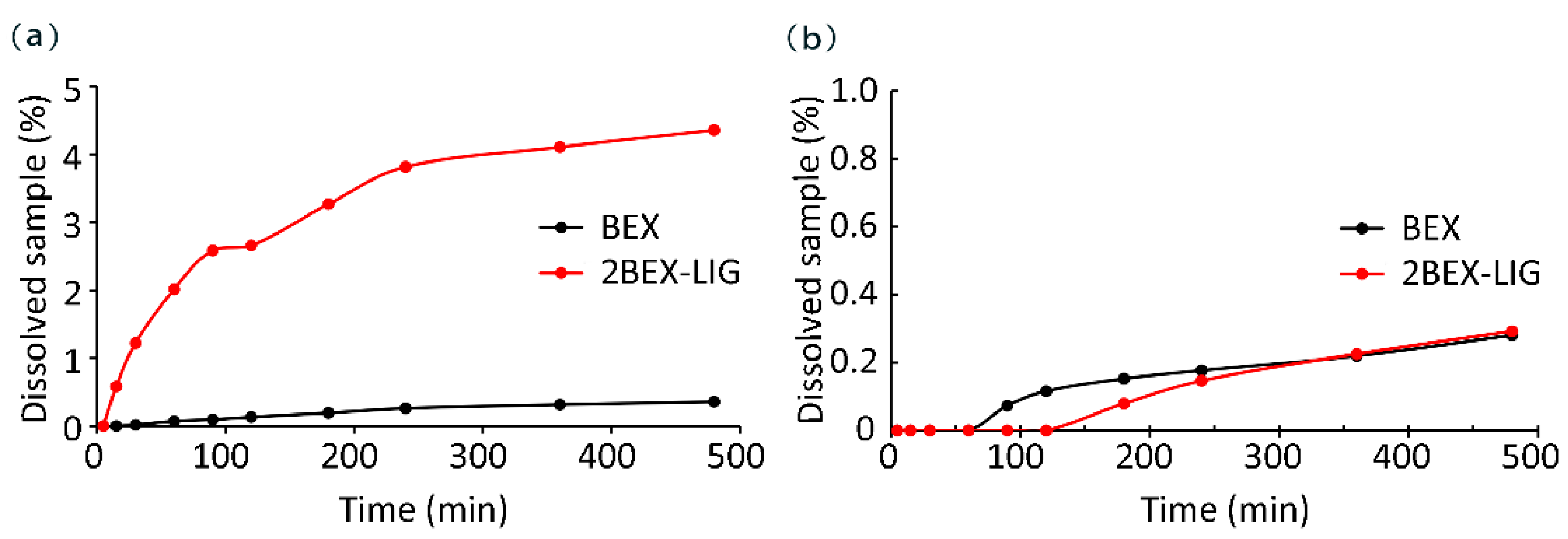
2.6. Dissolution Measurements
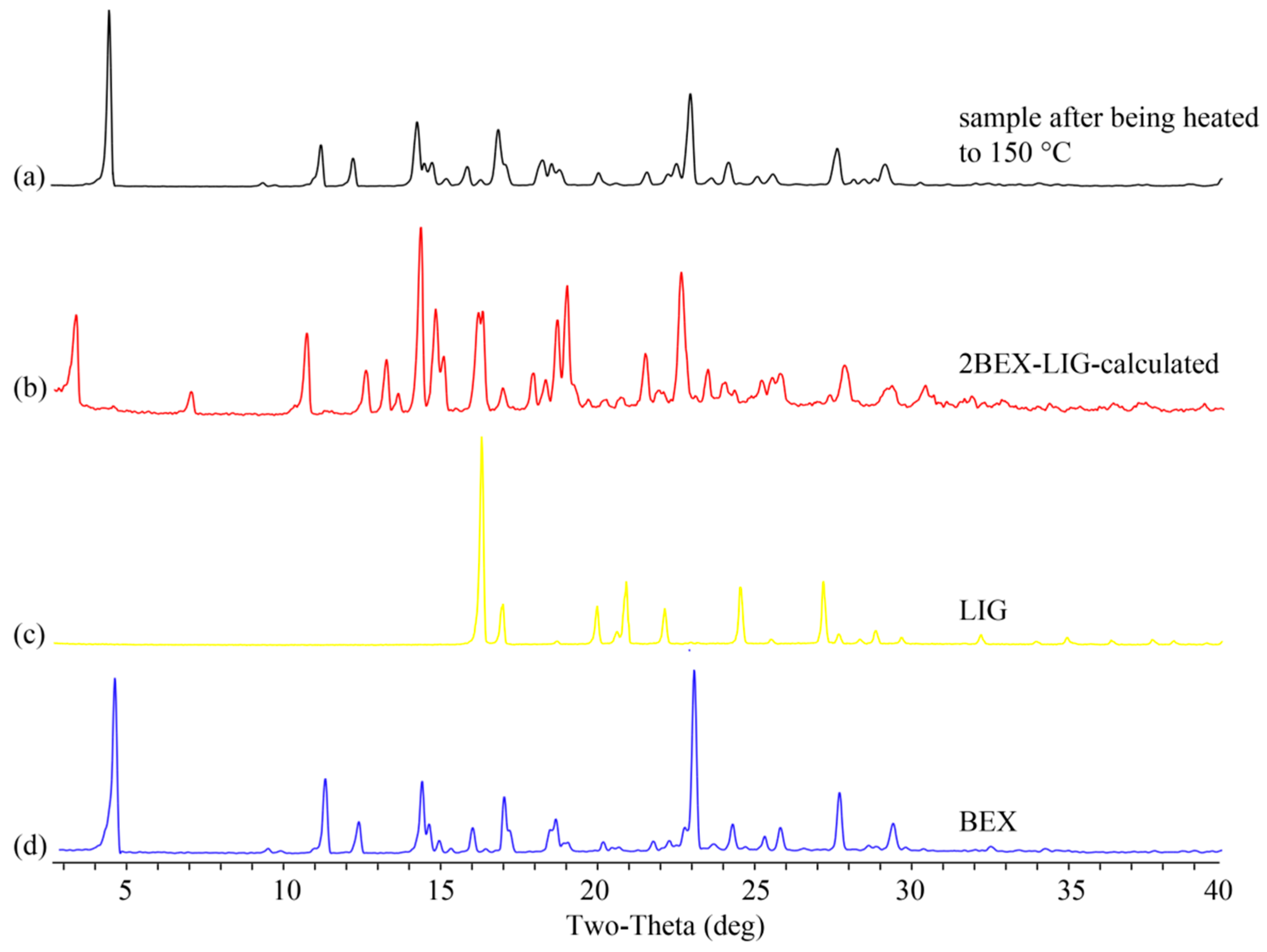
2.7. Stability Study
2.8. Pharmacokinetic Study of BEX in SD Rats
2.9. Tissue Distribution Study In Vivo
2.10. Detection of BEX Concentration Using LC-MS Method
2.11. Statistical Analysis
3. Results and Discussion
3.1. Construction of Co-Crystal Ternary Phase Diagram
3.2. Characterization of 2BEX-LIG
3.3. Powder Dissolution Measurements
3.4. Stability Study
3.5. Pharmacokinetic Study
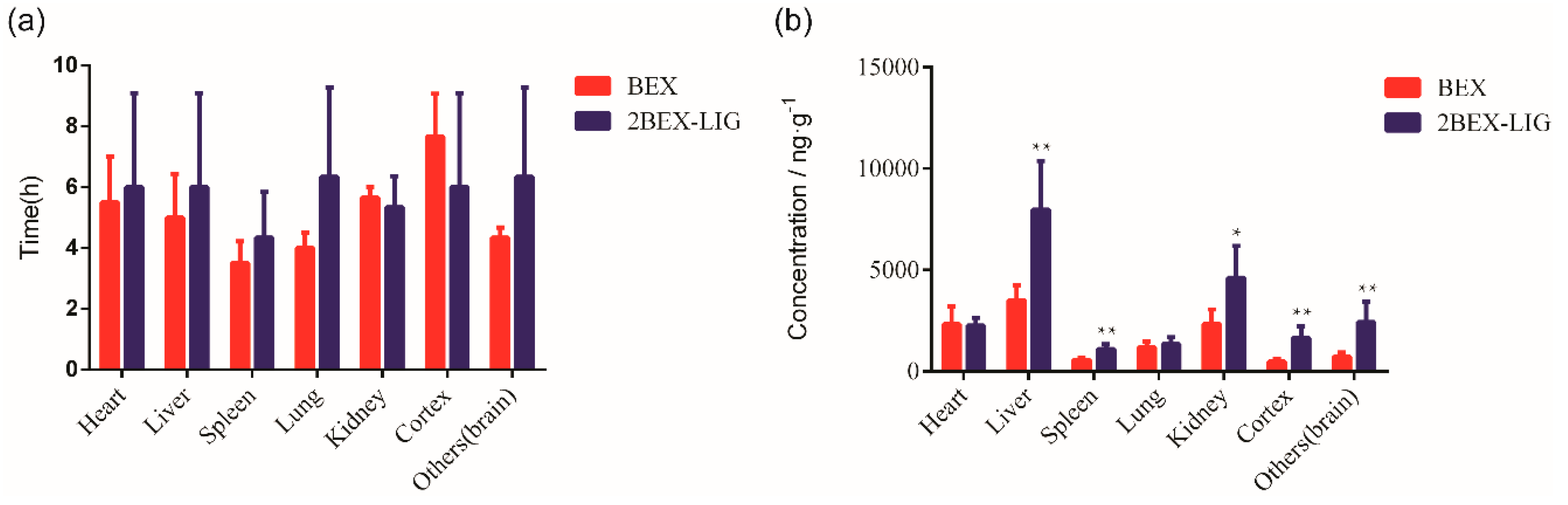
3.6. Tissue Distribution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Availability of Data and Material
References
- Nechipadappu, S.K.; Tekuri, V.; Trivedi, D.R. Pharmaceutical co-crystal of flufenamic acid: Synthesis and characterization of two novel drug-drug co-crystal. J. Pharm. Sci. 2017, 106, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- Bennion, J.C.; Siddiqi, Z.R.; Matzger, A.J. A melt castable energetic cocrystal. Chem. Commun. 2017, 53, 6065–6068. [Google Scholar] [CrossRef] [PubMed]
- Vioglio, P.C.; Chierotti, M.R.; Gobetto, R. Pharmaceutical aspects of salt and cocrystal forms of APIs and characterization challenges. Adv. Drug. Deliv. Rev. 2017, 117, 86–110. [Google Scholar] [CrossRef]
- Bommaka, M.K.; Mannava, M.C.; Suresh, K.; Gunnam, A.; Nangia, A. Entacapone: Improving aqueous solubility, diffusion permeability, and cocrystal stability with theophylline. Cryst. Growth Des. 2018, 18, 6061–6069. [Google Scholar] [CrossRef]
- Yu, Q.; Yan, Z.; Bao, J.; Wang, J.R.; Mei, X. Taming photo-induced oxidation degradation of dihydropyridine drugs through cocrystallization. Chem. Commun. 2017, 53, 12266–12269. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, J.R.; Zhang, Q.; Mei, X. Improving dissolution and photostability of vitamin K3 via cocrystallization with naphthoic acids and sulfamerazine. Cryst. Growth Des. 2016, 16, 483–492. [Google Scholar] [CrossRef]
- Bethune, S.J.; Huang, N.; Jayasankar, A.; Rodriguez-Hornedo, N. Understanding and predicting the effect of cocrystal components and pH on cocrystal solubility. Cryst. Growth Des. 2009, 9, 3976–3988. [Google Scholar] [CrossRef]
- Ren, S.Z.; Liu, M.Y.; Hong, C.M.; Li, G.B.; Sun, J.Y.; Wang, L.; Zhang, X.Y. The effects of pH, surfactant, ion concentration, coformer, and molecular arrangement on the solubility behavior of myricetin cocrystals. Acta Pharm. Sin. B 2019, 9, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Bavishi, D.D.; Borkhataria, C.H. Spring and parachute: How cocrystals enhance solubility. Prog. Cryst. Growth Charact. Mater. 2016, 62, 1–8. [Google Scholar] [CrossRef]
- Gopi, S.P.; Banik, M.; Desiraju, G.R. New cocrystals of hydrochlorothiazide: Optimizing solubility and membrane diffusivity. Cryst. Growth Des. 2017, 17, 308–316. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, Q.; Wang, J.R.; Mei, X. Cocrystals of baicalein with higher solubility and enhanced bioavailability. Cryst. Growth Des. 2017, 17, 1893–1901. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Yao, J.; Ma, Y.Y.; Chen, J.M.; Lu, T.B. Improving the solubility and bioavailability of apixaban via apixaban–oxalic acid cocrystal. Cryst. Growth Des. 2016, 16, 2923–2930. [Google Scholar] [CrossRef]
- Wang, C.; Tong, Q.; Hou, X.; Hu, S.; Fang, J.; Sun, C.C. Enhancing bioavailability of dihydromyricetin through inhibiting precipitation of soluble cocrystals by a crystallization inhibitor. Cryst. Growth Des. 2016, 16, 5030–5039. [Google Scholar] [CrossRef]
- Darwish, S.; Zeglinski, J.; Krishna, G.R.; Shaikh, R.; Khraishe, M.; Walker, G.M.; Croker, D. A New 1:1 Drug-Drug Cocrystal of Theophylline and Aspirin: Discovery, Characterization, and Construction of Ternary Phase Diagrams. Cryst. Growth Des. 2018, 18, 7526–7532. [Google Scholar] [CrossRef]
- Ai, X.; Mao, F.; Shen, S.; Shentu, Y.; Wang, J.; Lu, S. Bexarotene inhibits the viability of non-small cell lung cancer cells via slc10a2/PPARgamma/PTEN/ mTOR signaling pathway. BMC Cancer 2018, 18, 407. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Long, C.; Nguyen, J.; Kumar, D.; Lee, J. Discovering alkylamide derivatives of bexarotene as new therapeutic agents against triple-negative breast cancer. Bioorg. Med. Chem. Lett. 2018, 28, 420–424. [Google Scholar] [CrossRef]
- Heo, J.C.; Jung, T.H.; Lee, S.; Kim, H.; Choi, G.; Jung, M.; Jung, D.; Lee, H.K.; Lee, J.O.; Park, J.H.; et al. Effect of bexarotene on differentiation of glioblastoma multiforme compared with ATRA. Clin. Exp. Metastasis. 2016, 33, 417–429. [Google Scholar] [CrossRef]
- Haugen, B.R.; Larson, L.L.; Pugazhenthi, U.; Hays, W.R.; Klopper, J.P.; Kramer, C.A.; Sharma, V. Retinoic acid and retinoid X receptors are differentially expressed in thyroid cancer and thyroid carcinoma cell lines and predict response to treatment with retinoids. J. Clin. Endocrinol. Metab. 2004, 89, 272–280. [Google Scholar] [CrossRef]
- Chang, C.F.; Massey, J.; Osherov, A.; Angenendt da Costa, L.H.; Sansing, L.H. Bexarotene Enhances Macrophage Erythrophagocytosis and Hematoma Clearance in Experimental Intracerebral Hemorrhage. Stroke 2020, 51, 612–618. [Google Scholar] [CrossRef]
- Tu, L.; Yang, X.L.; Zhang, Q.; Wang, Q.; Tian, T.; Liu, D.; Qu, X.; Tian, J.Y. Bexarotene attenuates early brain injury via inhibiting micoglia activation through PPARγ after experimental subarachnoid hemorrhage. Neurol. Res. 2018, 40, 702–708. [Google Scholar] [CrossRef]
- Ghosal, K.; Haag, M.; Verghese, P.B.; West, T.; Veenstra, T.; Braunstein, J.B.; Bateman, R.J.; Holtzman, D.M.; Landreth, G.E. A randomized controlled study to evaluate the effect of bexarotene on amyloid-β and apolipoprotein E metabolism in healthy subjects. Alzheimers Dement. 2016, 2, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Pollinger, J.; Gellrich, L.; Schierle, S.; Kilu, W.; Schmidt, J.; Kalinowsky, L.; Ohrndorf, J.; Kaiser, A.; Heering, J.; Proschak, E.; et al. Tuning Nuclear Receptor Selectivity of Wy14,643 towards Selective Retinoid X Receptor Modulation. J. Med. Chem. 2019, 62, 2112–2126. [Google Scholar] [CrossRef] [PubMed]
- Vasile, A.; Ignat, M.; Zaltariov, M.F.; Sacarescu, L.; Stoleriu, I.; Draganescu, D.; Dumitras, M.; Ochiuz, L. Development of New Bexarotene-loaded Mesoporous Silica Systems for Topical Pharmaceutical Formulations. Acta. Chim. Slov. 2018, 65, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, Y.; Zhang, J.; Hao, L.; Guo, H.; Lou, H.; Zhang, D. Bexarotene nanocrystal-Oral and parenteral formulation development, characterization and pharmacokinetic evaluation. Eur. J. Pharm. Biopharm. 2014, 87, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Kim, T.H.; Feng, W.; Choi, H.G.; Zgair, A.; Shin, S.; Yoo, S.D.; Gershkovich, P.; Shin, B.S. Quantitative prediction of oral bioavailability of a lipophilic antineoplastic drug bexarotene administered in lipidic formulation using a combined in vitro lipolysis/microsomal metabolism approach. J. Pharm. Sci. 2019, 108, 1047–1052. [Google Scholar] [CrossRef]
- Branchu, S.; Rogueda, P.G.; Plumb, A.P.; Cook, W.G. A decision-support tool for the formulation of orally active, poorly soluble compounds. Eur. J. Pharm. Sci. 2007, 32, 128–139. [Google Scholar] [CrossRef]
- Cummings, J.L.; Zhong, K.; Kinney, J.W.; Heaney, C.; Joanne, M.T.; Joshi, A.; Joshi, M.; Pontecorvo, M.; Devous, A.; Tang, J.; et al. Double-blind, placebo-controlled, proof-of-concept trial of bexarotene in moderate Alzheimer’s disease. Alzheimers. Res. Ther. 2016, 8, 4. [Google Scholar] [CrossRef]
- Farol, L.T.; Hymes, K.B. Bexarotene: A clinical review. Expert. Rev. Anticancer. Ther. 2004, 4, 180–188. [Google Scholar] [CrossRef]
- Assaf, C.; Bagot, M.; Dummer, R.; Duvic, M.; Gniadecki, R.; Knobler, R.; Ranki, A.; Schwandt, P.; Whittaker, S. Minimizing adverse side-effects of oral bexarotene in cutaneous T-cell lymphoma: An expert opinion. Br. J. Dermatol. 2006, 155, 261–266. [Google Scholar] [CrossRef]
- Wang, Y.; Rong, J.; Zhang, J.; Liu, Y.; Meng, X.; Guo, H.; Liu, H.S.; Chen, L.J. Morphology, in vivo distribution and antitumor activity of bexarotene nanocrystals in lung cancer. Drug. Dev. Ind. Pharm. 2017, 43, 132–141. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Wang, J.; Chen, L.; Zhang, W.; Yan, X. Preparation, in vitro and in vivo evaluation of bexarotene nanocrystals with surface modification by folate-chitosan conjugates. Drug. Deliv. 2016, 23, 79–87. [Google Scholar] [CrossRef]
- Wu, J.Y.; Li, Y.J.; Yang, L.; Hu, Y.Y.; Hu, X.B.; Tang, T.T.; Wang, J.M.; Liu, X.Y.; Xaing, D.X. Borneol and Alpha-asarone as adjuvant agents for improving blood-brain barrier permeability of puerarin and tetramethylpyrazine by activating adenosine receptors. Drug. Deliv. 2018, 25, 1858–1864. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Gao, P.; Hao, X.; Xu, H.; Zhan, P.; Liu, X. Recent progress in the structural modification and pharmacological activities of ligustrazine derivatives. Eur. J. Med. Chem. 2018, 147, 150–162. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, T.; Li, N.; Wu, L.; Gu, J.; Li, C.; Zhao, C.; Liu, W.; Shan, L.C.; Yu, P.; et al. Tetramethylpyrazine nitrone activates the BDNF/Akt/CREB pathway to promote post-ischaemic neuroregeneration and recovery of neurological functions in rats. Br. J. Pharmacol. 2018, 175, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Wu, P.; Wang, X.; Jin, M.; Liu, S.; Ma, X.; Shi, H.Z. Tetramethylpyrazine Protects Against Early Brain Injury and Inhibits the PERK/Akt Pathway in a Rat Model of Subarachnoid Hemorrhage. Neurochem. Res. 2018, 43, 1650–1659. [Google Scholar] [CrossRef]
- Zhao, T.; Fu, Y.; Sun, H.; Liu, X. Ligustrazine suppresses neuron apoptosis via the Bax/Bcl-2 and caspase-3 pathway in PC12 cells and in rats with vascular dementia. IUBMB Life 2018, 70, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Seliger, J.; Zagar, V. Nuclear Quadrupole Resonance Investigation of Hydrogen Bonding in Some Cocrystals of 2,3,5,6-Tetramethylpyrazine and Carboxylic Acids. J. Phys. Chem. B 2014, 118, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Sreekanth, B.R.; Vishweshwar, P.; Vyas, K. Supramolecular synthon polymorphism in 2: 1 co-crystal of 4-hydroxybenzoic acid and 2,3,5,6-tetramethylpyrazine. Chem. Commun. 2007, 23, 2375–2377. [Google Scholar] [CrossRef]
- Zhu, X.L.; Xiong, L.Z.; Wang, Q.; Liu, Z.G.; Ma, X.; Zhu, Z.H.; Hu, S.; Gong, G.; Chen, S.Y. Therapeutic time window and mechanism of tetramethylpyrazine on transient focal cerebral ischemia/reperfusion injury in rats. Neurosci. Lett. 2009, 449, 24–27. [Google Scholar] [CrossRef]
- Zeng, M.F.; Pan, L.M.; Qi, S.M.; Cao, Y.T.; Zhu, H.X.; Guo, L.W.; Zhou, J. Systematic review of recent advances in pharmacokinetics of four classical Chinese medicines used for the treatment of cerebrovascular disease. Fitoterapia 2013, 88, 50–75. [Google Scholar] [CrossRef]
- Dissociation Constants of Bexarotene. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/82146#section=Dissociation-Constants (accessed on 10 August 2020).
- Agnes, S.C.; Trimble, R.F. Acidbase properties of some pyrazines. J. Phys. Chem. 1961, 65, 863–866. [Google Scholar]
- Good, D.J.; Rodriguez-Hornedo, N. Solubility advantage of pharmaceutical cocrystals. Cryst. Growth Des. 2009, 9, 2252–2264. [Google Scholar] [CrossRef]
- Ren, S.Y.; Jiao, L.T.; Yu, H.Y.; Wang, J.R.; Song, J.K.; Lv, T.T.; Lu, Y.; Yang, S.Y.; Sun, L.; Du, G.H. Preparation of a co-amorphous form of bexarotene-PVP-K30 and evaluation in rats. Acta Pharm. Sin. 2020, 1–16. [Google Scholar]
- Ferner, R.E.; Chambers, J. Alcohol intake: Measure for measure. BMJ Br. Med. J. 2001, 323, 1439–1440. [Google Scholar] [CrossRef]
- Jia, Q.; Zhang, J.; Zhang, S.; Lei, D.; Xu, Y.; Kou, K. Investigation of the Phase Behavior of a HNIW TNT Cocrystal System and Construction of Ternary Phase Diagrams. Cryst. Growth Des. 2019, 19, 6370–6376. [Google Scholar] [CrossRef]
- Song, L.; Robeyns, K.; Leyssens, T. Crystallizing ionic cocrystals: Structural characteristics, thermal behavior, and crystallization development of a piracetam-CaCl2 cocrystallization process. Cryst. Growth Des. 2018, 18, 3215–3221. [Google Scholar] [CrossRef]
- Kissel, P.; Murray, D.J.; Wulftange, W.J.; Catalano, V.J.; King, B.T. A nanoporous two-dimensional polymer by single-crystal-to-single-crystal photopolymerization. Nat. Chem. 2014, 6, 774–778. [Google Scholar] [CrossRef]
- Wang, J.R.; Yu, Q.; Dai, W.; Mei, X. Drug–drug co-crystallization presents a new opportunity for the development of stable vitamins. Chem. Commun. 2016, 52, 3572–3575. [Google Scholar] [CrossRef]
- Childs, S.L.; Hardcastle, K.I. Cocrystals of Piroxicam with Carboxylic Acids. Cryst. Growth Des. 2007, 7, 1291–1304. [Google Scholar] [CrossRef]
- Seki, T.; Takamatsu, Y.; Ito, H. A Screening Approach for the Discovery of Mechanochromic Gold(I) Isocyanide Complexes with Crystal-to-Crystal Phase Transitions. J. Am. Chem. Soc. 2016, 138, 6252–6260. [Google Scholar] [CrossRef]
- Daiss, J.O.; Burschka, C.; Mills, J.S.; Montana, J.G.; Showell, G.A.; Fleming, I.; Gaudon, C.; Ivanova, D.; Gronemeyer, H.; Tacke, R. Synthesis, crystal structure analysis, and pharmacological characterization of disila-bexarotene, a disila-analogue of the RXR-selective retinoid agonist bexarotene. Organometallics 2005, 24, 3192–3199. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Z.; Chen, Y.; Chen, Z.; Chen, H.; Pui, Y.; Feng, Q. Oral bioavailability enhancement of β-lapachone, a poorly soluble fast crystallizer, by cocrystal, amorphous solid dispersion, and crystalline solid dispersion. Eur. J. Pharm. Biopharm. 2018, 124, 73–81. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; Chinese Pharmacopoeia Commission, The Medicine Science and Technology Press of China: Beijing, China, 2015; Volume 4, pp. 354–356. [Google Scholar]
- Desai, P.P.; Patravale, V.B. Curcumin Cocrystal Micelles-Multifunctional Nanocomposites for Management of Neurodegenerative Ailments. J. Pharm. Sci. 2017, 107, 1143–1156. [Google Scholar] [CrossRef]
- Abosede, O.O.; Gordon, A.T.; Dembaremba, T.O.; Lorentino, C.M.A.; Frota, H.F.; Santos, A.L.S.; Hosten, E.C.; Ogunlaja, A.S. Trimesic acid–Theophylline and Isopthalic acid–Caffeine Cocrystals: Synthesis, Characterization, Solubility, Molecular Docking, and Antimicrobial Activity. Cryst. Growth Des. 2020, 20, 3510–3522. [Google Scholar] [CrossRef]
- Hariprasad, V.M.; Nechipadappu, S.K.; Trivedi, D.R. Co-Crystals of Ethenzamide: Study of Structural and Physico-Chemical Properties. Cryst. Growth Des. 2016, 16, 4473–4481. [Google Scholar] [CrossRef]
- Cadden, J.; Klooster, W.T.; Coles, S.J.; Aitipamula, S. Cocrystals of Leflunomide: Design, Structural and Physicochemical Evaluation. Cryst. Growth Des. 2019, 19, 3923–3933. [Google Scholar] [CrossRef]
- Bolla, G.; Sanphui, P.; Nangia, A. Solubility Advantage of Tenoxicam Phenolic Cocrystals Compared to Salts. Cryst. Growth Des. 2013, 13, 1988–2003. [Google Scholar] [CrossRef]
- Zhang, G.; Xiao, X.; Zhang, L.; Ren, G.L.; Zhang, S.Q. Hydrates and Solvates of Acotiamide Hydrochloride: Crystallization, Structure, Stability, and Solubility. Cryst. Growth Des. 2019, 19, 768–779. [Google Scholar] [CrossRef]
- Du, R.; Xu, J.; Zhang, L.; Ning, L.; Li, S. Ethinyl estradiol cocrystals assembled by chain structures: Improvement in stability and solubility. New J. Chim. 2019, 43, 16889–16897. [Google Scholar] [CrossRef]
- Khan, T.; Ranjan, R.; Dogra, Y.; Pandya, S.M.; Shafi, H.; Singh, S.K.; Yadav, P.N.; Misra, A. Intranasal Eutectic Powder of Zolmitriptan with Enhanced Bioavailability in the Rat Brain. Mol. Pharm. 2016, 13, 3234–3240. [Google Scholar] [CrossRef]
- Cui, W.X.; He, Z.; Zhang, Y.T.; Fan, Q.Y.; Feng, N.P. Naringenin Cocrystals Prepared by Solution Crystallization Method for Improving Bioavailability and Anti-hyperlipidemia Effects. AAPS PharmSciTech 2019, 20, 115–126. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, H.; Yang, C.; Wang, J. Preparation, characterization, and evaluation of dipfluzine-benzoic acid co-crystals with improved physicochemical properties. Pharm. Res. 2014, 31, 566–578. [Google Scholar] [CrossRef]





| Weight (mg) | Concentration (mg/mL) | Weight (mg) | Concentration (mg/mL) | ||||
|---|---|---|---|---|---|---|---|
| LIG | BEX | LIG | BEX | LIG | BEX | LIG | BEX |
| 0.00 | 40.00 | 0.00 | 7.01 | 120.00 | 40.00 | 39.09 | 9.44 |
| 3.00 | 40.00 | 0.50 | 6.57 | 140.00 | 40.00 | 46.32 | 11.74 |
| 6.00 | 40.00 | 2.49 | 6.54 | 160.00 | 40.00 | 47.51 | 12.78 |
| 9.00 | 40.00 | 4.00 | 7.84 | 180.00 | 40.00 | 47.89 | 12.03 |
| 12.00 | 40.00 | 5.38 | 7.57 | 200.00 | 40.00 | 48.11 | 12.12 |
| 15.00 | 40.00 | 5.87 | 6.77 | 220.00 | 40.00 | 48.55 | 12.71 |
| 18.00 | 40.00 | 6.91 | 7.50 | 240.00 | 40.00 | 51.16 | 11.76 |
| 21.00 | 40.00 | 7.07 | 7.09 | 260.00 | 40.00 | 51.86 | 13.06 |
| 24.00 | 40.00 | 8.44 | 7.36 | 280.00 | 40.00 | 55.50 | 14.42 |
| 27.00 | 40.00 | 9.46 | 6.81 | 300.00 | 40.00 | 56.14 | 13.55 |
| 30.00 | 40.00 | 9.58 | 7.47 | 320.00 | 40.00 | 57.04 | 13.77 |
| 40.00 | 40.00 | 11.85 | 8.90 | 340.00 | 40.00 | 58.51 | 14.31 |
| 50.00 | 40.00 | 14.75 | 8.19 | 360.00 | 40.00 | 61.60 | 14.87 |
| 60.00 | 40.00 | 17.84 | 9.81 | 380.00 | 40.00 | 66.26 | 15.99 |
| 70.00 | 40.00 | 19.88 | 9.45 | 400.00 | 40.00 | 74.23 | 14.25 |
| 80.00 | 40.00 | 22.98 | 9.40 | 600.00 | 40.00 | 236.13 | 16.47 |
| 90.00 | 40.00 | 30.46 | 10.68 | 800.00 | 40.00 | 249.90 | 13.29 |
| 100.00 | 40.00 | 30.58 | 10.13 | 1400.00 | 40.00 | 312.43 | 10.96 |
| 110.00 | 40.00 | 34.54 | 10.07 | 3000.00 | 0.00 | 332.64 | 0.00 |
| Crystallographic Data | 2BEX-LIG |
|---|---|
| Empirical formula | C28H34NO2 |
| Temperature (K) | 293 |
| Crystal system | monoclinic |
| Space group | C2/c |
| a (Å) | 49.281 |
| b (Å) | 8.517 |
| c (Å) | 11.787 |
| α (deg) | 90.00 |
| β (deg) | 100.41 |
| γ (deg) | 90.00 |
| Volume (Å3) | 4866 |
| Z | 8 |
| Calculated density (g/cm3) | 1.137 |
| Absorption coefficient. (mm−1) | 0.546 |
| F (000) | 1800 |
| Crystal size (mm) | 0.2 × 0.2 × 0.2 |
| Rint | 0.0710 |
| R1 [I>2σ(I)] | 0.0621 |
| wR2 (all data) | 0.1693 |
| GOF | 0.976 |
| Samples | Retention Time (min) | Peak Area | Concentration (mg/mL) | Concentration (mmol/mL) |
|---|---|---|---|---|
| BEX | 22.52 | 3.46 × 103 | 0.28 | 7.99 × 10−4 |
| LIG | 3.07 | 1.16 × 103 | 0.42 | 3.11 × 10−3 |
| BEX in co-crystal | 22.22 | 3.47 × 103 | 0.28 | 8.02 × 10−4 |
| LIG in co-crystal | 3.07 | 1.52 × 102 | 0.06 | 4.07 × 10−4 |
| Parameter | Unit | Oral Dose (30 mg/kg) | Injection Dose (5 mg/kg) | |
|---|---|---|---|---|
| BEX | 2BEX-LIG | BEX | ||
| AUC(0–t) | (×103) μg/L h | 7.03 ± 2.27 | 13.05 ± 3.85 ** | 5.14 ± 0.96 |
| AUC(0–∞) | (×103) μg/L·h | 7.24 ± 2.27 | 13.57 ± 3.98 | 5.27 ± 0.97 |
| T1/2 | h | 7.81 ± 2.21 | 7.92 ± 3.18 | 4.45 ± 1.53 |
| Tmax | h | 7.33 ± 2.58 | 9.58 ± 7.81 | 0.033 |
| Cmax | (×103) μg/L | 0.63 ± 0.30 | 1.24 ± 0.60 * | 5.14 ± 0.79 |
| MRT(0–t) | h | 11.33 ±1.30 | 11.00 ±4.03 | 2.50 ± 0.69 |
| FAUC(0–∞) % | 22.89 | 42.86 ** | / | |
| Kp (Ctissues/Cplasma) | BEX | 2BEX-LIG |
|---|---|---|
| Kp (Heart) | 3.70 | 1.82 |
| Kp (Liver) | 5.58 | 6.42 |
| Kp (Spleen) | 0.91 | 0.88 |
| Kp (Lung) | 1.88 | 1.08 |
| Kp (Kidney) | 3.66 | 3.71 |
| Kp (Cortex) | 0.76 | 1.33 |
| Kp (Others (Brain)) | 1.14 | 1.96 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, S.; Jiao, L.; Yang, S.; Zhang, L.; Song, J.; Yu, H.; Wang, J.; Lv, T.; Sun, L.; Lu, Y.; et al. A Novel Co-Crystal of Bexarotene and Ligustrazine Improves Pharmacokinetics and Tissue Distribution of Bexarotene in SD Rats. Pharmaceutics 2020, 12, 906. https://doi.org/10.3390/pharmaceutics12100906
Ren S, Jiao L, Yang S, Zhang L, Song J, Yu H, Wang J, Lv T, Sun L, Lu Y, et al. A Novel Co-Crystal of Bexarotene and Ligustrazine Improves Pharmacokinetics and Tissue Distribution of Bexarotene in SD Rats. Pharmaceutics. 2020; 12(10):906. https://doi.org/10.3390/pharmaceutics12100906
Chicago/Turabian StyleRen, Shuyue, Lingtai Jiao, Shiying Yang, Li Zhang, Junke Song, Haoying Yu, Jingrong Wang, Tingting Lv, Lan Sun, Yang Lu, and et al. 2020. "A Novel Co-Crystal of Bexarotene and Ligustrazine Improves Pharmacokinetics and Tissue Distribution of Bexarotene in SD Rats" Pharmaceutics 12, no. 10: 906. https://doi.org/10.3390/pharmaceutics12100906
APA StyleRen, S., Jiao, L., Yang, S., Zhang, L., Song, J., Yu, H., Wang, J., Lv, T., Sun, L., Lu, Y., & Du, G. (2020). A Novel Co-Crystal of Bexarotene and Ligustrazine Improves Pharmacokinetics and Tissue Distribution of Bexarotene in SD Rats. Pharmaceutics, 12(10), 906. https://doi.org/10.3390/pharmaceutics12100906








