Nanolipid-Trehalose Conjugates and Nano-Assemblies as Putative Autophagy Inducers
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis-General
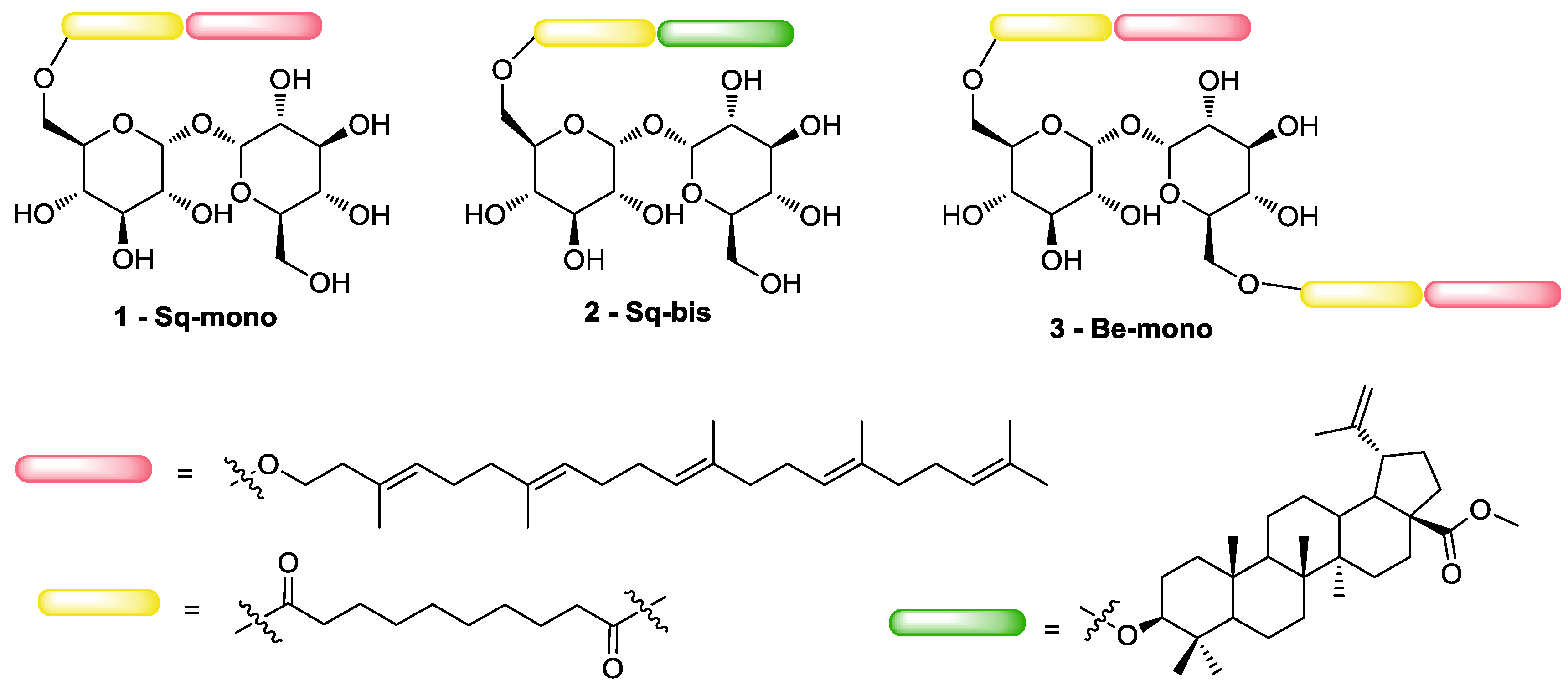
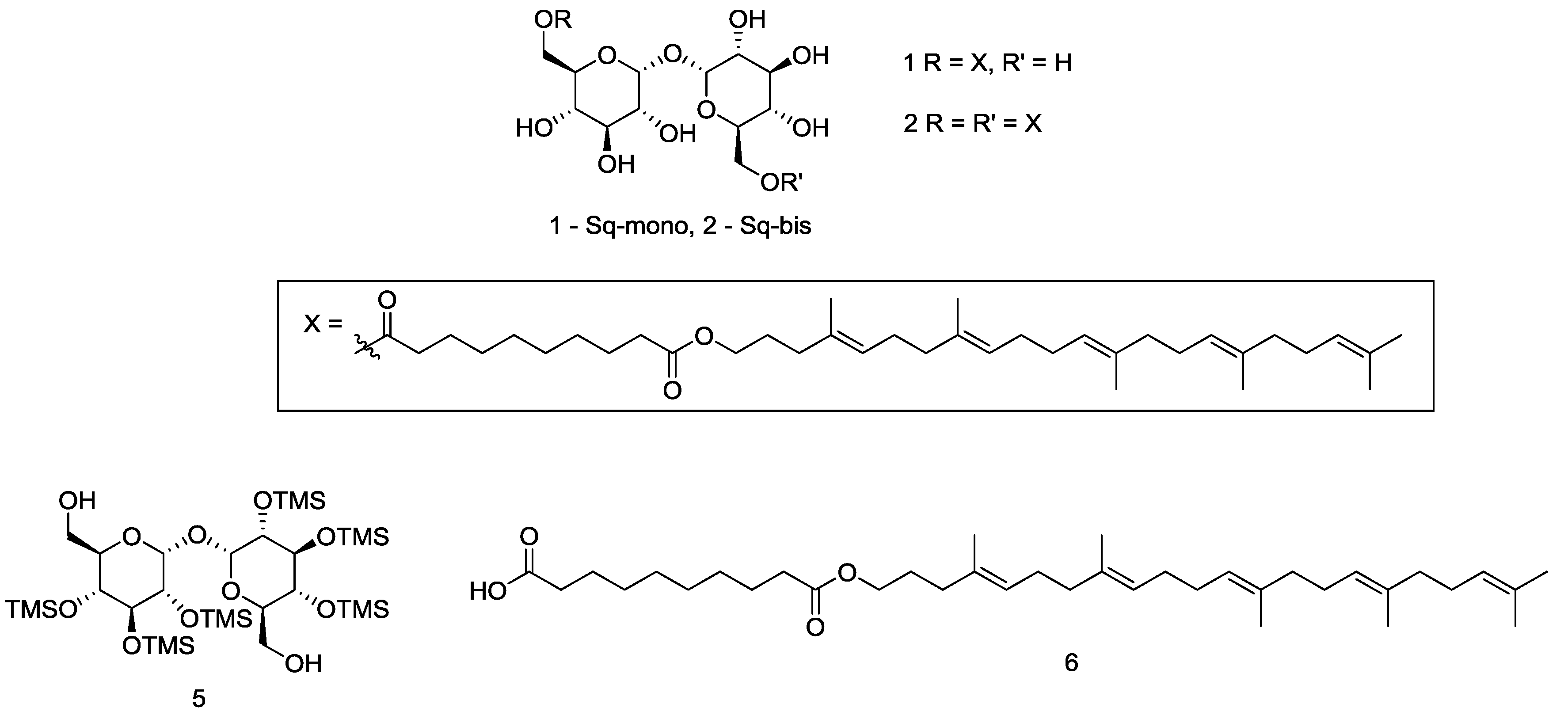
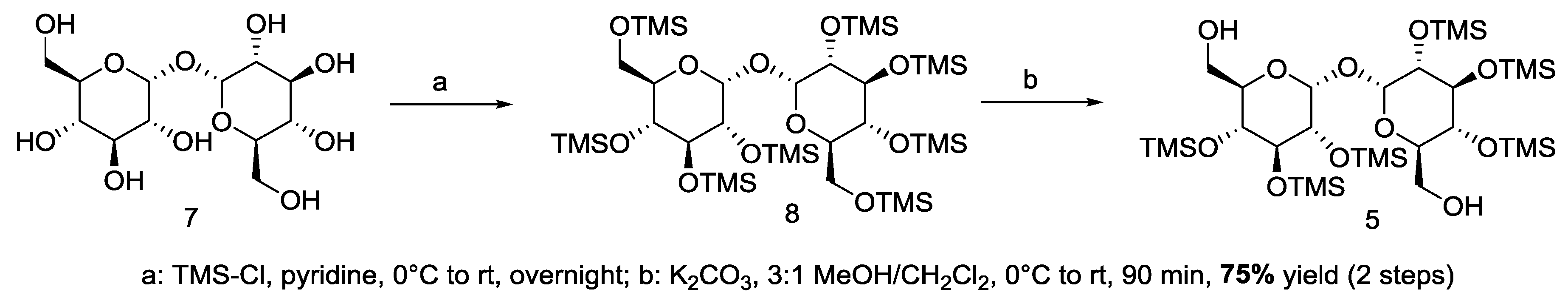
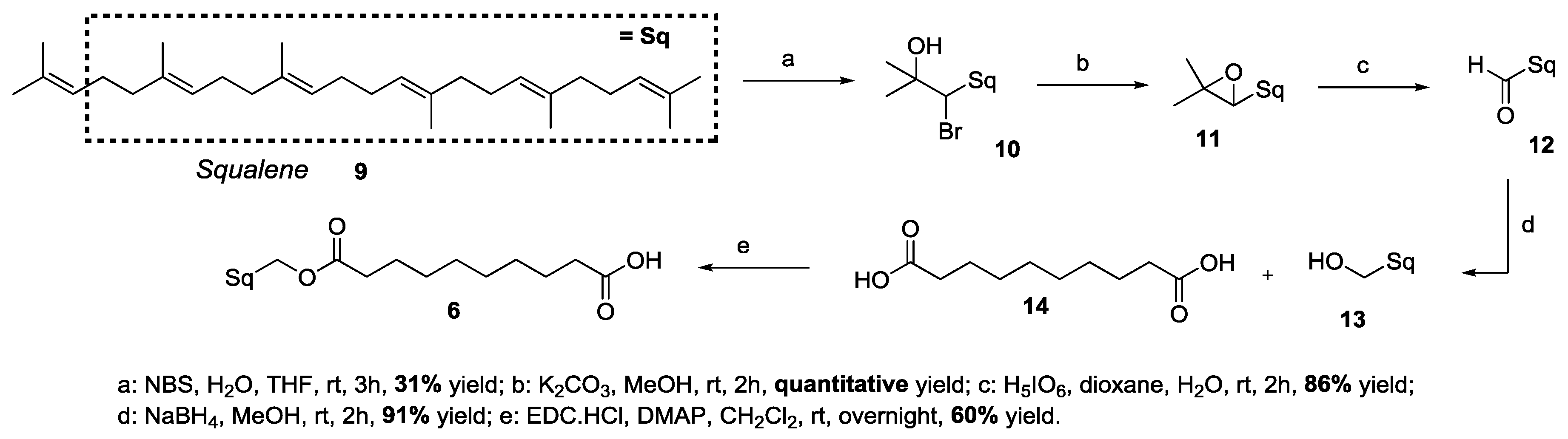
2.2. Synthesis-Squalene-Trehalose Conjugates 1-Sq-Mono and 2-Sq-Bis
2.3. Synthesis—Betulinic Acid-trehalose Conjugate 3–Be-mono
2.4. NA Assembly and Characterization
2.5. Biology
2.6. Statistical Analysis
3. Results
3.1. Synthesis of Target 1-mono and 2-bis Squalene-Trehalose Conjugates
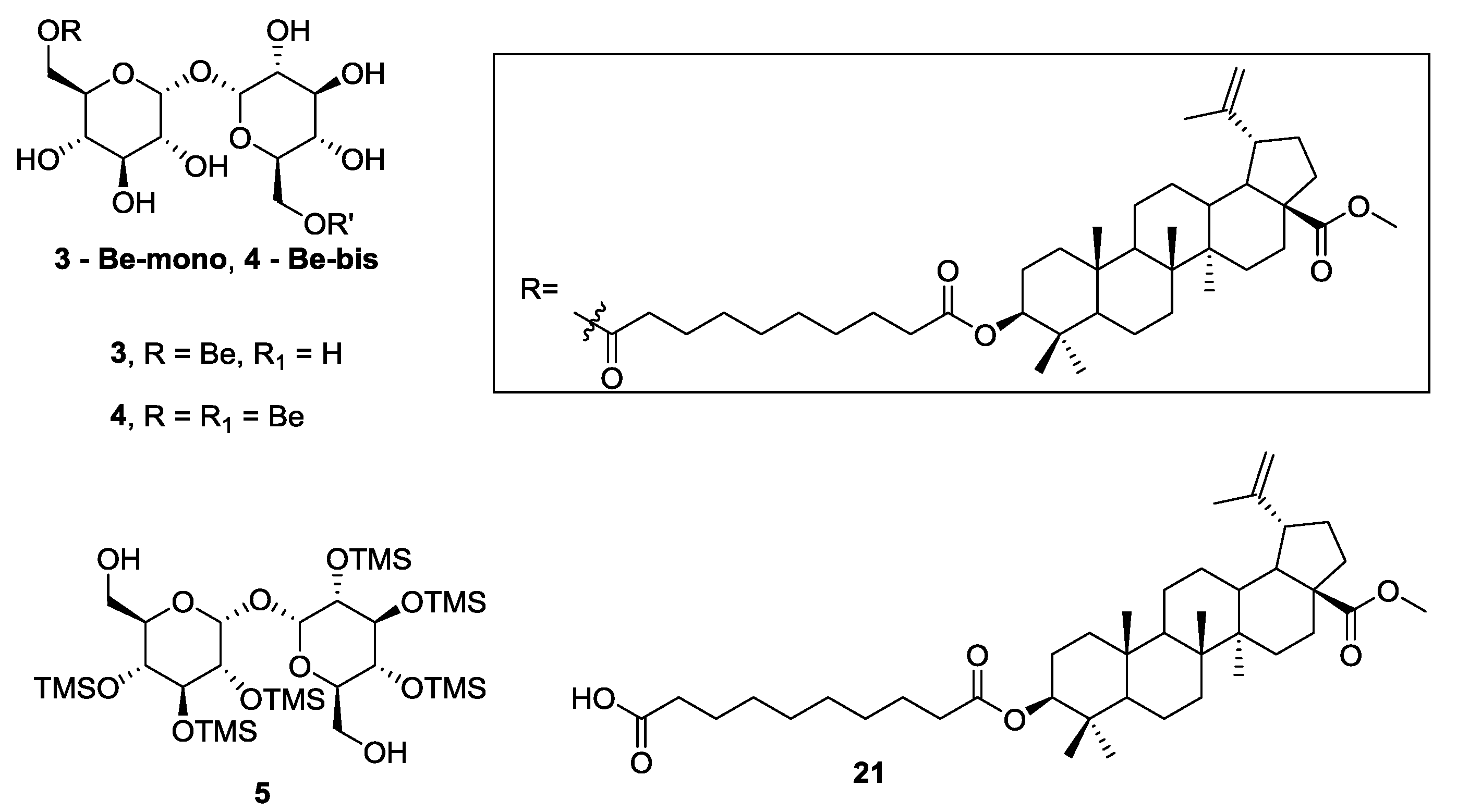
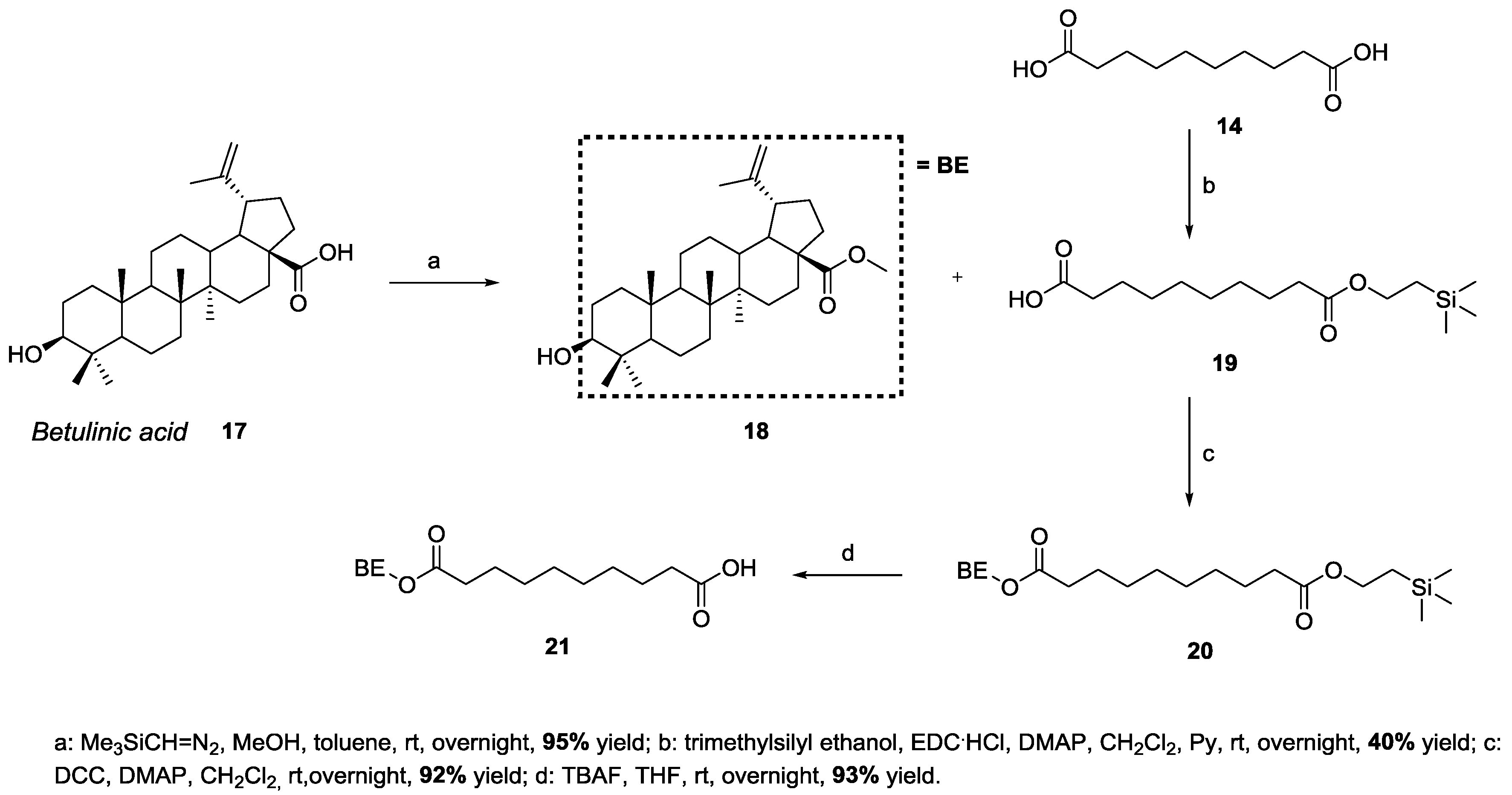
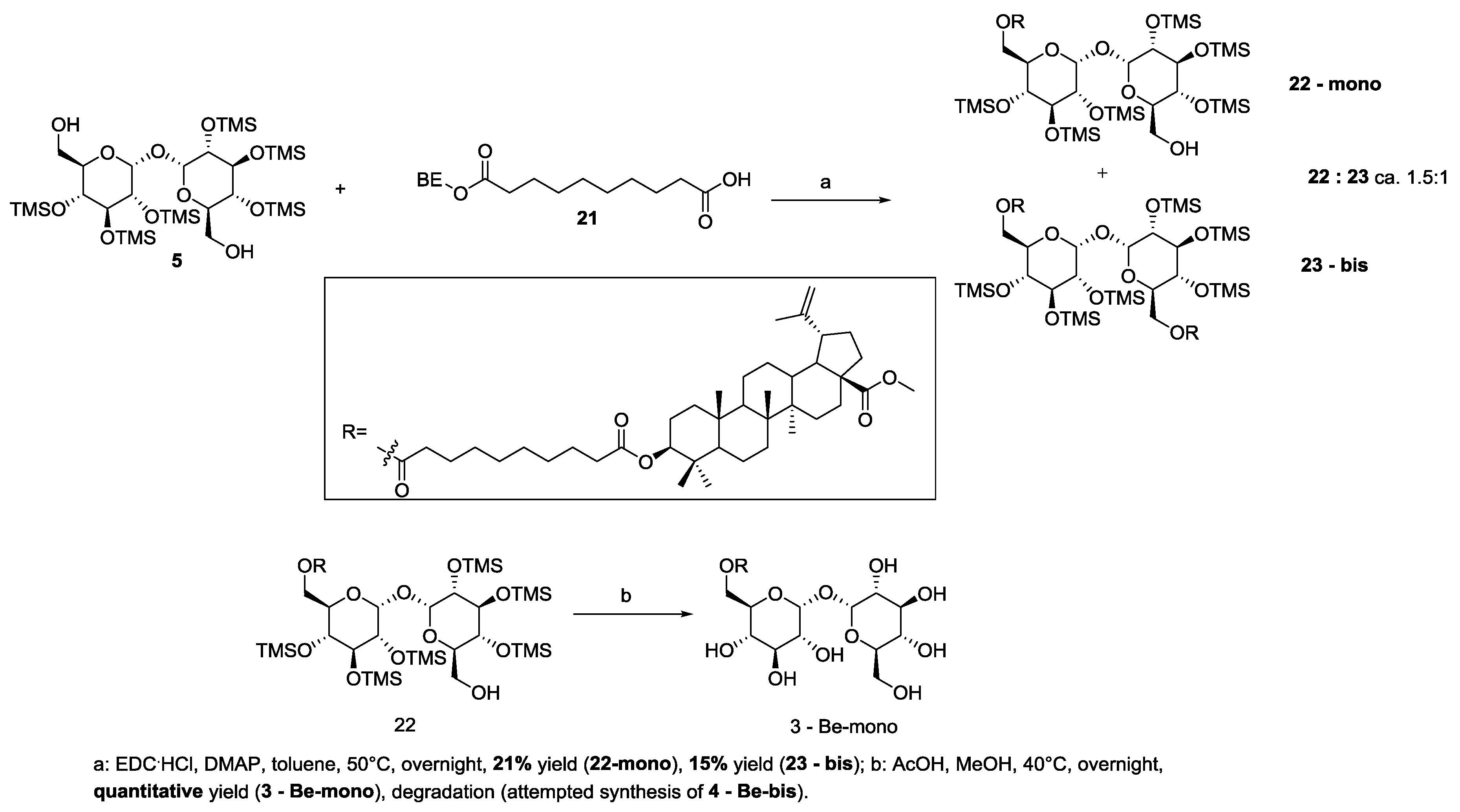
3.2. Synthesis of Target 3-Be-mono Betulinic Acid-Trehalose Conjugate, Attempted Synthesis of Target 4-Be bis Betulinic Acid-Trehalose Conjugate
3.3. NA Assembly and Structural Characterization
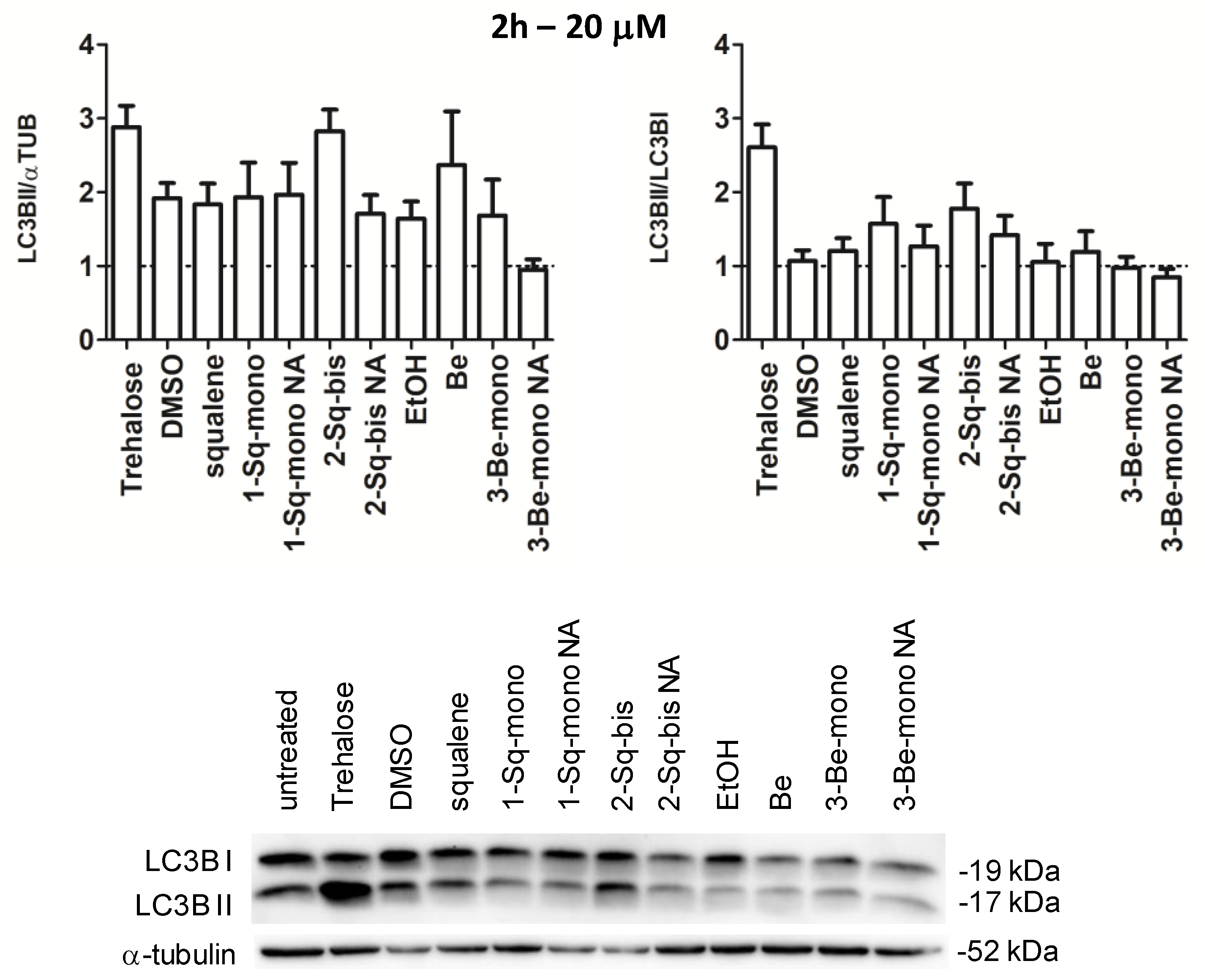
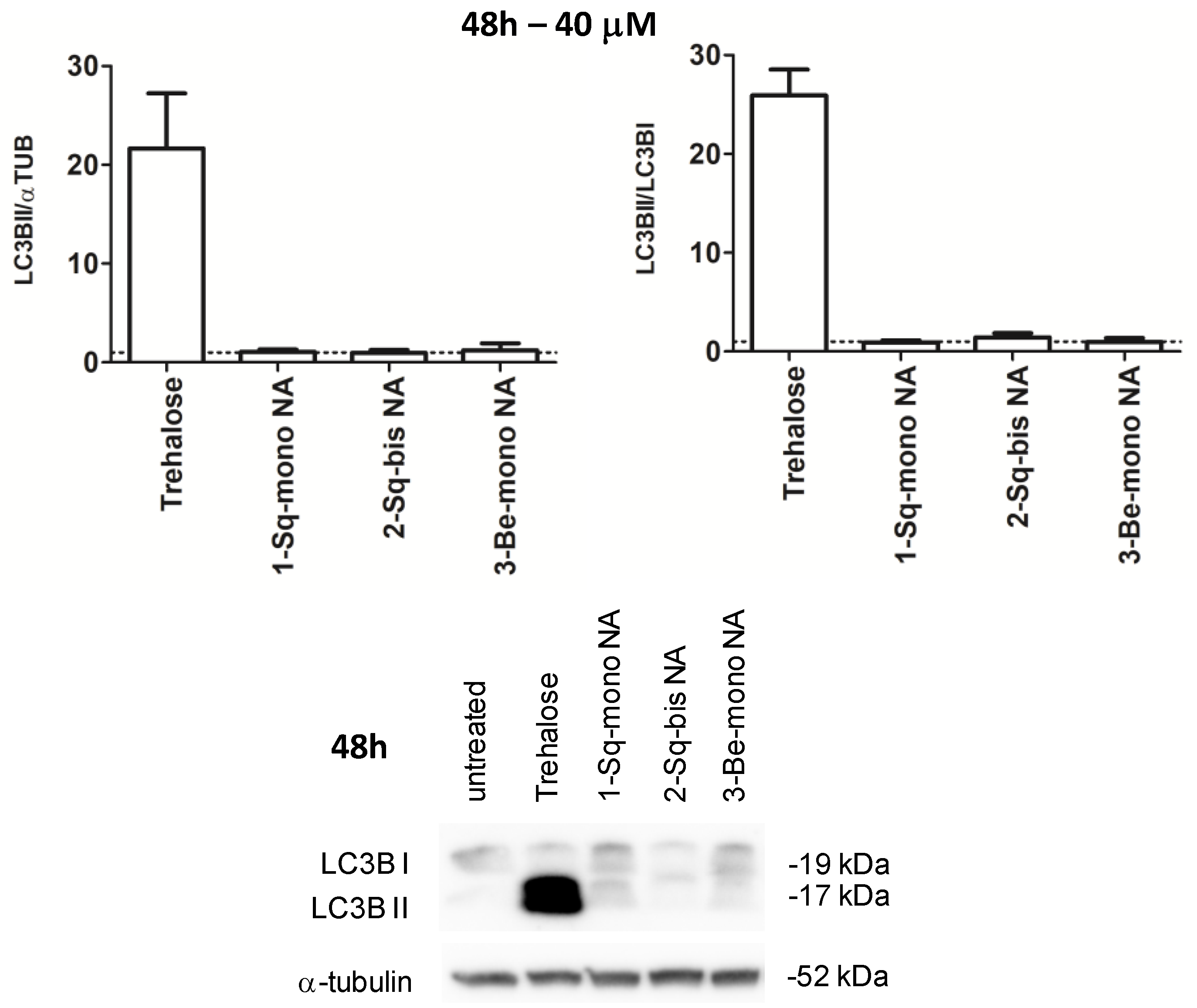
3.4. Biological Profiling
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Farjadian, F.; Ghasemi, A.; Gohari, O.; Roointan, A.; Karimi, M.; Hamblin, M.R. Nanopharmaceuticals and nanomedicines currently on the market: Challenges and opportunities. Nanomedicine 2019, 14, 93–126. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Progress in nanomedicine: Approved and investigational nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Hirst, D.; O’Sullivan, J. Gold nanoparticles as novel agents for cancer therapy. Br. J. Radiol. 2012, 85, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous silica nanoparticles in target drug-delivery system: A review. Int. J. Pharm. Investig. 2015, 5, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Farjadian, F.; Moradi, S.; Hosseini, M. Thin chitosan films containing super-paramagnetic nanoparticles with contrasting capability in magnetic resonance imaging. J. Mater. Sci. Mater. Med. 2017, 28, 47. [Google Scholar] [CrossRef] [PubMed]
- Maier-Hauff, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neuro-Oncol. 2011, 103, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Schwenk, M.H. Ferumoxytol: A new intravenous iron preparation for the treatment of iron deficiency anemia in patients with chronic kidney disease. Pharmacotherapy 2010, 33, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Lasic, D.D. Doxorubicin in sterically stabilized liposomes. Nature 1996, 380, 561–562. [Google Scholar] [CrossRef] [PubMed]
- Buster, J.E. Transdermal menopausal hormone therapy: Delivery through skin changes the rules. Expert Opin. Pharmacother. 2010, 11, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Turecek, P.L.; Bossard, M.J.; Schoetens, F.; Ivens, I.A. PEGylation of biopharmaceuticals: A review of chemistry and nonclinical safety information of approved drugs. J. Pharm. Sci. 2016, 105, 460–475. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, R.; Roseblade, A.; Hansbro, P.M.; Rathbone, M.J.; Dua, K.; Bebawy, M. Nanoparticles in cancer treatment: Opportunities and obstacles. Curr. Drug Targets 2018, 69, 1696–1709. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M.; Eslamifar, M.; Khezri, K.; Dizaj, S.M. Applications of nanotechnology in drug delivery to the central nervous system. Biomed. Pharmacother. 2019, 111, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Miller, L.; Richman, S.; Hitchman, S.; Glick, G.; Liu, S.; Zhu, Y.; Crossman, M.; Nestorov, I.; Gronke, R.S.; et al. A novel PEGylated interferon beta- 1a for multiple sclerosis: Safety, pharmacology, and biology. J. Clin. Pharmacol. 2012, 52, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Birch, G.G. Trehaloses. Adv. Carbohydr. Chem. 1963, 18, 201–225. [Google Scholar] [PubMed]
- Richards, A.B.; Krakowka, S.; Dexter, L.B.; Schmid, H.; Wolterbeek, A.P.; Waalkens-Berendsen, D.H.; Shigoyuki, A.; Kurimoto, M. Trehalose: A review of properties, history of use and human tolerance, and results of multiple safety studies. Food Chem. Toxicol. 2003, 40, 871–898. [Google Scholar] [CrossRef]
- Sussman, A.S.; Lingappa, B.T. Role of trehalose in ascaspores of Neurospora tetrasperma. Science 1959, 130, 1343. [Google Scholar] [CrossRef] [PubMed]
- Van Dijck, P.; Colavizza, D.; Smet, P.; Thevelein, J.M. Differential importance of trehalose in stress resistance in fermenting and nonfermenting Saccharomyces cerevisiae cells. Appl. Environ. Microbiol. 1995, 61, 109–115. [Google Scholar] [PubMed]
- Mardones, P.; Rubinsztein, D.C.; Hetz, C. Mystery solved: Trehalose kickstarts autophagy by blocking glucose transport. Sci. Signal. 2016, 9, fs2. [Google Scholar] [CrossRef]
- Tanaka, M.; Machida, Y.; Niu, S.; Ikeda, T.; Jana, N.R.; Doi, H.; Kurosawa, M.; Nekooki, M.; Nukina, N. Trehalose alleviates polyglutamine-mediated pathology in a mouse model of Huntington disease. Nat. Med. 2004, 10, 148–154. [Google Scholar] [CrossRef]
- Jain, N.K.; Roy, I. Effect of trehalose on protein structure. Protein Sci. 2009, 18, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Barkhordarian, H.; Emadi, S.; Park, C.B.; Sierks, M.R. Trehalose differentially inhibits aggregation and neurotoxicity of beta-amyloid 40 and 42. Neurobiol. Dis. 2005, 20, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Kalf, G.F.; Rieder, S.V. The purification and properties of trehalase. J. Biol. Chem. 1958, 230, 691–698. [Google Scholar] [PubMed]
- Adhikari, P.; Pal, P.; Das, A.K.; Ray, S.; Bhattacharjee, A.; Mazumder, B. Nano lipid-drug conjugate: An integrated review. Int. J. Pharm. 2017, 529, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Couvreur, P.; Stella, B.; Reddy, L.H.; Mangenot, S.; Poupaert, J.H.; Desmaele, D.; Lepetre-Mouelhi, S.; Rocco, F.; Dereuddre-Bosquet, N.; Clayette, P.; et al. Squalenoyl nanomedicines as potential therapeutics. Nano Lett. 2006, 6, 2544–2548. [Google Scholar] [CrossRef] [PubMed]
- Desmaele, D.; Gref, R.; Couvreur, P. Squalenoylation: A generic platform for nanoparticular drug delivery. J. Control. Release 2012, 161, 609–618. [Google Scholar] [CrossRef]
- Semiramoth, N.; Di Meo, C.; Zouhiri, F.; Said-Hassane, F.; Valetti, S.; Gorges, R.; Nicolas, V.; Poupaert, J.H.; Chollet-Martin, S.; Desmaele, D.; et al. Selfassembled squalenoylated penicillin bioconjugates: An original approach for the treatment of intracellular infections. ACS Nano 2012, 6, 3820–3831. [Google Scholar] [CrossRef]
- Buchy, E.; Valetti, S.; Mura, S.; Mougin, J.; Troufflard, C.; Couvreur, P.; Desmaële, D. Synthesis and cytotoxic activity of self-assembling squalene conjugates of 3-[(pyrrol-2-yl)methylidene]-2,3-dihydro-1H-indol-2-one anticancer agents. Eur. J. Org. Chem. 2015. [Google Scholar] [CrossRef]
- Dash, S.K.; Giri, B. Self-assembled betulinic acid: A better alternative form of betulinic acid for anticancer therapy. J. Exp. Med. Biol. 2019, 1, 1–2. [Google Scholar]
- Dash, S.K.; Dash, S.S.; Chattopadhyay, S.; Ghosh, T.; Tripathy, S.; Mahapatra, S.K.; Bag, B.G.; Das, D.; Roy, S. Folate decorated delivery of self-assembled betulinic acid nano fibers: A biocompatible antileukemic therapy. RCS Adv. 2015, 5, 24144. [Google Scholar] [CrossRef]
- Fumagalli, G.; Marucci, C.; Christodoulou, M.S.; Stella, B.; Dosio, F.; Passarella, D. Self-assembly drug conjugates for anticancer treatment. Drug Discov. Today 2016, 21, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, S.; Christodoulou, M.S.; Ficarra, I.; Silvani, A.; Cappelletti, G.; Cartelli, D.; Damia, G.; Ricci, F.; Zucchetti, M.; Dosio, F.; et al. New class of squalene-based releasable nanoassemblies of paclitaxel, podophyllotoxin, camptothecin and epothilone A. Eur. J. Med. Chem. 2014, 85, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, S.; Cartelli, D.; Secundo, F.; Fumagalli, G.; Christodoulou, M.S.; Borroni, A.; Perdicchia, D.; Dosio, F.; Milla, P.; Cappelletti, G.; et al. Self-Assembled Squalene-based Fluorescent Heteronanoparticles. ChemPlusChem 2015, 80, 47–49. [Google Scholar] [CrossRef]
- Fumagalli, G.; Mazza, D.; Christodoulou, M.S.; Damia, G.; Ricci, F.; Perdicchia, D.; Stella, B.; Dosio, F.; Sotiropoulou, P.A.; Passarella, D. Cyclopamine-paclitaxel-containing nanoparticles: Internalization in cells detected by confocal and super-resolution microscopy. ChemPlusChem 2015, 80, 1380–1383. [Google Scholar] [CrossRef]
- Fumagalli, G.; Stella, B.; Pastushenko, I.; Ricci, F.; Christodoulou, M.S.; Damia, G.; Mazza, D.; Arpicco, S.; Giannini, C.; Morosi, L.; et al. Heteronanoparticles by self-assembly of doxorubicin and cyclopamine conjugates. ACS Med. Chem. Lett. 2017, 8, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, G.; Giorgi, G.; Vágvölgyi, M.; Colombo, E.; Christodoulou, M.S.; Collico, V.; Prosperi, D.; Dosio, F.; Hunyadi, A.; Montopoli, M.; et al. Hetero-nanoparticles by self-assembly of ecdysteroid and doxorubicin conjugates to overcome cancer resistance. ACS Med. Chem. Lett. 2018, 9, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, G.; Christodoulou, M.S.; Riva, B.; Revuelta, I.; Marucci, C.; Collico, V.; Prosperi, D.; Riva, S.; Perdicchia, D.; Bassanini, I.; et al. Self-assembled 4-(1,2-diphenylbut-1-en-1-yl)aniline based nanoparticles: Podophyllotoxin and aloin as building blocks. Org. Biomol. Chem. 2017, 15, 1106–1109. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, G.; Polito, L.; Colombo, E.; Foschi, F.; Christodoulou, M.S.; Galeotti, F.; Perdicchia, D.; Bassanini, I.; Riva, S.; Seneci, P.; et al. Self-assembling releasable thiocolchicine-diphenylbutenylaniline conjugates. ACS Med. Chem. Lett. 2019, 10, 611–614. [Google Scholar] [CrossRef]
- Guo, F.; Liu, X.; Cai, H.; Le, W. Autophagy in neurodegenerative diseases: Pathogenesis and therapy. Brain Pathol. 2018, 28, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Sarpe, A.V.; Kulkarni, S.S. Synthesis of maradolipid. J. Org. Chem. 2011, 76, 6866–6870. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, L.; Gallarate, M. Lipid nanoparticles: State of the art, new preparation methods and challenges in drug delivery. Expert Opin. Drug Deliv. 2012, 9, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Von Smoluchowski, M. Contribution à la théorie de l’endosmose électrique et de quelques phenomènes. Pisma Mariana Smoluchowskiego 1924, 1, 403. [Google Scholar]
- Klionski, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Acevedo Arozena, A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef] [PubMed]
- Fic, E.; Kedracka-Krok, S.; Jankowska, U.; Pirog, A.; Dziedzicka-Wasylewska, M. Comparison of protein precipitation methods for various rat brain structures prior of proteomic analysis. Electrophoresis 2010, 31, 3573–3579. [Google Scholar] [CrossRef] [PubMed]
- Emanuele, E. Can trehalose prevent neurodegeneration? Insights from experimental studies. Curr. Drug Targets 2014, 15, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Rusmini, P.; Cortese, K.; Crippa, V.; Cristofani, R.; Cicardi, M.E.; Ferrari, V.; Vezzoli, G.; Tedesco, B.; Meroni, M.; Messi, E.; et al. Trehalose induces autophagy via lysosomal-mediated TFEB activation in models of motoneuron degeneration. Autophagy 2019, 15, 631–651. [Google Scholar] [CrossRef] [PubMed]

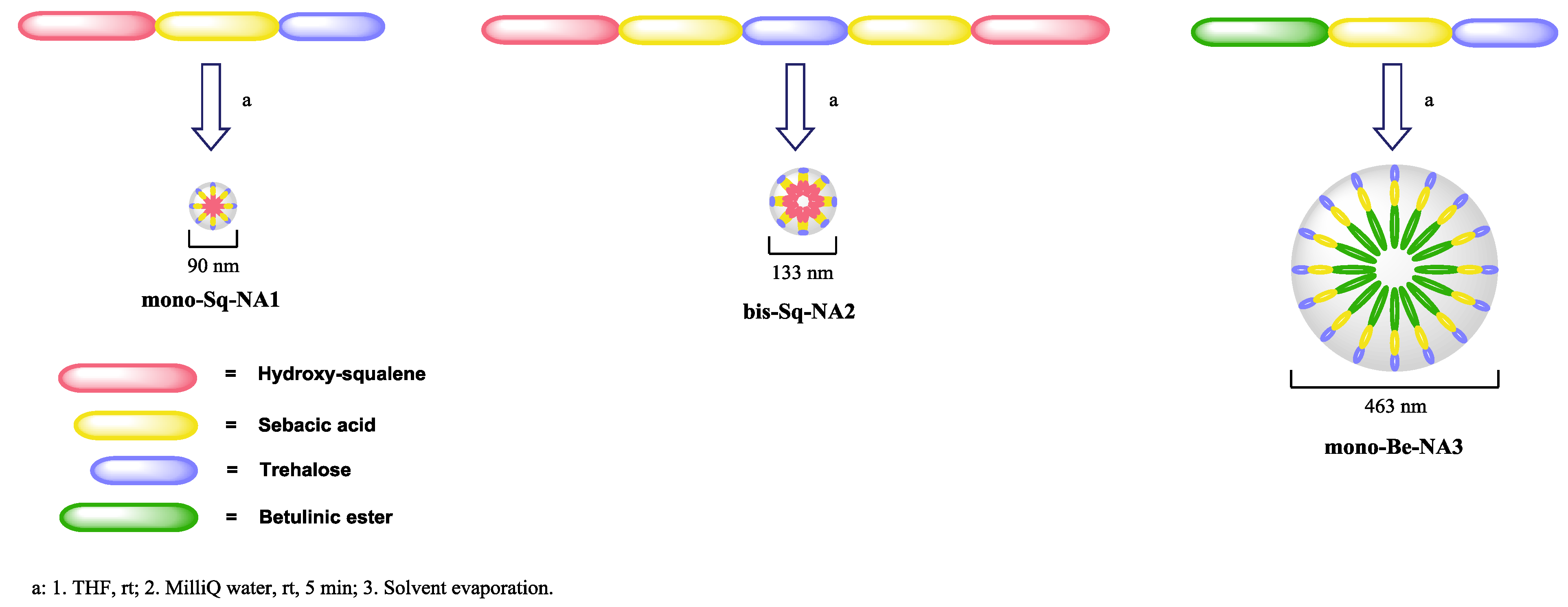

| Nanovector/Test | Hydrodynamic Diameter (HD, nm) | ζ-Potential (mV) | Polydispersity Index |
|---|---|---|---|
| mono-Sq-NA1 | 90.4 ± 0.7 | −25.12 ± 0.79 | 0.121 ± 0.019 |
| bis-Sq-NA2 | 132.8 ± 0.9 | −25.43 ± 0.69 | 0.072 ± 0.010 |
| mono-Be-NA3 | 463 ±29 | −23.53± 0.29 | 0.126 ± 0.010 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombo, E.; Biocotino, M.; Frapporti, G.; Randazzo, P.; Christodoulou, M.S.; Piccoli, G.; Polito, L.; Seneci, P.; Passarella, D. Nanolipid-Trehalose Conjugates and Nano-Assemblies as Putative Autophagy Inducers. Pharmaceutics 2019, 11, 422. https://doi.org/10.3390/pharmaceutics11080422
Colombo E, Biocotino M, Frapporti G, Randazzo P, Christodoulou MS, Piccoli G, Polito L, Seneci P, Passarella D. Nanolipid-Trehalose Conjugates and Nano-Assemblies as Putative Autophagy Inducers. Pharmaceutics. 2019; 11(8):422. https://doi.org/10.3390/pharmaceutics11080422
Chicago/Turabian StyleColombo, Eleonora, Michele Biocotino, Giulia Frapporti, Pietro Randazzo, Michael S. Christodoulou, Giovanni Piccoli, Laura Polito, Pierfausto Seneci, and Daniele Passarella. 2019. "Nanolipid-Trehalose Conjugates and Nano-Assemblies as Putative Autophagy Inducers" Pharmaceutics 11, no. 8: 422. https://doi.org/10.3390/pharmaceutics11080422
APA StyleColombo, E., Biocotino, M., Frapporti, G., Randazzo, P., Christodoulou, M. S., Piccoli, G., Polito, L., Seneci, P., & Passarella, D. (2019). Nanolipid-Trehalose Conjugates and Nano-Assemblies as Putative Autophagy Inducers. Pharmaceutics, 11(8), 422. https://doi.org/10.3390/pharmaceutics11080422







