Application of Direct Sonoporation from a Defined Surface Area of the Peritoneum: Evaluation of Transfection Characteristics in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. pDNA
2.2. Preparation of Nanobubbles
2.3. Animals
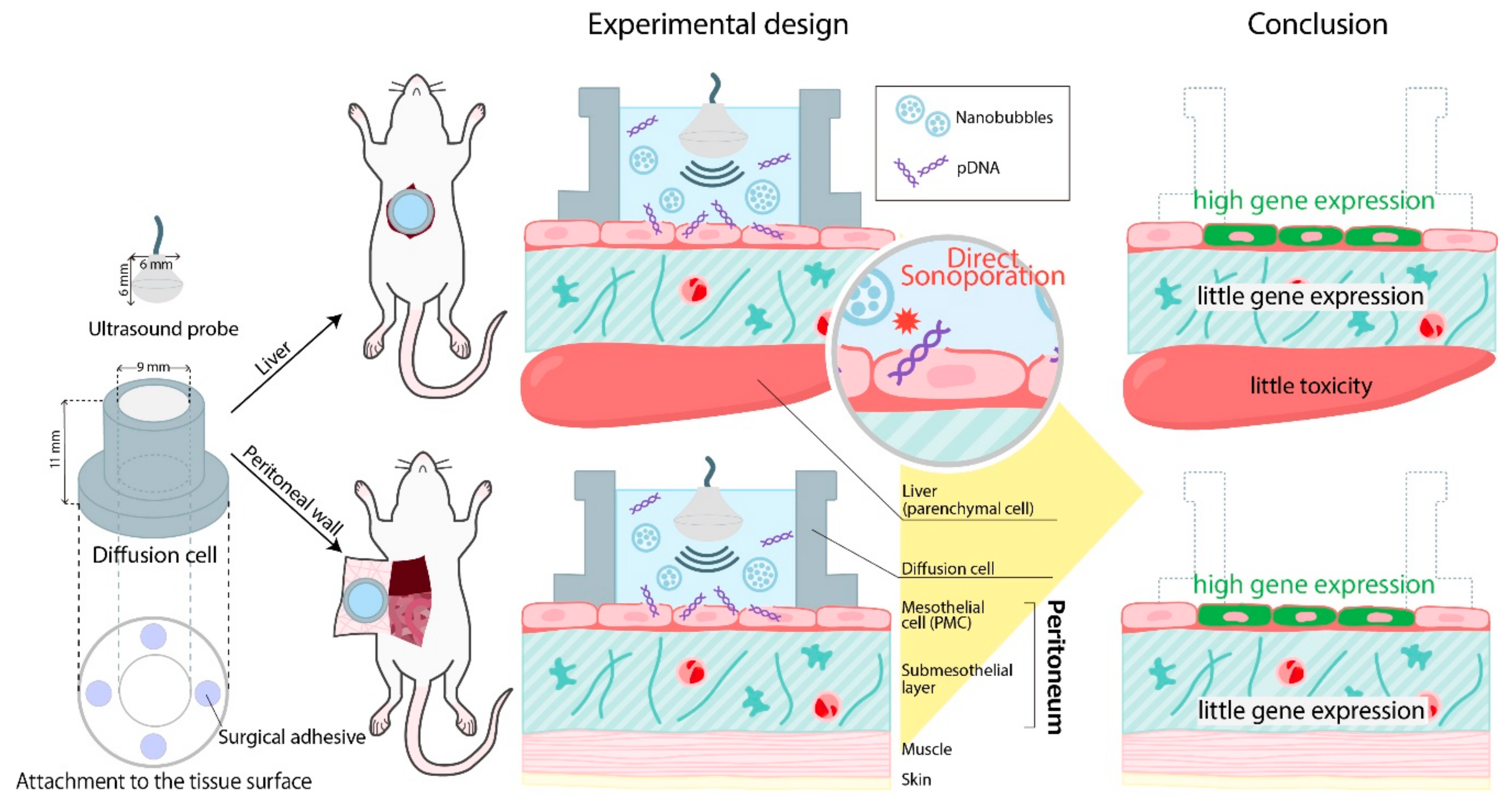
2.4. Direct Sonoporation Method into the Peritoneum from the Peritoneal Tissue Surface
2.5. Luciferase Assay
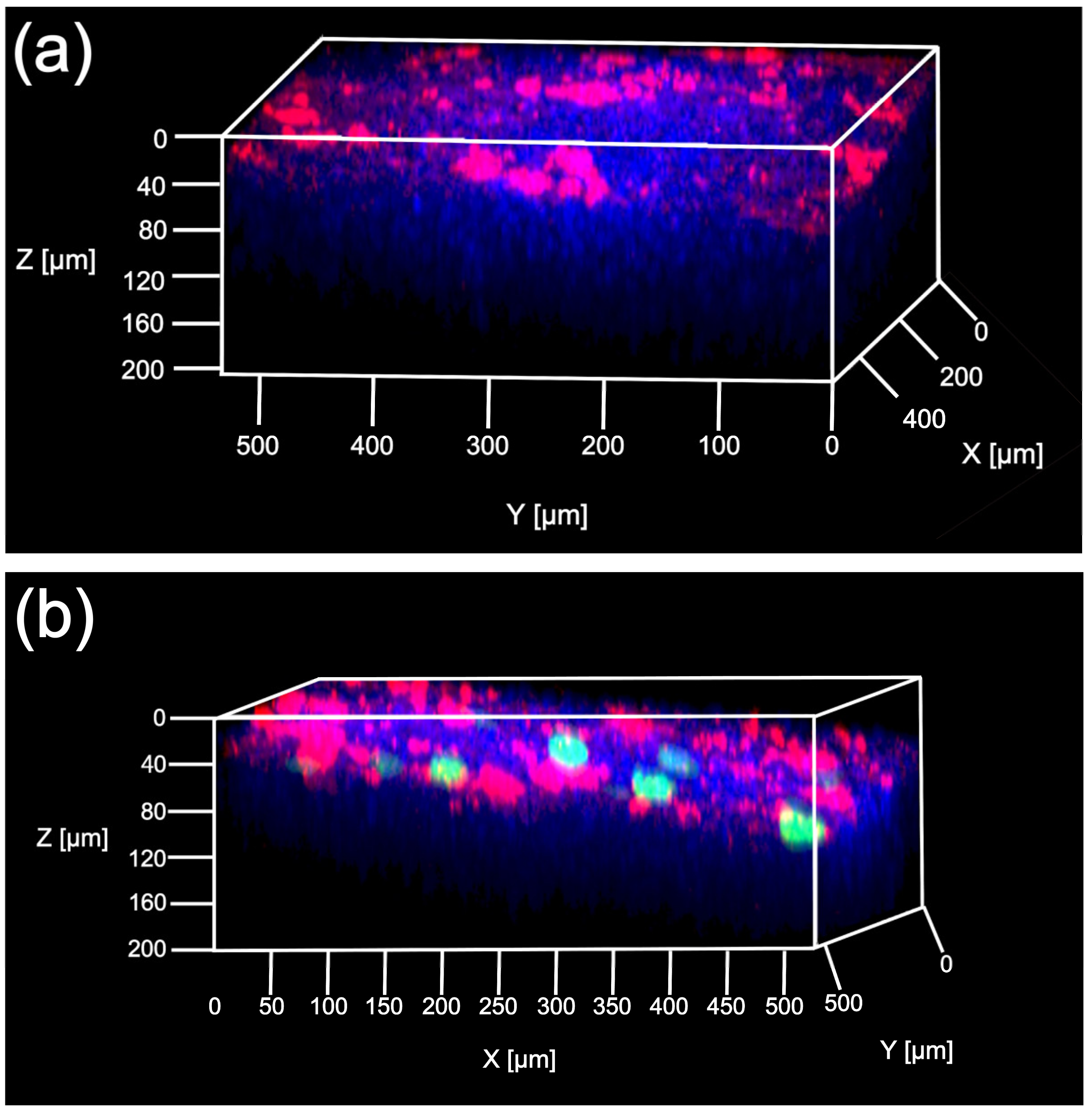
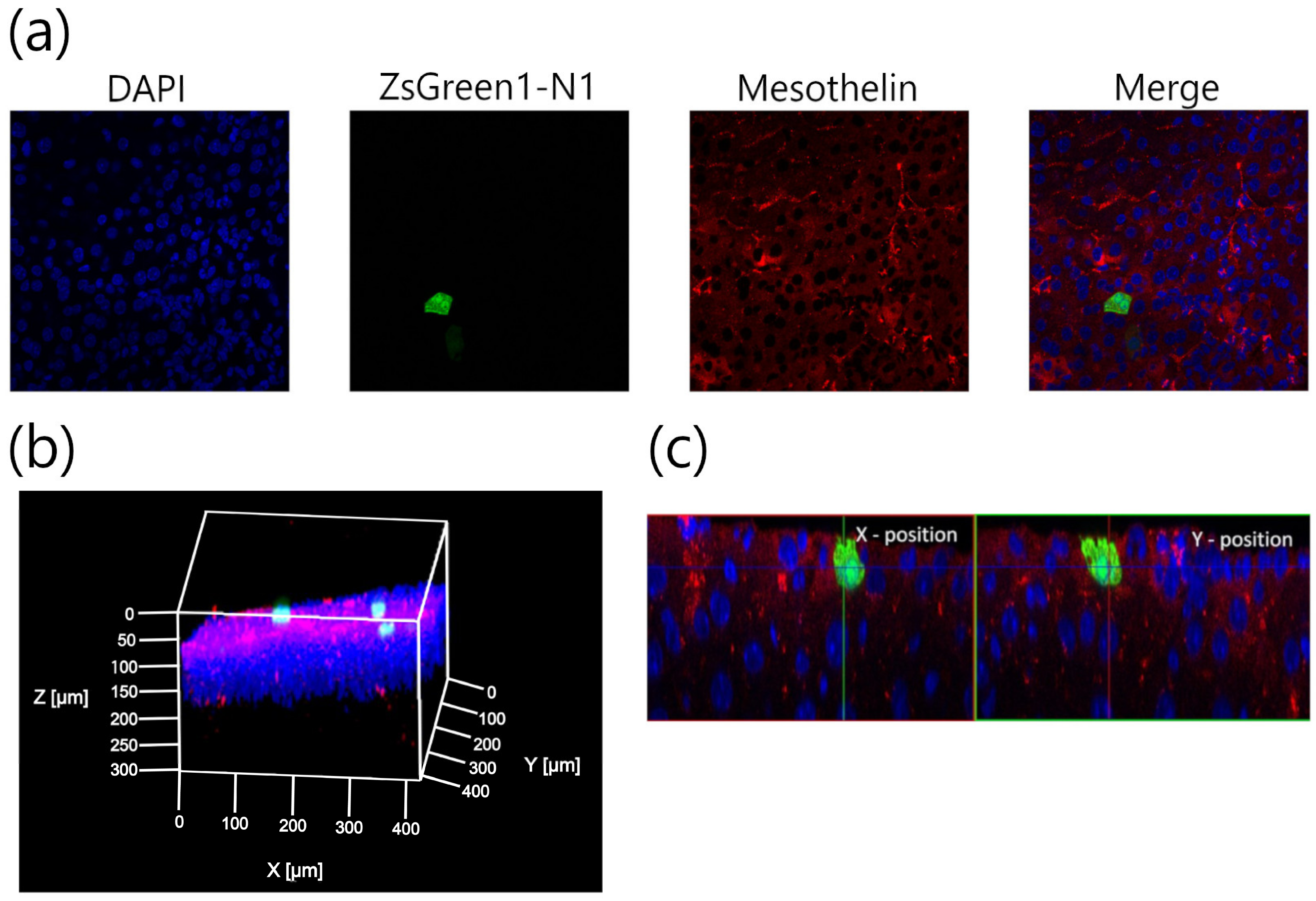
2.6. Multicolor Deep Imaging Analysis
2.7. Histological Evaluation
2.8. Assessment of Serum Alanine Aminotransferase (ALT) Level
2.9. Statistical Analysis
3. Results
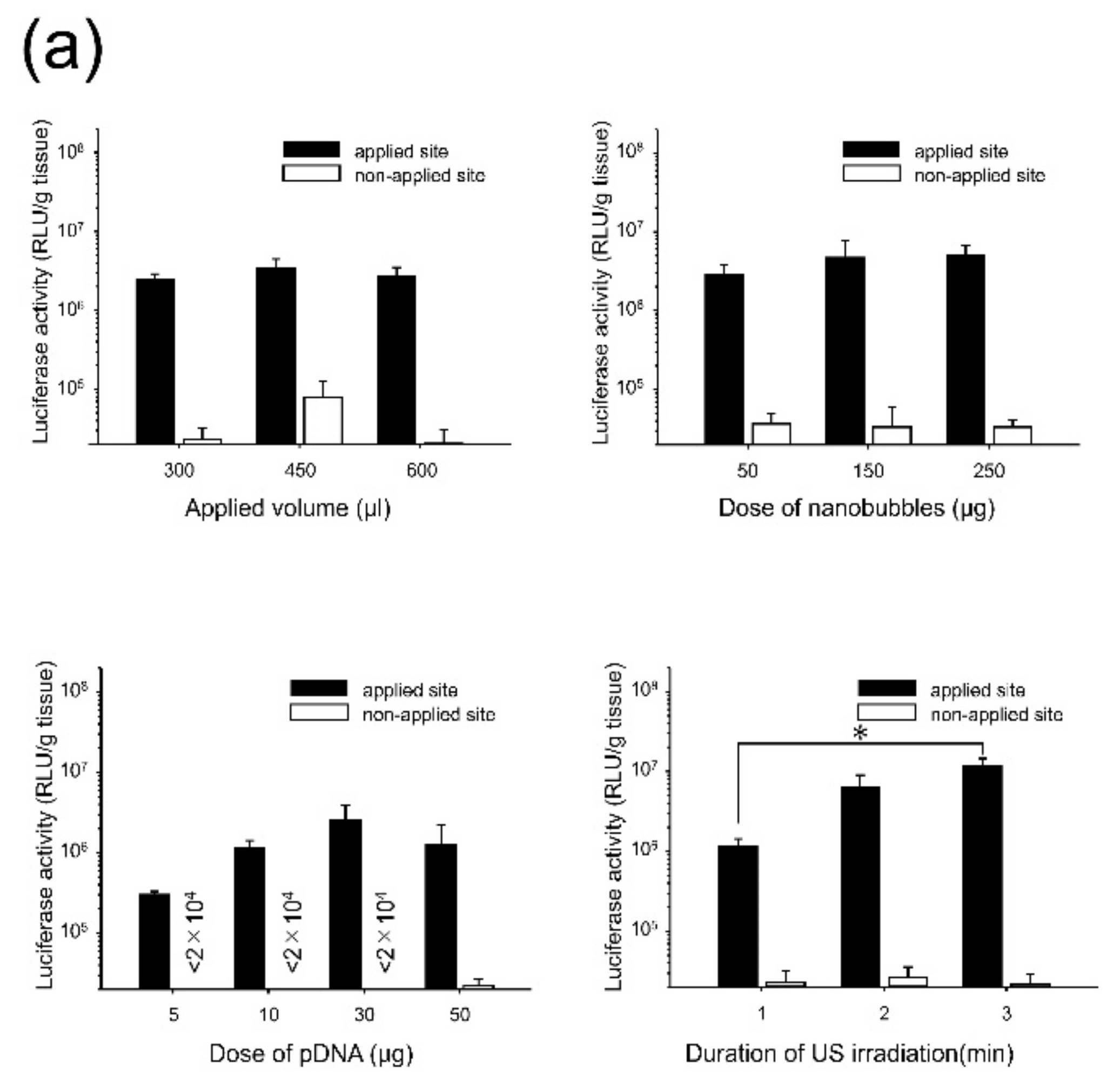
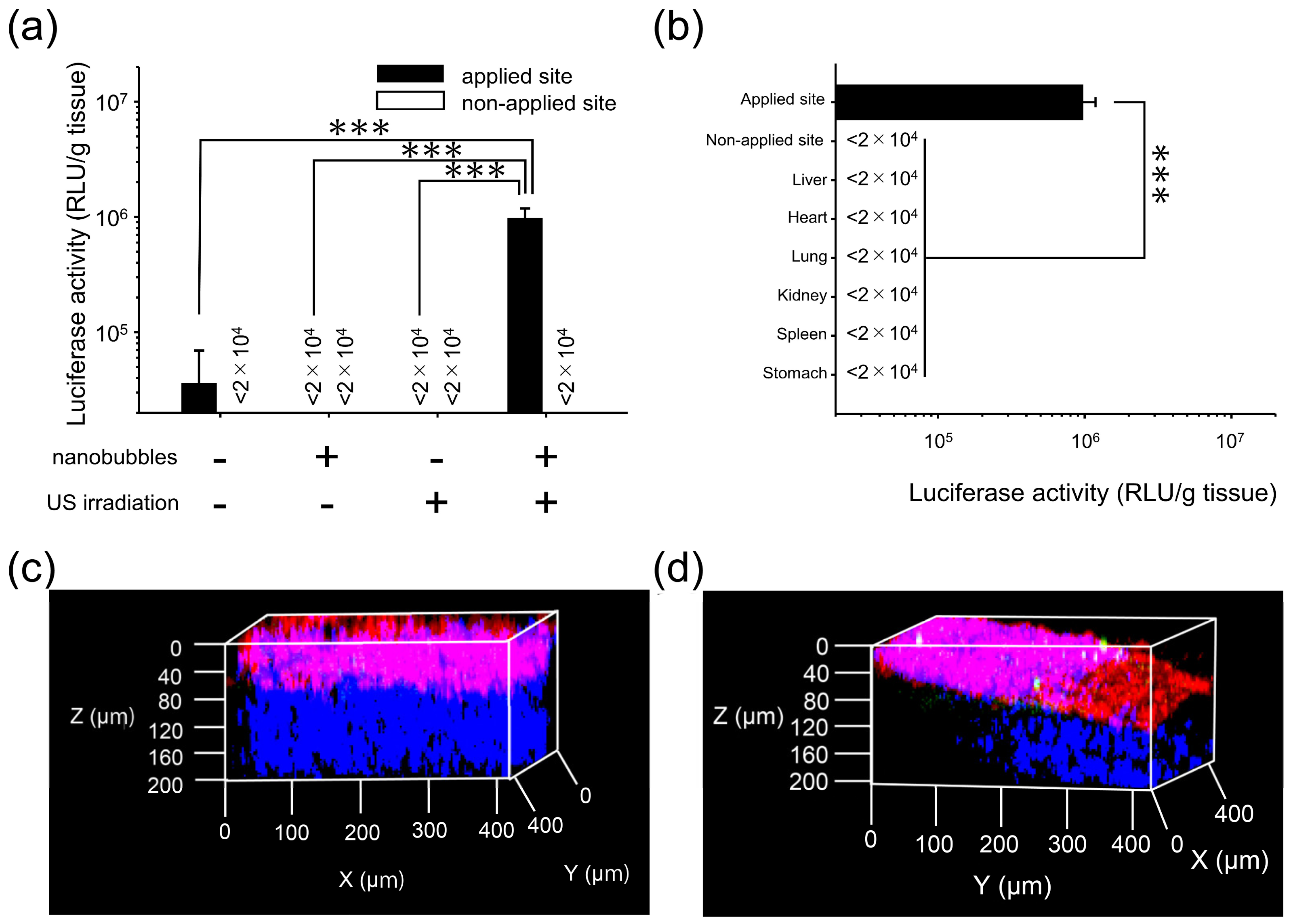
3.1. Effects of Direct Sonoporation to the Visceral Peritoneum from the Liver Surface
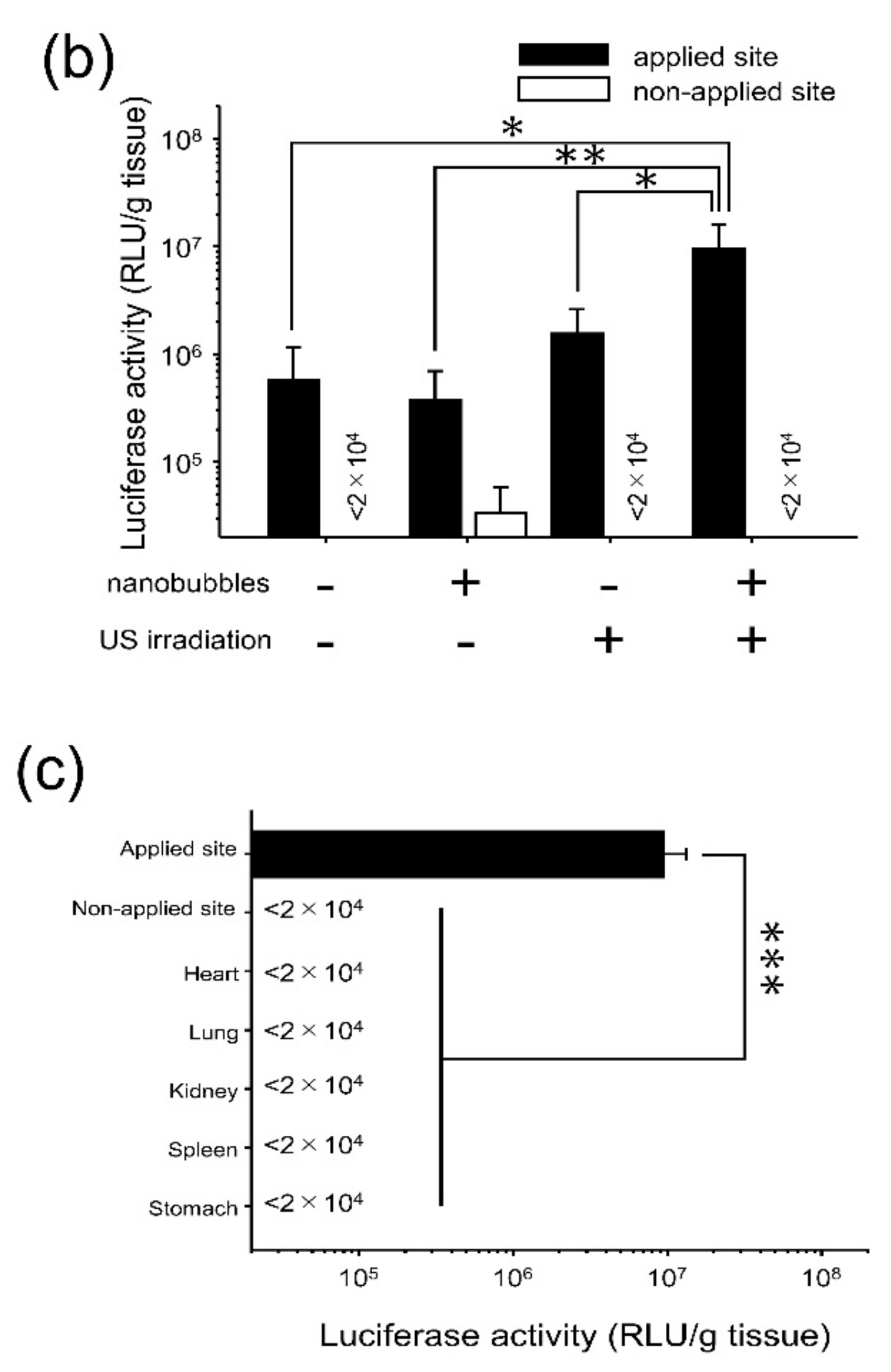
3.2. Characterization of Transfection Expression by Direct Sonoporation to the Visceral Peritoneum from the Liver Surface
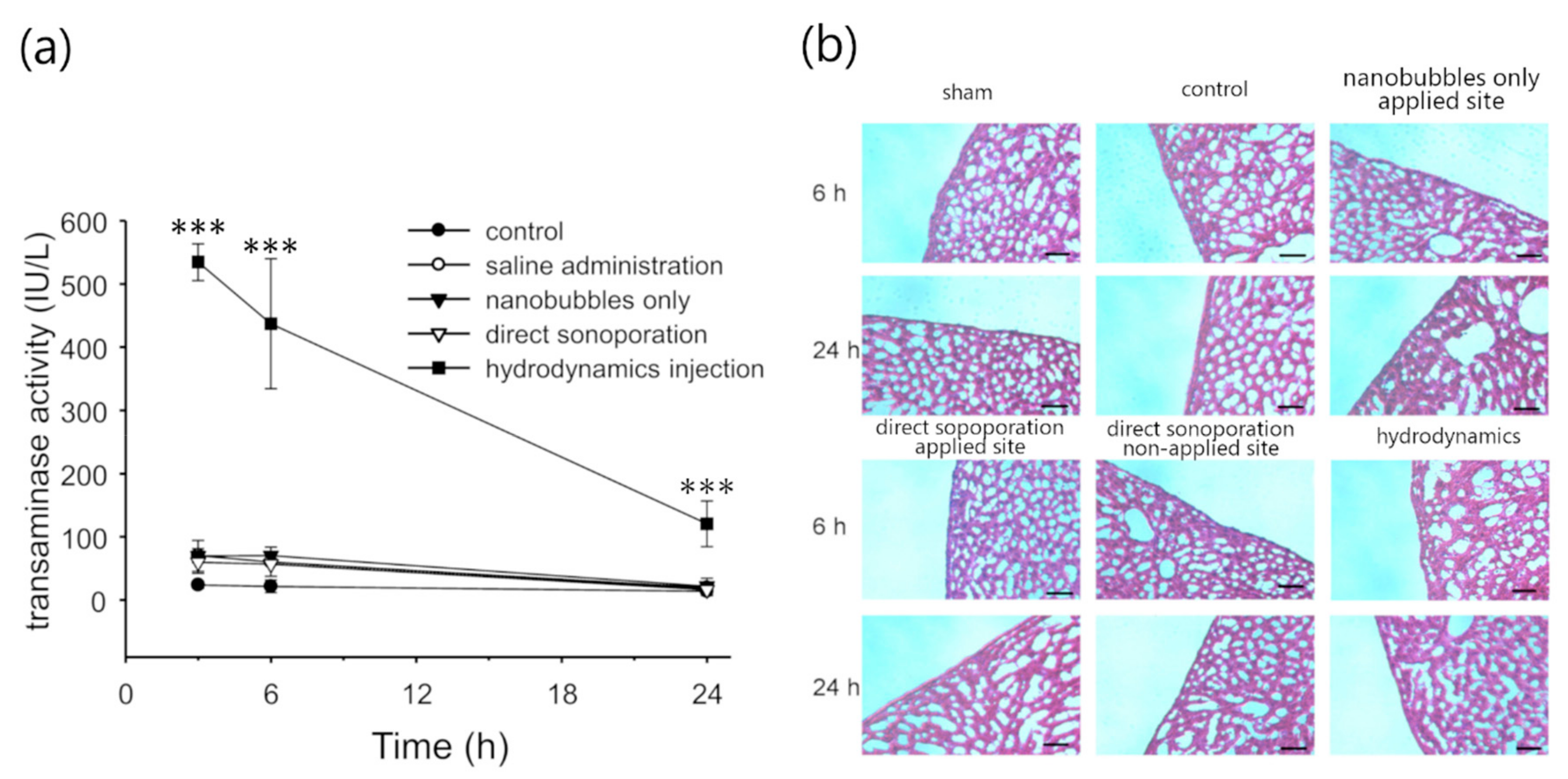
3.3. Assessment of Hepatic Toxicity Caused by Direct Sonoporation to the Visceral Peritoneum from the Liver Surface
3.4. Characterization of Transfection Expression by Direct Sonoporation to The Parietal Peritoneum from the Peritoneal Wall Surface
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Devuyst, O.; Margetts, P.J.; Topley, N. The pathophysiology of the peritoneal membrane. J. Am. Soc. Nephrol. 2010, 21, 1077–1085. [Google Scholar] [CrossRef]
- Mehrotra, R.; Devuyst, O.; Davies, S.J.; Johnson, D.W. The current state of peritoneal dialysis. J. Am. Soc. Nephrol. 2016, 27, 3238–3252. [Google Scholar] [CrossRef]
- Clifford, J.H.; Dirk, F. Peritoneal dialysis solution biocompatibility: Definitions and evaluation strategies. Kidney Int. 2003, 64, S50–S56. [Google Scholar]
- Selgas, R.; Bajo, A.; Jiménez-Heffernan, J.A.; Sánchez-Tomero, J.A.; Del Peso, G.; Aguilera, A.; López-Cabrera, M. Epithelial-to-mesenchymal transition of the mesothelial cell--its role in the response of the peritoneum to dialysis. Nephrol. Dial. Transplant. 2006, 21, ii2–ii7. [Google Scholar] [CrossRef]
- Osada, S.; Ebihara, I.; Setoguchi, Y.; Takahashi, H.; Tomino, Y.; Koide, H. Gene therapy for renal anemia in mice with polycystic kidney using an adenovirus vector encoding the human erythropoietin gene. Kidney Int. 1999, 55, 1234–1240. [Google Scholar] [CrossRef][Green Version]
- Setoguchi, Y.; Danel, C.; Crystal, R.G. Stimulation of erythropoiesis by in vivo gene therapy: Physiologic consequences of transfer of the human erythropoietin gene to experimental animals using an adenovirus vector. Blood 1994, 84, 2946–2953. [Google Scholar] [PubMed]
- Lee, M.J.; Cho, S.S.; You, J.R.; Lee, Y.; Kang, B.D.; Choi, J.S.; Park, J.W.; Suh, Y.L.; Kim, J.A.; Kim, D.K.; et al. Intraperitoneal gene delivery mediated by a novel cationic liposome in a peritoneal disseminated ovarian cancer model. Gene Ther. 2002, 9, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Yoshida, T.; Sugimura, T.; Terada, M. Liposome-mediated in vivo gene transfer of antisense K-ras construct inhibits pancreatic tumor dissemination in the murine peritoneal cavity. Cancer Res. 1995, 55, 3810–3816. [Google Scholar]
- Louis, M.H.; Dutoit, S.; Denoux, Y.; Erbacher, P.; Deslandes, E.; Behr, J.P.; Gauduchon, P.; Poulain, L. Intraperitoneal linear polyethylenimine (L-PEI)-mediated gene delivery to ovarian carcinoma nodes in mice. Cancer Gene Ther. 2006, 13, 367–374. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ogawa, K.; Fuchigami, Y.; Hagimori, M.; Fumoto, S.; Maruyama, K.; Kawakami, S. Ultrasound-responsive nanobubble-mediated gene transfection in the cerebroventricular region by intracerebroventricular administration in mice. Eur. J. Pharm. Biopharm. 2019, 137, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Manta, S.; Renault, G.; Delalande, A.; Couture, O.; Lagoutte, I.; Seguin, J.; Lager, F.; Houzé, P.; Midoux, P.; Bessodes, M.; et al. Cationic microbubbles and antibiotic-free miniplasmid for sustained ultrasound–mediated transgene expression in liver. J. Control Release 2017, 262, 170–181. [Google Scholar] [CrossRef]
- Kurosaki, T.; Kawakami, S.; Higuchi, Y.; Suzuki, R.; Maruyama, K.; Sasaki, H.; Yamashita, F.; Hashida, M. Kidney-selective gene transfection using anionic bubble lipopolyplexes with renal ultrasound irradiation in mice. Nanomedicine 2014, 10, 1829–1838. [Google Scholar] [CrossRef]
- Suzuki, R.; Takizawa, T.; Negishi, Y.; Hagisawa, K.; Tanaka, K.; Sawamura, K.; Utoguchi, N.; Nishioka, T.; Maruyama, K. Gene delivery by combination of novel liposomal bubbles with perfluoropropane and ultrasound. J. Control Release 2007, 117, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wan, J.M.; Yu, A.C. Membrane perforation and recovery dynamics in microbubble-mediated sonoporation. Ultrasound Med. Biol. 2013, 39, 2393–2405. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Fumoto, S.; Fuchigami, Y.; Hagimori, M.; Maruyama, K.; Kawakami, S. Effective intraperitoneal gene transfection system using nanobubbles and ultrasound irradiation. Drug Deliv. 2017, 24, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Leung, J.C.; Chan, L.Y.; Tsang, A.W.; Lam, M.F.; Lan, H.Y.; Lai, K.N. Ultrasound-contrast agent mediated naked gene delivery in the peritoneal cavity of adult rat. Gene Ther. 2007, 14, 1712–1720. [Google Scholar] [CrossRef]
- Machi, J.; Schwartz, J.H.; Zaren, H.A.; Noritomi, T.; Sigel, B. Technique of laparoscopic ultrasound examination of the liver and pancreas. Surg. Endosc. 1996, 10, 684–689. [Google Scholar] [CrossRef]
- Letterie, G.S.; Marshall, L. Evaluation of real-time imaging using a laparoscopic ultrasound probe during operative endoscopic procedures. Ultrasound Obstet. Gynecol. 2000, 16, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.A.; Shin, K.S.; Kim, J.H.; Kim, Y.I.; Chung, S.S.; Park, S.H.; Kim, Y.L.; Kang, D.H. HGF and BMP-7 ameliorate high glucose-induced epithelial-to-mesenchymal transition of peritoneal mesothelium. J. Am. Soc. Nephrol. 2009, 20, 567–581. [Google Scholar] [CrossRef]
- Matsuoka, T.; Maeda, Y.; Matsuo, K.; Naiki, Y.; Tamai, Y.; Sakaguchi, M.; Hasegawa, H.; Funauchi, M.; Kanamaru, A. Hepatocyte growth factor prevents peritoneal fibrosis in an animal model of encapsulating peritoneal sclerosis. J. Nephrol. 2008, 21, 64–73. [Google Scholar]
- Hirayama, R.; Nishida, K.; Fumoto, S.; Nakashima, M.; Sasaki, H.; Nakamura, J. Liver site-specific gene transfer following the administration of naked plasmid DNA to the liver surface in mice. Biol. Pharm. Bull. 2004, 27, 1697–1699. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Hewage, S.A.; Batagoda, J.H. Stability of nanobubbles. Environ. Eng. Sci. 2018, 35, 1216–1227. [Google Scholar] [CrossRef]
- Wei, X.; Shao, B.; He, Z.; Ye, T.; Luo, M.; Sang, Y.; Liang, X.; Wang, W.; Luo, S.; Yang, S.; et al. Cationic nanocarriers induce cell necrosis through impairment of Na(+)/K(+)-ATPase and cause subsequent inflammatory response. Cell Res. 2015, 25, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Peruzzi, G.; Sinibaldi, G.; Silvani, G.; Ruocco, G.; Casciola, C.M. Perspectives on cavitation enhanced endothelial layer permeability. Colloids Surf. B Biointerfaces 2018, 168, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Nhan, T.; Burgess, A.; Cho, E.E.; Stefanovic, B.; Lilge, L.; Hynynen, K. Drug delivery to the brain by focused ultrasound induced blood–brain barrier disruption: Quantitative evaluation of enhanced permeability of cerebral vasculature using two-photon microscopy. J. Control Release 2013, 172, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.L.; Smith, N.B.; Bailey, M.R.; Czarnota, G.J.; Hynynen, K.; Makin, I.R. Overview of therapeutic ultrasound applications and safety considerations. J. Ultrasound Med. 2012, 31, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, S.; Hirayama, R.; Shoji, K.; Kawanami, R.; Nishida, K.; Nakashima, M.; Sasaki, H.; Sakaeda, T.; Nakamura, J. Liver- and lobe-selective gene transfection following the instillation of plasmid DNA to the liver surface in mice. Biochem. Biophys. Res. Commun. 2002, 294, 46–50. [Google Scholar] [CrossRef]
- Kawai, S.; Takagi, Y.; Kaneko, S.; Kurosawa, T. Effect of three types of mixed anesthetic agents alternate to ketamine in mice. Exp. Anim. 2011, 60, 481–487. [Google Scholar] [CrossRef]
- Un, K.; Kawakami, S.; Suzuki, R.; Maruyama, K.; Yamashita, F.; Hashida, M. Development of an ultrasound-responsive and mannose-modified gene carrier for DNA vaccine therapy. Biomaterials 2010, 31, 7813–7826. [Google Scholar] [CrossRef]
- Fumoto, S.; Nishimura, K.; Nishida, K.; Kawakami, S. Three-dimensional imaging of the intracellular fate of plasmid DNA and transgene expression: ZsGreen1 and tissue clearing method CUBIC are an optimal combination for multicolor deep imaging in murine tissues. PLoS ONE 2016, 11, e0148233. [Google Scholar] [CrossRef]
- Hama, H.; Hioki, H.; Namiki, K.; Hoshida, T.; Kurokawa, H.; Ishidate, F.; Kaneko, T.; Akagi, T.; Saito, T.; Saido, T.; et al. ScaleS: An optical clearing palette for biological imaging. Nat. Neurosci. 2015, 18, 1518–1529. [Google Scholar] [CrossRef]
- Tainaka, K.; Kubota, S.I.; Suyama, T.Q.; Susaki, E.A.; Perrin, D.; Ukai-Tadenuma, M.; Ukai, H.; Ueda, H.R. Whole-body imaging with single-cell resolution by tissue decolorization. Cell 2014, 159, 911–924. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Miyamoto, H.; Shimokawa, K.; Nakashima, M.; Nakayama, M.; Fumoto, S.; Nishida, K. Novel diagnostic method of peritoneal injury using dual macromolecular markers. Biol. Pharm. Bull. 2014, 37, 262–267. [Google Scholar] [CrossRef][Green Version]
- Liu, F.; Song, Y.; Liu, D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999, 6, 1258–1266. [Google Scholar] [CrossRef]
- Oda, Y.; Suzuki, R.; Mori, T.; Takahashi, H.; Natsugari, H.; Omata, D.; Unga, J.; Uruga, H.; Sugii, M.; Kawakami, S.; et al. Development of fluorous lipid-based nanobubbles for efficiently containing perfluoropropane. Int. J. Pharm. 2015, 487, 64–71. [Google Scholar] [CrossRef]
- Song, S.; Shen, Z.; Chen, L.; Brayman, A.A.; Miao, C.H. Explorations of high-intensity therapeutic ultrasound and microbubble-mediated gene delivery in mouse liver. Gene Ther. 2011, 18, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Luo, Y.; Zhang, Y.; Cui, W.; Zhang, D.; Wu, J.; Zhang, J.; Tu, J. The correlation between acoustic cavitation and sonoporation involved in ultrasound-mediated DNA transfection with Polyethylenimine (PEI) in vitro. J. Control Release 2010, 145, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Haslam, D.B. A quantitative and highly sensitive luciferase-based assay for bacterial toxins that inhibit protein synthesis. J. Med. Microbiol. 2005, 54, 1023–1030. [Google Scholar] [CrossRef]
- Simionescu, M.; Simionescu, N. Organization of cell junctions in the peritoneal mesothelium. J. Cell Biol. 1977, 74, 98–110. [Google Scholar] [CrossRef]
- Shimizu, K.; Kawakami, S.; Hayashi, K.; Kinoshita, H.; Kuwahara, K.; Nakao, K.; Hashida, M.; Konishi, S. In vivo site-specific transfection of naked plasmid DNA and siRNAs in mice by using a tissue suction device. PLoS ONE 2012, 7, e41319. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Kawakami, S.; Fuchigami, Y.; Oyama, N.; Yamashita, F.; Konishi, S.; Shimizu, K.; Hashida, M. Optimization of renal transfection using a renal suction-mediated transfection method in mice. J. Drug Target 2016, 24, 450–456. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimura, K.; Yonezawa, K.; Fumoto, S.; Miura, Y.; Hagimori, M.; Nishida, K.; Kawakami, S. Application of Direct Sonoporation from a Defined Surface Area of the Peritoneum: Evaluation of Transfection Characteristics in Mice. Pharmaceutics 2019, 11, 244. https://doi.org/10.3390/pharmaceutics11050244
Nishimura K, Yonezawa K, Fumoto S, Miura Y, Hagimori M, Nishida K, Kawakami S. Application of Direct Sonoporation from a Defined Surface Area of the Peritoneum: Evaluation of Transfection Characteristics in Mice. Pharmaceutics. 2019; 11(5):244. https://doi.org/10.3390/pharmaceutics11050244
Chicago/Turabian StyleNishimura, Koyo, Keita Yonezawa, Shintaro Fumoto, Yusuke Miura, Masayori Hagimori, Koyo Nishida, and Shigeru Kawakami. 2019. "Application of Direct Sonoporation from a Defined Surface Area of the Peritoneum: Evaluation of Transfection Characteristics in Mice" Pharmaceutics 11, no. 5: 244. https://doi.org/10.3390/pharmaceutics11050244
APA StyleNishimura, K., Yonezawa, K., Fumoto, S., Miura, Y., Hagimori, M., Nishida, K., & Kawakami, S. (2019). Application of Direct Sonoporation from a Defined Surface Area of the Peritoneum: Evaluation of Transfection Characteristics in Mice. Pharmaceutics, 11(5), 244. https://doi.org/10.3390/pharmaceutics11050244






