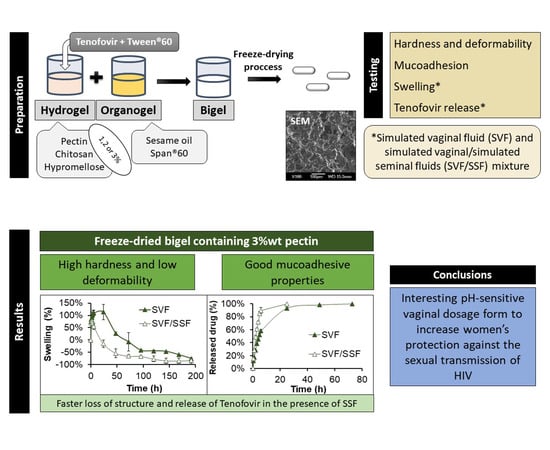
Smart Freeze-Dried Bigels for the Prevention of the Sexual Transmission of HIV by Accelerating the Vaginal Release of Tenofovir during Intercourse
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Fresh Formulations
2.3. Freeze-Drying the Fresh Systems to Obtain the Final Formulations
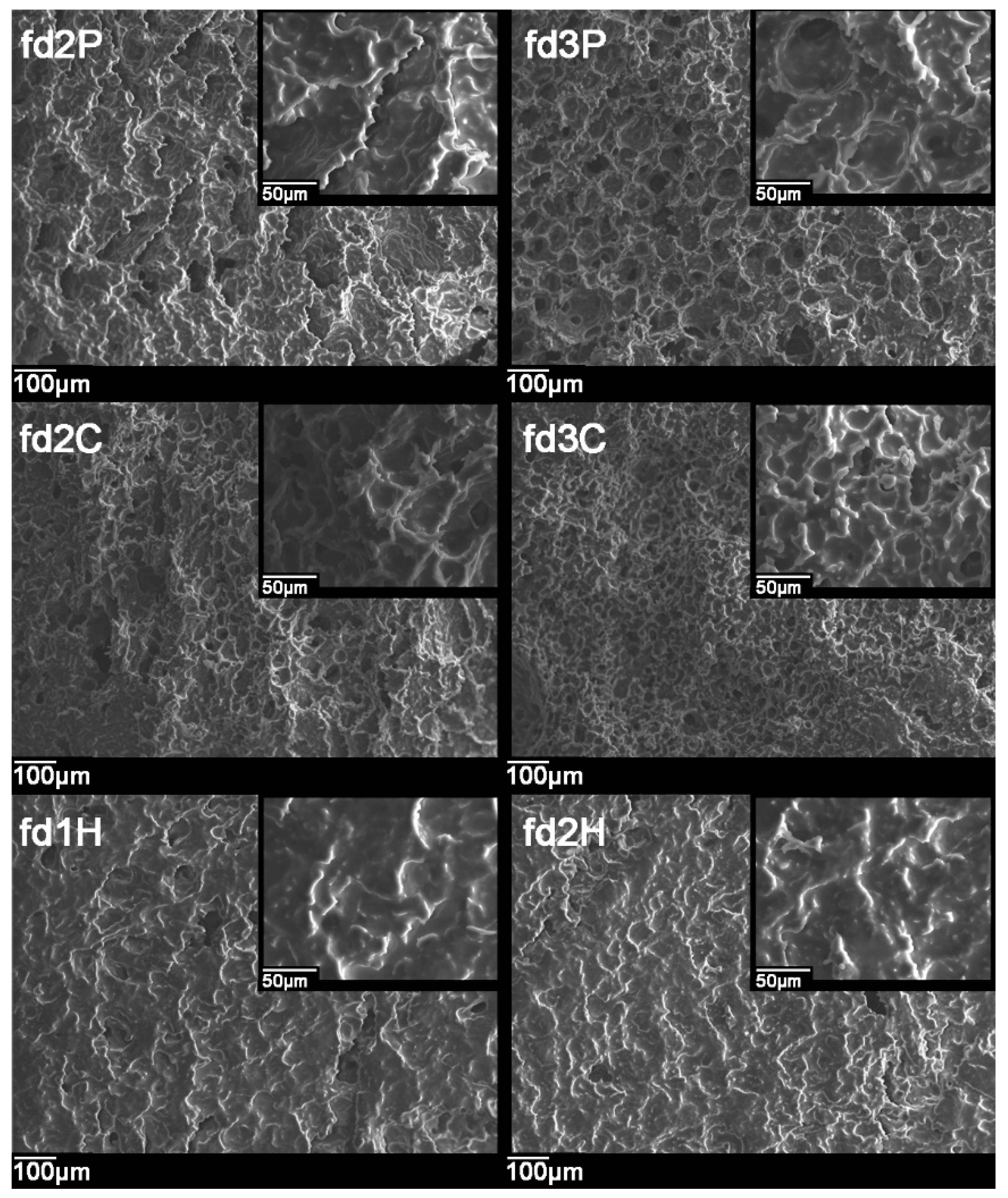
2.4. Scanning Electron Microscopy (SEM)
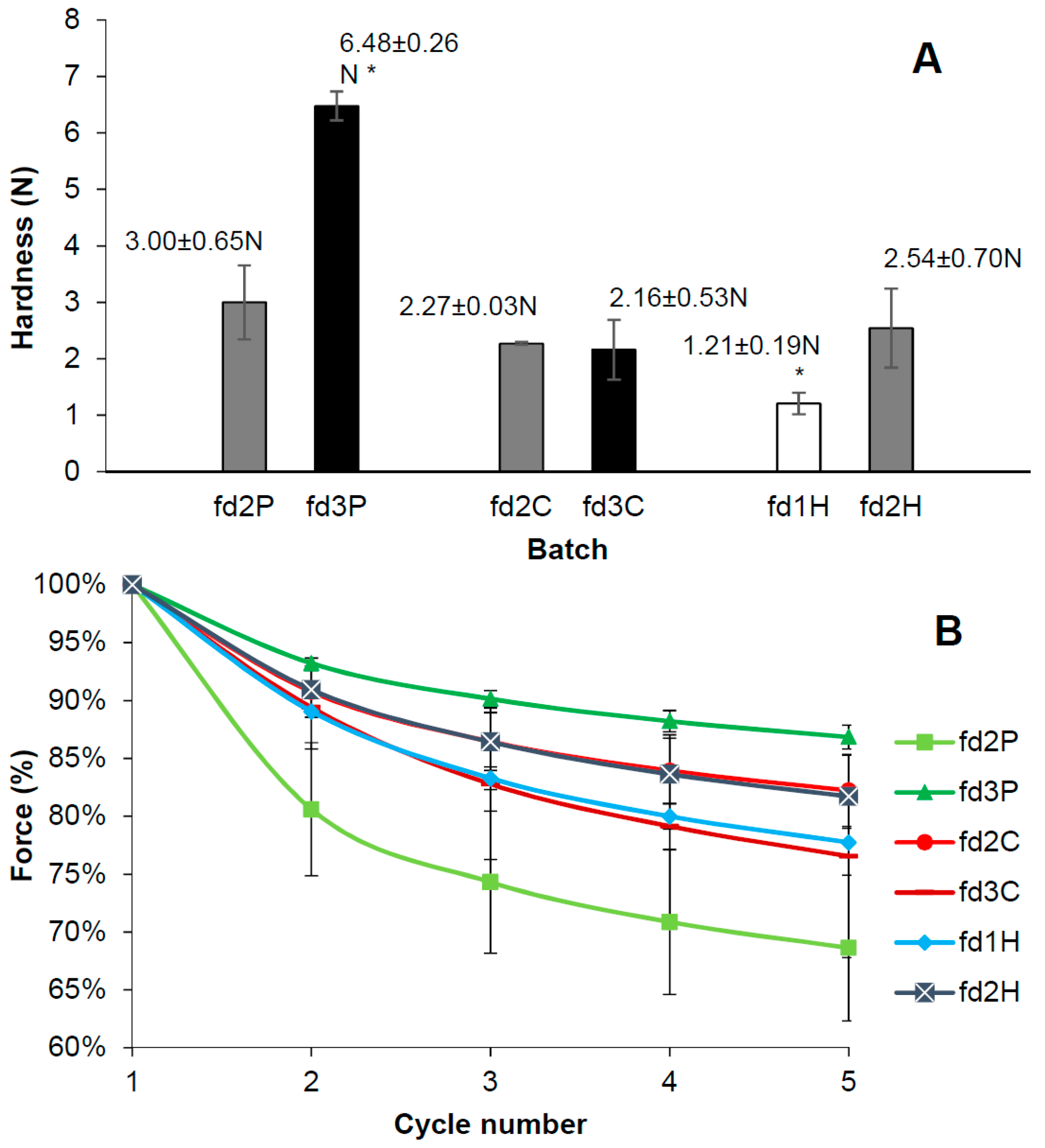
2.5. Hardness and Deformability Test
2.6. Mucoadhesion Test
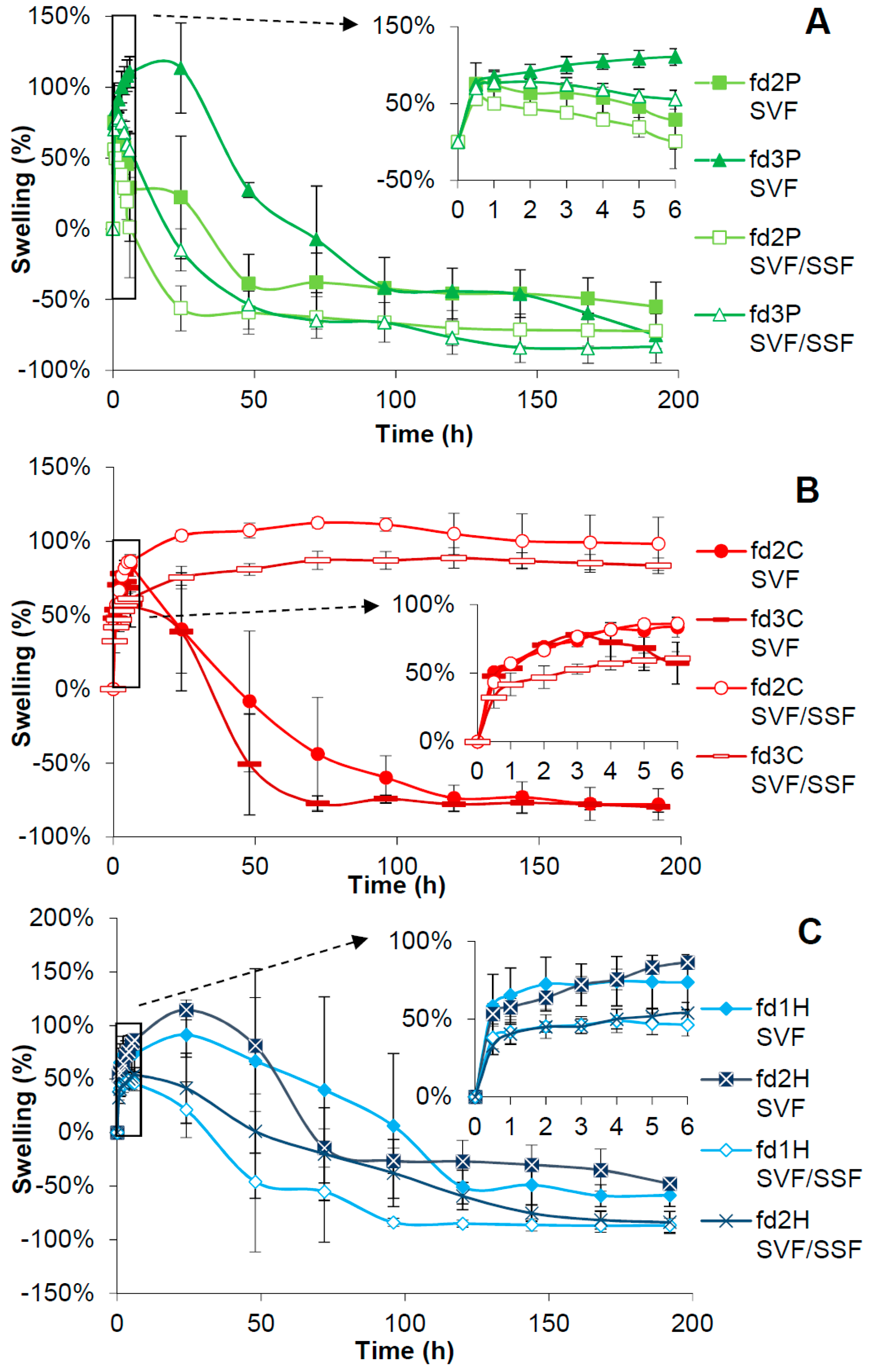
2.7. Swelling Test
2.8. Drug Release Test
2.8.1. Korsmeyer-Peppas kinetic
2.8.2. Higuchi kinetic
3. Results and Discussion
3.1. Preparing the Fresh Formulations
3.2. Freeze-Drying the Fresh Systems to Obtain the Final Formulations
3.3. Scanning Electron Microscopy (SEM)
3.4. Hardness and Deformability Test
3.5. Mucoadhesion Test
3.6. Swelling Test
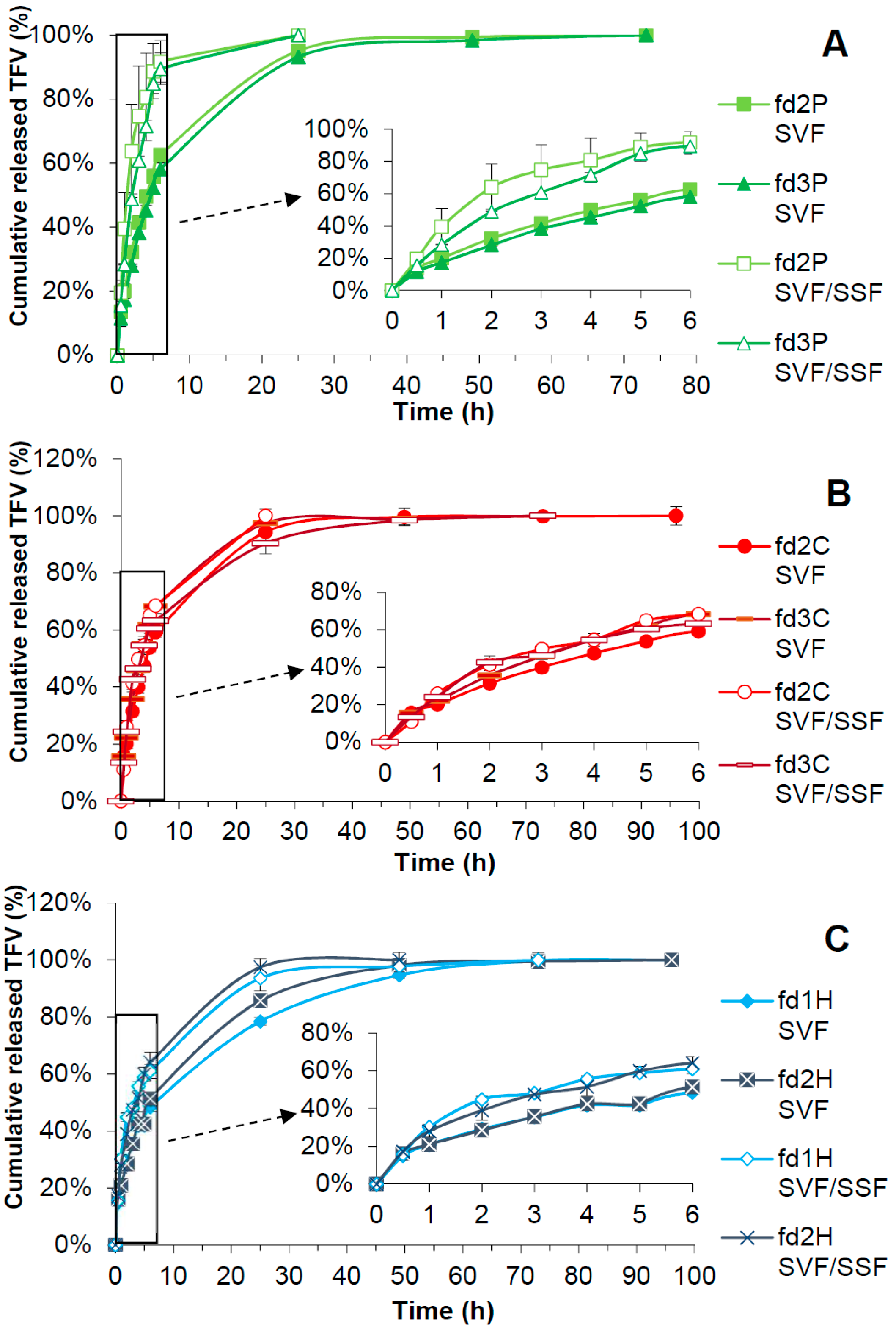
3.7. Drug Release Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- UNAIDS Data 2018 | UNAIDS. Available online: http://www.unaids.org/sites/default/files/media_asset/unaids-data-2018_en.pdf (accessed on 13 January 2019).
- Nicol, M.R.; Corbino, J.A.; Cottrell, M.L. Pharmacology of Antiretrovirals in the Female Genital Tract for HIV Prevention. J. Clin. Pharmacol. 2018, 58, 1381–1395. [Google Scholar] [CrossRef]
- Stein, Z.A. HIV prevention: The need for methods women can use. Am. J. Public Health 1990, 80, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Krakower, D.S.; Mayer, K.H. Pre-Exposure Prophylaxis to Prevent HIV Infection: Current Status, Future Opportunities and Challenges. Drugs 2015, 75, 243–251. [Google Scholar] [CrossRef]
- Notario-Pérez, F.; Ruiz-Caro, R.; Veiga-Ochoa, M.D. Historical development of vaginal microbicides to prevent sexual transmission of HIV in women: From past failures to future hopes. Drug Des. Devel. Ther. 2017, 11, 1767–1787. [Google Scholar] [CrossRef] [PubMed]
- Cutler, B.; Justman, J. Vaginal microbicides and the prevention of HIV transmission. Lancet Infect. Dis. 2008, 8, 685–697. [Google Scholar] [CrossRef]
- Delany-Moretlwe, S.; Lombard, C.; Baron, D.; Bekker, L.-G.; Nkala, B.; Ahmed, K.; Sebe, M.; Brumskine, W.; Nchabeleng, M.; Palanee-Philips, T.; et al. Tenofovir 1% vaginal gel for prevention of HIV-1 infection in women in South Africa (FACTS-001): A phase 3, randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 2018, 18, 1241–1250. [Google Scholar] [CrossRef]
- Timur, S.S.; Şahin, A.; Aytekin, E.; Öztürk, N.; Polat, K.H.; Tezel, N.; Gürsoy, R.N.; Çalış, S. Design and in vitro evaluation of tenofovir-loaded vaginal gels for the prevention of HIV infections. Pharm. Dev. Technol. 2018, 23, 301–310. [Google Scholar] [CrossRef]
- Notario-Pérez, F.; Cazorla-Luna, R.; Martín-Illana, A.; Ruiz-Caro, R.; Tamayo, A.; Rubio, J.; Veiga, M.D. Optimization of tenofovir release from mucoadhesive vaginal tablets by polymer combination to prevent sexual transmission of HIV. Carbohydr. Polym. 2018, 179, 305–316. [Google Scholar] [CrossRef]
- Clark, M.R.; Melissa Peet, M.; Davis, S.; Doncel, G.F.; Friend, D.R. Evaluation of rapidly disintegrating vaginal tablets of tenofovir, emtricitabine and their combination for HIV-1 prevention. Pharmaceutics 2014, 6, 616–631. [Google Scholar] [CrossRef] [PubMed]
- Marzinke, M.A.; Moncla, B.J.; Hendrix, C.W.; Richardson-Harman, N.; Dezzutti, C.S.; Schwartz, J.L.; Spiegel, H.M.L.; Hillier, S.L.; Bunge, K.E.; Meyn, L.A.; et al. FAME-04: A Phase 1 trial to assess the safety, acceptability, pharmacokinetics and pharmacodynamics of film and gel formulations of tenofovir. J. Int. AIDS Soc. 2018, 21, e25156. [Google Scholar]
- Akil, A.; Agashe, H.; Dezzutti, C.S.; Moncla, B.J.; Hillier, S.L.; Devlin, B.; Shi, Y.; Uranker, K.; Rohan, L.C. Formulation and characterization of polymeric films containing combinations of antiretrovirals (ARVs) for HIV prevention. Pharm. Res. 2015, 32, 458–468. [Google Scholar] [CrossRef]
- Johnson, T.J.; Clark, M.R.; Albright, T.H.; Nebeker, J.S.; Tuitupou, A.L.; Clark, J.T.; Fabian, J.; McCabe, R.T.; Chandra, N.; Doncel, G.F.; et al. A 90-day tenofovir reservoir intravaginal ring for mucosal HIV prophylaxis. Antimicrob. Agents Chemother. 2012, 56, 6272–6283. [Google Scholar] [CrossRef]
- Moss, J.A.; Malone, A.M.; Smith, T.J.; Butkyavichene, I.; Cortez, C.; Gilman, J.; Kennedy, S.; Kopin, E.; Nguyen, C.; Sinha, P.; et al. Safety and pharmacokinetics of intravaginal rings delivering tenofovir in pig-tailed macaques. Antimicrob. Agents Chemother. 2012, 56, 5952–5960. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Machado, A.; Cunha-Reis, C.; Araújo, F.; Nunes, R.; Seabra, V.; Ferreira, D.; das Neves, J.; Sarmento, B. Development and in vivo safety assessment of tenofovir-loaded nanoparticles-in-film as a novel vaginal microbicide delivery system. Acta Biomater. 2016, 44, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.A.; Baum, M.M.; Easley, J.T.; Cox, D.M.; Smith, T.J. An intravaginal ring for real-time evaluation of adherence to therapy. PLoS ONE 2017, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Shattock, R.J.; Rosenberg, Z. Microbicides: Topical Prevention against HIV. Cold Spring Harb. Perspect. Med. 2012, 2, a007385. [Google Scholar] [CrossRef] [PubMed]
- Valenta, C. The use of mucoadhesive polymers in vaginal delivery. Adv. Drug Deliv. Rev. 2005, 57, 1692–1712. [Google Scholar] [CrossRef] [PubMed]
- Brannon-Peppas, L.; Peppas, N.A. Equilibrium swelling behavior of pH-sensitive hydrogels. Chem. Eng. Sci. 1991, 46, 715–722. [Google Scholar] [CrossRef]
- Notario-Pérez, F.; Martín-Illana, A.; Cazorla-Luna, R.; Ruiz-Caro, R.; Bedoya, L.M.; Tamayo, A.; Rubio, J.; Veiga, M.D. Influence of chitosan swelling behaviour on controlled release of tenofovir from mucoadhesive vaginal systems for prevention of sexual transmission of HIV. Mar. Drugs 2017, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Cazorla-Luna, R.; Notario-Pérez, F.; Martín-Illana, A.; Tamayo, A.; Rubio, J.; Ruiz-Caro, R.; Veiga, M.D. Chitosan-Based Mucoadhesive Vaginal Tablets for Controlled Release of the Anti-HIV Drug Tenofovir. Pharmaceutics 2019, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.E.; Singletary, T.; Martin, A.; Dinh, C.T.; Deyounks, F.; Holder, A.; McNicholl, J.; Buckheit, K.W.; Buckheit, R.W.; Ham, A.; et al. Effects of gel volume on pharmacokinetics for vaginal and rectal applications of combination DuoGel-IQB4012, a dual chamber-dual drug HIV microbicide gel, in pigtailed macaques. Drug Deliv. Transl. Res. 2018, 8, 1180–1190. [Google Scholar] [CrossRef]
- Chatterjee, A.; Bhowmik, B.B.; Thakur, Y.S. Formulation, In Vitro and In Vivo Pharmacokinetics of Anti-HIV Vaginal Bioadhesive Gel. J. Young Pharm. 2011, 3, 83–89. [Google Scholar] [CrossRef]
- Baldino, N.; Lupi, F.R.; Gabriele, D.; Shakeel, A.; Greco, V.; Oliviero Rossi, C. A rheological and microstructural characterisation of bigels for cosmetic and pharmaceutical uses. Mater. Sci. Eng. C 2016, 69, 358–365. [Google Scholar]
- Rehman, K.; Mohd Amin, M.C.I.; Zulfakar, M.H. Development and Physical Characterization of Polymer-Fish Oil Bigel (Hydrogel/Oleogel) System as a Transdermal Drug Delivery Vehicle. J. Oleo Sci. 2014, 63, 961–970. [Google Scholar] [CrossRef]
- Lupi, F.R.; Gentile, L.; Gabriele, D.; Mazzulla, S.; Baldino, N.; de Cindio, B. Olive oil and hyperthermal water bigels for cosmetic uses. J. Colloid Interface Sci. 2015, 459, 70–78. [Google Scholar] [CrossRef]
- Sagiri, S.S.; Singh, V.K.; Kulanthaivel, S.; Banerjee, I.; Basak, P.; Battachrya, M.K.; Pal, K. Stearate organogel–gelatin hydrogel based bigels: Physicochemical, thermal, mechanical characterizations and in vitro drug delivery applications. J. Mech. Behav. Biomed. Mater. 2015, 43, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Banerjee, I.; Agarwal, T.; Pramanik, K.; Bhattacharya, M.K.; Pal, K. Guar gum and sesame oil based novel bigels for controlled drug delivery. Colloids Surfaces B Biointerfaces 2014, 123, 582–592. [Google Scholar] [CrossRef]
- Shakeel, A.; Farooq, U.; Iqbal, T.; Yasin, S.; Lupi, F.R.; Gabriele, D. Key characteristics and modelling of bigels systems: A review. Mater. Sci. Eng. C 2019, 97, 932–953. [Google Scholar] [CrossRef]
- Rehman, K.; Zulfakar, M.H. Novel Fish Oil-based Bigel System for Controlled Drug Delivery and its Influence on Immunomodulatory Activity of Imiquimod Against Skin Cancer. Pharm. Res. 2017, 34, 36–48. [Google Scholar] [CrossRef]
- Singh, V.K.; Anis, A.; Banerjee, I.; Pramanik, K.; Bhattacharya, M.K.; Pal, K. Preparation and characterization of novel carbopol based bigels for topical delivery of metronidazole for the treatment of bacterial vaginosis. Mater. Sci. Eng. C 2014, 44, 151–158. [Google Scholar] [CrossRef]
- Martín-Illana, A.; Cazorla-Luna, R.; Notario-Pérez, F.; Bedoya, L.M.; Ruiz-Caro, R.; Veiga, M.D. Freeze-dried bioadhesive vaginal bigels for controlled release of Tenofovir. Eur. J. Pharm. Sci. 2019, 127, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sturgis, T.F.; Youan, B.-B.C. pH-responsive nanoparticles releasing tenofovir intended for the prevention of HIV transmission. Eur. J. Pharm. Biopharm. 2011, 79, 526–536. [Google Scholar] [CrossRef]
- Gupta, K.M.; Barnes, S.R.; Tangaro, R.A.; Roberts, M.C.; Owen, D.H.; Katz, D.F.; Kiser, P.F. Temperature and pH Sensitive Hydrogels: An Approach Towards Smart Semen-Triggered Vaginal Microbicidal Vehicles. J. Pharm. Sci. 2007, 96, 670–681. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, C.; Agrahari, V.; Murowchick, J.B.; Oyler, N.A.; Youan, B.C. Spray drying tenofovir loaded mucoadhesive and pH-sensitive microspheres intended for HIV prevention. Antiviral Res. 2013, 97, 334–346. [Google Scholar] [CrossRef]
- Bazban-Shotorbani, S.; Hasani-Sadrabadi, M.M.; Karkhaneh, A.; Serpooshan, V.; Jacob, K.I.; Moshaverinia, A.; Mahmoudi, M. Revisiting structure-property relationship of pH-responsive polymers for drug delivery applications. J. Control. Release 2017, 253, 46–63. [Google Scholar] [CrossRef]
- Jalil, A.; Sarwar, S.; Saher, F.; Naeem, F.; Riaz, A.; Khan, S.; Haider, M.S.; Ranjha, N.M.; Afzal, S. pH responsive cross-linked polymeric matrices based on natural polymers: effect of process variables on swelling characterization and drug delivery properties. BioImpacts 2017, 7, 177–192. [Google Scholar]
- Markov, P.A.; Popov, S.V.; Krachkovsky, N.S.; Durnev, E.A.; Martinson, E.A.; Litvinets, S.G. Mechanical properties, structure, bioadhesion, and biocompatibility of pectin hydrogels. J. Biomed. Mater. Res. Part A 2017, 105, 2572–2581. [Google Scholar] [CrossRef]
- Sheshala, R.; Quah, S.Y.; Tan, G.C.; Meka, V.S.; Jnanendrappa, N.; Sahu, P.S. Investigation on solution-to-gel characteristic of thermosensitive and mucoadhesive biopolymers for the development of moxifloxacin-loaded sustained release periodontal in situ gels. Drug Deliv. Transl. Res. 2019, 9, 434–443 40. [Google Scholar] [CrossRef]
- Ozturk, T.; Cig, T.; Bostan, M.S.; Eroglu, M.S.; Senol, M.; Peker, I.; Goren, A.C. Controlled release of 5-aminosalicylicacid from chitosan based pH and temperature sensitive hydrogels. Int. J. Biol. Macromol. 2012, 52, 177–183. [Google Scholar]
- Viridén, A.; Larsson, A.; Abrahmsén-Alami, S.; Caccavo, D.; Barba, A.A.; Lamberti, G. Effects of HPMC substituent pattern on water up-take, polymer and drug release: An experimental and modelling study. Int. J. Pharm. 2017, 528, 705–713. [Google Scholar]
- Li, C.L.; Martini, L.G.; Ford, J.L.; Roberts, M. The use of hypromellose in oral drug delivery. J. Pharm. Pharmacol. 2005, 57, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Conway, B.R.; Larhrib, H.; Tetteh, J.; Asare-Addo, K.; Levina, M.; Rajabi-Siahboomi, A.R.; Nokhodchi, A.; Boateng, J. The effect of pH and ionic strength of dissolution media on in-vitro release of two model drugs of different solubilities from HPMC matrices. Colloids Surfaces B Biointerfaces 2013, 111, 384–391. [Google Scholar]
- Cazorla-Luna, R.; Martín-Illana, A.; Notario-Pérez, F.; Bedoya, L.-M.; Bermejo, P.; Ruiz-Caro, R.; Veiga, M.-D. Dapivirine Bioadhesive Vaginal Tablets Based on Natural Polymers for the Prevention of Sexual Transmission of HIV. Polymers 2019, 11, 483. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Woolfson, A.D.; Umrethia, M.L.; Kett, V.L.; Malcolm, R.K. Freeze-dried, mucoadhesive system for vaginal delivery of the HIV microbicide, dapivirine: Optimisation by an artificial neural network. Int. J. Pharm. 2010, 388, 136–143. [Google Scholar] [CrossRef] [PubMed]
- do Vale Morais, A.R.; do Nascimento Alencar, É.; Xavier Júnior, F.H.; De Oliveira, C.M.; Marcelino, H.R.; Barratt, G.; Fessi, H.; do Egito, E.S.T.; Elaissari, A. Freeze-drying of emulsified systems: A review. Int. J. Pharm. 2016, 503, 102–114. [Google Scholar] [CrossRef]
- Ruiz-Caro, R.; Veiga-Ochoa, M.D. Characterization and dissolution study of chitosan freeze-dried systems for drug controlled Release. Molecules 2009, 14, 4370–4386. [Google Scholar] [CrossRef]
- Furst, T.; Dakwar, G.R.; Zagato, E.; Lechanteur, A.; Remaut, K.; Evrard, B.; Braeckmans, K.; Piel, G. Freeze-dried mucoadhesive polymeric system containing pegylated lipoplexes: Towards a vaginal sustained released system for siRNA. J. Control. Release 2016, 236, 68–78. [Google Scholar] [CrossRef]
- Owen, D.H.; Katz, D.F. A vaginal fluid simulant. Contraception 1999, 59, 91–95. [Google Scholar] [CrossRef]
- Owen, D.H.; Katz, D.F. A review of the physical and chemical properties of human semen and the formulation of a semen simulant. J. Androl. 2005, 26, 459–469. [Google Scholar] [CrossRef]
- Mamani, P.L.; Ruiz-Caro, R.; Veiga, M.D. Matrix Tablets: The Effect of Hydroxypropyl Methylcellulose/Anhydrous Dibasic Calcium Phosphate Ratio on the Release Rate of a Water-Soluble Drug Through the Gastrointestinal Tract I. In Vitro Tests. AAPS PharmSciTech 2012, 13, 1073–1083. [Google Scholar] [CrossRef]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Murdan, S.; van den Bergh, B.; Gregoriadis, G.; Florence, A.T. Water-in-sorbitan monostearate organogels (water-in-oil gels). J. Pharm. Sci. 1999, 88, 615–619. [Google Scholar] [CrossRef]
- Luppi, B.; Bigucci, F.; Abruzzo, A.; Corace, G.; Cerchiara, T.; Zecchi, V. Freeze-dried chitosan/pectin nasal inserts for antipsychotic drug delivery. Eur. J. Pharm. Biopharm. 2010, 75, 381–387. [Google Scholar] [CrossRef]
- Shen, X.; Shamshina, J.L.; Berton, P.; Gurau, G.; Rogers, R.D. Hydrogels based on cellulose and chitin: Fabrication, properties, and applications. Green Chem. 2015, 18, 53–75. [Google Scholar] [CrossRef]
- Furst, T.; Piette, M.; Lechanteur, A.; Evrard, B.; Piel, G. Mucoadhesive cellulosic derivative sponges as drug delivery system for vaginal application. Eur. J. Pharm. Biopharm. 2015, 95, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.F.; Matsuura, T. (Eds.) Membrane Technology for Water and Wastewater Treatment, Energy and Environment; Taylor & Francis: London, UK, 2016. [Google Scholar]
- Smart, J.D. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef]
- Thirawong, N.; Kennedy, R.A.; Sriamornsak, P. Viscometric study of pectin-mucin interaction and its mucoadhesive bond strength. Carbohydr. Polym. 2008, 71, 170–179. [Google Scholar] [CrossRef]
- Sriamornsak, P.; Wattanakorn, N.; Takeuchi, H. Study on the mucoadhesion mechanism of pectin by atomic force microscopy and mucin-particle method. Carbohydr. Polym. 2010, 79, 54–59. [Google Scholar] [CrossRef]
- Liu, L.; Yao, W.D.; Rao, Y.F.; Lu, X.Y.; Gao, J.Q. pH-responsive carriers for oral drug delivery: Challenges and opportunities of current platforms. Drug Deliv. 2017, 24, 569–581. [Google Scholar] [CrossRef]
- Dumitriu, S. (Ed.) Polymeric Biomaterials, Revised and Expanded, 2nd ed.; Taylor & Francis: London, UK, 2001; ISBN 9780824705695-CAT#DK1811. [Google Scholar]
- Agarwal, S.; Murthy, R.S.R. Effect of different polymer concentration on drug release rate and physicochemical properties of mucoadhesive gastroretentive tablets. Indian J. Pharm. Sci. 2016, 77, 705. [Google Scholar] [CrossRef]
- Rojewska, M.; Olejniczak-Rabinek, M.; Bartkowiak, A.; Snela, A.; Prochaska, K.; Lulek, J. The wettability and swelling of selected mucoadhesive polymers in simulated saliva and vaginal fluids. Colloids Surfaces B Biointerfaces 2017, 156, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Elsamaligy, S.; Aleanizy, F.S.; Mahmoud, H.A.; Bayomi, M.A.; Fitaihi, R.A. Role of chitosan on controlling the characteristics and antifungal activity of bioadhesive fluconazole vaginal tablets. Saudi Pharm. J. 2017, 26, 151–161. [Google Scholar]
- Notario-Pérez, F.; Martín-Illana, A.; Cazorla-Luna, R.; Ruiz-Caro, R.; Peña, J.; Veiga, M.D. Improvement of Tenofovir vaginal release from hydrophilic matrices through drug granulation with hydrophobic polymers. Eur. J. Pharm. Sci. 2018, 117, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Begum, R.; Yusof, Y.A.; Aziz, M.G.; Uddin, M.B. Structural and functional properties of pectin extracted from jackfruit (Artocarpus heterophyllus) waste: Effects of drying. Int. J. Food Prop. 2017, 20, S190–S201. [Google Scholar] [CrossRef]
- Sundar Raj, A.A.; Rubila, S.; Jayabalan, R.; Ranganathan, T.V. A Review on Pectin: Chemistry due to General Properties of Pectin and its Pharmaceutical Uses. Open Access Sci. Rep. 2012. [Google Scholar] [CrossRef]
- Hemant, K.S.Y.; Shivakumar, H.G. Development of chitosan acetate films for transdermal delivery of propranolol hydrochloride. Trop. J. Pharm. Res. 2010, 9, 197–203. [Google Scholar] [CrossRef]
- Karim, S.S.A.; Kashuba, A.D.; Werner, L.; Karim, Q.A. Drug concentrations after topical and oral antiretroviral pre-exposure prophylaxis: implications for HIV prevention in women. Lancet 2011, 378, 279–281. [Google Scholar] [CrossRef]


| Batch | Organogel | Hydrogel | Polymer in the Hydrogel | Tween®60 | |
|---|---|---|---|---|---|
| Sesame Oil | Span®60 | ||||
| 1P | 37 | 5.8 | 55.5 | 1 (pectin) | 1.7 |
| 2P | 37 | 5.8 | 55.5 | 2 (pectin) | 1.7 |
| 3P | 37 | 5.8 | 55.5 | 3 (pectin) | 1.7 |
| 1C | 37 | 5.8 | 55.5 | 1 (chitosan) | 1.7 |
| 2C | 37 | 5.8 | 55.5 | 2 (chitosan) | 1.7 |
| 3C | 37 | 5.8 | 55.5 | 3 (chitosan) | 1.7 |
| 1H | 37 | 5.8 | 55.5 | 1 (HPMC) | 1.7 |
| 2H | 37 | 5.8 | 55.5 | 2 (HPMC) | 1.7 |
| 3H | 37 | 5.8 | 55.5 | 3 (HPMC) | 1.7 |
| Batch (Medium) | Kinetics | |||||||
|---|---|---|---|---|---|---|---|---|
| Korsmeyer-Peppas | Higuchi | |||||||
| r2 | n | SSR × 102 | AIC | r2 | KH | SSR × 102 | AIC | |
| fd2P (SVF) | 0.9988 | 0.6243 | 0.1828 | −33.8282 | 0.9918 | 0.2623 | 0.2752 | −45.1627 |
| fd3P (SVF) | 0.9987 | 0.6618 | 0.2843 | −37.0410 | 0.9857 | 0.2457 | 0.4250 | −41.6863 |
| fd2P (SVF/SSF) | 0.9887 | 1.0020 | 4.7743 | −5.1257 | 0.9769 | 0.4360 | 1.2005 | −24.5348 |
| fd3P (SVF/SSF) | 0.9990 | 0.8251 | 0.0626 | −18.1277 | 0.9814 | 0.3896 | 1.0660 | −29.7891 |
| fd2C (SVF) | 0.9938 | 0.5563 | 0.9602 | −28.5206 | 0.9952 | 0.2451 | 0.1412 | −50.5031 |
| fd3C (SVF) | 0.9965 | 0.6158 | 0.3830 | −23.8249 | 0.9925 | 0.2871 | 0.3030 | −44.3930 |
| fd2C (SVF/SSF) | 0.9528 | 0.7640 | 8.2576 | −8.4702 | 0.9839 | 0.2990 | 0.7085 | −37.5984 |
| fd3C (SVF/SSF) | 0.9731 | 0.6718 | 3.5662 | −12.6684 | 0.9838 | 0.2756 | 0.6056 | −38.8534 |
| fd1H (SVF) | 0.9945 | 0.4693 | 0.6021 | −31.7872 | 0.9705 | 0.1558 | 1.1887 | −37.8910 |
| fd2H (SVF) | 0.9901 | 0.4605 | 1.0454 | −27.9251 | 0.9914 | 0.2006 | 0.1686 | −49.0818 |
| fd1H (SVF/SSF) | 0.9471 | 0.5522 | 6.6216 | −12.2890 | 0.9721 | 0.2614 | 0.9530 | −35.2264 |
| fd2H (SVF/SSF) | 0.9885 | 0.5219 | 1.2367 | −22.3566 | 0.9963 | 0.2643 | 0.1259 | −51.4211 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Illana, A.; Notario-Pérez, F.; Cazorla-Luna, R.; Ruiz-Caro, R.; Veiga, M.D. Smart Freeze-Dried Bigels for the Prevention of the Sexual Transmission of HIV by Accelerating the Vaginal Release of Tenofovir during Intercourse. Pharmaceutics 2019, 11, 232. https://doi.org/10.3390/pharmaceutics11050232
Martín-Illana A, Notario-Pérez F, Cazorla-Luna R, Ruiz-Caro R, Veiga MD. Smart Freeze-Dried Bigels for the Prevention of the Sexual Transmission of HIV by Accelerating the Vaginal Release of Tenofovir during Intercourse. Pharmaceutics. 2019; 11(5):232. https://doi.org/10.3390/pharmaceutics11050232
Chicago/Turabian StyleMartín-Illana, Araceli, Fernando Notario-Pérez, Raúl Cazorla-Luna, Roberto Ruiz-Caro, and María Dolores Veiga. 2019. "Smart Freeze-Dried Bigels for the Prevention of the Sexual Transmission of HIV by Accelerating the Vaginal Release of Tenofovir during Intercourse" Pharmaceutics 11, no. 5: 232. https://doi.org/10.3390/pharmaceutics11050232
APA StyleMartín-Illana, A., Notario-Pérez, F., Cazorla-Luna, R., Ruiz-Caro, R., & Veiga, M. D. (2019). Smart Freeze-Dried Bigels for the Prevention of the Sexual Transmission of HIV by Accelerating the Vaginal Release of Tenofovir during Intercourse. Pharmaceutics, 11(5), 232. https://doi.org/10.3390/pharmaceutics11050232