The Interactions between Ciprofloxacin and Parenteral Nutrition Admixtures
Abstract
1. Introduction
2. Materials and Methods
2.1. Composition of PN Admixtures
2.2. Preparation of PN Admixtures and CF–PN Samples
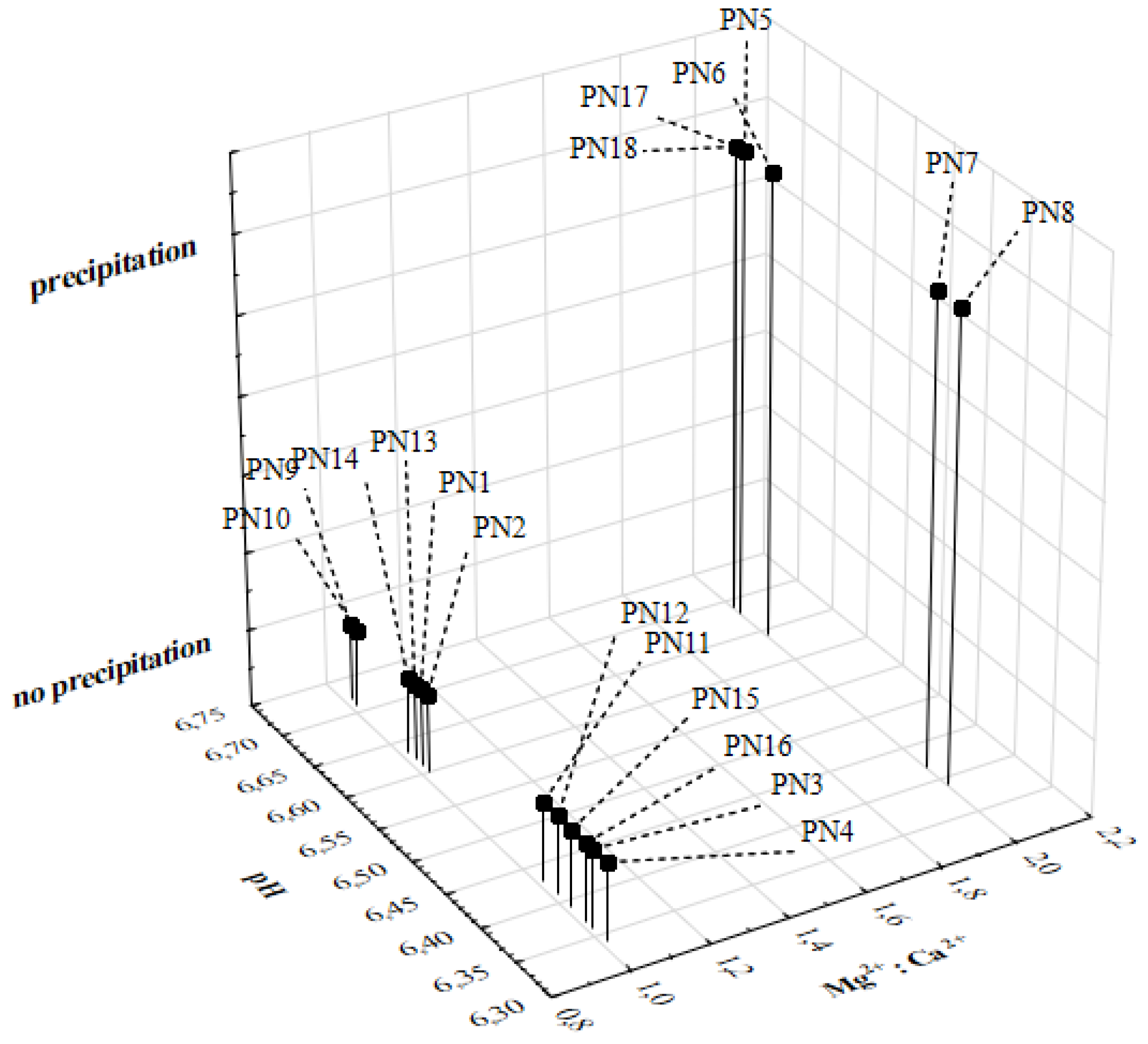
2.3. Visual Inspection
2.4. pH Evaluation
2.5. Determination of MDD of Lipid Emulsion and Zeta Potential
2.6. Osmolality Determination
2.7. Fourier Transform Infrared Spectroscopy (FT-IR)
2.8. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vincent, J.L.; Rello, J.; Marshall, J.; Silva, E.; Anzueto, A.; Martin, C.D.; Moreno, R.; Lipman, J.; Gomersall, C.; Sakr, Y.; et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009, 302, 2323–2329. [Google Scholar] [CrossRef] [PubMed]
- Van Zanten, A.R.; Polderman, K.H.; van Geijlswijk, I.M.; van der Meer, G.Y.; Schouten, M.A.; Girbes, A.R. Ciprofloxacin pharmacokinetics in critically ill patients: A prospective cohort study. J. Crit. Care 2008, 23, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Taxis, K.; Barber, N. Ethnographic study of incidence and severity of intravenous drug errors. BMJ 2003, 326, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Taxis, K.; Barber, N. Incidence and severity of intravenous drug errors in a German hospital. Eur. J. Clin. Pharmacol. 2004, 59, 815–817. [Google Scholar] [PubMed]
- Taxis, K.; Barber, N. Causes of intravenous medication errors: An ethnographic study. Qual. Saf. Health Care 2003, 12, 343–348. [Google Scholar] [CrossRef]
- Allen, L.V.; Levinson, R.S.; Phisutsinthop, D. Compatibility of various admixtures with secondary additives at Y-injection sites of intravenous administration sets. Am. J. Hosp. Pharm. 1977, 34, 939–943. [Google Scholar] [CrossRef]
- Staven, V.; Iqbal, H.; Wang, S.; Grønlie, I.; Tho, I. Physical compatibility of total parenteral nutrition and drugs in Y-site administration to children from neonates to adolescents. J. Pharm. Pharmacol. 2017, 69, 448–462. [Google Scholar] [CrossRef]
- Watson, D. Piggyback compatibility of antibiotics with pediatric parenteral nutrition solutions. J. Parenter. Enter. 1985, 9, 220–224. [Google Scholar] [CrossRef]
- Stawny, M.; Olijarczyk, R.; Jaroszkiewicz, E.; Jelińska, A. Pharmaceutical point of view on parenteral nutrition. Sci. World J. 2013, 2013, 415310. [Google Scholar] [CrossRef]
- Slattery, E.; Rumore, M.M.; Douglas, J.S.; Seres, D.S. 3-in-1 vs 2-in-1 parenteral nutrition in adults: A review. Nutr. Clin. Pract. 2014, 29, 631–635. [Google Scholar] [CrossRef]
- Kanji, S.; Lam, J.; Johanson, C.; Singh, A.; Goddard, R.; Fairbairn, J.; Lloyd, T.; Monsour, D.; Kakal, J. Systematic review of physical and chemical compatibility of commonly used medications administered by continuous infusion in intensive care units. Crit. Care Med. 2010, 38, 1890–1898. [Google Scholar] [CrossRef] [PubMed]
- Newton, D.W. Drug incompatibility chemistry. Am. J. Health Syst. Pharm. 2009, 66, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.E.; Heldman, L.S.; Goo, E.D.; Whippo, P.E.; Perkinson, J.C. Fatal microvascular pulmonary emboli from precipitation of a total nutrient admixture solution. J. Parenter. Enter. Nutr. 1996, 20, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.S.; Wassel, R.T.; Lee, L.; Nambiar, S. Intravenous ceftriaxone and calcium in the neonate: Assessing the risk for cardiopulmonary adverse events. Pediatrics 2009, 123, 609–613. [Google Scholar] [CrossRef]
- Driscoll, D.F.; Ling, P.R.; Bistrian, B.R. Pathological consequences to reticuloendothelial system organs following infusion of unstable all-in-one mixtures in rats. Clin. Nutr. 2006, 25, 842–850. [Google Scholar] [CrossRef]
- Driscoll, D.F.; Nehne, J.; Peterss, H.; Klutsch, K.; Bistrian, B.R.; Niemann, W. Physical assessments of lipid injectable emulsions via microscopy: A comparison to methods proposed in united states pharmacopeia chapter 729. Int. J. Pharm. Compd. 2006, 10, 309–315. [Google Scholar]
- Driscoll, D.F.; Ling, P.R.; Silvestri, A.P.; Bistrian, B.R. Fine vs. coarse complete all-in-one admixture infusions over 96 hours in rats: Fat globule size and hepatic function. Clin. Nutr. 2008, 27, 889–894. [Google Scholar] [CrossRef]
- Trissel, L.A.; Gilbert, D.L.; Martinez, J.F.; Baker, M.B.; Walter, W.V.; Mirtallo, J.M. Compatibility of parenteral nutrient solutions with selected drugs during simulated Y-site administration. Am. J. Hosp. Pharm. 1997, 54, 1295–1300. [Google Scholar] [CrossRef]
- Trissel, L.A.; Gilbert, D.L.; Martinez, J.F.; Baker, M.B.; Walter, W.V.; Mirtallo, J.M. Compatibility of medications with 3-in-1 parenteral nutrition admixtures. J. Parenter. Enter. Nutr. 1999, 23, 67–74. [Google Scholar] [CrossRef]
- European Directorate for Quality in Medicines and Healthcare (EDQM). Chromatographic separation techniques. In European Pharmacopoeia 9.0; EDQM: Strasburg, France, 2017; Chapter 2.2.46; pp. 74–80. [Google Scholar]
- Stawny, M.; Gostyńska, A.; Dettlaff, K.; Jelińska, A.; Główka, E.; Ogrodowczyk, M. Effect of lipid emulsion on stability of ampicillin in total parenteral nutrition. Nutrients 2019, 11, 559. [Google Scholar] [CrossRef]
- The United States Pharmacopeia and National Formulary, 38th ed.; United States Pharmacopeial Convention: Rockville, MD, USA, 2015.
- Völgyi, G.; Ruiz, R.; Box, K.; Comer, J.; Bosch, E.; Takács-Novák, K. Potentiometric and spectrophotometric pKa determination of water-insoluble compounds: Validation study in a new cosolvent system. Anal. Chim. Acta 2007, 583, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Breda, S.A.; Jimenez-Kairuz, A.F.; Manzo, R.H.; Olivera, M.E. Solubility behavior and biopharmaceutical classification of novel high-solubility ciprofloxacin and norfloxacin pharmaceutical derivatives. Int. J. Pharm. 2009, 17, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Stojković, A.; Tajber, L.; Paluch, K.J.; Djurić, Z.; Parojčić, J. Corrigan OI. Biopharmaceutical characterization of ciprofloxacin-metallic ion interactions: Comparative study into the effect of aluminium, calcium, zinc and iron on drug solubility and dissolution. Acta Pharm. 2014, 64, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Eboka, C.J.; Okeri, H.A. Aqueous solubility of ciprofloxacin in the presence of metal cations. Trop. J. Pharm. Res. 2005, 4, 349–354. [Google Scholar] [CrossRef]
- Turel, I.; Leban, I.; Zupancic, M.; Bukovec, P.; Gruber, K. An adduct of magnesium sulfate with a member of the quinolone family (Ciprofloxacin). Acta Crystallogr. 1996, 52, 2443–2445. [Google Scholar] [CrossRef]
- Japanese Pharmacopoeia, 17th ed.; Pharmaceuticals and Medical Devices Agency: Tokyo, Japan, 2016.
- Omotani, S.; Aoe, M.; Esaki, S.; Nagai, K.; Hatsuda, Y.; Mukai, J.; Teramachi, H.; Myotoku, M. Compatibility of intravenous fat emulsion with antibiotics for secondary piggyback infusion. Ann. Nutr. Metab. 2018, 73, 227–233. [Google Scholar] [CrossRef] [PubMed]
| Ingredient/Parameter | Unit | PN1 | PN2 | PN3 | PN4 | PN5 | PN6 | PN7 | PN8 | PN9 | PN10 | PN11 | PN12 | PN13 | PN14 | PN15 | PN16 | PN17 | PN18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amino Acid | g | 27 | 27 | 63 | 63 | 27 | 27 | 63 | 63 | 27 | 27 | 63 | 63 | 27 | 27 | 63 | 63 | 27 | 27 |
| Glucose | 180 | 180 | 180 | 180 | 180 | 180 | 180 | 180 | 180 | 180 | 180 | 180 | 180 | 180 | 180 | 180 | 180 | 180 | |
| Lipid Emulsion | 9 a | 45 a | 9 a | 45 a | 9 a | 45 a | 9 a | 45 a | 9 a | 45 a | 9 a | 45 a | 9 b | 45 b | 9 b | 45 b | 45 b | 9 b | |
| Water | 893 | 713 | 549 | 369 | 928 | 748 | 583 | 403 | 966 | 789 | 611 | 434 | 894 | 714 | 549 | 369 | 748 | 928 | |
| Sodium | mmol | 117 | 117 | 117 | 117 | 117 | 117 | 117 | 117 | 72 | 66 | 82 | 77 | 117 | 117 | 117 | 117 | 117 | 117 |
| Potassium | 99 | 99 | 99 | 99 | 99 | 99 | 99 | 99 | 7 | 7 | 16 | 16 | 99 | 99 | 99 | 99 | 99 | 99 | |
| Magnesium | 7 | 7 | 7 | 7 | 2 | 2 | 2 | 2 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 2 | 2 | |
| Calcium | 7 | 7 | 7 | 7 | 1 | 1 | 1 | 1 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 1 | 1 | |
| Phosphate | 56 | 56 | 89 | 89 | 56 | 56 | 56 | 56 | 89 | 89 | 56 | 56 | 56 | 56 | 89 | 89 | 56 | 56 | |
| Total Energy | kcal | 909 | 1233 | 1053 | 1377 | 909 | 1233 | 2021 | 1697 | 2567 | 2248 | 2072 | 1753 | 900 | 1233 | 1233 | 1377 | 1233 | 909 |
| Theoretical Osmolarity | mOsm/L | 919 | 959 | 1093 | 1134 | 919 | 959 | 1094 | 1134 | 757 | 791 | 956 | 989 | 918 | 956 | 1093 | 1131 | 956 | 918 |
| Total Volume | mL | 1800 | 1800 | 1800 | 1800 | 1800 | 1800 | 1800 | 1800 | 1800 | 1800 | 1800 | 1800 | 1800 | 1800 | 1800 | 1800 | 1800 | 1800 |
| PN Admixture | pH ± SD | Osmolality ± SD (mOsm/kg H2O) | Zeta Potential ± SD (mV) | MDD ± SD (nm) | ||||
|---|---|---|---|---|---|---|---|---|
| 0 h | 4 h | 0 h | 4 h | 0 h | 4 h | 0 h | 4 h | |
| PN1 | 6.61 ± 0.01 | 6.60 ± 0.00 | 996 ± 5 | 997 ± 3 | −11.12 ± 1.09 | −11.53 ± 0.85 | 219.7 ± 1.9 | 215.7 ± 2.9 |
| PN2 | 6.60 ± 0.00 | 6.61 ± 0.01 | 1076 ± 4 | 1076 ± 5 | −11.40 ± 1.00 | −11.33 ± 0.85 | 220.0 ± 1.4 | 218.3 ± 1.5 |
| PN3 | 6.36 ± 0.00 | 6.37 ± 0.01 | 1217 ± 3 | 1217 ± 2 | −9.19 ± 0.43 | −9.89 ± 0.09 | 219.2 ± 1.1 | 218.7 ± 1.9 |
| PN4 | 6.34 ± 0.01 | 6.34 ± 0.01 | 1281 ± 3 | 1281 ± 5 | −8.38 ± 0.15 | −7.88 ± 0.58 | 220.6 ± 1.6 | 221.7 ± 0.9 |
| PN5 | 6.68 ± 0.00 | 6.69 ± 0.01 | 992 ± 3 | 992 ± 4 | −17.50 ± 1.05 | −14.73 ± 1.46 | 216.5 ± 2.4 | 217.0 ± 3.5 |
| PN6 | 6.64 ± 0.00 | 6.65 ± 0.01 | 1057 ± 6 | 1057 ± 7 | −16.37 ± 0.75 | −15.77 ± 0.32 | 219.4 ± 2.6 | 220.8 ± 2.1 |
| PN7 | 6.42 ± 0.01 | 6.40 ± 0.01 | 1172 ± 4 | 1172 ± 5 | −11.58 ± 2.92 | −10.30 ± 2.51 | 222.0 ± 1.5 | 223.9 ± 3.7 |
| PN8 | 6.39 ± 0.01 | 6.38 ± 0.01 | 1278 ± 3 | 1278 ± 4 | −13.87 ± 2.46 | −13.87 ± 1.85 | 224.7 ± 3.6 | 221.3 ± 2.5 |
| PN9 | 6.72 ± 0.01 | 6.73 ± 0.01 | 848 ± 1 | 848 ± 3 | −13.00 ± 0.10 | −13.50 ± 1.13 | 221.0 ± 1.0 | 216.8 ± 2.0 |
| PN10 | 6.71 ± 0.01 | 6.70 ± 0.00 | 900 ± 5 | 899 ± 5 | −12.87 ± 1.90 | −14.67 ± 0.67 | 223.4 ± 0.4 | 221.9 ± 0.9 |
| PN11 | 6.43 ± 0.01 | 6.42 ± 0.01 | 1042 ± 1 | 1042 ± 3 | −9.83 ± 0.99 | −7.42 ± 1.88 | 220.4 ± 1.4 | 219.3 ± 0.9 |
| PN12 | 6.41 ± 0.01 | 6.40 ± 0.00 | 1136 ± 7 | 1135 ± 5 | −10.00 ± 0.78 | −9.91 ± 0.25 | 221.8 ± 2.1 | 219.6 ± 0.8 |
| PN13 | 6.62 ± 0.01 | 6.61 ± 0.01 | 994 ± 3 | 993 ± 3 | −17.97 ± 0.81 | −19.17 ± 2.31 | 336.7 ± 3.6 | 334.5 ± 4.4 |
| PN14 | 6.63 ± 0.00 | 6.63 ± 0.00 | 1053 ± 3 | 1053 ± 2 | −18.37 ± 3.26 | −16.80 ± 1.15 | 335.0 ± 1.9 | 329.5 ± 4.4 |
| PN15 | 6.39 ± 0.01 | 6.39 ± 0.00 | 1197 ± 2 | 1196 ± 6 | −12.70 ± 0.60 | −13.10 ± 0.61 | 338.8 ± 3.4 | 334.8 ± 3.6 |
| PN16 | 6.37 ± 0.01 | 6.38 ± 0.01 | 1271 ± 3 | 1271 ± 2 | −11.27 ± 0.31 | −13.00 ± 0.89 | 330.9 ± 4.9 | 330.4 ± 3.1 |
| PN17 | 6.69 ± 0.01 | 6.69 ± 0.00 | 1047 ± 3 | 1048 ± 5 | −15.83 ± 1.10 | −18.33 ± 1.12 | 329.0 ± 2.8 | 326.1 ± 5.5 |
| PN18 | 6.69 ± 0.00 | 6.69 ± 0.01 | 979 ± 4 | 978 ± 6 | −22.47 ± 0.81 | −23.80 ± 1.31 | 328.6 ± 3.2 | 335.0 ± 6.4 |
| PN Admixture | CF:PN Volume Ratio | pH ± SD | Osmolality ± SD (mOsm/kg H2O) | Zeta Potential ± SD (mV) | MDD ± SD (nm) | ||||
|---|---|---|---|---|---|---|---|---|---|
| 0 h | 4 h | 0 h | 4 h | 0 h | 4 h | 0 h | 4 h | ||
| PN1 | 1:1 | 6.24 ± 0.01 | 6.24 ± 0.01 | 607 ± 3 | 601 ± 5 | −7.26 ± 0.47 | −8.22 ± 0.35 | 212.9 ± 2.8 | 212.7 ± 2.7 |
| PN1 | 2:1 | 6.04 ± 0.01 | 6.04 ± 0.00 | 494 ± 3 | 495 ± 3 | −5.72 ± 0.73 | −5.54 ± 0.23 | 218.7 ± 2.3 | 210.0 ± 3.3 * |
| PN2 | 1:1 | 6.22 ± 0.01 | 6.22 ± 0.00 | 633 ± 3 | 629 ± 3 | −6.49 ± 0.20 | −7.07 ± 0.61 | 216.4 ± 2.7 | 214.0 ± 1.4 |
| PN2 | 2:1 | 6.03 ± 0.01 | 6.03 ± 0.01 | 511 ± 2 | 510 ± 1 | −3.63 ± 0.51 | −4.43 ± 0.09 | 213.4 ± 0.8 | 212.4 ± 2.7 |
| PN3 | 1:1 | 6.14 ± 0.00 | 6.14 ± 0.00 | 701 ± 2 | 712 ± 3 | −5.87 ± 0.16 | −7.04 ± 0.42 | 215.0 ± 2.5 | 212.8 ± 1.7 |
| PN3 | 2:1 | 6.00 ± 0.01 | 6.00 ± 0.01 | 548 ± 7 | 545 ± 3 | −3.31 ± 0.27 | −3.41 ± 0.31 | 214.3 ± 2.5 | 212.4 ± 1.7 |
| PN4 | 1:1 | 6.09 ± 0.01 | 6.09 ± 0.01 | 724 ± 2 | 725 ± 1 | −4.86 ± 0.31 | −5.41 ± 0.51 | 214.5 ± 3.7 | 214.4 ± 2.0 |
| PN4 | 2:1 | 6.00 ± 0.01 | 5.93 ± 0.02 * | 554 ± 4 | 555 ± 3 | −2.50 ± 0.15 | −2.44 ± 0.54 | 215.3 ± 0.6 | 212.5 ± 2.2 |
| PN5 | 1:1 | 6.32 ± 0.01 | 6.31 ± 0.01 | 601 ± 1 | 608 ± 1 | −9.02 ± 0.80 | −10.26 ± 0.33 | 214.3 ± 0.9 | 211.0 ± 0.6 |
| PN5 | 2:1 | 6.15 ± 0.01 | 5.95 ± 0.01 * | 493 ± 6 | 492 ± 3 | −4.91 ± 0.60 | −7.27 ± 0.09 | 215.6 ± 2.8 | 209.7 ± 1.6 |
| PN6 | 1:1 | 6.29 ± 0.01 | 6.29 ± 0.01 | 634 ± 4 | 638 ± 5 | −8.46 ± 0.48 | −10.31 ± 0.55 | 214.6 ± 2.3 | 213.2 ± 3.0 |
| PN6 | 2:1 | 6.12 ± 0.01 | 5.98 ± 0.01 * | 511 ± 6 | 511 ± 3 | −4.72 ± 0.63 | −6.10 ± 0.19 | 215.2 ± 2.3 | 210.2 ± 0.8 |
| PN7 | 1:1 | 6.21 ± 0.01 | 6.12 ± 0.00 * | 689 ± 4 | 686 ± 1 | −8.91 ± 0.62 | −12.80 ± 0.56 * | 216.3 ± 2.2 | 214.4 ± 2.4 |
| PN7 | 2:1 | 6.08 ± 0.00 | 5.88 ± 0.01 * | 540 ± 2 | 547 ± 5 | −7.63 ± 0.23 | −10.77 ± 0.32 | 214.5 ± 2.1 | 212.4 ± 2.4 |
| PN8 | 1:1 | 6.17 ± 0.01 | 6.10 ± 0.00 * | 730 ± 5 | 715 ± 2 * | −7.93 ± 0.62 | −9.20 ± 0.41 | 218.6 ± 3.1 | 216.1 ± 4.3 |
| PN8 | 2:1 | 6.04 ± 0.02 | 5.87 ± 0.01 * | 558 ± 1 | 564 ± 1 | −5.26 ± 0.31 | −6.01 ± 0.23 | 216.6 ± 4.1 | 214.2 ± 4.0 |
| PN9 | 1:1 | 6.26 ± 0.01 | 6.28 ± 0.01 | 535 ± 5 | 531 ± 8 | −10.00 ± 0.36 | −12.37 ± 0.32 | 215.8 ± 2.9 | 211.7 ± 3.6 |
| PN9 | 2:1 | 6.06 ± 0.01 | 6.04 ± 0.01 | 441 ± 6 | 441 ± 5 | −6.66 ± 0.96 | −8.52 ± 0.91 | 214.5 ± 3.7 | 214.9 ± 2.7 |
| PN10 | 1:1 | 6.23 ± 0.01 | 6.24 ± 0.01 | 553 ± 1 | 555 ± 5 | −8.72 ± 0.23 | −8.77 ± 0.63 | 217.7 ± 4.6 | 214.2 ± 3.1 |
| PN10 | 2:1 | 6.05 ± 0.01 | 6.00 ± 0.02 * | 453 ± 5 | 455 ± 5 | −5.41 ± 0.67 | −5.68 ± 0.52 | 215.0 ± 4.4 | 214.7 ± 4.3 |
| PN11 | 1:1 | 6.06 ± 0.01 | 6.06 ± 0.01 | 634 ± 0 | 626 ± 3 | −7.33 ± 0.26 | −7.46 ± 0.72 | 220.6 ± 4.0 | 216.1 ± 1.6 |
| PN11 | 2:1 | 5.90 ± 0.00 | 5.90 ± 0.01 | 503 ± 7 | 502 ± 6 | −6.22 ± 0.43 | −5.82 ± 0.35 | 212.5 ± 2.2 | 211.9 ± 1.9 |
| PN12 | 1:1 | 6.13 ± 0.01 | 6.15 ± 0.02 | 654 ± 4 | 653 ± 5 | −7.13 ± 0.47 | −6.81 ± 0.19 | 218.4 ± 4.7 | 213.1 ± 3.1 |
| PN12 | 2:1 | 6.03 ± 0.01 | 5.88 ± 0.02 * | 521 ± 4 | 520 ± 3 | −4.79 ± 0.54 | −5.32 ± 0.14 | 214.5 ± 3.7 | 214.7 ± 3.3 |
| PN13 | 1:1 | 6.29 ± 0.01 | 6.25 ± 0.02 | 613 ± 4 | 610 ± 5 | −9.93 ± 0.70 | −15.80 ± 0.40 * | 323.8 ± 2.7 | 323.6 ± 3.6 |
| PN13 | 2:1 | 6.08 ± 0.01 | 6.04 ± 0.00 | 497 ± 7 | 497 ± 2 | −14.43 ± 0.49 | −14.13 ± 1.63 | 325.7 ± 7.0 | 330.0 ± 8.3 |
| PN14 | 1:1 | 6.26 ± 0.01 | 6.26 ± 0.01 | 628 ± 1 | 629 ± 4 | –12.97 ± 0.55 | −11.27 ± 1.21 | 326.5 ± 6.4 | 326.7 ± 5.2 |
| PN14 | 2:1 | 6.06 ± 0.01 | 6.05 ± 0.01 | 503 ± 1 | 506 ± 3 | –10.47 ± 1.14 | −11.57 ± 1.31 | 322.4 ± 7.0 | 317.9 ± 1.7 |
| PN15 | 1:1 | 6.13 ± 0.01 | 6.12 ± 0.00 | 681 ± 3 | 675 ± 3 | −11.47 ± 0.85 | −11.13 ± 0.76 | 323.9 ± 2.2 | 319.9 ± 5.2 |
| PN15 | 2:1 | 5.99 ± 0.01 | 5.99 ± 0.01 | 537 ± 3 | 533 ± 3 | −11.30 ± 0.30 | −9.10 ± 0.93 | 325.5 ± 5.0 | 320.5 ± 5.3 |
| PN16 | 1:1 | 6.13 ± 0.01 | 6.14 ± 0.00 | 723 ± 6 | 717 ± 2 | −10.45 ± 0.70 | −8.85 ± 0.91 | 323.4 ± 5.4 | 324.3 ± 1.1 |
| PN16 | 2:1 | 5.99 ± 0.01 | 6.00 ± 0.02 | 563 ± 4 | 559 ± 1 | −11.17 ± 0.75 | −6.75 ± 1.25 * | 321.8 ± 4.8 | 324.7 ± 3.5 |
| PN17 | 1:1 | 6.33 ± 0.02 | 6.27 ± 0.01 * | 624 ± 1 | 626 ± 5 | −16.27 ± 0.71 | −14.63 ± 0.55 | 329.0 ± 10.6 | 325.2 ± 4.5 |
| PN17 | 2:1 | 6.17 ± 0.02 | 5.89 ± 0.01 * | 501 ± 6 | 503 ± 3 | −15.27 ± 1.02 | −11.83 ± 0.75 | 328.4 ± 1.7 | 328.5 ± 4.3 |
| PN18 | 1:1 | 6.32 ± 0.02 | 6.22 ± 0.02 * | 602 ± 5 | 597 ± 8 | −26.30 ± 0.56 | −18.13 ± 0.40 * | 326.4 ± 4.1 | 332.7 ± 7.2 |
| PN18 | 2:1 | 6.14 ± 0.01 | 5.82 ± 0.02 * | 484 ± 1 | 484 ± 5 | −21.03 ± 2.06 | −12.53 ± 0.7 * | 325.8 ± 6.0 | 329.5 ± 8.3 |
| Precipitate | Reference Sample | Peak Assignment |
|---|---|---|
| Wavenumbers (cm−1) | ||
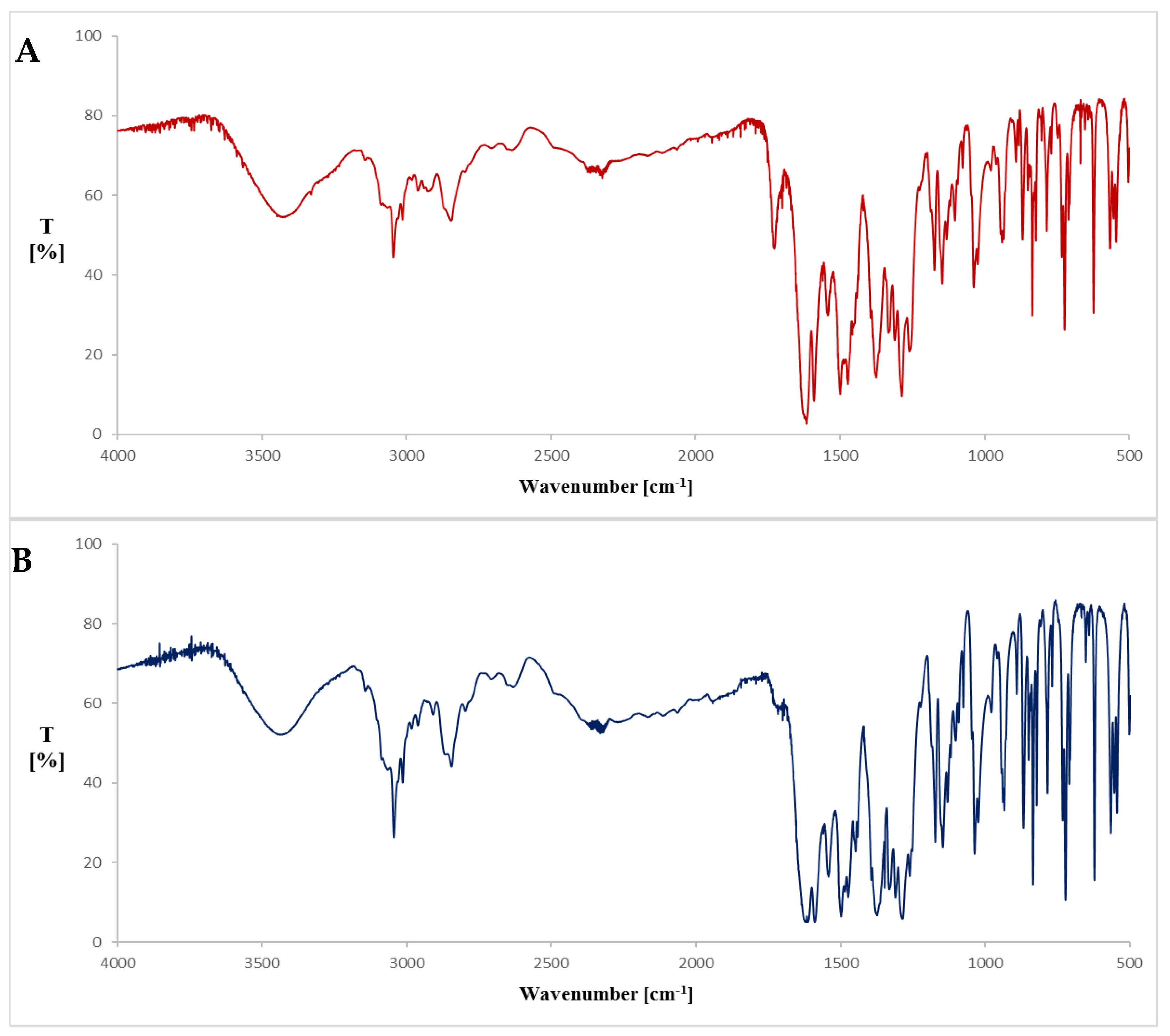
| 3436.21 | 3444.15 | –OH stretching vibration |
| 1621.67 | 1616.36 | C=O stretching vibration |
| 1587.91 | 1592.21 | C=C stretching vibration |
| 1284.60 | 1282.67 | C–N stretching vibration |
| 1036.26 | 1035.78 | C–F stretching vibration |
| 722.34 | 722.34 | –NH vibration |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gostyńska, A.; Stawny, M.; Dettlaff, K.; Jelińska, A. The Interactions between Ciprofloxacin and Parenteral Nutrition Admixtures. Pharmaceutics 2020, 12, 27. https://doi.org/10.3390/pharmaceutics12010027
Gostyńska A, Stawny M, Dettlaff K, Jelińska A. The Interactions between Ciprofloxacin and Parenteral Nutrition Admixtures. Pharmaceutics. 2020; 12(1):27. https://doi.org/10.3390/pharmaceutics12010027
Chicago/Turabian StyleGostyńska, Aleksandra, Maciej Stawny, Katarzyna Dettlaff, and Anna Jelińska. 2020. "The Interactions between Ciprofloxacin and Parenteral Nutrition Admixtures" Pharmaceutics 12, no. 1: 27. https://doi.org/10.3390/pharmaceutics12010027
APA StyleGostyńska, A., Stawny, M., Dettlaff, K., & Jelińska, A. (2020). The Interactions between Ciprofloxacin and Parenteral Nutrition Admixtures. Pharmaceutics, 12(1), 27. https://doi.org/10.3390/pharmaceutics12010027