A Low-Molecular-Weight Polyethylenimine/pDNA-VEGF Polyplex System Constructed in a One-Pot Manner for Hindlimb Ischemia Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
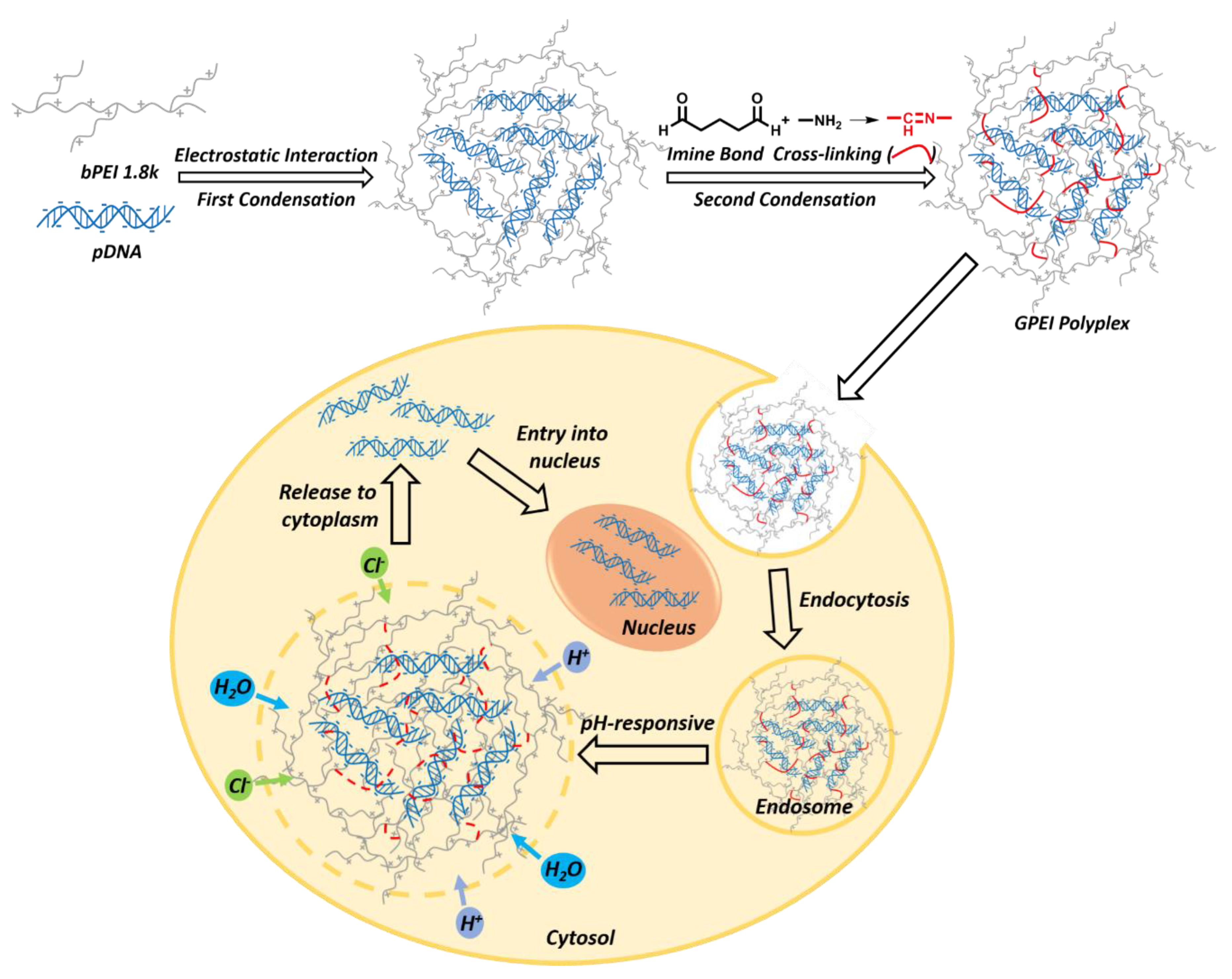
2.3. Preparation of GPEI Polyplexes
2.4. Characterization of Polyplexes
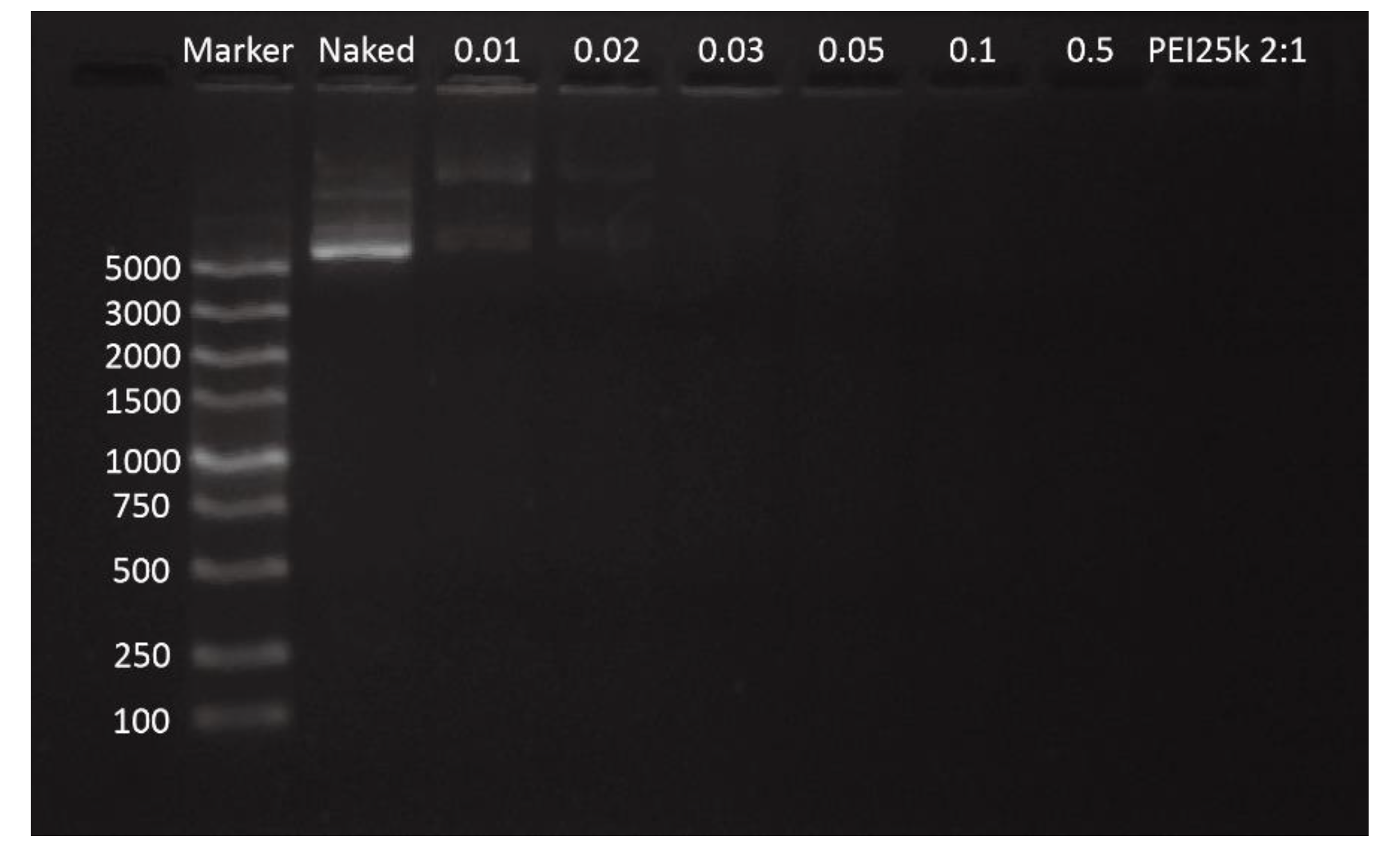
2.4.1. Agarose Gel Electrophoresis (AGE)
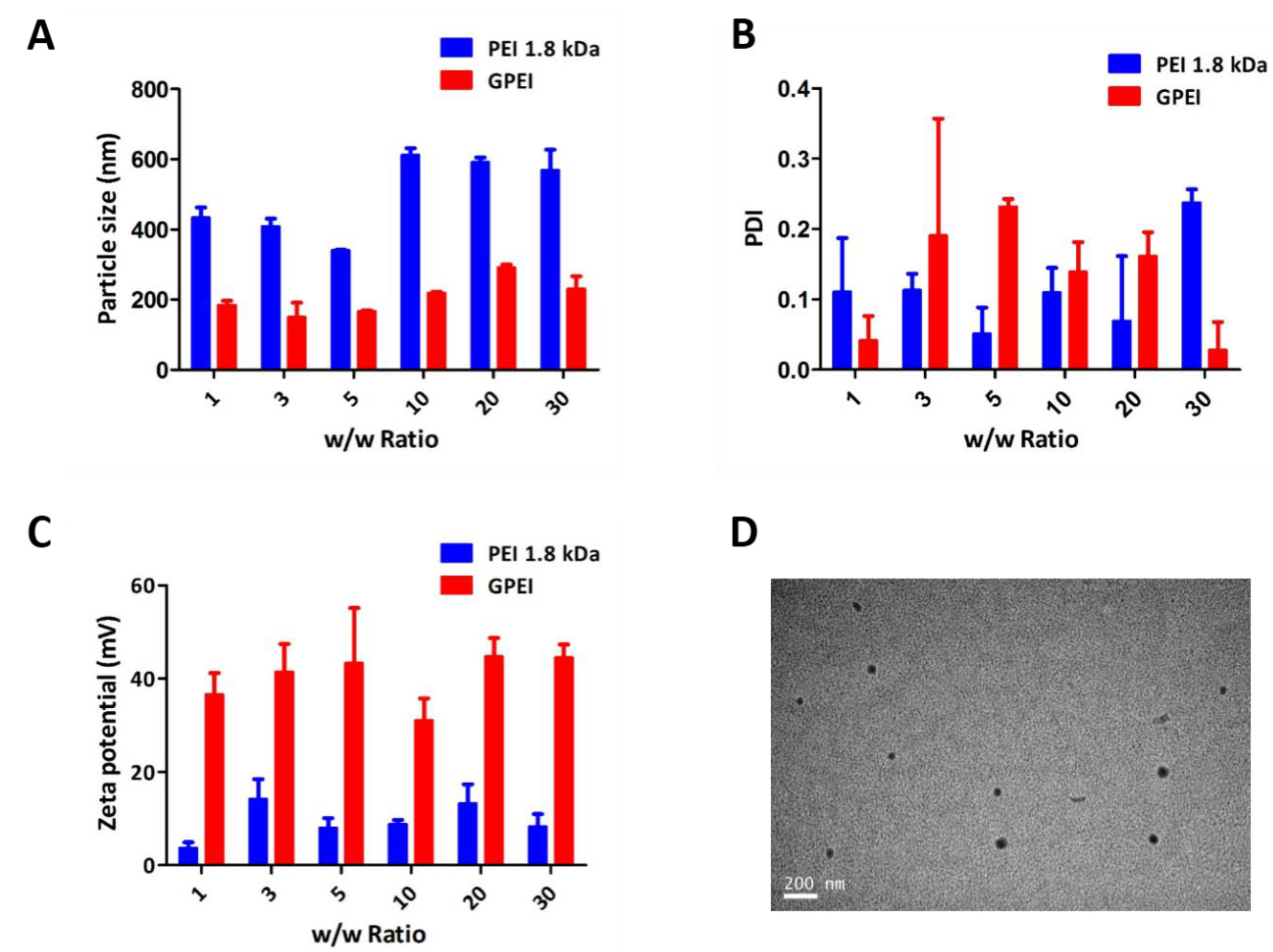
2.4.2. Particle Size, Zeta Potential and Morphology Measurements
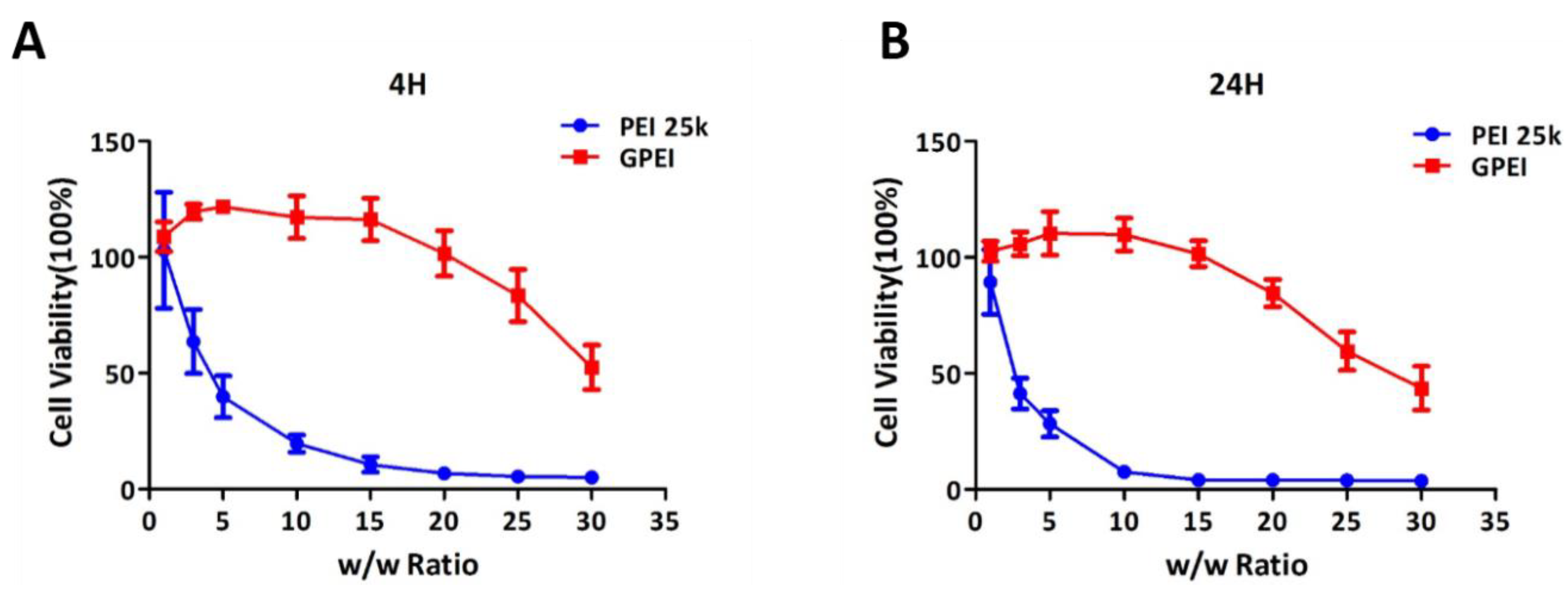
2.5. In Vitro Cytotoxicity
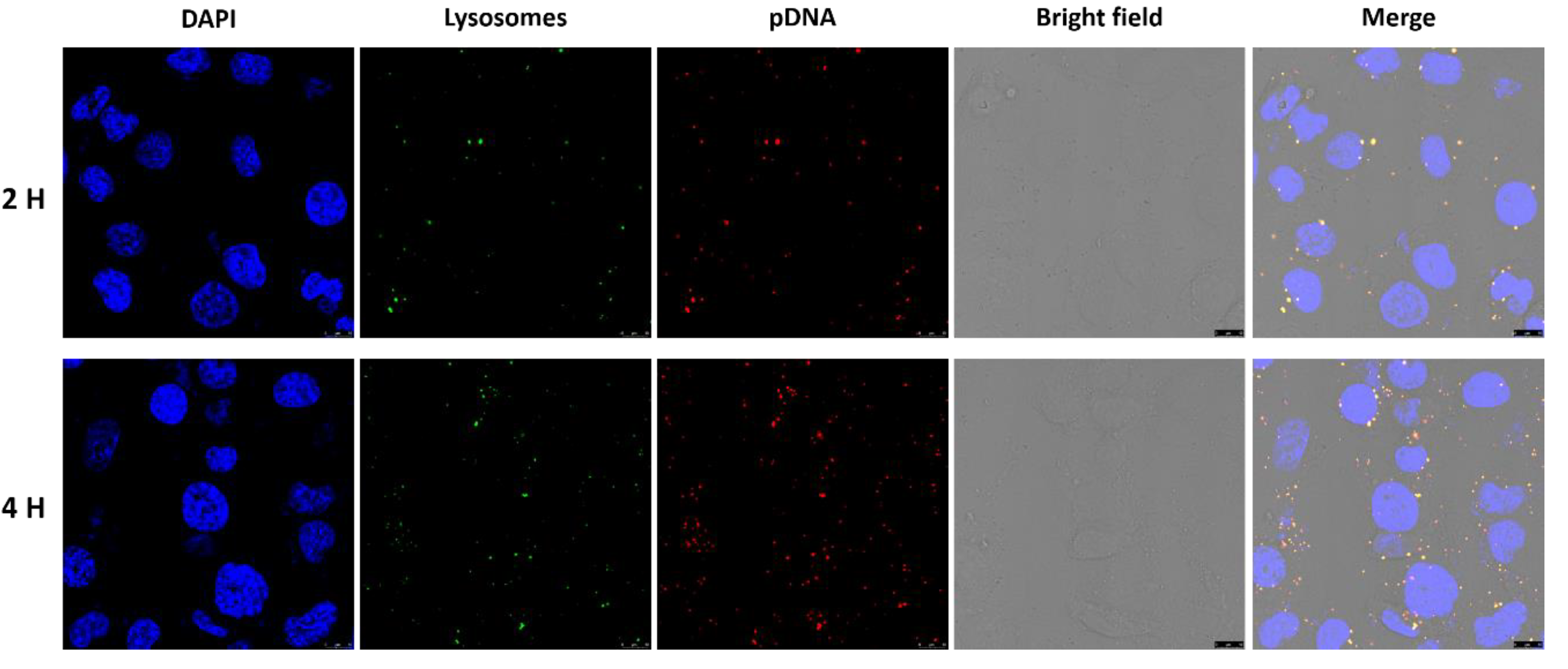
2.6. Intracellular Uptake
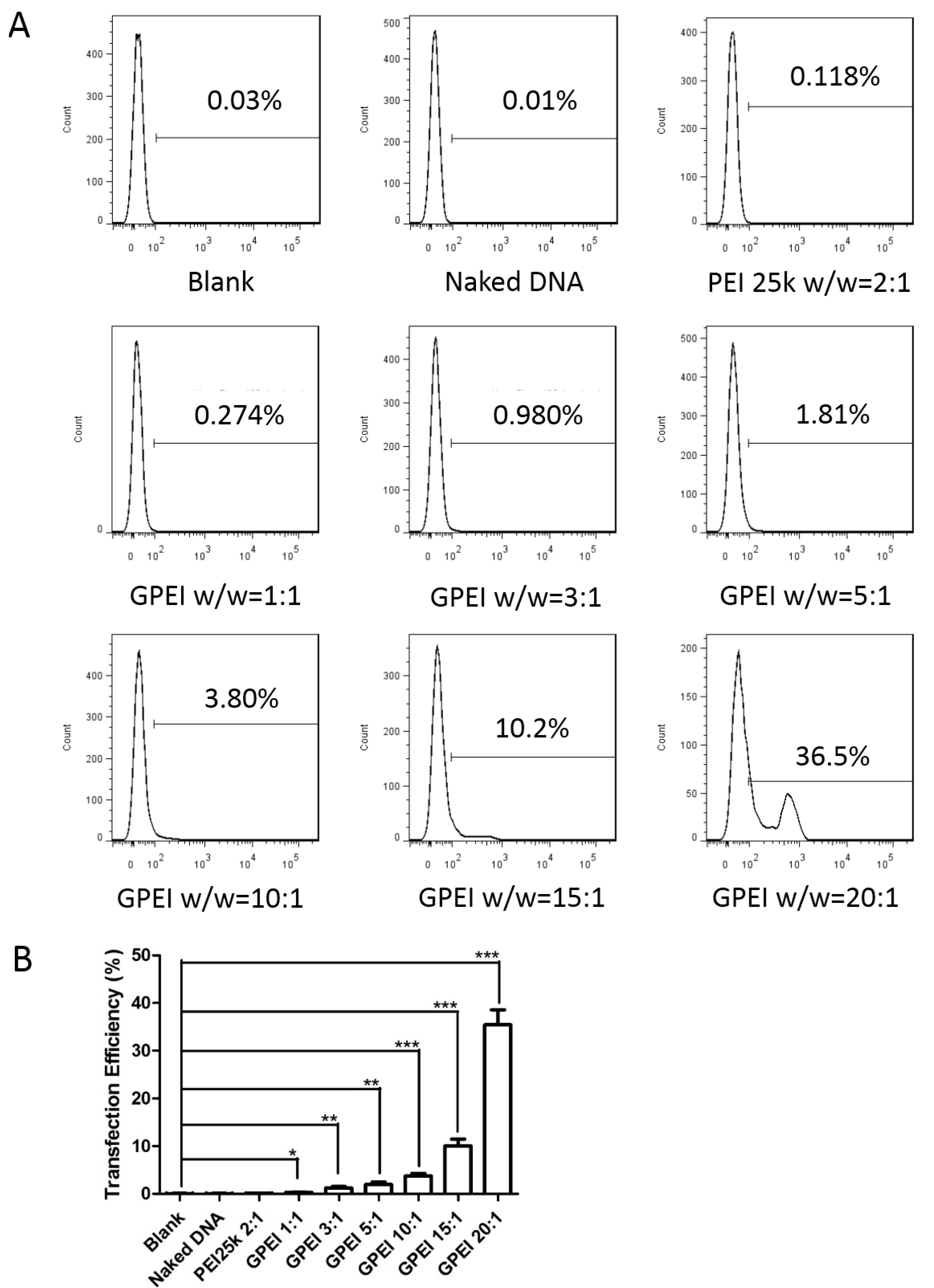
2.7. In Vitro Cell Transfection
2.8. Hindlimb Ischemia Model Study
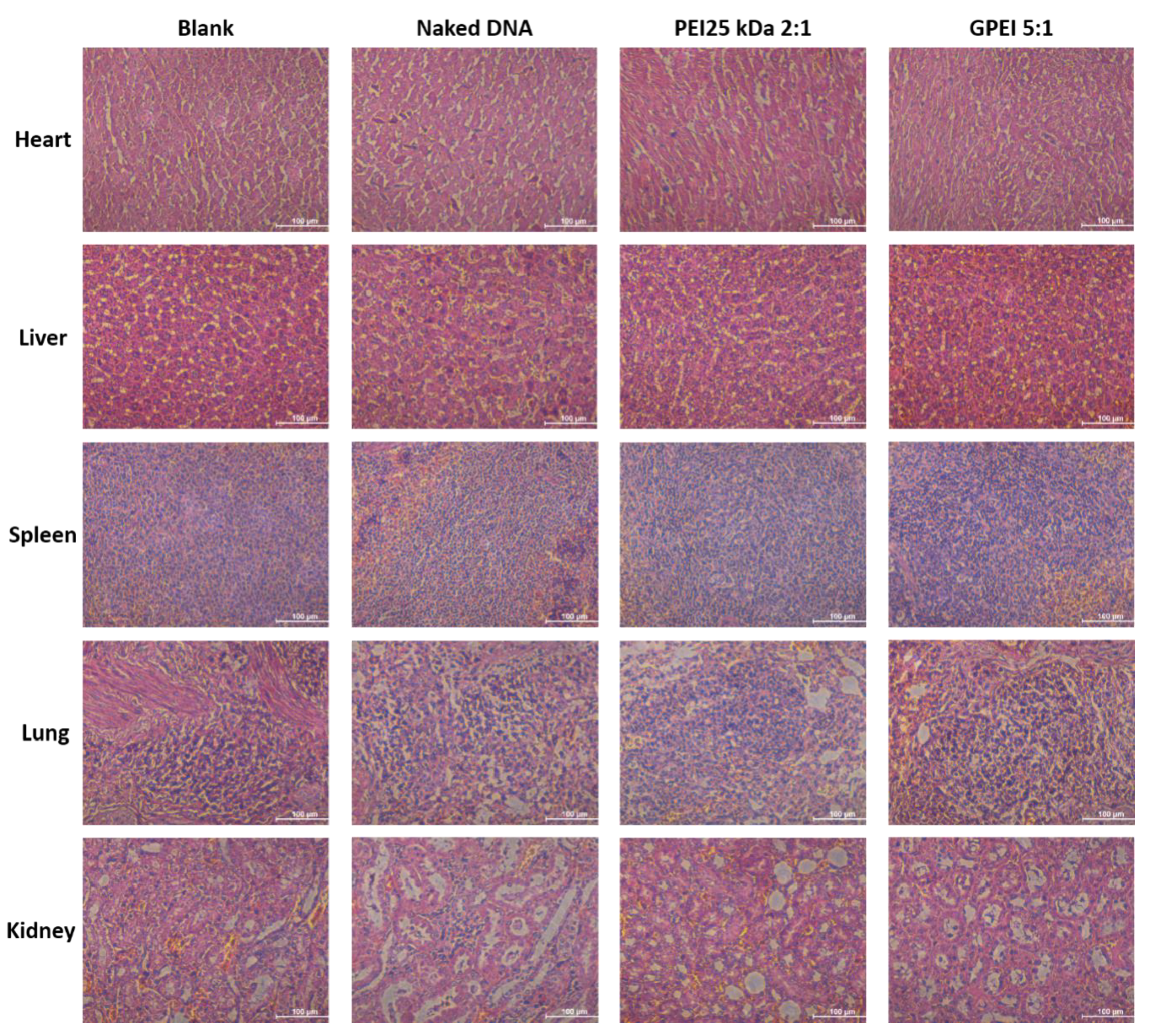
2.9. In Vivo Cytotoxicity
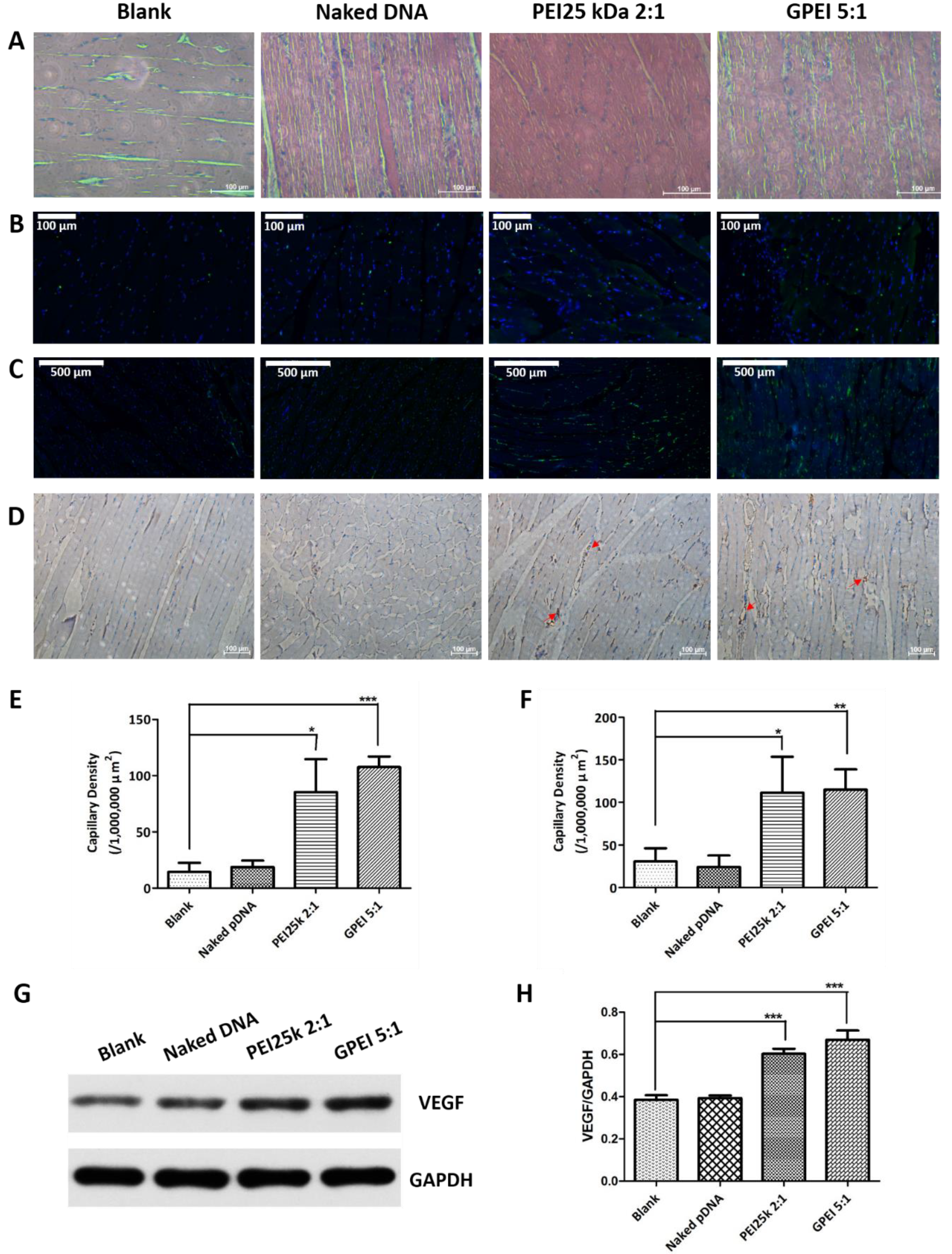
2.10. In Vivo Therapeutic Effects
2.11. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Polyplexes
3.1.1. Agarose Gel Electrophoresis (AGE)
3.1.2. Particle Size, Zeta Potential, and Morphology Measurements
3.2. In Vitro Cytotoxicity
3.3. Intracellular Uptake
3.4. In Vitro Cell Transfection
3.5. In Vivo Cytotoxicity
3.6. In Vivo Therapeutic Effects
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ross, R. The Pathogenesis of Atherosclerosis—An Update. N. Engl. Mech. Ageing Dev. 1986, 9, 435–440. [Google Scholar] [CrossRef]
- Jude, E.B.; Oyibo, S.O.; Chalmers, N.; Boulton, A.J. Peripheral arterial disease in diabetic and nondiabetic patients: A comparison of severity and outcome. Diabetes Care 2001, 24, 1433–1437. [Google Scholar] [CrossRef] [PubMed]
- Fowkes, F.G.; Housley, E.; Riemersma, R.A.; Macintyre, C.C.; Cawood, E.H.; Prescott, R.J.; Ruckley, C.V. Smoking, lipids, glucose intolerance, and blood pressure as risk factors for peripheral atherosclerosis compared with ischemic heart disease in the Edinburgh Artery Study. Am. J. Epidemiol. 1992, 135, 331–340. [Google Scholar] [CrossRef]
- Krystyna, Z.; Bozena, S.R.; Rajmund, A. Chronic lower limb ischemia–clinical symptom element of atherosclerosis–actual guideline. Polski Merkuriusz Lekarski 2010, 28, 71–74. [Google Scholar]
- Weinberg, I.; Weinberg, M.D. Non-Atherosclerotic Arterial Disorders of the Lower Extremities. Circulation 2012, 126, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.-I.; Shimamura, M.; Suda, H.; Wakayama, K.; Kumagai, H.; Ikeda, Y.; Akazawa, H.; Isobe, M.; Komuro, I.; Morishita, R. Current therapies and investigational drugs for peripheral arterial disease. Hypertens. Res. 2016, 39, 183–191. [Google Scholar] [CrossRef]
- Shalhoub, J.; Davies, A.H.; Franklin, I.J. Cilostazol may improve outcome in critical limb ischemia. Int. Angiol. 2009, 28, 363–366. [Google Scholar]
- Antoniou, G.A.; Fisher, R.K.; Georgiadis, G.S.; Antoniou, S.A.; Torella, F. Statin therapy in lower limb peripheral arterial disease: Systematic review and meta-analysis. Vasc. Pharmacol. 2014, 63, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.C.; Shi, G.D.; Huang, W.L. Effect of sarpogrelate (Anplag) versus cilostazol on the peripheral artery disease in patients with type 2 diabetes: A randomized and controlled trial. Chin. J. Diabetes 2010, 18, 607–610. [Google Scholar]
- Raval, Z.; Losordo, D.W. Cell Therapy of Peripheral Arterial Disease From Experimental Findings to Clinical Trials. Circ. Res. 2013, 112, 1288–1302. [Google Scholar] [CrossRef]
- Forster, R.; Liew, A.; Bhattacharya, V.; Shaw, J.; Stansby, G. Gene therapy for peripheral arterial disease. Cochrane Database Syst. Rev. 2018. [Google Scholar] [CrossRef]
- Tateishi-Yuyama, E.; Matsubara, H.; Murohara, T.; Ikeda, U.; Shintani, S.; Masaki, H.; Amano, K.; Kishimoto, Y.; Yoshimoto, K.; Akashi, H.; et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: A pilot study and a randomised controlled trial. Lancet 2002, 360, 427–435. [Google Scholar] [CrossRef]
- Shimamura, M.; Nakagami, H.; Taniyama, Y.; Morishita, R. Gene therapy for peripheral arterial disease. Expert Opin. Biol. Ther. 2014, 14, 1175–1184. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Thomas, C.E.; Ehrhardt, A.; Kay, M.A. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 2003, 4, 346–358. [Google Scholar] [CrossRef]
- Mintzer, M.A.; Simanek, E.E. Nonviral Vectors for Gene Delivery. Chem. Rev. 2009, 109, 259–302. [Google Scholar] [CrossRef]
- Fischer, D.; Li, Y.X.; Ahlemeyer, B.; Krieglstein, J.; Kissel, T. In vitro cytotoxicity testing of polycations: Influence of polymer structure on cell viability and hemolysis. Biomaterials 2003, 24, 1121–1131. [Google Scholar] [CrossRef]
- Kim, Y.H.; Park, J.H.; Lee, M.; Kim, Y.H.; Park, T.G.; Kim, S.W. Polyethylenimine with acid-labile linkages as a biodegradable gene carrier. J. Control. Release 2005, 103, 209–219. [Google Scholar] [CrossRef]
- Che, J.; Tao, A.; Chen, S.; Li, X.; Yi, Z.; Yuan, W. Biologically responsive carrier-mediated anti-angiogenesis shRNA delivery for tumor treatment. Sci. Rep. 2016, 6, 35661. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, X.; Cheng, Y.; Zhao, X.; Fang, Z.; Luo, Y.; Xia, S.; Feng, Y.; Chen, J.; Yuan, W.-E. pH-Responsive Cross-Linked Low Molecular Weight Polyethylenimine as an Efficient Gene Vector for Delivery of Plasmid DNA Encoding Anti-VEGF-shRNA for Tumor Treatment. Front. Oncol. 2018, 8, 354. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Qian, Y.; Cheng, Y.; Guo, X.; Yuan, W.-E. One-pot construction of a twice-condensed pDNA polyplex system for peripheral nerve crush injury therapy. Biomater. Sci. 2018, 6, 2059–2072. [Google Scholar] [CrossRef]
- Patrick, M.; Gilles, B.; Jean Pierre, G.; Chantal, P. Polymer-based gene delivery: A current review on the uptake and intracellular trafficking of polyplexes. Curr. Gene Ther. 2008, 8, 335–352. [Google Scholar]
- Niiyama, H.; Huang, N.F.; Rollins, M.D.; Cooke, J.P. Murine model of hindlimb ischemia. J. Vis. Exp. JoVE 2009, 23, e1035. [Google Scholar] [CrossRef]
- Huang, X. Application of BrdU label technique for studying proliferating cells in rat endolymphatic sac. Chin. J. Histochem. Cytochem. 2002, 11, 403–404, 508. [Google Scholar]
- Akihiko, T.; Toshihiro, S.; Hidekazu, T.; Takayoshi, K.; Hiroyuki, N.; Hiroo, Y.; Yoshitane, T.; Hiroyuki, I.; Yoshihiro, F.; Stern, D.M.; et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J. Clin. Investig. 2004, 114, 330–338. [Google Scholar]
- Kim, S.; Kim, J.J.; Wook-Kim, S.; Maya, M.; Zhang, F.; He, J.; Fan, D.; Langley, R.; Fidler, I. Circulating monocytes expressing CD31: Implications for acute and chronic angiogenesis. Am. J. Pathol. 2009, 174, 1972–1980. [Google Scholar] [CrossRef]
- Che, J.; Xue, Y.; Feng, J.; Chen, S.; Bai, G.; Yuan, W. Comparison biological responses of polymers-based on imine- and disulfide-backbones for siRNA delivery. ACS Appl. Mater. Interfaces 2018, 10, 5196–5202. [Google Scholar] [CrossRef]
- Ge, X.; Feng, J.; Chen, S.; Zhang, C.; Ouyang, Y.; Liu, Z.; Yuan, W. Biscarbamate cross-linked low molecular weight Polyethylenimine polycation as an efficient intra-cellular delivery cargo for cancer therapy. J. Nanobiotechnol. 2014, 12, 13. [Google Scholar] [CrossRef]
- Chen, S.; Feng, J.; Ma, L.; Liu, Z.; Yuan, W. RNA interference technology for anti-VEGF treatment. Expert Opin. Drug Deliv. 2014, 11, 1471–1480. [Google Scholar] [CrossRef]
- Kim, J.; Mirando, A.C.; Popel, A.S.; Green, J.J. Gene delivery nanoparticles to modulate angiogenesis. Adv. Drug Deliv. Rev. 2016, 119, 20–43. [Google Scholar] [CrossRef]
- Wang, X.; Niu, D.; Hu, C.; Li, P. Polyethyleneimine-Based Nanocarriers for Gene Delivery. Curr. Pharm. Des. 2015, 21, 6140–6156. [Google Scholar] [CrossRef]
- Guo, G.; Tortorella, M.; Zhang, B.; Wang, Y. Disassembly of micelle-like polyethylenimine nanocomplexes for siRNA delivery: High transfection efficiency and reduced toxicity achieved by simple reducible lipid modification. J. Colloid Interface Sci. 2017, 504, 633–644. [Google Scholar] [CrossRef]
- Merkel, O.M.; Beyerle, A.; Beckmann, B.M.; Zheng, M.; Hartmann, R.K.; Stöger, T.; Kissel, T.H. Polymer-related off-target effects in non-viral siRNA delivery. Biomaterials 2011, 32, 2388–2398. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Jung, Y.; Choi, H.; Yang, J.; Suh, J.S.; Huh, Y.M.; Kim, K.; Haam, S. Prostate cancer cell death produced by the co-delivery of Bcl-xL shRNA and doxorubicin using an aptamer-conjugated polyplex. Biomaterials 2010, 31, 4592–4599. [Google Scholar] [CrossRef]
- Lv, J.; Chang, H.; Wang, Y.; Wang, M.; Xiao, J.; Zhang, Q.; Cheng, Y. Fluorination on polyethylenimine allows efficient 2D and 3D cell culture gene delivery. J. Mater. Chem. B 2015, 3, 642–650. [Google Scholar] [CrossRef]
- Wang, M.; Cheng, Y. The effect of fluorination on the transfection efficacy of surface-engineered dendrimers. Biomaterials 2014, 35, 6603–6613. [Google Scholar] [CrossRef]
| w/w Ratio | pDNA (μL) | PEI 1.8 kDa (μL) | GA (μL) | H2O (μL) |
|---|---|---|---|---|
| 1 | 1000 | 10 | 7 | 983 |
| 3 | 1000 | 30 | 21 | 949 |
| 5 | 1000 | 50 | 35 | 915 |
| 10 | 1000 | 100 | 70 | 830 |
| 15 | 1000 | 150 | 105 | 745 |
| 20 | 1000 | 200 | 140 | 660 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Yuan, Z.; Xu, Y.; Zhao, X.; Fang, Z.; Yuan, W.-E. A Low-Molecular-Weight Polyethylenimine/pDNA-VEGF Polyplex System Constructed in a One-Pot Manner for Hindlimb Ischemia Therapy. Pharmaceutics 2019, 11, 171. https://doi.org/10.3390/pharmaceutics11040171
Guo X, Yuan Z, Xu Y, Zhao X, Fang Z, Yuan W-E. A Low-Molecular-Weight Polyethylenimine/pDNA-VEGF Polyplex System Constructed in a One-Pot Manner for Hindlimb Ischemia Therapy. Pharmaceutics. 2019; 11(4):171. https://doi.org/10.3390/pharmaceutics11040171
Chicago/Turabian StyleGuo, Xiaoshuang, Zihan Yuan, Yang Xu, Xiaotian Zhao, Zhiwei Fang, and Wei-En Yuan. 2019. "A Low-Molecular-Weight Polyethylenimine/pDNA-VEGF Polyplex System Constructed in a One-Pot Manner for Hindlimb Ischemia Therapy" Pharmaceutics 11, no. 4: 171. https://doi.org/10.3390/pharmaceutics11040171
APA StyleGuo, X., Yuan, Z., Xu, Y., Zhao, X., Fang, Z., & Yuan, W.-E. (2019). A Low-Molecular-Weight Polyethylenimine/pDNA-VEGF Polyplex System Constructed in a One-Pot Manner for Hindlimb Ischemia Therapy. Pharmaceutics, 11(4), 171. https://doi.org/10.3390/pharmaceutics11040171





