Alginate/Chitosan Particle-Based Drug Delivery Systems for Pulmonary Applications
Abstract
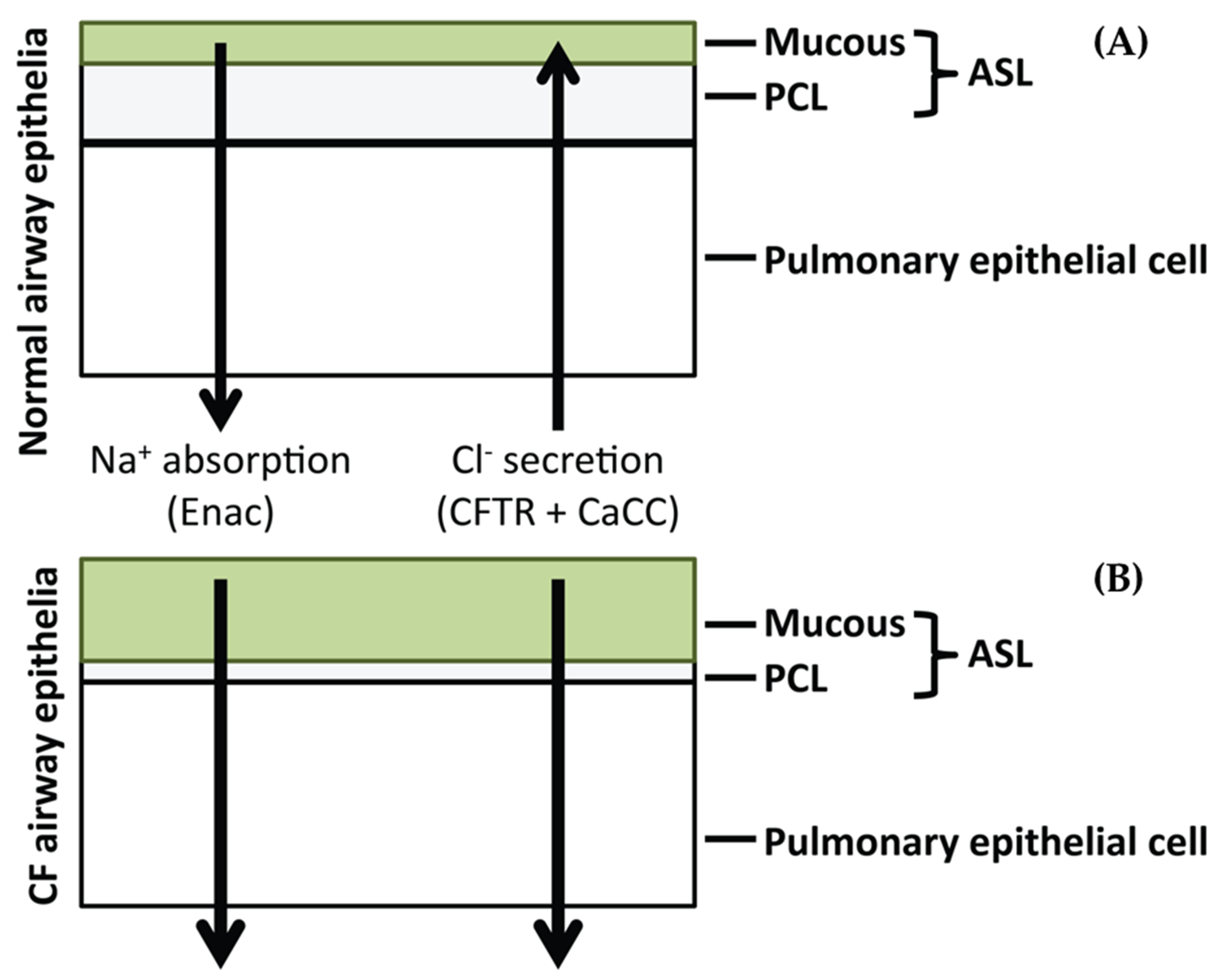
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Tobramycin Loaded Alginate/Chitosan Particles
2.3. Particle Characterisation
2.4. Conjugation of SLPI to Chitosan
2.5. Quantification of SLPI Conjugation
2.6. Analytical Methodology for Detection of Tobramycin Sulphate
2.7. Quantification of Tobramycin Release
2.8. Minimum Inhibitory Concentration Effect of Tobramycin on P. aeruginosa
2.9. Inhibition of Neutrophil Elastase by SLPI Functionalised Alginate/Chitosan Particles
2.10. Preparation of Rhodamine 6G Loaded Alginate/Chitosan Particles
2.11. Penetration of SLPI Functionalised Particles in CF Mucus
2.12. Statistical Analysis
3. Results and Discussion
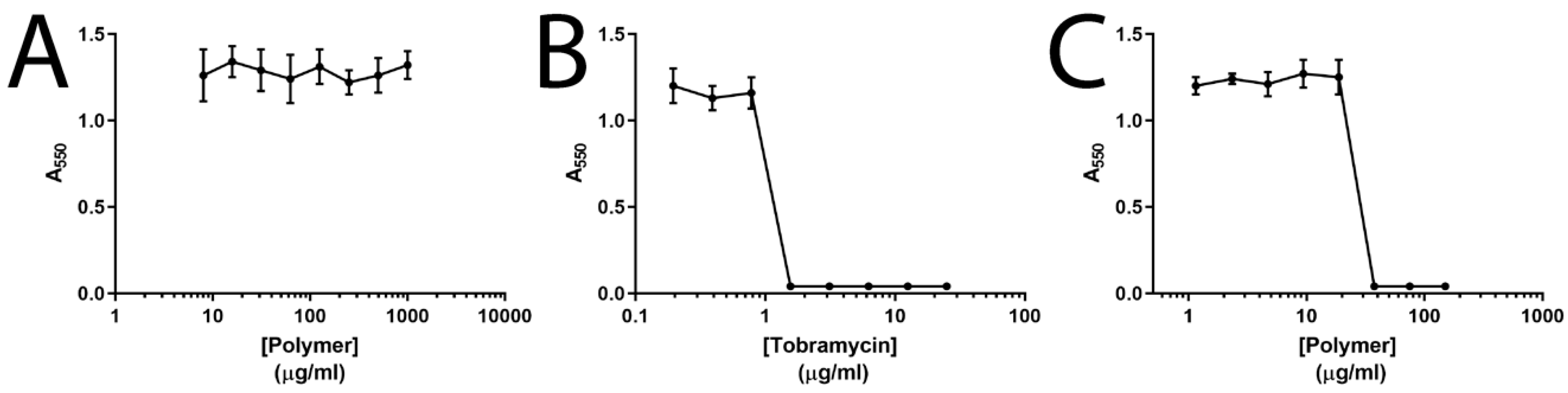
3.1. Particle Preparation and Tobramycin Loading and Release
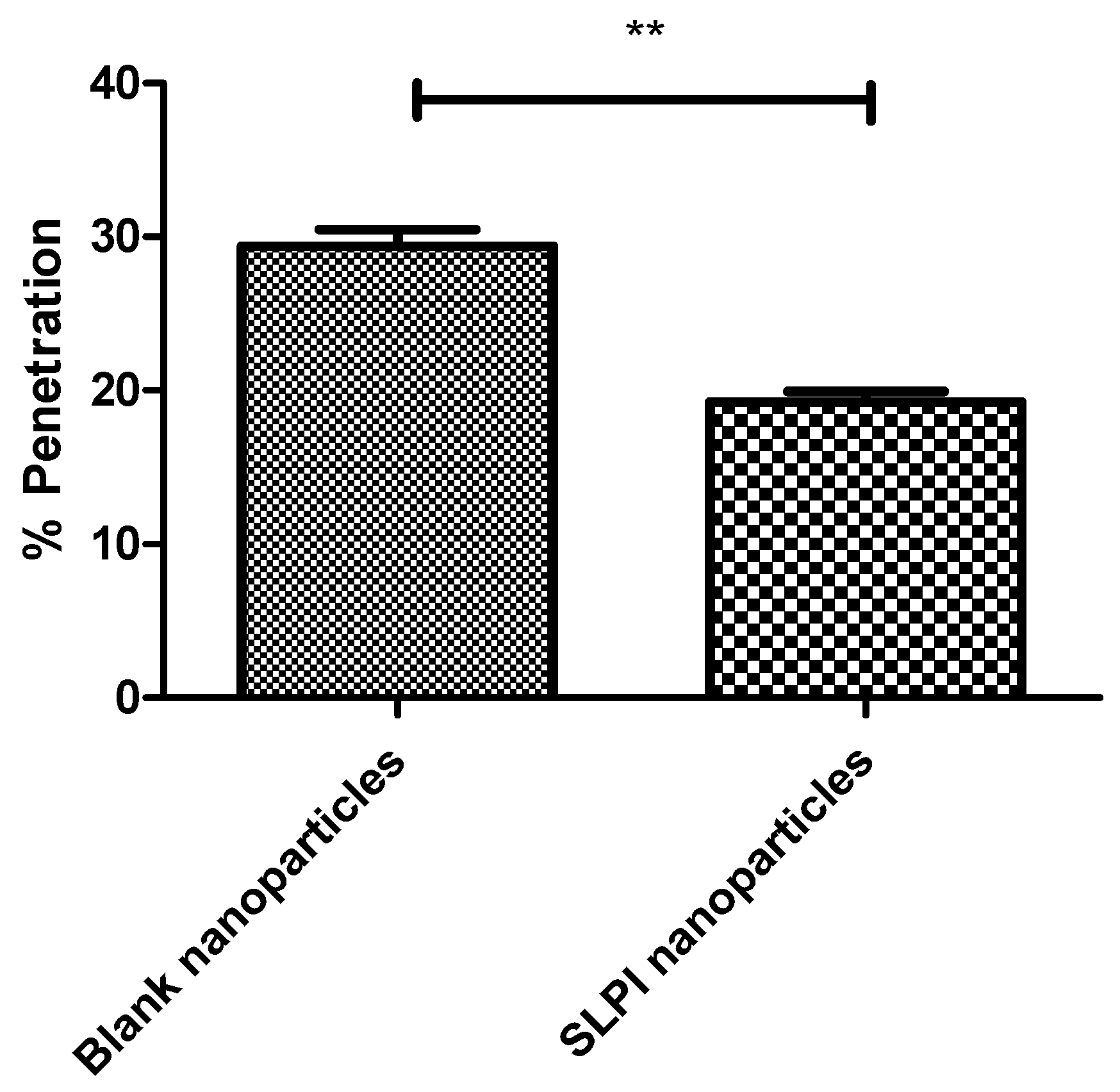
3.2. SLPI-Conjugated Particle Preparation and Interactions with Model Biological Milieu
3.3. Applicability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dulhanty, A.M.; Chang, X.B.; Riordan, J.R. Mutation of potential phosphorylation sites in the recombinant R domain of the cystic fibrosis transmembrane conductance regulator has significant effects on domain conformation. Biochem. Biophys. Res. Commun. 1995, 206, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Riordan, J.R.; Rommens, J.M.; Kerem, B.; Alon, N.; Rozmahel, R.; Grzelczak, Z.; Chou, J.L. Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science 1989, 245, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Marino, C.R.; Matovcik, L.M.; Gorelick, F.S.; Cohn, J.A. Localization of the cystic fibrosis transmembrane conductance regulator in pancreas. J. Clin. Investig. 1991, 88, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Jacquot, J.; Puchelle, E.; Hinnrasky, J.; Fuchey, C.; Bettinger, C.; Spilmont, C.; Pavirani, A. Localization of the cystic fibrosis transmembrane conductance regulator in airway secretory glands. Eur. Resp. J. 1993, 6, 169–176. [Google Scholar]
- Sbarbati, A.; Bertini, M.; Catassi, C.; Gagliardini, R.; Osculati, F. Ultrastructural lesions in the small bowel of patients with cystic fibrosis. Ped. Res. 1998, 43, 234–239. [Google Scholar] [CrossRef]
- Kreda, S.M.; Mall, M.; Mengos, A.; Rochelle, L.; Yankaskas, J.; Riordan, J.R.; Boucher, R.C. Characterization of wild-type and deltaF508 cystic fibrosis transmembrane regulator in human respiratory epithelia. Mol. Biol. Cell 2005, 16, 2154–2167. [Google Scholar] [CrossRef]
- Krouse, M.E. Is cystic fibrosis lung disease caused by abnormal ion composition or abnormal volume? J. Gen. Physiol. 2001, 118, 219–222. [Google Scholar] [CrossRef]
- Stenbit, A.E.; Flume, P.A. Pulmonary exacerbations in cystic fibrosis. Curr. Opin. Pulm. Med. 2011, 17, 442–447. [Google Scholar] [CrossRef]
- Mall, M.; Boucher, R. Pathogenesis of Pulmonary Disease in Cystic Fibrosis. In Cystic Fibrosis in the 21st Century; Bush, A., Alton, E.W.F.W., Davies, J.C., Griesenbach, U., Jaffe, A., Eds.; Prog. Respir. Res. Karger: Basel, Switzerland, 2006; Volume 34, pp. 116–121. [Google Scholar] [CrossRef]
- Haley, C.L.; Colmer-Hamood, J.A.; Hamood, A.N. Characterization of biofilm-like structures formed by Pseudomonas aeruginosa in a synthetic mucus medium. BMC Microbiol. 2012, 12, 181. [Google Scholar] [CrossRef]
- May, T.B.; Shinabarger, D.; Maharaj, R.; Kato, J.; Chu, L.; DeVault, J.D.; Rothmel, R.K. Alginate synthesis by Pseudomonas aeruginosa: A key pathogenic factor in chronic pulmonary infections of cystic fibrosis patients. Clin. Microbiol. Rev. 1991, 4, 191–206. [Google Scholar] [CrossRef]
- Quinn, D.J.; Weldon, S.; Taggart, C.C. Antiproteases as therapeutics to target inflammation in cystic fibrosis. Open Respir. Med. J. 2010, 4, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Ciofu, O.; Bjarnsholt, T. Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol. 2010, 5, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Cystic Fibrosis: Diagnosis and Management. Available online: https://www.nice.org.uk/guidance/ng78/chapter/Recommendations#pulmonary-monitoring-assessment-and-management (accessed on 30 June 2019).
- Tobramycin. Available online: https://bnf.nice.org.uk/drug/tobramycin.html (accessed on 30 June 2019).
- Omri, A.; Beaulac, C.; Bouhajib, M.; Montplaisir, S.; Sharkawi, M.; Lagacé, J. Pulmonary retention of free and liposome-encapsulated tobramycin after intratracheal administration in uninfected rats and rats infected with Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1994, 38, 1090–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smyth, A.; Walters, S. Prophylactic antibiotics for cystic fibrosis. Cochrane Database Syst. Rev. 2003, 3, CD001912. [Google Scholar] [CrossRef]
- Girón Moreno, R.M.; Salcedo Posadas, A.; Mar Gómez-Punter, R. Inhaled antibiotic therapy in cystic fibrosis. Arch. De Bronconeumol. 2011, 47, 14–18. [Google Scholar] [CrossRef]
- IM2 Cystic Fibrosis Patient Adherence (Adult). Available online: https://www.england.nhs.uk/wp-content/uploads/2016/11/im2-cystic-fibrosis-patient-adherence.pdf (accessed on 30 June 2019).
- Selimoglu, E. Aminoglycoside-induced ototoxicity. Curr. Pharm. Des. 2007, 13, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Kumin, G.D. Clinical Nephrotoxicity of Tobramycin and Gentamicin. JAMA 1980, 244, 1808. [Google Scholar] [CrossRef] [PubMed]
- Tobramycin 300 mg/5 mL Nebuliser Solution. Available online: https://www.medicines.org.uk/emc/product/2683/smpc (accessed on 30 June 2019).
- Ramphal, R.; Lhermitte, M.; Filliat, M.; Roussel, P. The binding of anti-pseudomonal antibiotics to macromolecules from cystic fibrosis sputum. J. Antimicrob. Chemother. 1988, 22, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Mall, M.; Grubb, B.R.; Harkema, J.R.; O’Neal, W.K.; Boucher, R.C. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat. Med. 2004, 10, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Heeckeren, A.; Walenga, R.; Konstan, M.W.; Bonfield, T.; Davis, P.B.; Ferkol, T. Excessive inflammatory response of cystic fibrosis mice to bronchopulmonary infection with Pseudomonas aeruginosa. J. Clin. Invest. 1997, 100, 2810–2815. [Google Scholar] [CrossRef] [PubMed]
- King, A.E.; Critchley, H.O.; Kelly, R.W. Presence of secretory leukocyte protease inhibitor in human endometrium and first trimester decidua suggests an antibacterial protective role. Mol. Hum. Reprod. 2000, 6, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Appelhans, B.; Ender, B.; Sachse, G.; Nikiforov, T.; Appelhans, H.; Ebert, W. Secretion of antileucoprotease from a human lung tumor cell line. FEBS Lett. 1987, 224, 14–18. [Google Scholar] [CrossRef] [Green Version]
- Franken, C.; Meijer, C.J.; Dijkman, J.H. Tissue distribution of antileukoprotease and lysozyme in humans. J. Histochem. Cytochem. 1989, 37, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Heinzel, R.; Appelhans, H.; Gassen, G.; Seemüller, U.; Machleidt, W.; Fritz, H.; Steffens, G. Molecular cloning and expression of cDNA for human antileukoprotease from cervix uterus. Eur. J. Biochem. 1986, 160, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Igarashi, Y.; Hohman, R.J.; Kaulbach, H.; White, M.V.; Kaliner, M.A. Distribution of secretory leukoprotease inhibitor in the human nasal airway. Am. Rev. Respir. Dis. 1993, 147, 710–716. [Google Scholar] [CrossRef]
- Thompson, R.C.; Ohlsson, K. Isolation, properties, and complete amino acid sequence of human secretory leukocyte protease inhibitor, a potent inhibitor of leukocyte elastase. Proc. Natl. Acad. Sci. USA 1986, 83, 6692–6696. [Google Scholar] [CrossRef]
- McElvaney, N.G.; Nakamura, H.; Birrer, P.; Hébert, C.A.; Wong, W.L.; Alphonso, M.; Crystal, R.G. Modulation of airway inflammation in cystic fibrosis. In vivo suppression of interleukin-8 levels on the respiratory epithelial surface by aerosolization of recombinant secretory leukoprotease inhibitor. J. Clin. Invest. 1992, 90, 1296–1301. [Google Scholar] [CrossRef]
- McElvaney, N.G.; Doujaiji, B.; Moan, M.J.; Burnham, M.R.; Wu, M.C.; Crystal, R.G. Pharmacokinetics of recombinant secretory leukoprotease inhibitor aerosolized to normals and individuals with cystic fibrosis. Am. Rev. Respir. Dis. 1993, 148, 1056–1060. [Google Scholar] [CrossRef]
- Taggart, C.C.; Cryan, S.-A.; Weldon, S.; Gibbons, A.; Greene, C.M.; Kelly, E.; McElvaney, N.G. Secretory leucoprotease inhibitor binds to NF-kappaB binding sites in monocytes and inhibits p65 binding. J. Exp. Med. 2005, 202, 1659–1668. [Google Scholar] [CrossRef]
- Hill, M.; Cunningham, R.N.; Hathout, R.M.; Johnston, C.; Hardy, J.G.; Migaud, M.E. Formulation of antimicrobial tobramycin loaded PLGA nanoparticles via complexation with AOT. J. Funct. Biomater. 2019, 10, 26. [Google Scholar] [CrossRef]
- Mansour, H.M.; Rhee, Y.-S.; Wu, X. Nanomedicine in pulmonary delivery. Int. J. Nanomed. 2009, 4, 299–319. [Google Scholar] [CrossRef] [Green Version]
- Benson, J.R.; Hare, P.E. O-phthalaldehyde: Fluorogenic detection of primary amines in the picomole range. Comparison with fluorescamine and ninhydrin. Proc. Natl. Acad. Sci. USA 1975, 72, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Weldon, S.; McNally, P.; McElvaney, N.G.; Elborn, J.S.; McAuley, D.F.; Wartelle, J.; Taggart, C.C. Decreased levels of secretory leucoprotease inhibitor in the Pseudomonas-infected cystic fibrosis lung are due to neutrophil elastase degradation. J. Immunol. 2009, 183, 8148–8156. [Google Scholar] [CrossRef] [PubMed]
- Baltimore, R.S.; Christie, C.D.; Smith, G.J. Immunohistopathologic localization of Pseudomonas aeruginosa in lungs from patients with cystic fibrosis. Implications for the pathogenesis of progressive lung deterioration. Am. Rev. Respir. Dis. 1989, 140, 1650–1661. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M.; Kamada, M.; Maegawa, M.; Gima, H.; Aono, T. Binding of human secretory leukocyte protease inhibitor in uterine cervical mucus to immunoglobulins: Pathophysiology in immunologic infertility and local immune defense. Fertil. Steril. 1999, 71, 1108–1114. [Google Scholar] [CrossRef]
- Ungaro, F.; d’Angelo, I.; Coletta, C.; d’Emmanuele di Villa Bianca, R.; Sorrentino, R.; Perfetto, B.; Quaglia, F. Dry powders based on PLGA nanoparticles for pulmonary delivery of antibiotics: Modulation of encapsulation efficiency, release rate and lung deposition pattern by hydrophilic polymers. J. Control. Release 2012, 157, 149–159. [Google Scholar] [CrossRef]
- Yamamoto, H.; Kuno, Y.; Sugimoto, S.; Takeuchi, H.; Kawashima, Y. Surface-modified PLGA nanosphere with chitosan improved pulmonary delivery of calcitonin by mucoadhesion and opening of the intercellular tight junctions. J. Control. Release 2005, 102, 373–381. [Google Scholar] [CrossRef]
- Daniels, T.; Mills, N.; Whitaker, P. Nebuliser systems for drug delivery in cystic fibrosis. Cochrane Database Syst. Rev. 2013, 4, CD007639. [Google Scholar] [CrossRef]
- Moreno-Sastre, M.; Pastor, M.; Salomon, C.J.; Esquisabel, A.; Pedraz, J.L. Pulmonary drug delivery: A review on nanocarriers for antibacterial chemotherapy. J. Antimicrob. Chemother. 2015, 70, 2945–2955. [Google Scholar] [CrossRef]
- Ratjen, F.; Brockhaus, F.; Angyalosi, G. Aminoglycoside therapy against Pseudomonas aeruginosa in cystic fibrosis: A review. J. Cystic Fibrosis. 2009, 8, 361–369. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, E.; Giron, R.M.; Gómez-Punter, R.M.; García-Castillo, E.; Valenzuela, C.; Cisneros, C.; Zamora, E.; García-Pérez, F.J.; Ancochea, J. Long-term safety and efficacy of tobramycin in the management of cystic fibrosis. Ther. Clin. Risk Manag. 2015, 11, 407–415. [Google Scholar] [CrossRef]
- Uttley, L.; Harnan, S.; Cantrell, A.; Taylor, C.; Walshaw, M.; Brownlee, K.; Tappenden, P. Systematic review of the dry powder inhalers colistimethate sodium and tobramycin in cystic fibrosis. Eur. Resp. Rev. 2013, 22, 476–486. [Google Scholar] [CrossRef] [Green Version]
- McKeage, K. Tobramycin inhalation powder: A review of its use in the treatment of chronic Pseudomonas aeruginosa infection in patients with cystic fibrosis. Drugs. 2013, 73, 1815–1827. [Google Scholar] [CrossRef]
- Hagerman, J.K.; Knechtel, S.A.; Klepser, M.E. Tobramycin solution for inhalation in cystic fibrosis patients: A review of the literature. Expert Opin. Pharmacother. 2007, 8, 467–475. [Google Scholar] [CrossRef]
- Dodd, M.E.; Webb, A.K. Understanding non-compliance with treatment in adults with cystic fibrosis. J. R. Soc. Med. 2000, 93, 2–8. [Google Scholar]
- Fisher, J.T.; Zhang, Y.; Engelhardt, J.F. Comparative biology of cystic fibrosis animal models. Methods Mol. Biol. 2011, 742, 311–334. [Google Scholar] [CrossRef]
- Pezzulo, A.A.; Tang, X.X.; Hoegger, M.J.; Alaiwa, M.H.A.; Ramachandran, S.; Moninger, T.O.; Zabner, J. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature 2012, 487, 109–113. [Google Scholar] [CrossRef]

| Alginate (w/w Ratio) | Chitosan (w/w Ratio) | Tobramycin (w/w Ratio) | CaCl2 (w/w Ratio) | Aggregation |
|---|---|---|---|---|
| 9 | 1.5 | 1.5 | 3 | Yes |
| 9 | 1.5 | 1.5 | 0.8 | Yes |
| 9 | 0.8 | 0.8 | 0 | No |
| 9 | 1 | 3 | 0 | Yes |
| 9 | 1 | 1.5 | 0 | No |
| Particle Size (nm) | PDI | Zeta Potential (mV) | Tobramycin Loading in Particles (µg/mg) | % Entrapment |
|---|---|---|---|---|
| 437.5 ± 22.3 | 0.27 ± 0.07 | 21.6 ± 1.1 | 74.2 ± 3.4 | 44.5 ± 2.0 |
| Crosslinker (EDC) | Particle Size (nm) | Polydispersity Index (PDI) | Zeta Potential (mV) | Conjugated SLPI (µg) in Particles (mg), (µg/mg) |
|---|---|---|---|---|
| No | 437.5 ± 26.5 | 0.26 ± 0.09 | −22.9 ± 3.1 | 0.2 ± 0.3 |
| Yes | 458.0 ± 31.1 | 0.31 ± 0.12 | −19.2 ± 2.1 | 11.2 ± 2.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hill, M.; Twigg, M.; Sheridan, E.A.; Hardy, J.G.; Elborn, J.S.; Taggart, C.C.; Scott, C.J.; Migaud, M.E. Alginate/Chitosan Particle-Based Drug Delivery Systems for Pulmonary Applications. Pharmaceutics 2019, 11, 379. https://doi.org/10.3390/pharmaceutics11080379
Hill M, Twigg M, Sheridan EA, Hardy JG, Elborn JS, Taggart CC, Scott CJ, Migaud ME. Alginate/Chitosan Particle-Based Drug Delivery Systems for Pulmonary Applications. Pharmaceutics. 2019; 11(8):379. https://doi.org/10.3390/pharmaceutics11080379
Chicago/Turabian StyleHill, Marcus, Matthew Twigg, Emer A. Sheridan, John G. Hardy, J. Stuart Elborn, Clifford C. Taggart, Christopher J. Scott, and Marie E. Migaud. 2019. "Alginate/Chitosan Particle-Based Drug Delivery Systems for Pulmonary Applications" Pharmaceutics 11, no. 8: 379. https://doi.org/10.3390/pharmaceutics11080379
APA StyleHill, M., Twigg, M., Sheridan, E. A., Hardy, J. G., Elborn, J. S., Taggart, C. C., Scott, C. J., & Migaud, M. E. (2019). Alginate/Chitosan Particle-Based Drug Delivery Systems for Pulmonary Applications. Pharmaceutics, 11(8), 379. https://doi.org/10.3390/pharmaceutics11080379