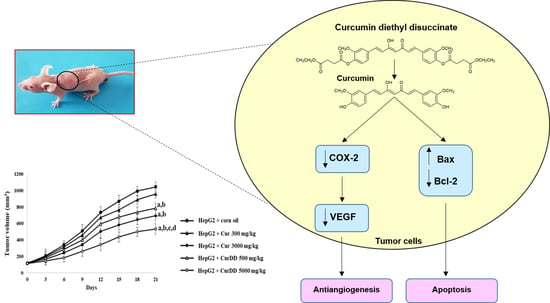
Scale-Up Synthesis and In Vivo Anti-Tumor Activity of Curcumin Diethyl Disuccinate, an Ester Prodrug of Curcumin, in HepG2-Xenograft Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Scale-Up Methods for Curcumin and CurDD Syntheses
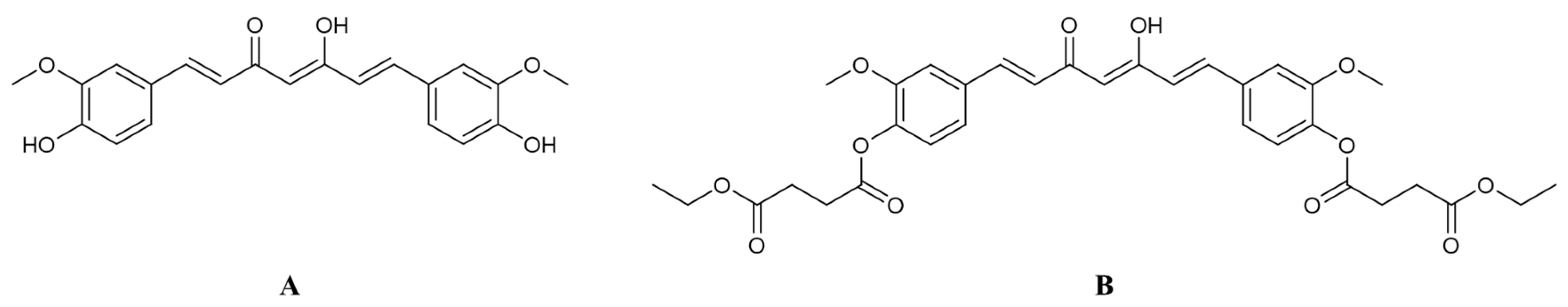
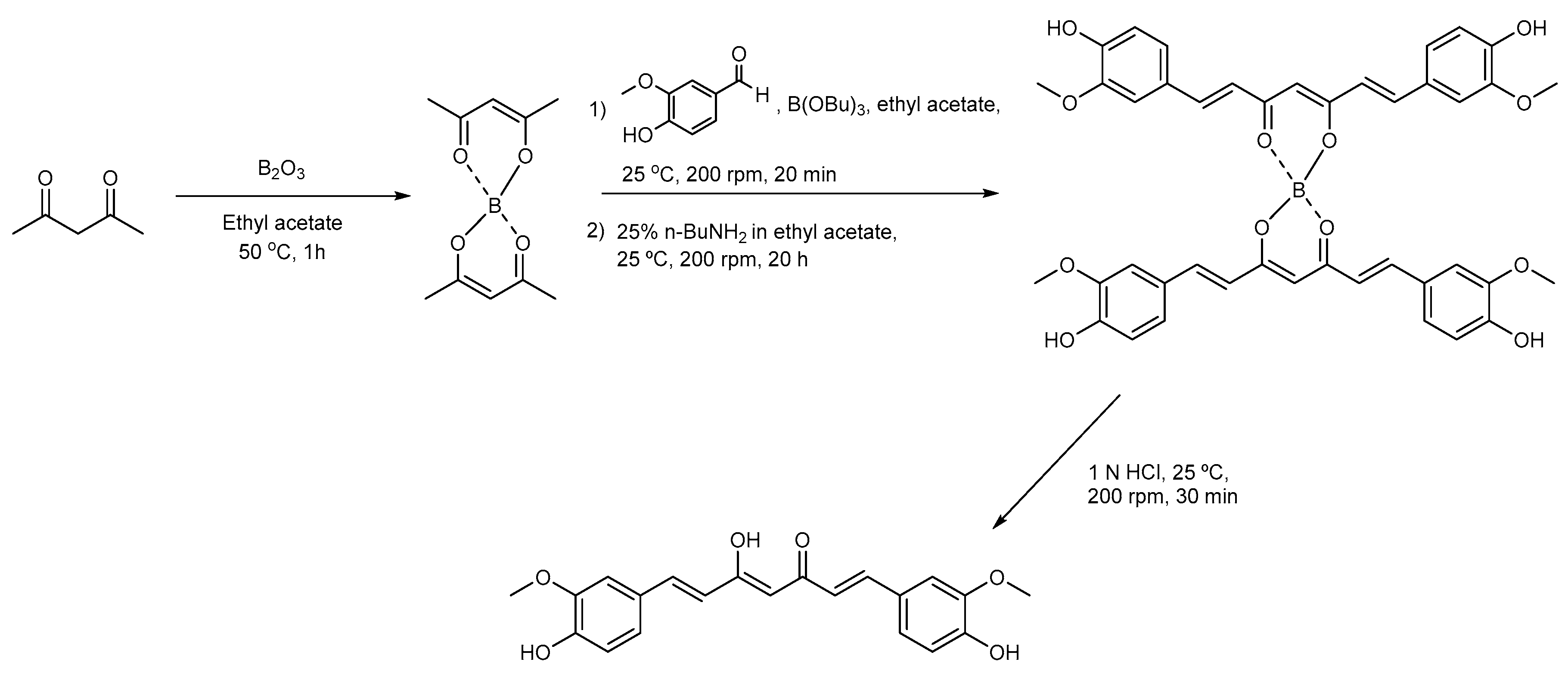
2.1.1. Synthesis of Curcumin
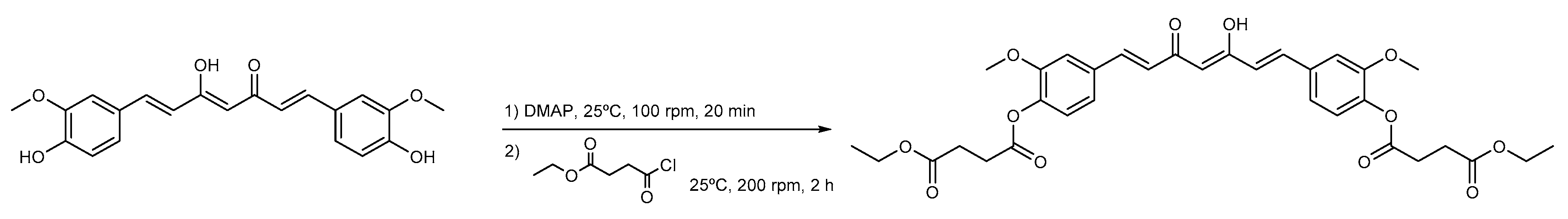
2.1.2. Synthesis of CurDD
2.2. Determination of Physical and Chemical Properties of Curcumin and CurDD Raw Materials
2.2.1. Solubility of Curcumin and CurDD
2.2.2. Partition Coefficient
2.2.3. Determination of Particle Size Distribution
2.2.4. X-Ray Diffraction
2.2.5. Melting Point Measurement
2.3. Animals
2.4. Implantation of Tumor Cells
2.5. Curcumin and CurDD Treatments
2.6. Aspartate Aminotransferase, Alanine Aminotransferase and VEGF Determination
2.7. Western Blot Analysis for Tumor Tissues
2.8. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of Curcumin and CurDD
3.2. Physical and Chemical Properties of Curcumin and CurDD
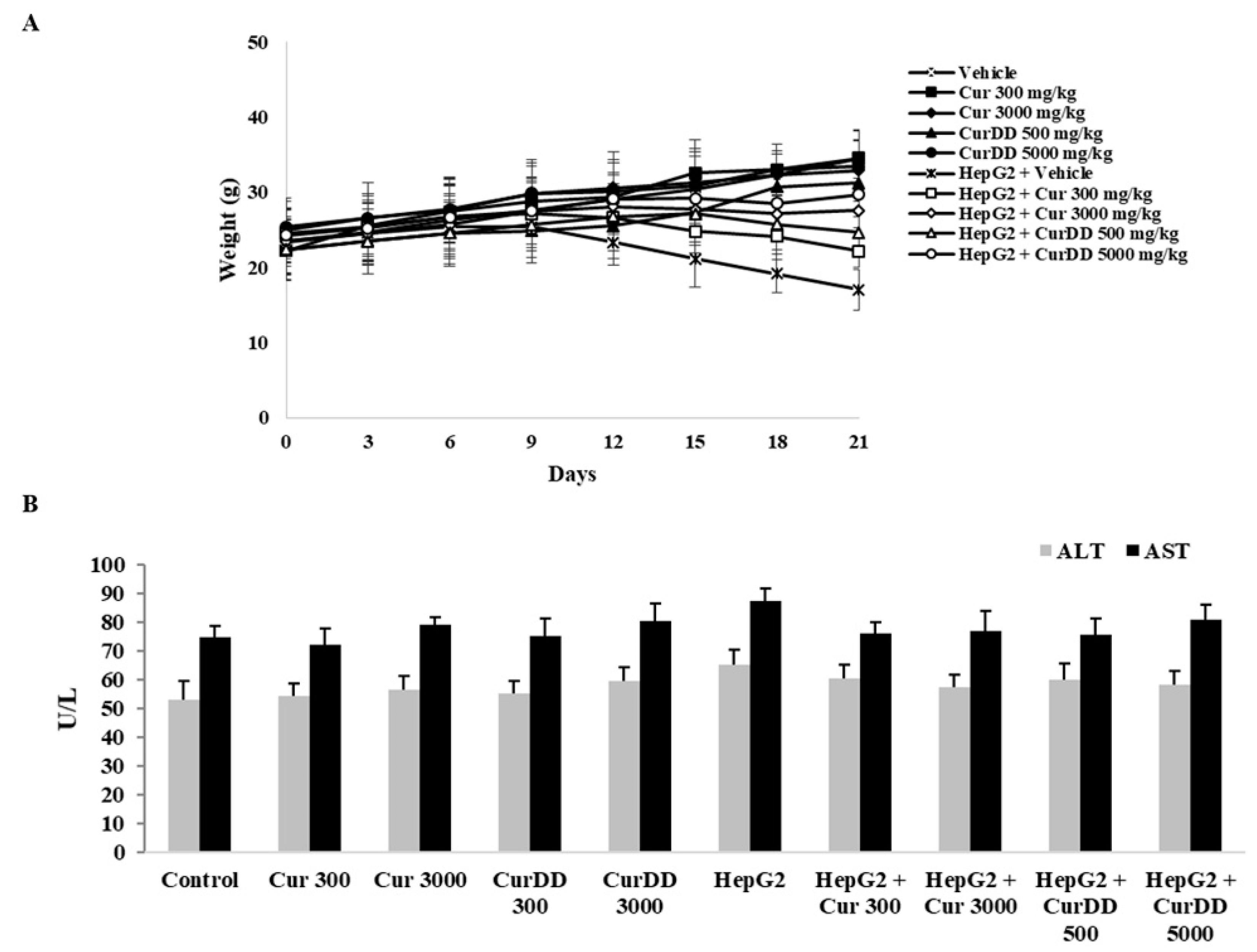
3.3. Effects of Curcumin and CurDD on Body Weight, AST and ALT Activities
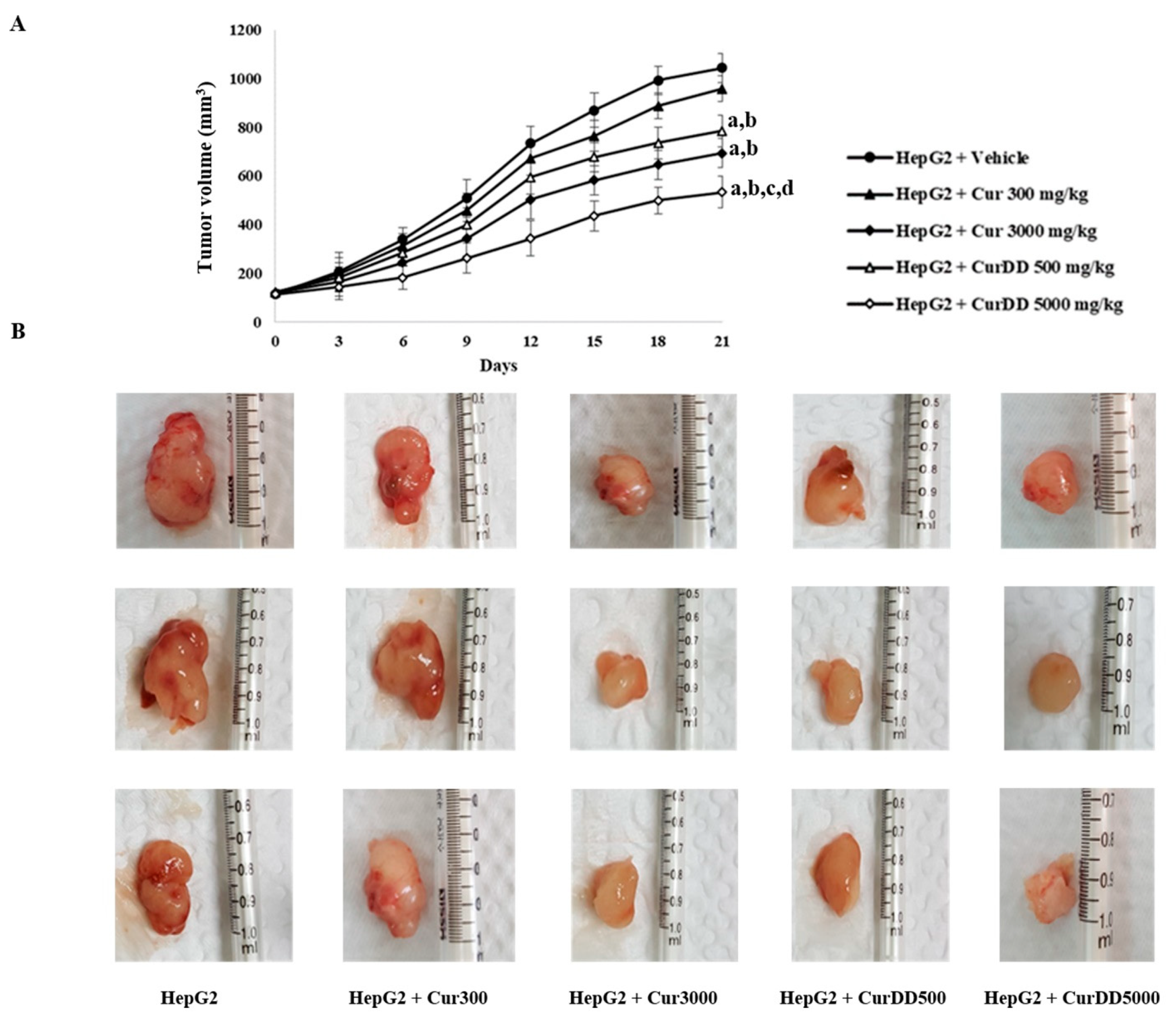
3.4. Inhibition of Tumor Growth by Curcumin and CurDD
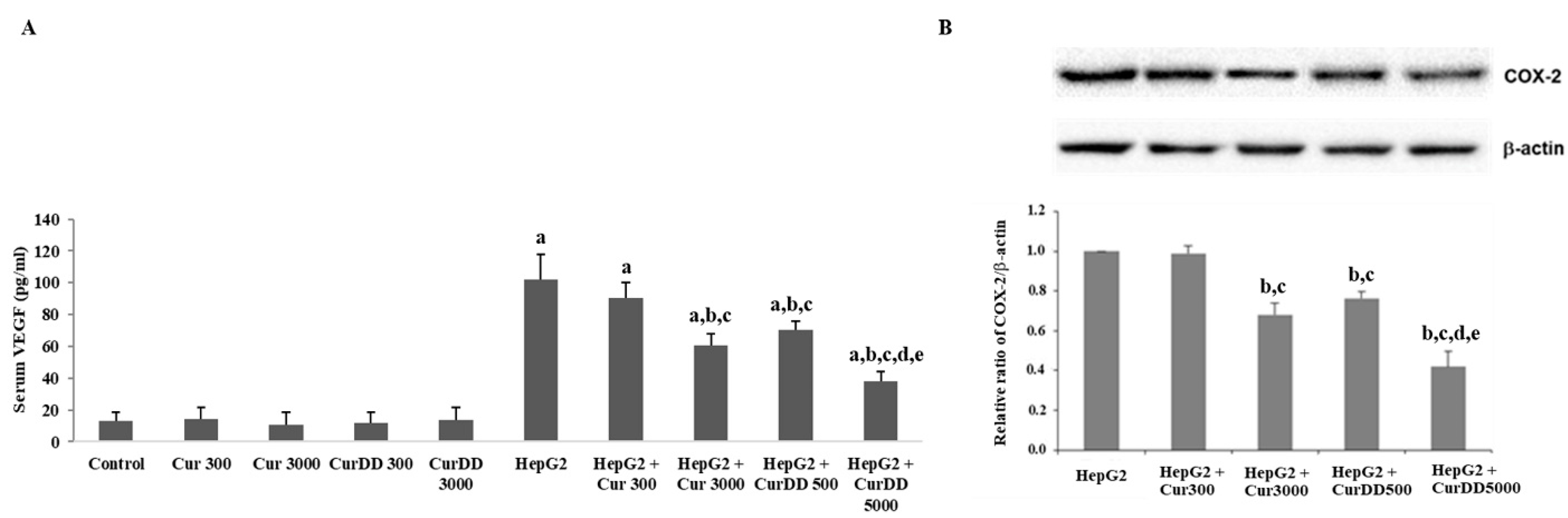
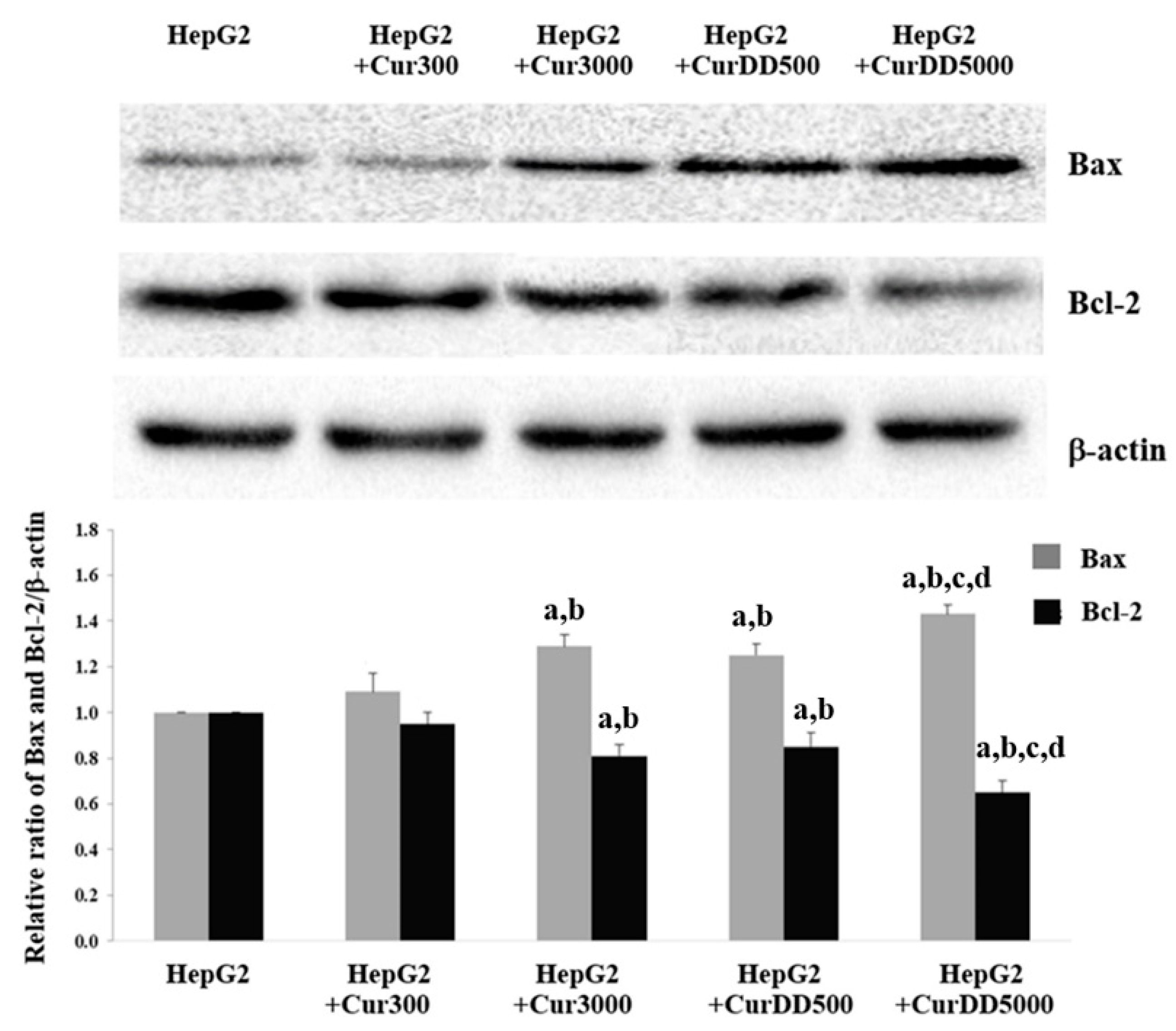
3.5. Effects of Curcumin and CurDD on VEGF Secretion, COX-2, Bax and Bcl-2 Expression
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nonn, L.; Duong, D.; Peehl, D.M. Chemopreventive anti-inflammatory activities of curcumin and other phytochemicals mediated by MAP kinase phosphatase-5 in prostate cells. Carcinogenesis 2007, 28, 1188–1196. [Google Scholar] [CrossRef]
- Basnet, P.; Skalko-Basnet, N. Curcumin: An anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules 2011, 16, 4567–4598. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Kinniry, P.A.; Arguiri, E.; Serota, M.; Kanterakis, S.; Chatterjee, S.; Solomides, C.C.; Javvadi, P.; Koumenis, C.; Cengel, K.A.; et al. Dietary curcumin increases antioxidant defenses in lung, ameliorates radiation-induced pulmonary fibrosis, and improves survival in mice. Radiat. Res. 2010, 173, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Yoysungnoen, P.; Wirachwong, P.; Changtam, C.; Suksamrarn, A.; Patumraj, S. Anti-cancer and anti-angiogenic effects of curcumin and tetrahydrocurcumin on implanted hepatocellular carcinoma in nude mice. World J. Gastroenterol. 2008, 14, 2003–2009. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Innao, V.; Russo, S.; Gerace, D.; Alonci, A.; Musolino, C. Anticancer activity of curcumin and its analogues: Preclinical and clinical studies. Cancer Investig. 2017, 35, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Karunagaran, D.; Rashmi, R.; Kumar, T.R.S. Induction of apoptosis by curcumin and its implications for cancer therapy. Curr. Cancer Drug Targets 2005, 5, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Eifes, S.; Dicato, M.; Aggarwal, B.B.; Diederich, M. Modulation of anti-apoptotic and survival pathways by curcumin as a strategy to induce apoptosis in cancer cells. Biochem. Pharmacol. 2008, 76, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, J.; Prasad, S.; Aggarwal, B.B. Curcumin and cancer cells: How many ways can curry kill tumor cells selectively? AAPS J. 2009, 11, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Mortezaee, K.; Salehi, E.; Mirtavoos-mahyari, H.; Motevaseli, E.; Najafi, M.; Farhood, B.; Rosengren, R.J.; Sahebkar, A. Mechanisms of apoptosis modulation by curcumin: Implications for cancer therapy. J. Cell. Physiol. 2019, 234, 12537–12550. [Google Scholar] [CrossRef]
- Ratnatilaka Na Bhuket, P.; El-Magboub, A.; Haworth, I.S.; Rojsitthisak, P. Enhancement of curcumin bioavailability via the prodrug approach: Challenges and prospects. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 341–353. [Google Scholar] [CrossRef]
- Wichitnithad, W.; Nimmannit, U.; Wacharasindhu, S.; Rojsitthisak, P. Synthesis, characterization and biological evaluation of succinate prodrugs of curcuminoids for colon cancer treatment. Molecules 2011, 16, 1888–1900. [Google Scholar] [CrossRef] [PubMed]
- Wongsrisakul, J.; Wichitnithad, W.; Rojsitthisak, P.; Towiwat, P. Antinociceptive effects of curcumin diethyl disuccinate in animal models. J. Health Res. 2010, 24, 175–180. [Google Scholar]
- Bangphumi, K.; Kittiviriyakul, C.; Towiwat, P.; Rojsitthisak, P.; Khemawoot, P. Pharmacokinetics of curcumin diethyl disuccinate, a prodrug of curcumin, in Wistar rats. Eur. J. Drug Metab. Pharmacokinet. 2016, 41, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Ratnatilaka Na Bhuket, P.; Niwattisaiwong, N.; Limpikirati, P.; Khemawoot, P.; Towiwat, P.; Ongpipattanakul, B.; Rojsitthisak, P. Simultaneous determination of curcumin diethyl disuccinate and its active metabolite curcumin in rat plasma by LC–MS/MS: Application of esterase inhibitors in the stabilization of an ester-containing prodrug. J. Chromatogr. B 2016, 1033–1034, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Ratnatilaka Na Bhuket, P.; Jithavech, P.; Ongpipattanakul, B.; Rojsitthisak, P. Interspecies differences in stability kinetics and plasma esterases involved in hydrolytic activation of curcumin diethyl disuccinate, a prodrug of curcumin. RSC Adv. 2019, 9, 4626–4634. [Google Scholar] [CrossRef]
- Aravalli, R.N.; Cressman, E.N.K.; Steer, C.J. Cellular and molecular mechanisms of hepatocellular carcinoma: An update. Arch. Toxicol. 2013, 87, 227–247. [Google Scholar] [CrossRef] [PubMed]
- Chitapanarux, T.; Phornphutkul, K. Risk factors for the development of hepatocellular carcinoma in Thailand. J. Clin. Transl. Hepatol. 2015, 3, 182–188. [Google Scholar] [CrossRef]
- Cabibbo, G.; Craxi, A. Epidemiology, risk factors and surveillance of hepatocellular carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 352–355. [Google Scholar]
- Srisuttee, R.; Koh, S.S.; Park, E.H.; Cho, I.R.; Min, H.J.; Jhun, B.H.; Yu, D.Y.; Park, S.; Park, D.Y.; Lee, M.O.; et al. Up-regulation of Foxo4 mediated by hepatitis B virus X protein confers resistance to oxidative stress-induced cell death. Inter. J. Mol. Med. 2011, 28, 255–260. [Google Scholar]
- Wang, M.; Ruan, Y.; Chen, Q.; Li, S.; Wang, Q.; Cai, J. Curcumin induced HepG2 cell apoptosis-associated mitochondrial membrane potential and intracellular free Ca2+ concentration. Eur. J. Pharmacol. 2011, 650, 41–47. [Google Scholar] [CrossRef]
- Shoji, M.; Nakagawa, K.; Watanabe, A.; Tsuduki, T.; Yamada, T.; Kuwahara, S.; Kimura, F.; Miyazawa, T. Comparison of the effects of curcumin and curcumin glucuronide in human hepatocellular carcinoma HepG2 cells. Food Chem. 2014, 151, 126–132. [Google Scholar] [CrossRef]
- Yoysungnoen, P.; Wirachwong, P.; Bhattarakosol, P.; Niimi, H.; Patumraj, S. Antiangiogenic activity of curcumin in hepatocellular carcinoma cells implanted nude mice. Clin. Hemorheol. Microcirc. 2005, 33, 127–135. [Google Scholar]
- OECD. Test No. 105: Water Solubility; OECD Publishing: Paris, France, 1995.
- Muangnoi, C.; Jithavech, P.; Ratnatilaka Na Bhuket, P.; Supasena, W.; Wichitnithad, W.; Towiwat, P.; Niwattisaiwong, N.; Haworth, I.; Rojsitthisak, P. A curcumin-diglutaric acid conjugated prodrug with improved water solubility and antinociceptive properties compared to curcumin. Biosci. Biotechnol. Biochem. 2018, 82, 1301–1308. [Google Scholar] [CrossRef]
- OECD. Test No. 117: Partition Coefficient (n-Octanol/Water), HPLC Method; OECD Publishing: Paris, France, 2004.
- Harris, R.E. Cyclooxygenase-2 (cox-2) blockade in the chemoprevention of cancers of the colon, breast, prostate, and lung. Inflammopharmacology 2009, 17, 55–67. [Google Scholar] [CrossRef]
- Yoysungnoen, P.; Wirachwong, P.; Bhattarakosol, P.; Niimi, H.; Patumraj, S. Effects of curcumin on tumor angiogenesis and biomarkers, COX-2 and VEGF, in hepatocellular carcinoma cell-implanted nude mice. Clin. Hemorheol. Microcirc. 2006, 34, 109–115. [Google Scholar]
- Olivera, A.; Moore, T.W.; Hu, F.; Brown, A.P.; Sun, A.; Liotta, D.C.; Snyder, J.P.; Yoon, Y.; Shim, H.; Marcus, A.I.; et al. Inhibition of the NF-κB signaling pathway by the curcumin analog, 3,5-Bis(2-pyridinylmethylidene)-4-piperidone (EF31): Anti-inflammatory and anti-cancer properties. Int. Immunopharmacol. 2012, 12, 368–377. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, T.; Wang, X.; Wei, X.; Chen, Y.; Guo, L.; Zhang, J.; Wang, C. Curcumin modulates macrophage polarization through the inhibition of the toll-like receptor 4 expression and its signaling pathways. Cell. Physiol. Biochem. 2015, 36, 631–641. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muangnoi, C.; Ratnatilaka Na Bhuket, P.; Jithavech, P.; Wichitnithad, W.; Srikun, O.; Nerungsi, C.; Patumraj, S.; Rojsitthisak, P. Scale-Up Synthesis and In Vivo Anti-Tumor Activity of Curcumin Diethyl Disuccinate, an Ester Prodrug of Curcumin, in HepG2-Xenograft Mice. Pharmaceutics 2019, 11, 373. https://doi.org/10.3390/pharmaceutics11080373
Muangnoi C, Ratnatilaka Na Bhuket P, Jithavech P, Wichitnithad W, Srikun O, Nerungsi C, Patumraj S, Rojsitthisak P. Scale-Up Synthesis and In Vivo Anti-Tumor Activity of Curcumin Diethyl Disuccinate, an Ester Prodrug of Curcumin, in HepG2-Xenograft Mice. Pharmaceutics. 2019; 11(8):373. https://doi.org/10.3390/pharmaceutics11080373
Chicago/Turabian StyleMuangnoi, Chawanphat, Pahweenvaj Ratnatilaka Na Bhuket, Ponsiree Jithavech, Wisut Wichitnithad, Onsiri Srikun, Chakkrapan Nerungsi, Suthiluk Patumraj, and Pornchai Rojsitthisak. 2019. "Scale-Up Synthesis and In Vivo Anti-Tumor Activity of Curcumin Diethyl Disuccinate, an Ester Prodrug of Curcumin, in HepG2-Xenograft Mice" Pharmaceutics 11, no. 8: 373. https://doi.org/10.3390/pharmaceutics11080373
APA StyleMuangnoi, C., Ratnatilaka Na Bhuket, P., Jithavech, P., Wichitnithad, W., Srikun, O., Nerungsi, C., Patumraj, S., & Rojsitthisak, P. (2019). Scale-Up Synthesis and In Vivo Anti-Tumor Activity of Curcumin Diethyl Disuccinate, an Ester Prodrug of Curcumin, in HepG2-Xenograft Mice. Pharmaceutics, 11(8), 373. https://doi.org/10.3390/pharmaceutics11080373