Enhanced Transepithelial Permeation of Gallic Acid and (−)-Epigallocatechin Gallate across Human Intestinal Caco-2 Cells Using Electrospun Xanthan Nanofibers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Electrospun Nanofibers
2.3. Morphology and FTIR Characterisation of the Nanofibers
2.4. In Vitro Release of Gallic Acid and (−)-Epigallocatechin Gallate from Electrospun Nanofibers
2.5. Caco-2 Cell Culture and Subculture
2.6. Compounds and Electrospun Nanofibers Tested with Caco-2 Cell Monolayers
2.7. Caco-2 Cell Viability Assay
2.8. Transepithelial Transport
2.9. Measurement of Transepithelial Electrical Resistance (TEER)
2.10. Quantification of Compounds
2.11. FLUO, LY, GA, and EGCG Distribution after Transport Experiments and Mass Balance
2.12. Calculation of the Apparent Permeability Coefficients, Papp,AB and Papp,BA
3. Results
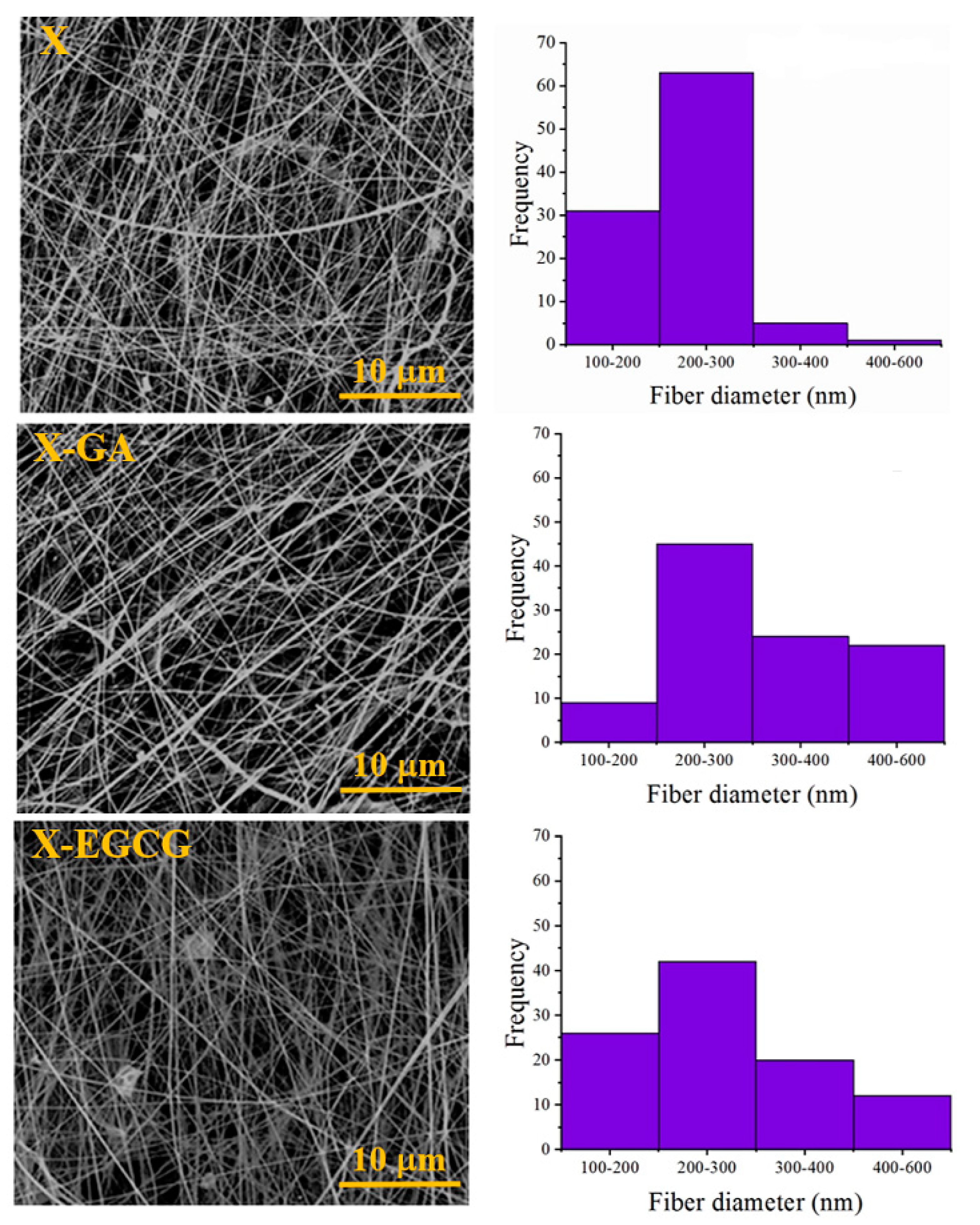
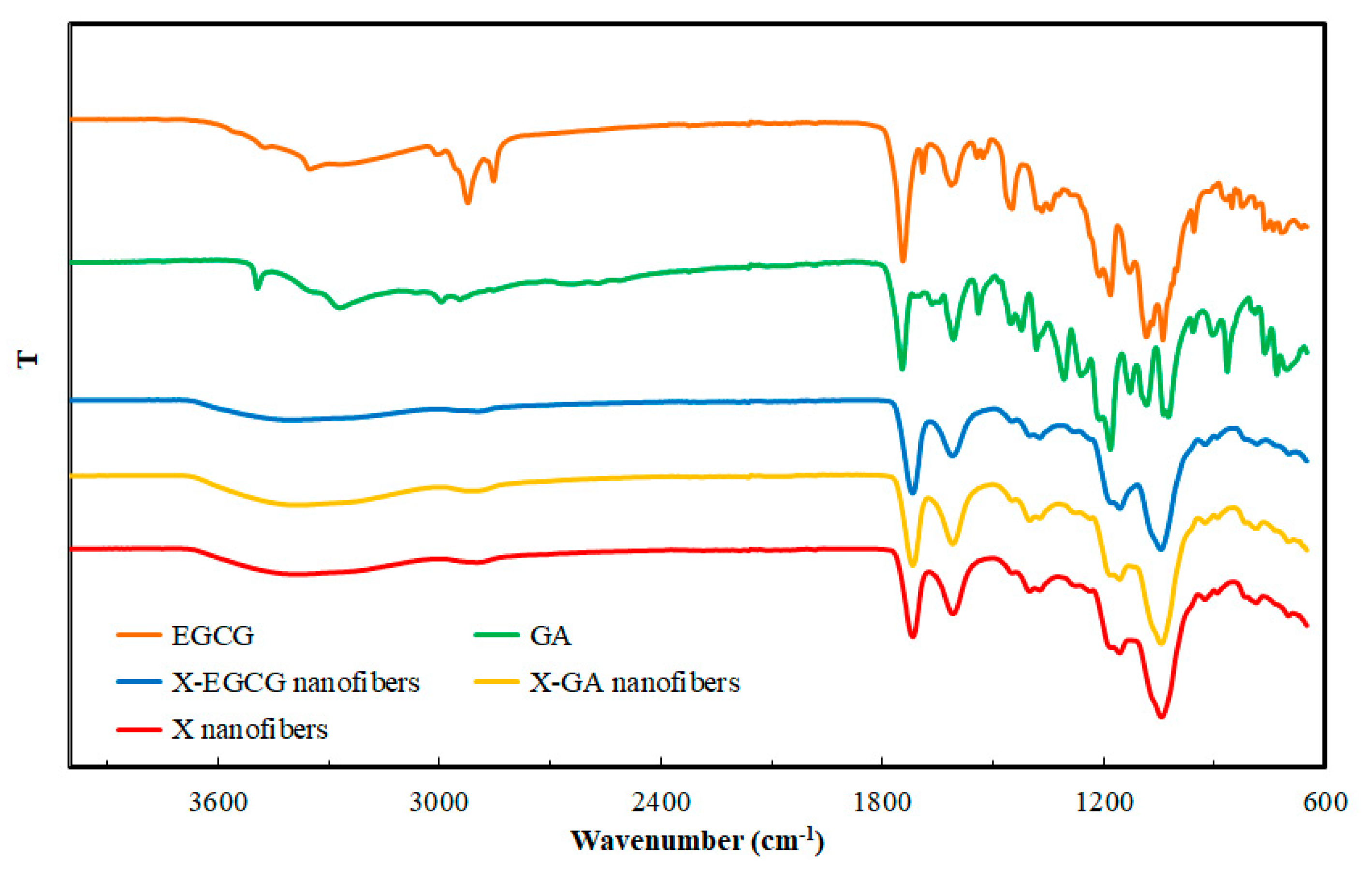
3.1. Morphological and FTIR Characterization of Nanofibers
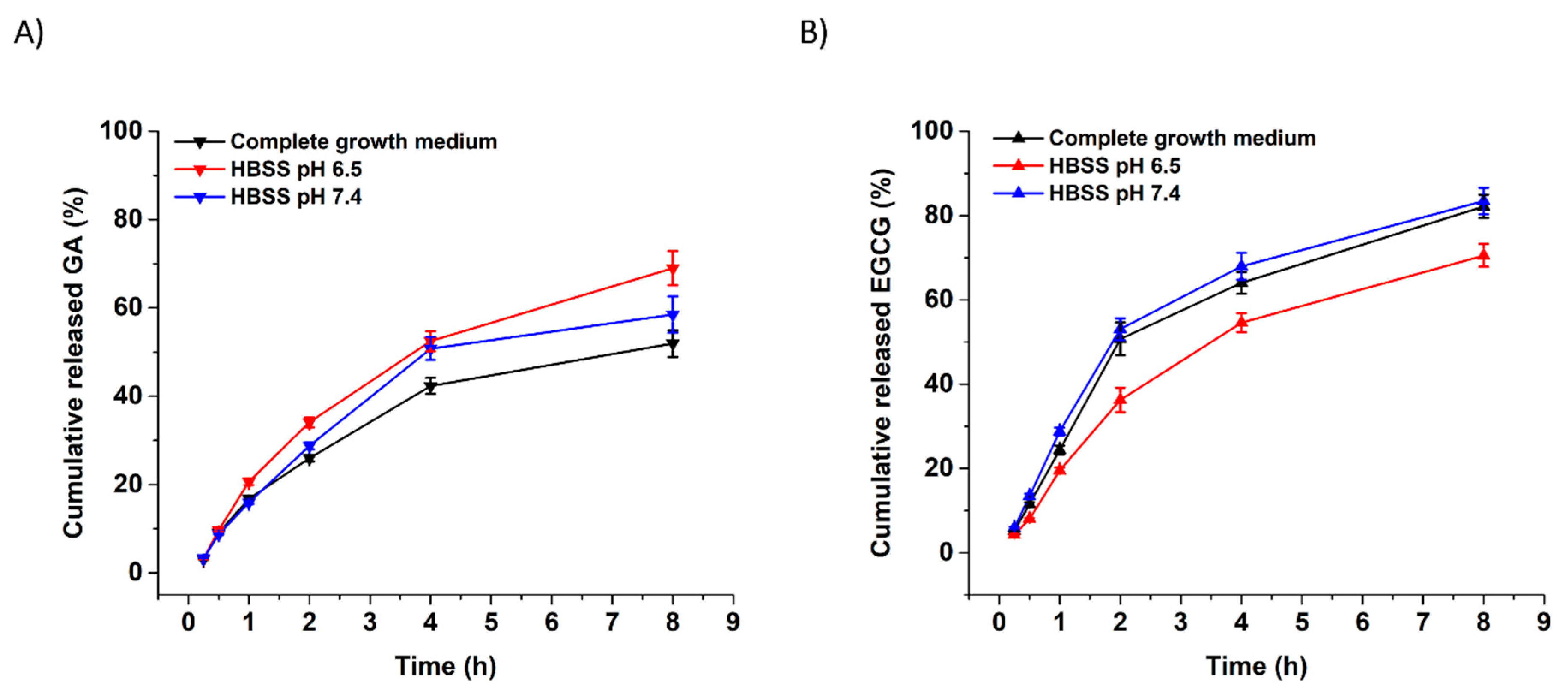
3.2. In Vitro Release of GA and EGCG from Xanthan Nanofibers
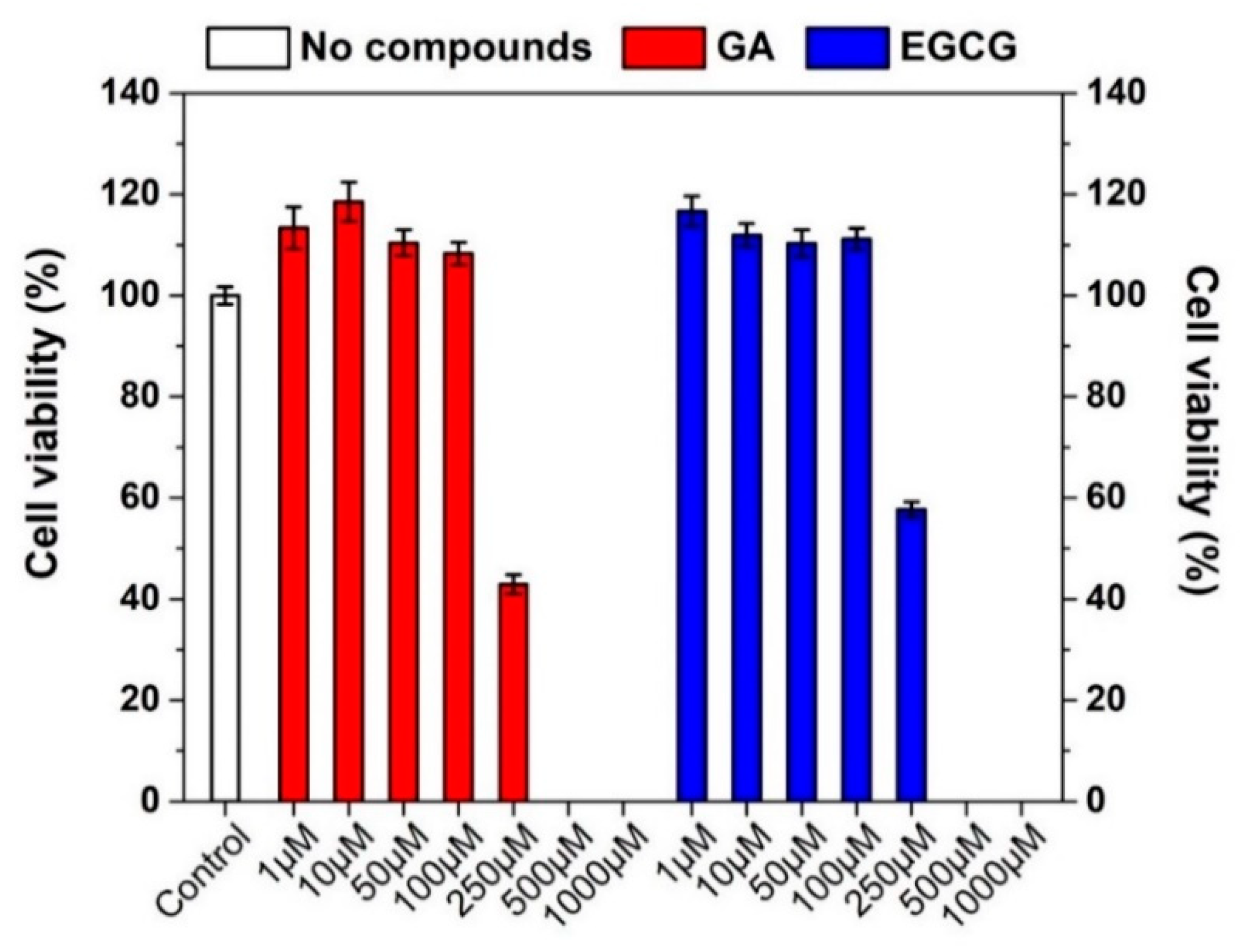
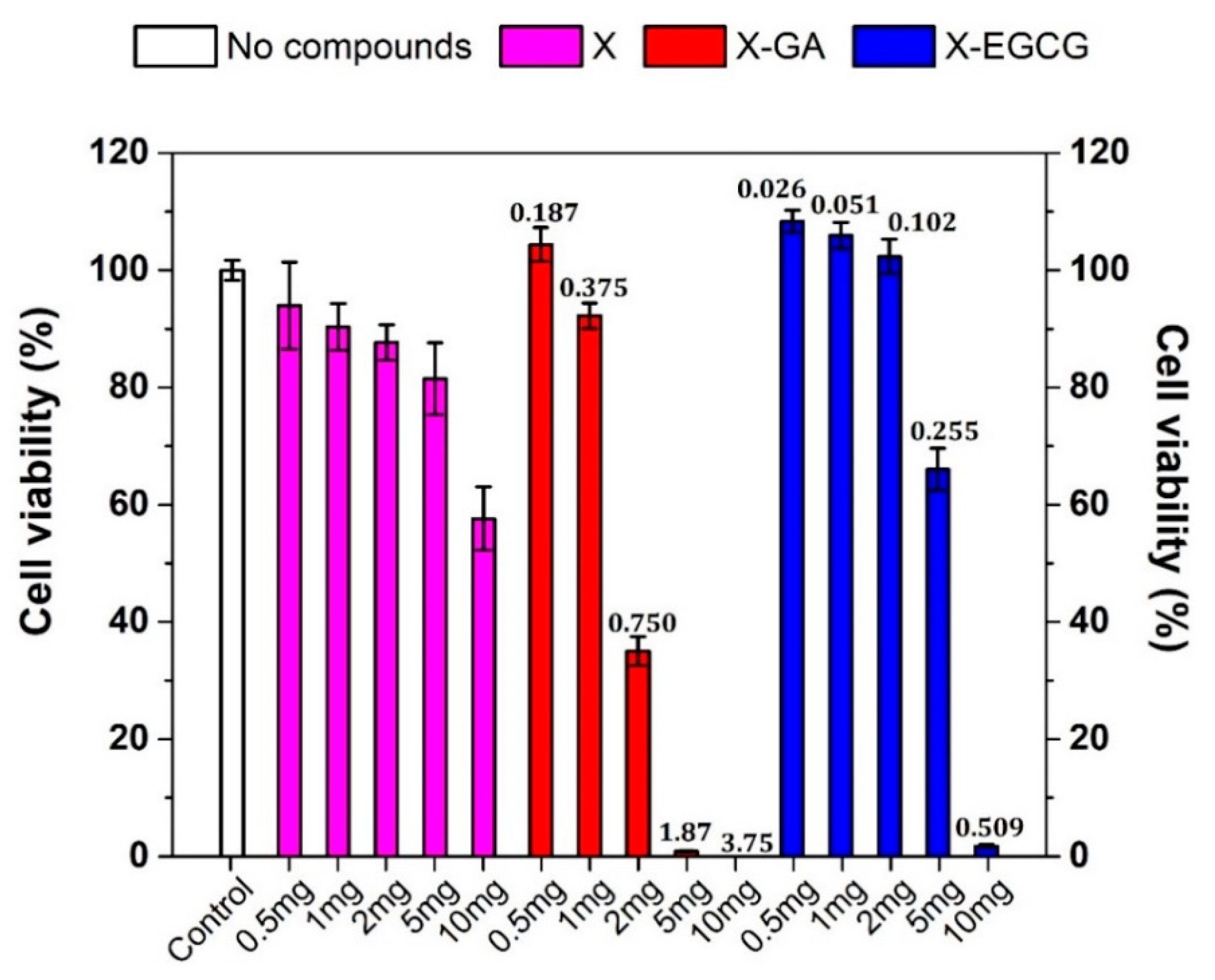
3.3. Effect of GA, EGCG, and Their Nanofiber Forms on Caco-2 Cell Viability
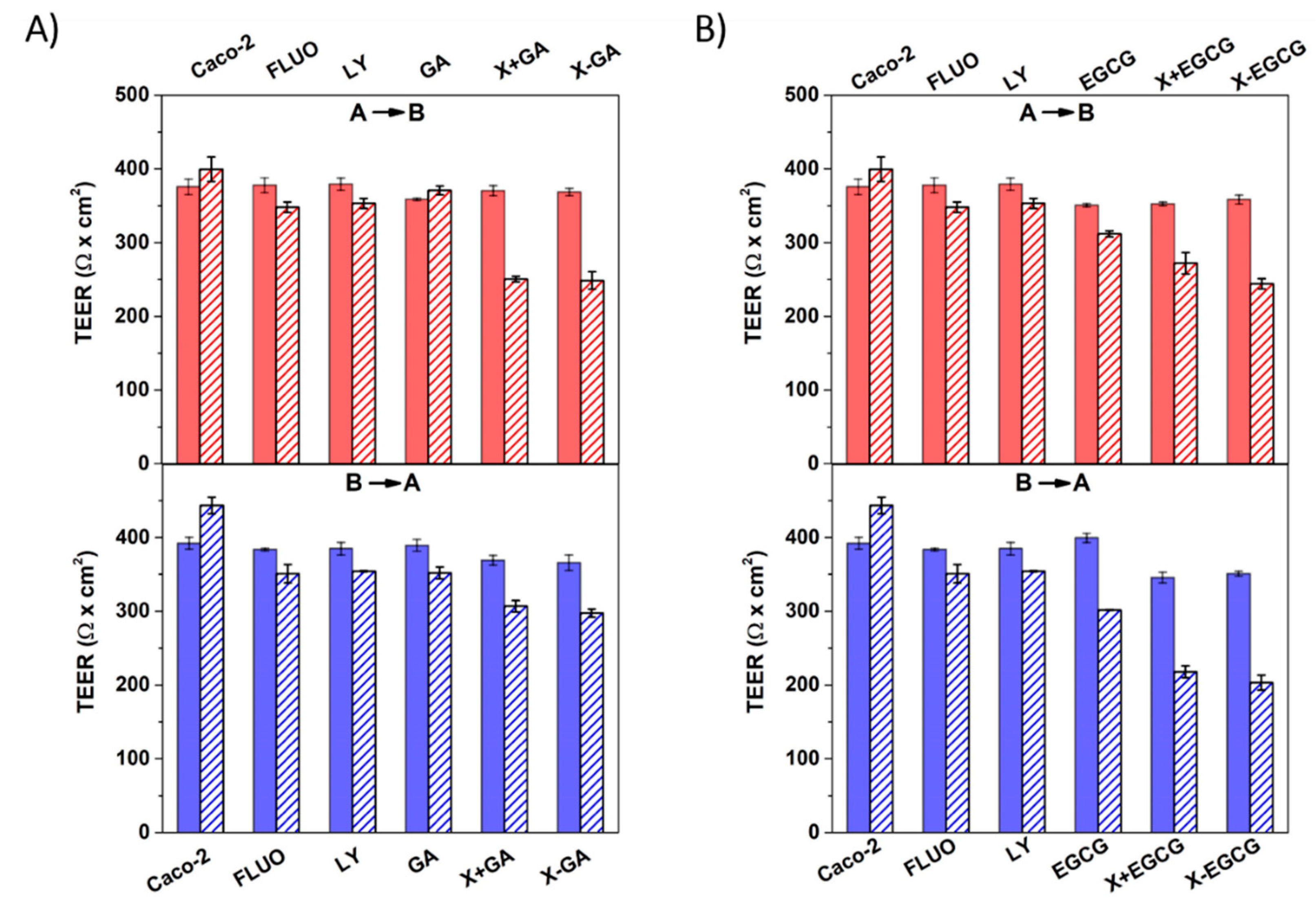
3.4. Assessment of Cell Monolayers’ Integrity
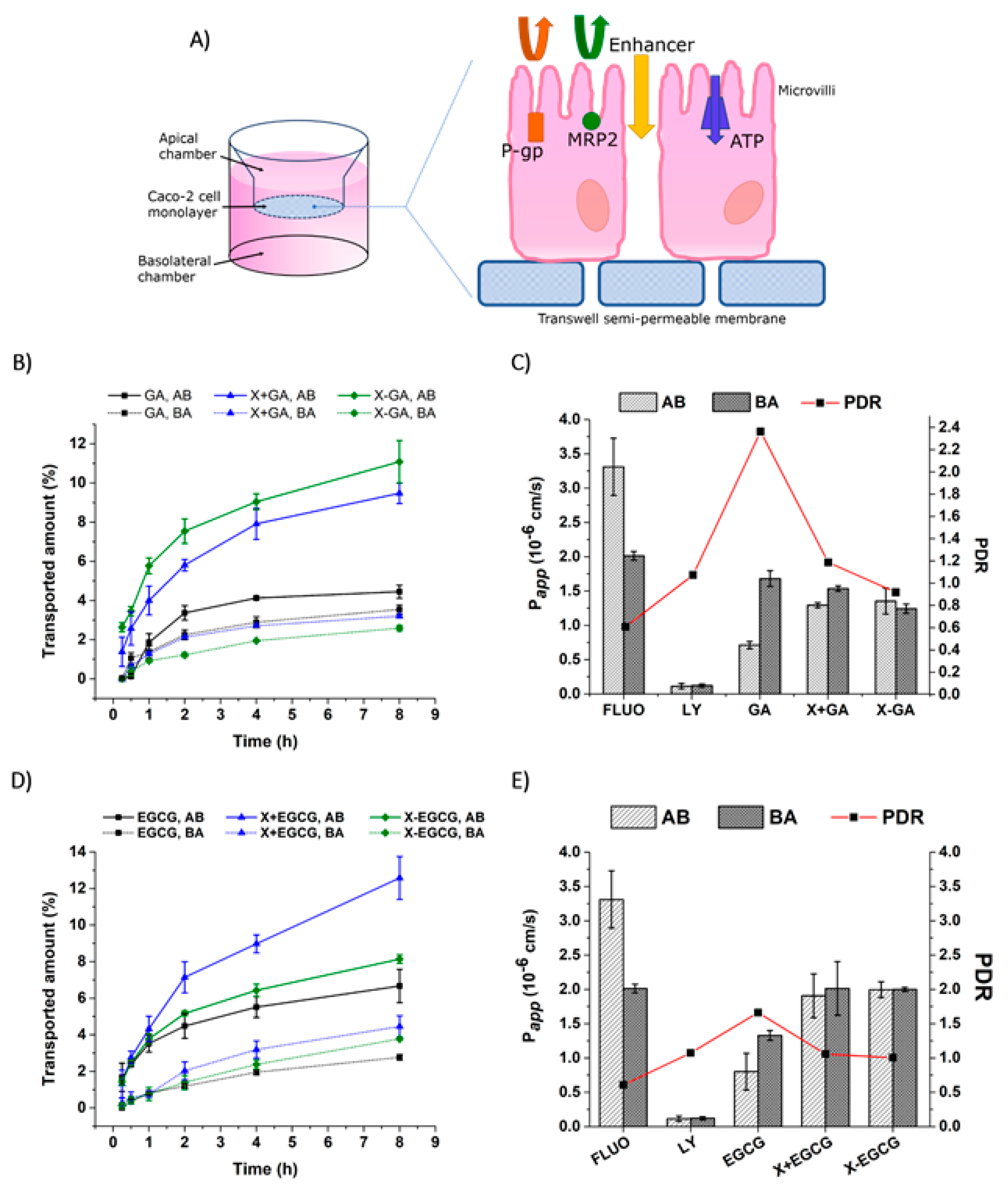
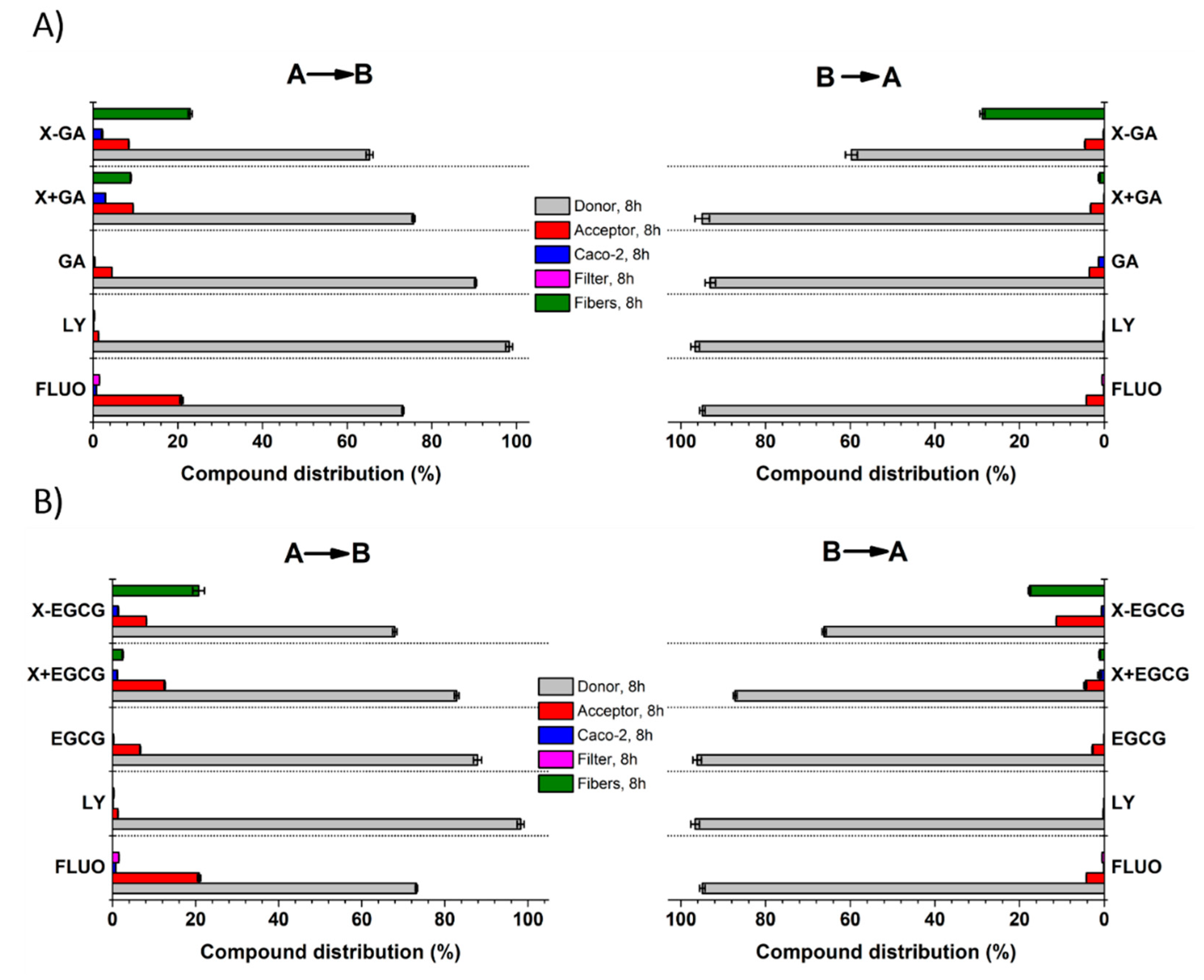
3.5. Transepithelial Transport and Distribution of Free GA, EGCG, and Their Nanofiber Forms
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Kühnau, J. The flavonoids. A class of semi-essential food components: Their role in human nutrition. World Rev. Nutr. Diet. 1976, 24, 117–191. [Google Scholar]
- Inoue, M.; Suzuki, R.; Koide, T.; Sakaguchi, N.; Ogihara, Y.; Yabu, Y. Antioxidant, gallic acid, induces apoptosis in HL-60RG cells. Biochem. Biophys. Res. Commun. 1994, 204, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Suzuki, R.; Sakaguchi, N.; Li, Z.; Takeda, T.; Ogihara, Y.; Jiang, B.Y.; Chen, Y. Selective induction of cell death in cancer cells by gallic acid. Biol. Pharm. Bull. 1995, 18, 1526–1530. [Google Scholar] [CrossRef]
- Gali, H.U.; Perchellet, E.M.; Perchellet, J.P. Inhibition of tumor promoter-induced ornithine decarboxylase activity by tannic acid and other polyphenols in mouse epidermis in vivo. Cancer Res. 1991, 51, 2820–2825. [Google Scholar]
- Gali, H.U.; Perchellet, E.M.; Klish, D.S.; Johnson, J.M.; Perchellet, J.P. Antitumor-promoting activities of hydrolyzable tannins in mouse skin. Carcinogenesis 1992, 13, 715–718. [Google Scholar] [CrossRef]
- Rashidi, L.; Vasheghani-Farahani, E.; Soleimani, M.; Atashi, A.; Rostami, K.; Gangi, F.; Fallahpour, M.; Tahouri, M.T. A cellular uptake and cytotoxicity properties study of gallic acid-loaded mesoporous silica nanoparticles on Caco-2 cells. J. Nanoparticle Res. 2014, 16, 2285. [Google Scholar] [CrossRef]
- Konishi, Y.; Kobayashi, S.; Shimizu, M. Transepithelial transport of p-coumaric acid and gallic acid in Caco-2 cell monolayers. Biosci. Biotechnol. Biochem. 2003, 67, 2317–2324. [Google Scholar] [CrossRef]
- Huang, P.-J.; Hseu, Y.-C.; Lee, M.-S.; Senthil Kumar, K.J.; Wu, C.-R.; Hsu, L.-S.; Liao, J.-W.; Cheng, I.-S.; Kuo, Y.-T.; Huang, S.-Y.; et al. In vitro and in vivo activity of gallic acid and toona sinensis leaf extracts against HL-60 human premyelocytic leukemia. Food Chem. Toxicol. 2012, 50, 3489–3497. [Google Scholar] [CrossRef]
- Inoue, M.; Sakaguchi, N.; Isuzugawa, K.; Tani, H.; Ogihara, Y. Role of reactive oxygen species in gallic acid-induced apoptosis. Biol. Pharm. Bull. 2000, 23, 1153–1157. [Google Scholar] [CrossRef]
- Kaur, M.; Velmurugan, B.; Rajamanickam, S.; Agarwal, R.; Agarwal, C. Gallic acid, an active constituent of grape seed extract, exhibits anti-proliferative, pro-apoptotic and anti-tumorigenic effects against prostate carcinoma xenograft growth in nude mice. Pharm. Res. 2009, 26, 2133–2140. [Google Scholar] [CrossRef] [PubMed]
- Pal, C.; Bindu, S.; Dey, S.; Alam, A.; Goyal, M.; Iqbal, M.S.; Maity, P.; Adhikari, S.S.; Bandyopadhyay, U. Gallic acid prevents nonsteroidal anti-inflammatory drug-induced gastropathy in rat by blocking oxidative stress and apoptosis. Free Radic. Biol. Med. 2010, 49, 258–267. [Google Scholar] [CrossRef]
- You, B.R.; Park, W.H. Gallic acid-induced lung cancer cell death is related to glutathione depletion as well as reactive oxygen species increase. Toxicol. Vitr. 2010, 24, 1356–1362. [Google Scholar] [CrossRef]
- You, B.R.; Moon, H.J.; Han, Y.H.; Park, W.H. Gallic acid inhibits the growth of HeLa cervical cancer cells via apoptosis and/or necrosis. Food Chem. Toxicol. 2010, 48, 1334–1340. [Google Scholar] [CrossRef] [PubMed]
- Munin, A.; Edwards-Lévy, F. Encapsulation of natural polyphenolic compounds; a review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Wu, L.-F.; Zhao, H.-J.; Liang, W.-Y.; Chen, W.-J.; Han, S.-X.; Qi, Q.; Cui, Y.-P.; Li, S.; Yang, G.-H.; et al. Transport of corilagin, gallic acid, and ellagic acid from fructus phyllanthi tannin fraction in Caco-2 cell monolayers. Evid.-Based Complement. Alternat. Med. 2016, 2016, 9205379. [Google Scholar] [CrossRef]
- Vaidyanathan, J.B.; Walle, T. Cellular uptake and efflux of the tea flavonoid (−)epicatechin-3-gallate in the human intestinal cell line Caco-2. J. Pharmacol. Exp. Ther. 2003, 307, 745–752. [Google Scholar] [CrossRef]
- Katiyar, S.; Mukhtar, H. Tea in chemoprevention of cancer. Int. J. Oncol. 1996, 8, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Li, D.; Zhou, Y.; Yang, J.; Yang, W.; Zhou, G.; Wen, J. Enhanced uptake and transport of (+)-catechin and (−)-epigallocatechin gallate in niosomal formulation by human intestinal Caco-2 cells. Int. J. Nanomed. 2014, 9, 2157. [Google Scholar] [CrossRef]
- Ji, B.T.; Chow, W.H.; Hsing, A.W.; McLaughlin, J.K.; Dai, Q.; Gao, Y.T.; Blot, W.J.; Fraumeni, J.F. Green tea consumption and the risk of pancreatic and colorectal cancers. Int. J. Cancer 1997, 70, 255–258. [Google Scholar] [CrossRef]
- Su, J.; Arab, L. Tea consumption and the reduced risk of colon cancer—results from a national prospective cohort study. Public Health Nutr. 2002, 5, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.H.; Cai, Y.; Alberts, D.S.; Hakim, I.; Dorr, R.; Shahi, F.; Crowell, J.A.; Yang, C.S.; Hara, Y. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol. Biomark. Prev. 2001, 10, 53–58. [Google Scholar]
- Warden, B.A.; Smith, L.S.; Beecher, G.R.; Balentine, D.A.; Clevidence, B.A. Catechins are bioavailable in men and women drinking black tea throughout the day. J. Nutr. 2001, 131, 1731–1737. [Google Scholar] [CrossRef]
- Teng, Z.; Yuan, C.; Zhang, F.; Huan, M.; Cao, W.; Li, K.; Yang, J.; Cao, D.; Zhou, S.; Mei, Q. Intestinal absorption and first-pass metabolism of polyphenol compounds in rat and their transport dynamics in Caco-2 cells. PLoS ONE 2012, 7, e29647. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, L.; Zhao, C.; Wu, X.; Wang, R.; Liu, C. Characterization of the intestinal absorption of seven flavonoids from the flowers of trollius chinensis using the Caco-2 cell monolayer model. PLoS ONE 2015, 10, e0119263. [Google Scholar] [CrossRef] [PubMed]
- Dube, A.; Ng, K.; Nicolazzo, J.A.; Larson, I. Effective use of reducing agents and nanoparticle encapsulation in stabilizing catechins in alkaline solution. Food Chem. 2010, 122, 662–667. [Google Scholar] [CrossRef]
- Caddeo, C.; Teskač, K.; Sinico, C.; Kristl, J. Effect of resveratrol incorporated in liposomes on proliferation and UV-B protection of cells. Int. J. Pharm. 2008, 363, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Chuysinuan, P.; Chimnoi, N.; Techasakul, S.; Supaphol, P. Gallic acid-loaded electrospun poly(l-lactic acid) fiber mats and their release characteristic. Macromol. Chem. Phys. 2009, 210, 814–822. [Google Scholar] [CrossRef]
- Neo, Y.P.; Ray, S.; Jin, J.; Gizdavic-Nikolaidis, M.; Nieuwoudt, M.K.; Liu, D.; Quek, S.Y. Encapsulation of food grade antioxidant in natural biopolymer by electrospinning technique: A physicochemical study based on zein-gallic acid system. Food Chem. 2013, 136, 1013–1021. [Google Scholar] [CrossRef]
- Phiriyawirut, M.; Phaechamud, T. Gallic acid-loaded cellulose acetate electrospun nanofibers: Thermal properties, mechanical properties, and drug release behavior. Open J. Polym. Chem. 2012, 02, 21–29. [Google Scholar] [CrossRef]
- Aytac, Z.; Kusku, S.I.; Durgun, E.; Uyar, T. Encapsulation of gallic acid/cyclodextrin inclusion complex in electrospun polylactic acid nanofibers: Release behavior and antioxidant activity of gallic acid. Mater. Sci. Eng. C 2016, 63, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lim, L.-T.; Kakuda, Y. Electrospun zein fibers as carriers to stabilize (−)-epigallocatechin gallate. J. Food Sci. 2009, 74, C233–C240. [Google Scholar] [CrossRef]
- Lee, E.J.; Lee, J.H.; Jin, L.; Jin, O.S.; Shin, Y.C.; Sang, J.O.; Lee, J.; Hyon, S.-H.; Han, D.-W. Hyaluronic acid/poly(lactic-co-glycolic acid) core/shell fiber meshes loaded with epigallocatechin-3-O-gallate as skin tissue engineering scaffolds. J. Nanosci. Nanotechnol. 2014, 14, 8458–8463. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Kwon, B.-J.; Koo, M.-A.; Jang, E.H.; Seon, G.M.; Park, J.-C. Exovascular application of epigallocatechin-3-O-gallate-releasing electrospun poly(l-lactide glycolic acid) fiber sheets to reduce intimal hyperplasia in injured abdominal aorta. Biomed. Mater. 2015, 10, 055010. [Google Scholar] [CrossRef]
- Ho, L.J.; Cheol, S.Y.; Jun, Y.W.; Chul, P.J.; Hyu, H.S.; Wook, H.D. Epigallocatechin-3-O-gallate-loaded poly(lactic-co-glycolic acid) fibrous sheets as anti-adhesion barriers. J. Biomed. Nanotechnol. 2014, 11, 1461–1471. [Google Scholar] [CrossRef]
- Tian, J.; Tu, H.; Shi, X.; Wang, X.; Deng, H.; Li, B.; Du, Y. Antimicrobial application of nanofibrous mats self-assembled with chitosan and epigallocatechin gallate. Colloids Surf. B Biointerfaces 2016, 145, 643–652. [Google Scholar] [CrossRef]
- Ayca, A.; Gulum, S.; Serpil, S. Fabrication of gallic acid loaded hydroxypropyl methylcellulose nanofibers by electrospinning technique as active packaging material. Carbohydr. Polym. 2019, 208, 241–250. [Google Scholar] [CrossRef]
- Shekarforoush, E.; Ajalloueian, F.; Zeng, G.; Mendes, A.C.; Chronakis, I.S. Electrospun xanthan-chitosan nanofibers as delivery carrier of hydrophobic bioactives. Mater. Lett. 2018, 228, 322–326. [Google Scholar] [CrossRef]
- Faralli, A.; Shekarforoush, E.; Ajalloueian, F.; Mendes, A.C.; Chronakis, I.S. In vitro permeability enhancement of curcumin across Caco-2 cells monolayers using electrospun xanthan-chitosan nanofibers. Carbohydr. Polym. 2019, 206, 38–47. [Google Scholar] [CrossRef]
- Shekarforoush, E.; Faralli, A.; Ndoni, S.; Mendes, A.C.; Chronakis, I.S. Electrospinning of xanthan polysaccharide. Macromol. Mater. Eng. 2017, 302, 1700067. [Google Scholar] [CrossRef]
- Hubatsch, I.; Ragnarsson, E.G.E.; Artursson, P. Determination of drug permeability and prediction of drug absorption in Caco-2 monolayers. Nat. Protoc. 2007, 2, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Amidon, G.L.; Lee, P.I.; Topp, E.M. Transport Processes in Pharmaceutical Systems; M. Dekker: New York, NY, USA, 2000; ISBN 9780824766108. [Google Scholar]
- Högerle, M.L.; Winne, D. Drug absorption by the rat jejunum perfused in situ. Dissociation from the pH-partition theory and role of microclimate-pH and unstirred layer. Naunyn. Schmiedebergs. Arch. Pharmacol. 1983, 322, 249–255. [Google Scholar]
- Bock, U.; Kolac, C.; Borchard, G.; Koch, K.; Fuchs, R.; Streichhan, P.; Lehr, C.M. Transport of proteolytic enzymes across Caco-2 cell monolayers. Pharm. Res. 1998, 15, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Hagiwara, K.; Shimizu, M. Transepithelial transport of fluorescein in Caco-2 Cell monolayers and use of such transport in in vitro evaluation of phenolic acid availability. Biosci. Biotechnol. Biochem. 2002, 66, 2449–2457. [Google Scholar] [CrossRef] [PubMed]
- Artursson, P.; Palm, K.; Luthman, K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv. Drug Deliv. Rev. 2001, 46, 27–43. [Google Scholar] [CrossRef]
- Yee, S. In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man—Fact or myth. Pharm. Res. 1997, 14, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Naruhashi, K.; Kurahashi, Y.; Fujita, Y.; Kawakita, E.; Yamasaki, Y.; Hattori, K.; Nishimura, A.; Shibata, N. Comparison of the expression and function of ATP binding cassette transporters in Caco-2 and T84 cells on stimulation by selected endogenous compounds and xenobiotics. Drug Metab. Pharmacokinet. 2011, 26, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Hoosain, F.G.; Choonara, Y.E.; Tomar, L.K.; Kumar, P.; Tyagi, C.; du Toit, L.C.; Pillay, V. Bypassing P-glycoprotein drug efflux mechanisms: Possible applications in pharmacoresistant schizophrenia therapy. BioMed Res. Int. 2015, 484963. [Google Scholar] [CrossRef] [PubMed]
- Werle, M. Expert review. Natural and synthetic polymers as inhibitors of drug efflux pumps. Pharm. Res. 2007, 25, 500–511. [Google Scholar] [CrossRef]
- Stephansen, K.; García-Díaz, M.; Jessen, F.; Chronakis, I.S.; Nielsen, H.M. Bioactive protein-based nanofibers interact with intestinal biological components resulting in transepithelial permeation of a therapeutic protein. Int. J. Pharm. 2015, 495, 58–66. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faralli, A.; Shekarforoush, E.; Mendes, A.C.; Chronakis, I.S. Enhanced Transepithelial Permeation of Gallic Acid and (−)-Epigallocatechin Gallate across Human Intestinal Caco-2 Cells Using Electrospun Xanthan Nanofibers. Pharmaceutics 2019, 11, 155. https://doi.org/10.3390/pharmaceutics11040155
Faralli A, Shekarforoush E, Mendes AC, Chronakis IS. Enhanced Transepithelial Permeation of Gallic Acid and (−)-Epigallocatechin Gallate across Human Intestinal Caco-2 Cells Using Electrospun Xanthan Nanofibers. Pharmaceutics. 2019; 11(4):155. https://doi.org/10.3390/pharmaceutics11040155
Chicago/Turabian StyleFaralli, Adele, Elhamalsadat Shekarforoush, Ana C. Mendes, and Ioannis S. Chronakis. 2019. "Enhanced Transepithelial Permeation of Gallic Acid and (−)-Epigallocatechin Gallate across Human Intestinal Caco-2 Cells Using Electrospun Xanthan Nanofibers" Pharmaceutics 11, no. 4: 155. https://doi.org/10.3390/pharmaceutics11040155
APA StyleFaralli, A., Shekarforoush, E., Mendes, A. C., & Chronakis, I. S. (2019). Enhanced Transepithelial Permeation of Gallic Acid and (−)-Epigallocatechin Gallate across Human Intestinal Caco-2 Cells Using Electrospun Xanthan Nanofibers. Pharmaceutics, 11(4), 155. https://doi.org/10.3390/pharmaceutics11040155






