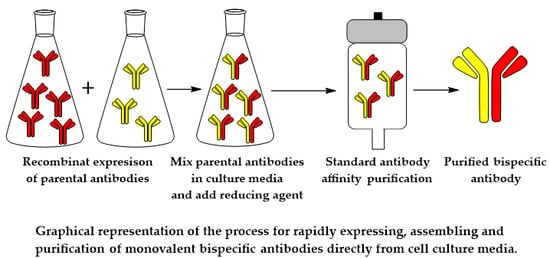
Fab-Arm Exchange Combined with Selective Protein A Purification Results in a Platform for Rapid Preparation of Monovalent Bispecific Antibodies Directly from Culture Media
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. Transient Expression of Parental Antibodies
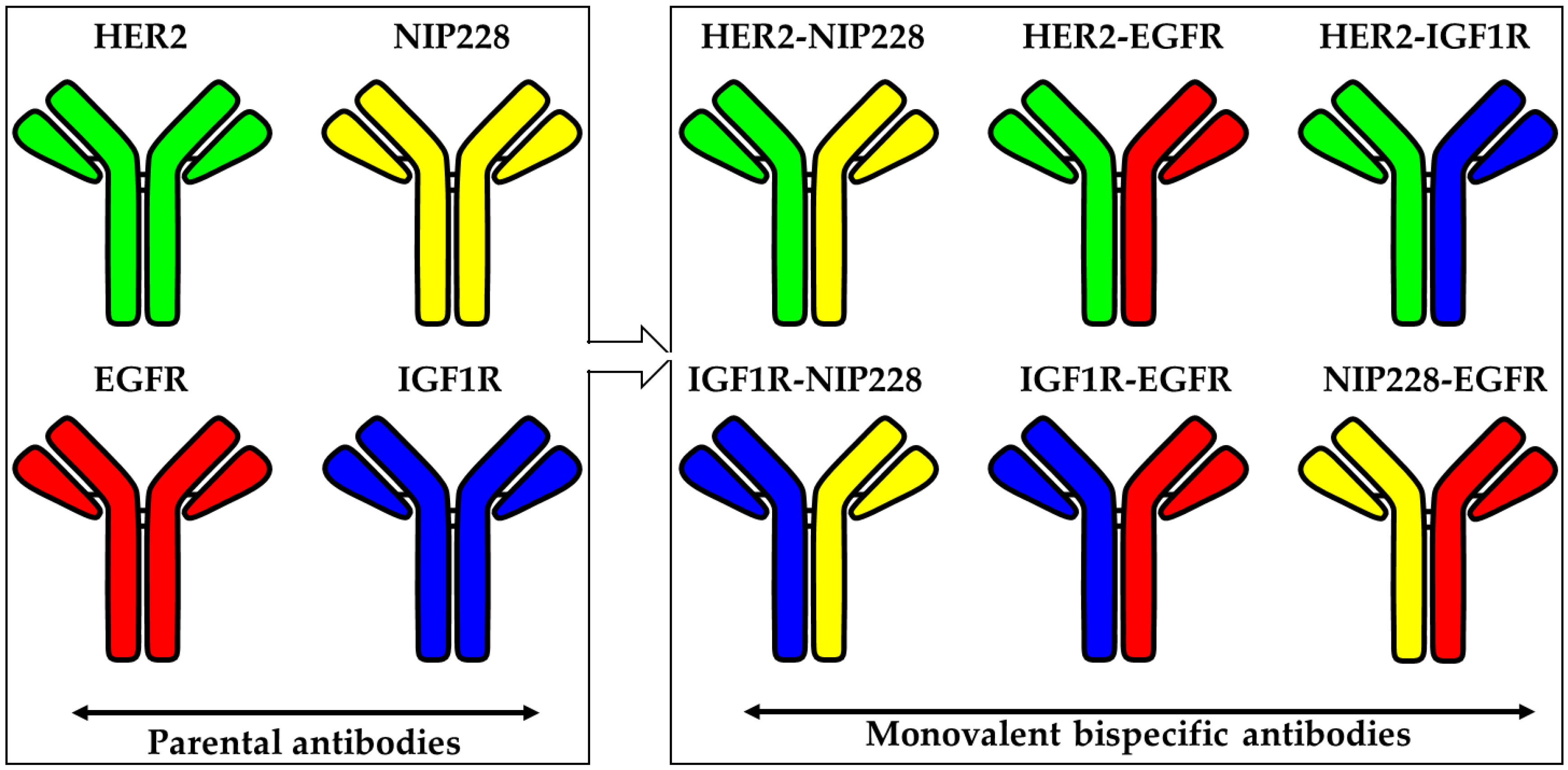
2.3. Generation of the Bispecific Antibodies (bsAb)
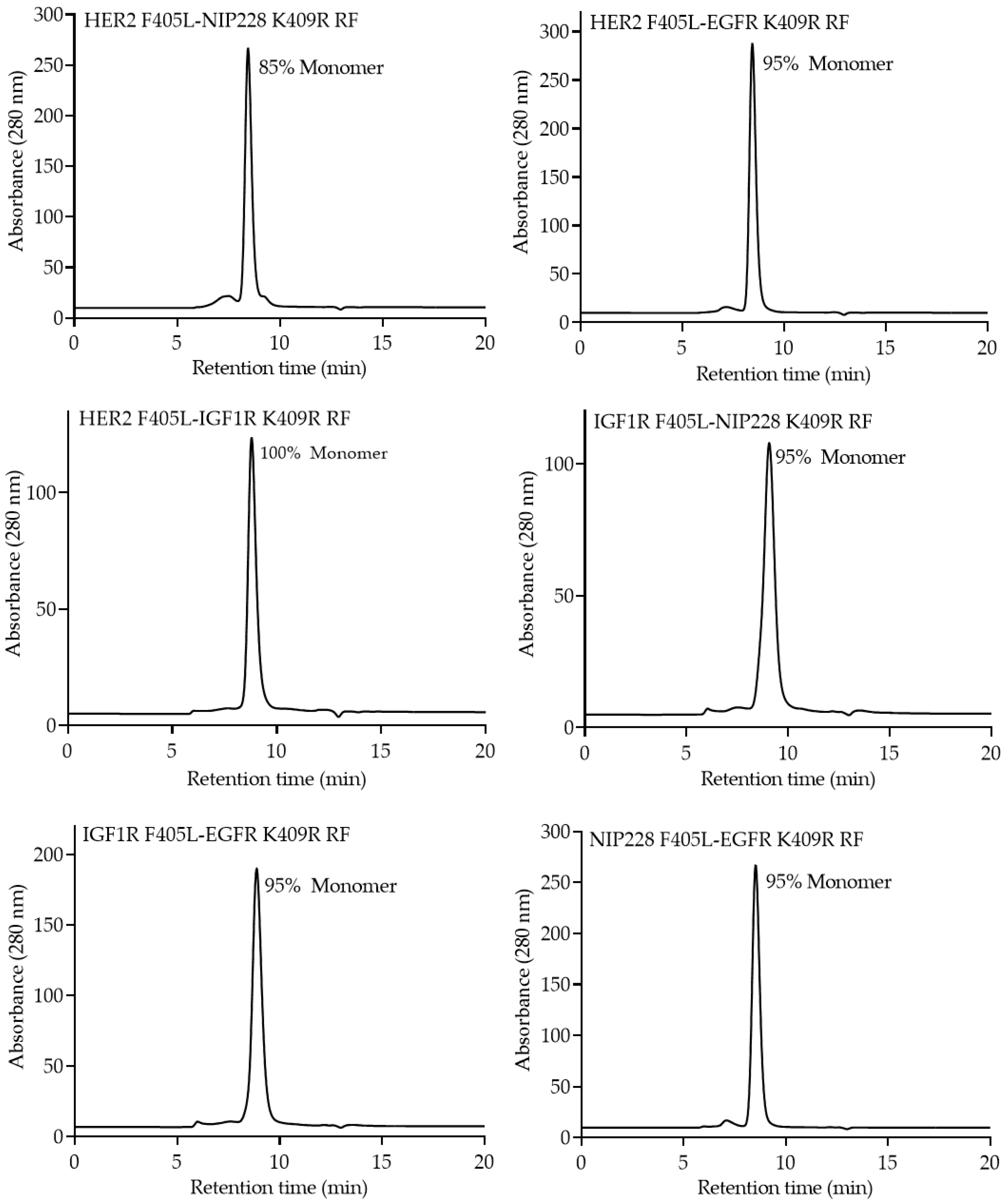
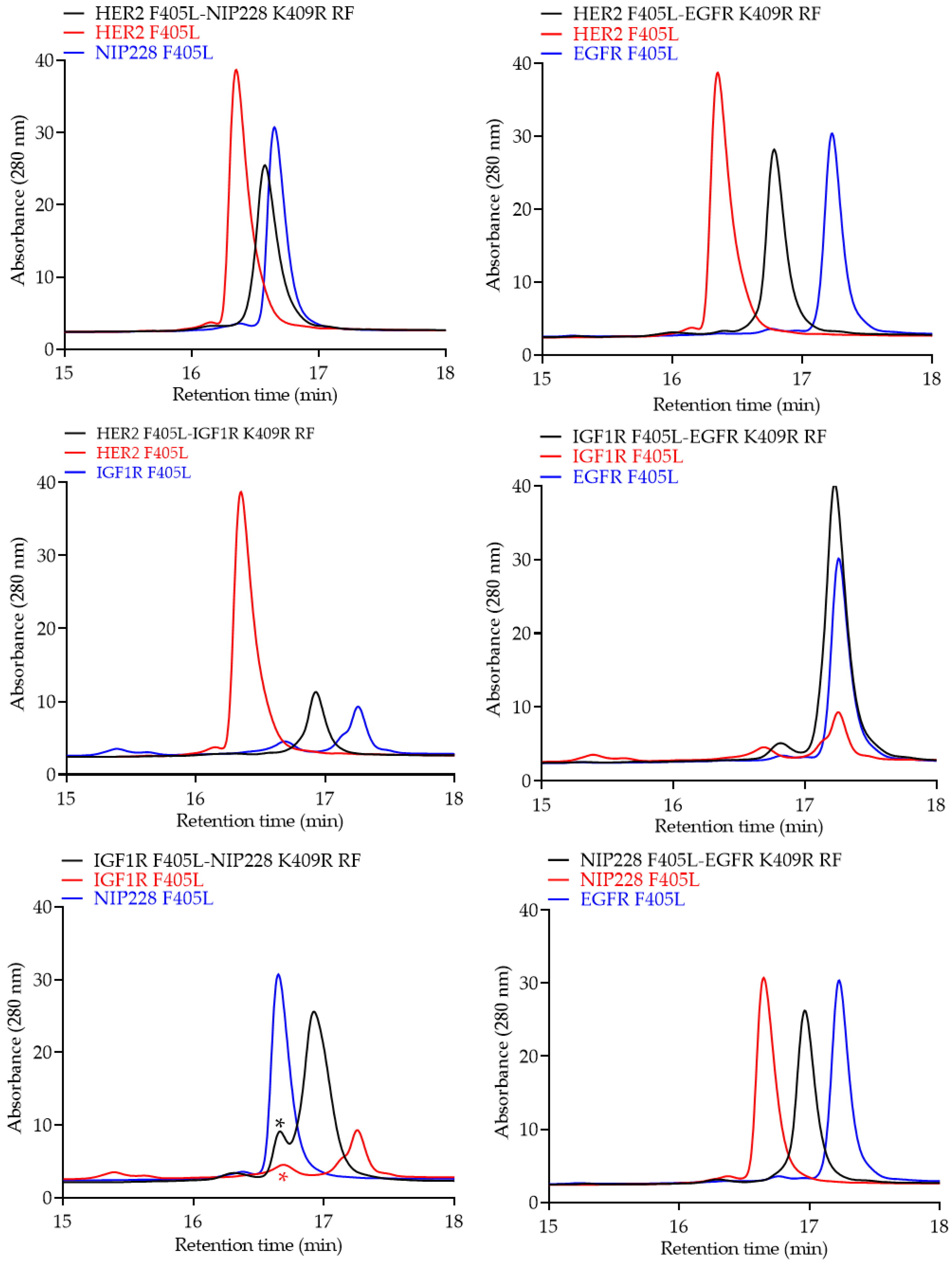
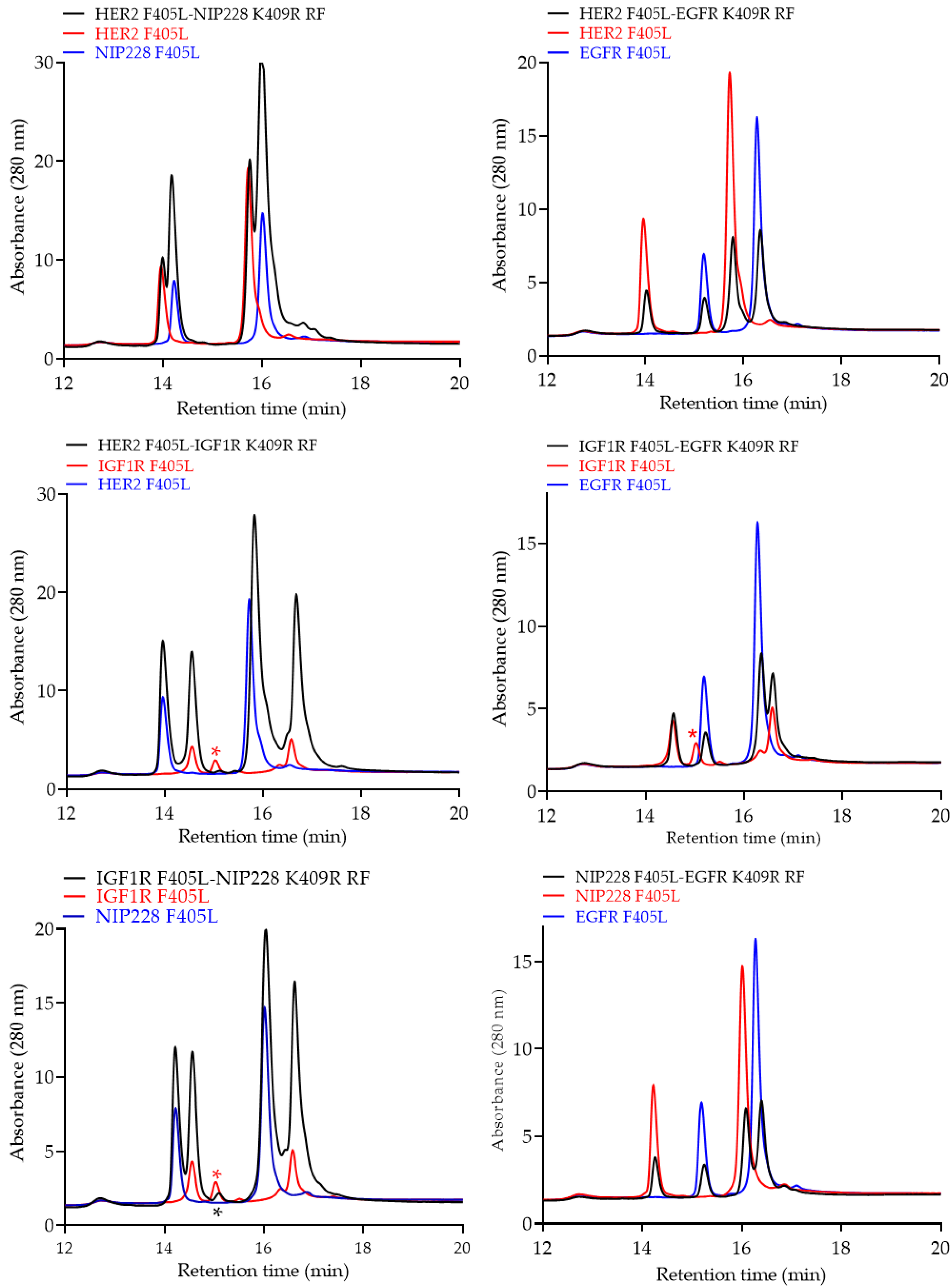
2.4. Analytical Characterization of the bsAb
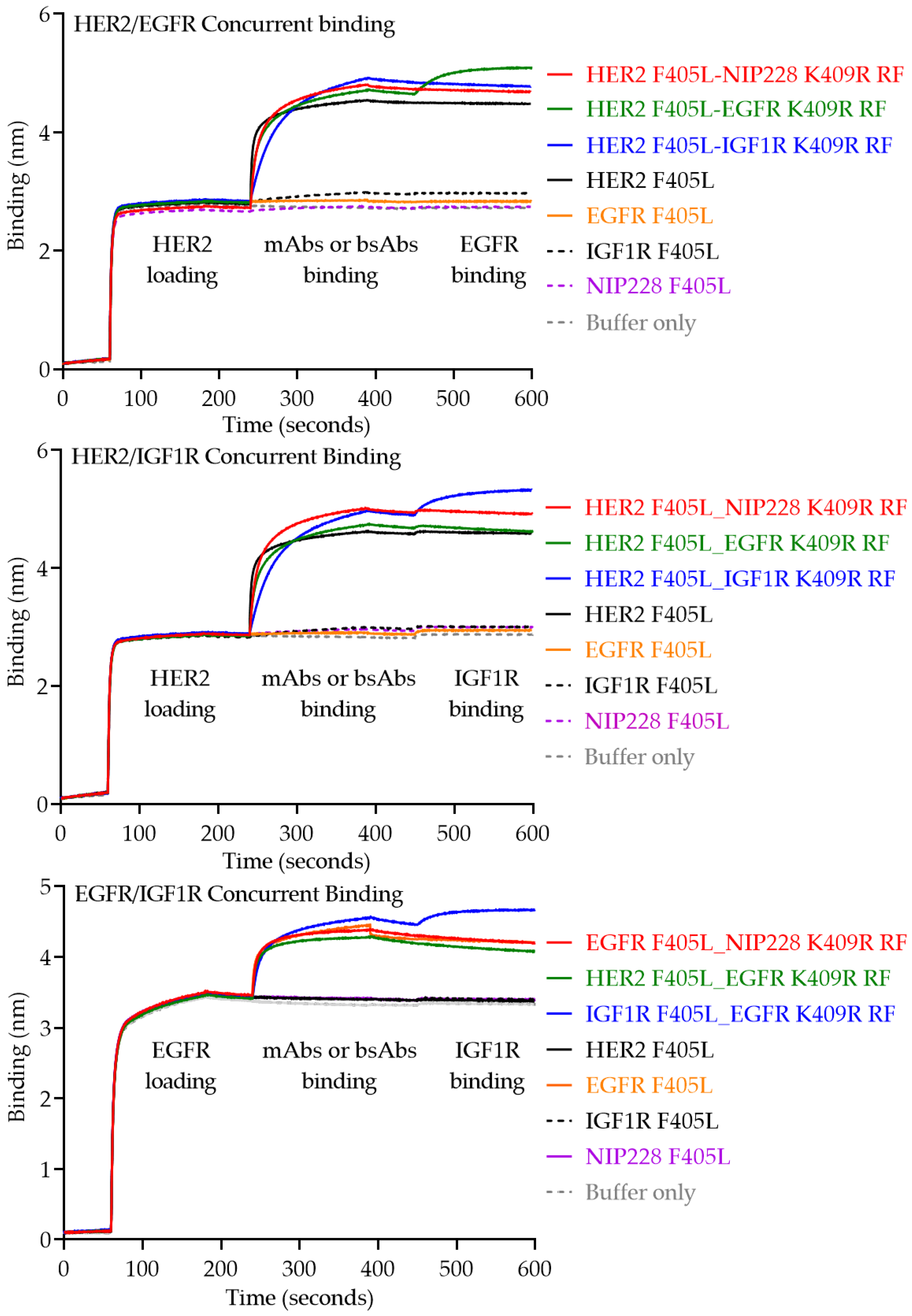
2.5. Cuncurrent Binding of the bsAb
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kohler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Steinitz, M.; Klein, G.; Koskimies, S.; Makel, O. EB virus-induced B lymphocyte cell lines producing specific antibody. Nature 1977, 269, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, K.; Ponten, J. Classification and biological nature of established human hematopoietic cell lines. Int. J. Cancer 1975, 15, 321–341. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.P. Filamentous fusion phage: Novel expression vectors that display cloned antigens on the virion surface. Science 1985, 228, 1315–1317. [Google Scholar] [CrossRef]
- Tiller, T.; Meffre, E.; Yurasov, S.; Tsuiji, M.; Nussenzweig, M.C.; Wardemann, H. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J. Immunol. Methods 2008, 329, 112–124. [Google Scholar] [CrossRef]
- Kung, P.; Goldstein, G.; Reinherz, E.L.; Schlossman, S.F. Monoclonal antibodies defining distinctive human T cell surface antigens. Science 1979, 206, 347–349. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, N.K.; Dwiwedi, P.; Charan, J.; Kaur, R.; Sidhu, P.; Chugh, V.K. Monoclonal antibodies: A review. Curr. Clin. Pharmacol. 2018, 13, 85–99. [Google Scholar] [CrossRef]
- Kaplon, H.; Reichert, J.M. Antibodies to watch in 2019. MAbs 2019, 11, 219–238. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Y.; Park, J.; Liu, X.; Hu, Y.; Wang, T.; McFarland, K.; Betenbaugh, M.J. Design and production of bispecific antibodies. Antibodies 2019, 8, 43. [Google Scholar] [CrossRef]
- Trabolsi, A.; Arumov, A.; Schatz, J.H. T cell-activating bispecific antibodies in cancer therapy. J. Immunol. 2019, 203, 585–592. [Google Scholar] [CrossRef]
- Krishnamurthy, A.; Jimeno, A. Bispecific antibodies for cancer therapy: A review. Pharmacol. Ther. 2018, 185, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Dibo, M.; Battocchio, E.C.; Dos Santos Souza, L.M.; Da Silva, M.D.V.; Banin-Hirata, B.K.; Sapla, M.M.M.; Marinello, P.; Rocha, S.P.D.; Faccin-Galhardi, L.C. Antibody therapy for the control of viral diseases: An update. Curr. Pharm. Biotechnol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Nyakatura, E.K.; Soare, A.Y.; Lai, J.R. Bispecific antibodies for viral immunotherapy. Hum. Vaccines Immunother. 2017, 13, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.S.; Ambrosino, D.M. History of passive antibody administration for prevention and treatment of infectious diseases. Curr. Opin. HIV AIDS 2015, 10, 129–134. [Google Scholar] [CrossRef]
- Kitazawa, T.; Igawa, T.; Sampei, Z.; Muto, A.; Kojima, T.; Soeda, T.; Yoshihashi, K.; Okuyama-Nishida, Y.; Saito, H.; Tsunoda, H.; et al. A bispecific antibody to factors IXa and X restores factor VIII hemostatic activity in a hemophilia A model. Nat. Med. 2012, 18, 1570–1574. [Google Scholar] [CrossRef]
- NPS MedicineWise. Emicizumab for haemophilia A. Aust. Prescr. 2019, 42, 42. [Google Scholar] [CrossRef]
- Kantarjian, H.; Stein, A.; Gokbuget, N.; Fielding, A.K.; Schuh, A.C.; Ribera, J.M.; Wei, A.; Dombret, H.; Foa, R.; Bassan, R.; et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N. Engl. J. Med. 2017, 376, 836–847. [Google Scholar] [CrossRef]
- Milstein, C.; Cuello, A.C. Hybrid hybridomas and the production of bi-specific monoclonal antibodies. Immunol. Today 1984, 5, 299–304. [Google Scholar] [CrossRef]
- Milstein, C.; Cuello, A.C. Hybrid hybridomas and their use in immunohistochemistry. Nature 1983, 305, 537–540. [Google Scholar] [CrossRef]
- Nisonoff, A.; Rivers, M.M. Recombination of a mixture of univalent antibody fragments of different specificity. Arch. Biochem. Biophys. 1961, 93, 460–462. [Google Scholar] [CrossRef]
- Lamoyi, E.; Nisonoff, A. Preparation of F(ab’)2 fragments from mouse IgG of various subclasses. J. Immunol. Methods 1983, 56, 235–243. [Google Scholar] [CrossRef]
- Glennie, M.J.; McBride, H.M.; Worth, A.T.; Stevenson, G.T. Preparation and performance of bispecific F(ab’ gamma)2 antibody containing thioether-linked Fab’ gamma fragments. J. Immunol. 1987, 139, 2367–2375. [Google Scholar] [PubMed]
- Brinkmann, U.; Kontermann, R.E. The making of bispecific antibodies. MAbs 2017, 9, 182–212. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.J.; Lee, J.A.; Wake, M.S.; Batt, K.V.; Wattam, T.A.; Hiles, I.D.; Batuwangala, T.D.; Ashman, C.I.; Steward, M. ‘In-Format’ screening of a novel bispecific antibody format reveals significant potency improvements relative to unformatted molecules. MAbs 2017, 9, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, A.; Umetsu, M.; Nakazawa, H.; Niide, T.; Onodera, T.; Hosokawa, K.; Hattori, S.; Asano, R.; Kumagai, I. A semi high-throughput method for screening small bispecific antibodies with high cytotoxicity. Sci. Rep. 2017, 7, 2862. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, A.; Umetsu, M.; Nakazawa, H.; Niide, T.; Asano, R.; Hattori, T.; Kumagai, I. High-throughput cytotoxicity and antigen-binding assay for screening small bispecific antibodies without purification. J. Biosci. Bioeng. 2018, 126, 153–161. [Google Scholar] [CrossRef]
- Dimasi, N.; Kumar, A.; Gao, C. Generation of bispecific antibodies using chemical conjugation methods. Drug Discov. Today Technol. 2020, in press. [Google Scholar]
- Ridgway, J.B.; Presta, L.G.; Carter, P. ‘Knobs-into-holes’ engineering of antibody CH3 domains for heavy chain heterodimerization. Protein Eng. 1996, 9, 617–621. [Google Scholar] [CrossRef]
- Klein, C.; Schaefer, W.; Regula, J.T. The use of CrossMAb technology for the generation of bi-and multispecific antibodies. MAbs 2016, 8, 1010–1020. [Google Scholar] [CrossRef]
- Krah, S.; Sellmann, C.; Rhiel, L.; Schröter, C.; Dickgiesser, S.; Beck, J.; Zielonka, S.; Toleikis, L.; Hock, B.; Kolmar, H.; et al. Engineering bispecific antibodies with defined chain pairing. New Biotechnol. 2017, 39 Pt B, 167–173. [Google Scholar] [CrossRef]
- Kim, H.S.; Dunshee, D.R.; Yee, A.; Tong, R.K.; Kim, I.; Farahi, F.; Hongo, J.A.; Ernst, J.A.; Sonoda, J.; Spiess, C. Tethered-variable CL bispecific IgG: An antibody platform for rapid bispecific antibody screening. Protein Eng. Des. Sel. PEDS 2017, 30, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Dimasi, N.; Fleming, R.; Sachsenmeier, K.F.; Bezabeh, B.; Hay, C.; Wu, J.; Sult, E.; Rajan, S.; Zhuang, L.; Cariuk, P.; et al. Guiding bispecific monovalent antibody formation through proteolysis of IgG1 single-chain. MAbs 2017, 9, 438–454. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Labrijn, A.F.; Meesters, J.I.; De Goeij, B.E.; Van Den Bremer, E.T.; Neijssen, J.; Van Kampen, M.D.; Strumane, K.; Verploegen, S.; Kundu, A.; Gramer, M.J.; et al. Efficient generation of stable bispecific IgG1 by controlled Fab-arm exchange. Proc. Natl. Acad. Sci. USA 2013, 110, 5145–5150. [Google Scholar] [CrossRef] [PubMed]
- Tustian, A.D.; Endicott, C.; Adams, B.; Mattila, J.; Bak, H. Development of purification processes for fully human bispecific antibodies based upon modification of protein A binding avidity. MAbs 2016, 8, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Dimasi, N.; Gao, C.; Fleming, R.; Woods, R.M.; Yao, X.T.; Shirinian, L.; Kiener, P.A.; Wu, H. The design and characterization of oligospecific antibodies for simultaneous targeting of multiple disease mediators. J. Mol. Biol. 2009, 393, 672–692. [Google Scholar] [CrossRef]
- Daramola, O.; Stevenson, J.; Dean, G.; Hatton, D.; Pettman, G.; Holmes, W.; Field, R. A high-yielding CHO transient system: Coexpression of genes encoding EBNA-1 and GS enhances transient protein expression. Biotechnol. Prog. 2014, 30, 132–141. [Google Scholar] [CrossRef]
- Dimasi, N.; Fleming, R.; Wu, H.; Gao, C. Molecular engineering strategies and methods for the expression and purification of IgG1-based bispecific bivalent antibodies. Methods 2019, 154, 77–86. [Google Scholar] [CrossRef]
- Collin, M.; Olsen, A. EndoS, a novel secreted protein from Streptococcus pyogenes with endoglycosidase activity on human IgG. EMBO J. 2001, 20, 3046–3055. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steinhardt, J.; Wu, Y.; Fleming, R.; Ruddle, B.T.; Patel, P.; Wu, H.; Gao, C.; Dimasi, N. Fab-Arm Exchange Combined with Selective Protein A Purification Results in a Platform for Rapid Preparation of Monovalent Bispecific Antibodies Directly from Culture Media. Pharmaceutics 2020, 12, 3. https://doi.org/10.3390/pharmaceutics12010003
Steinhardt J, Wu Y, Fleming R, Ruddle BT, Patel P, Wu H, Gao C, Dimasi N. Fab-Arm Exchange Combined with Selective Protein A Purification Results in a Platform for Rapid Preparation of Monovalent Bispecific Antibodies Directly from Culture Media. Pharmaceutics. 2020; 12(1):3. https://doi.org/10.3390/pharmaceutics12010003
Chicago/Turabian StyleSteinhardt, James, Yanli Wu, Ryan Fleming, Ben T. Ruddle, Pooja Patel, Herren Wu, Changshou Gao, and Nazzareno Dimasi. 2020. "Fab-Arm Exchange Combined with Selective Protein A Purification Results in a Platform for Rapid Preparation of Monovalent Bispecific Antibodies Directly from Culture Media" Pharmaceutics 12, no. 1: 3. https://doi.org/10.3390/pharmaceutics12010003
APA StyleSteinhardt, J., Wu, Y., Fleming, R., Ruddle, B. T., Patel, P., Wu, H., Gao, C., & Dimasi, N. (2020). Fab-Arm Exchange Combined with Selective Protein A Purification Results in a Platform for Rapid Preparation of Monovalent Bispecific Antibodies Directly from Culture Media. Pharmaceutics, 12(1), 3. https://doi.org/10.3390/pharmaceutics12010003