A Recombinant Enolase-Montanide™ PetGel A Vaccine Promotes a Protective Th1 Immune Response against a Highly Virulent Sporothrix schenckii by Toluene Exposure
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Microorganisms and Preparations
2.3. S. schenckii Growth in Toluene
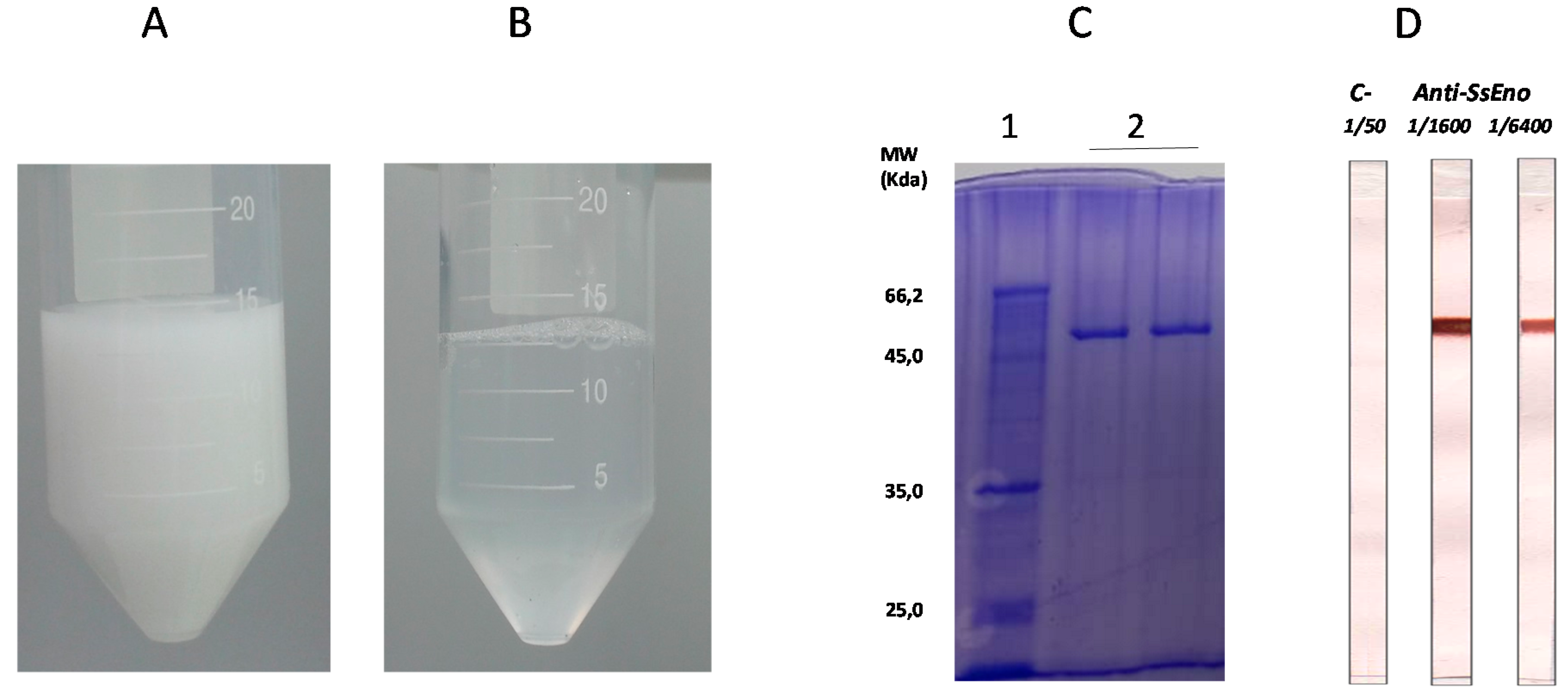
2.4. Expression and Purification of Recombinant S. schenckii Enolase (rSsEno)
2.5. Adjuvants and Vaccine Formulation
2.6. Immunization Schedule
2.7. Quantification of the rSsEno-Antibody Response by Enzyme-Linked Immunosorbent Assay (ELISA)
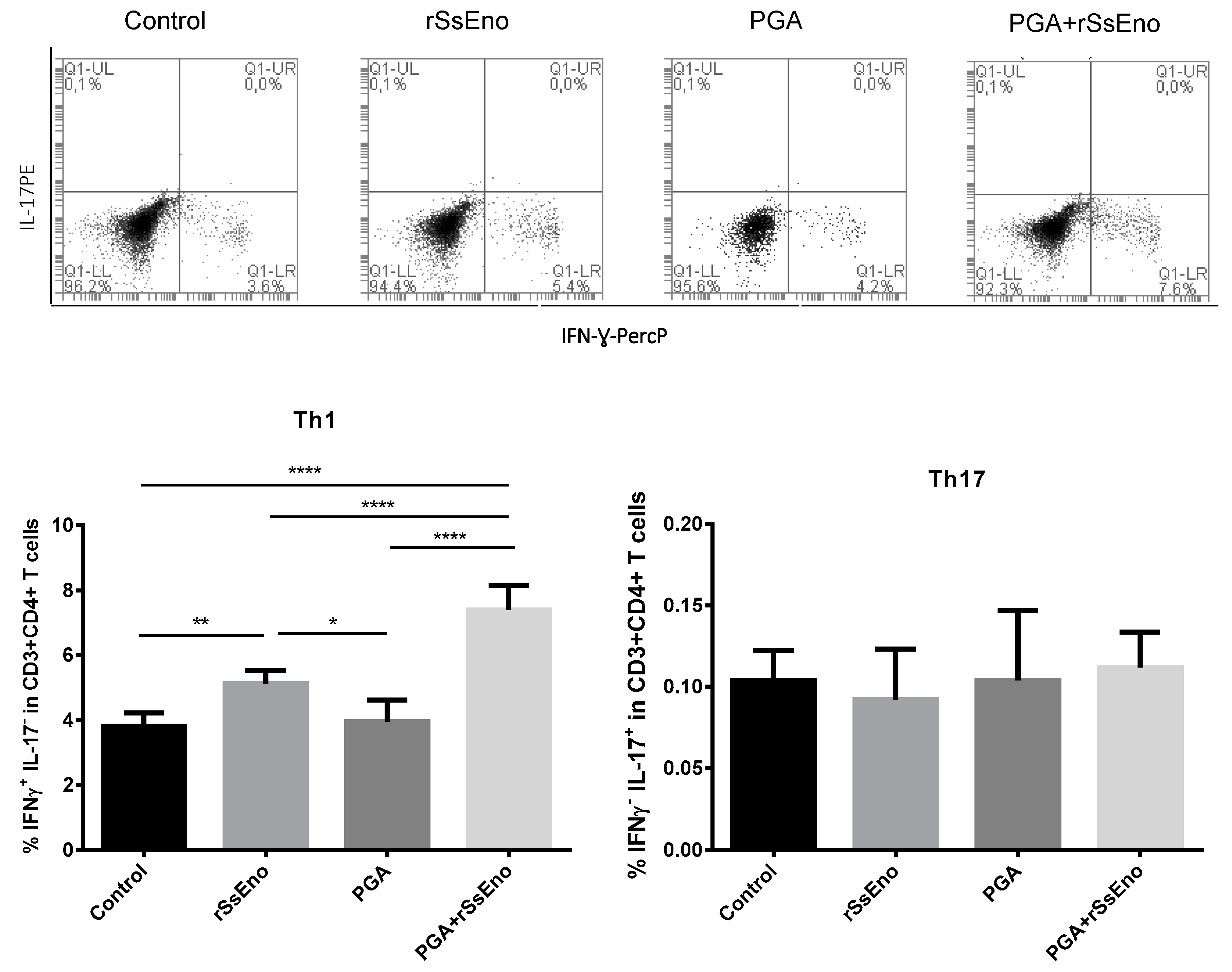
2.8. Th1-Th17 Phenotipagem
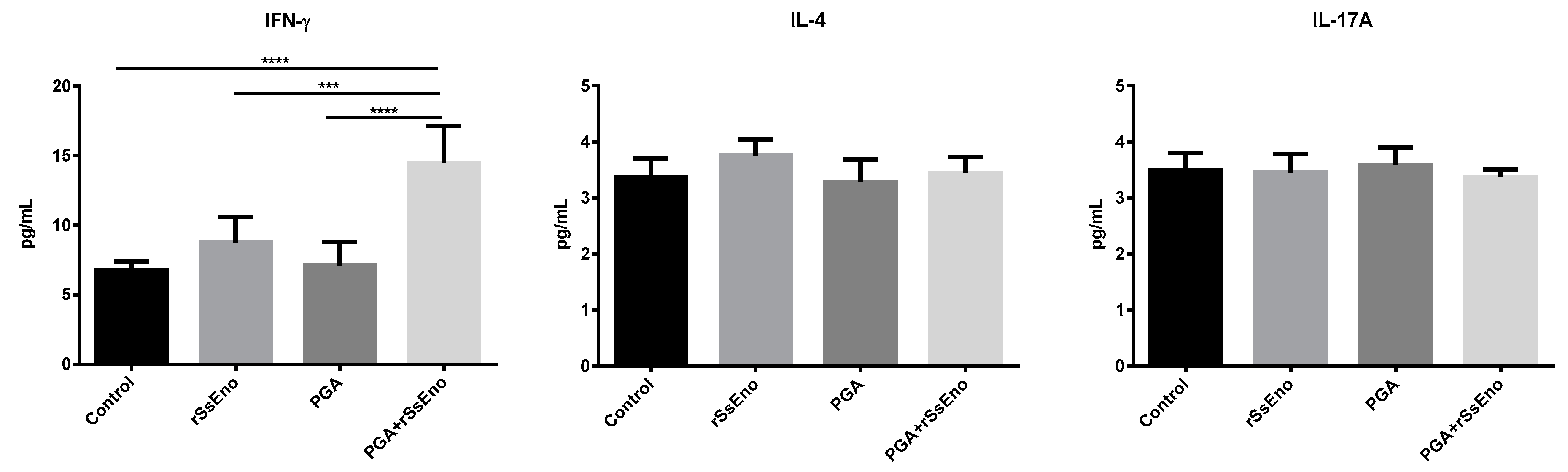
2.9. IFN-Ɣ, IL-4, and IL-17 Measurement in Supernatant of Splenocytes Culture
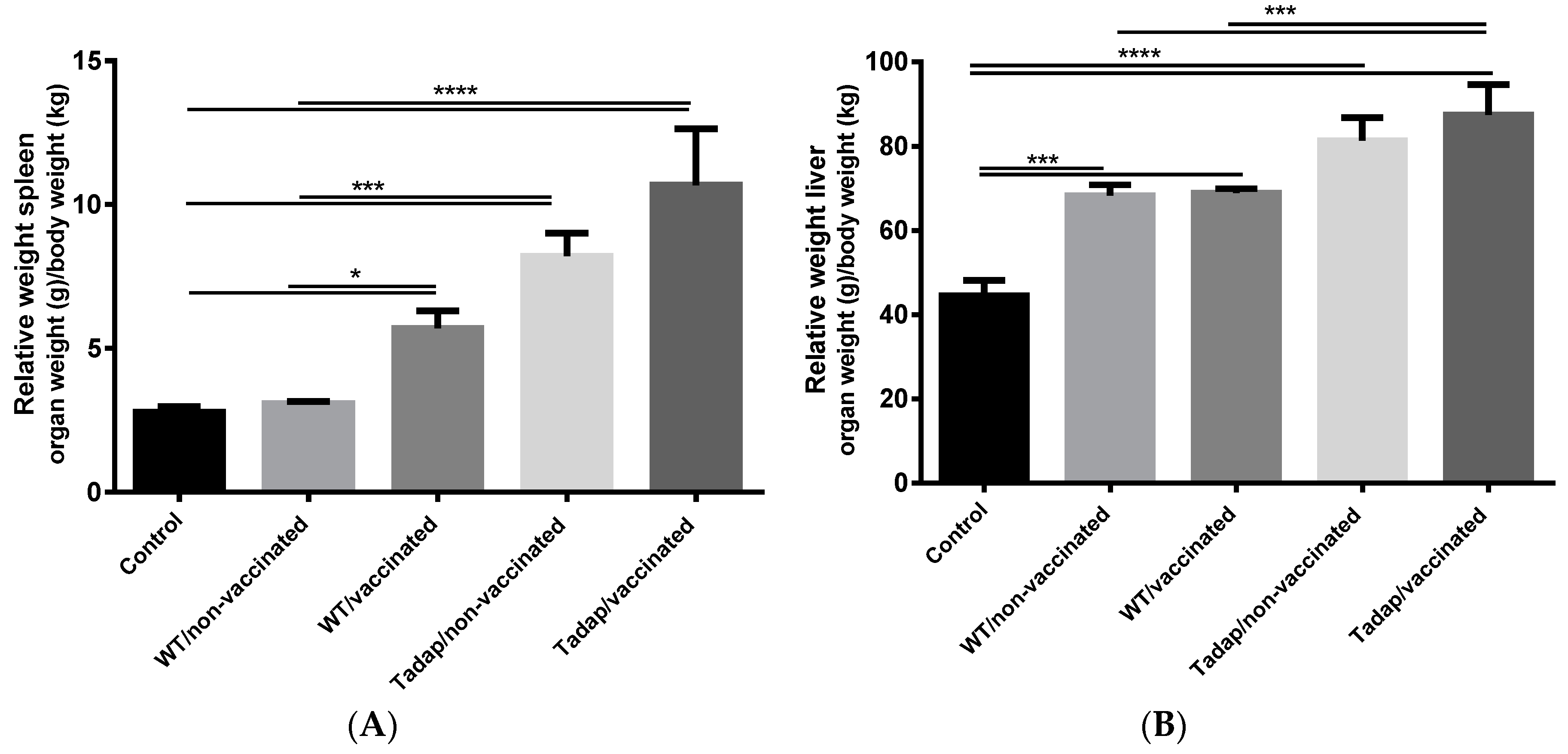
2.10. Fungal Challenge and Infection Assessment
2.11. Statistical Analysis
3. Results
3.1. Production and Purification of rSsEno
3.2. Post-Vaccination rSsEno-Antibody Response
3.3. Th1 and Th17 Response
3.4. Fungal Challenge and Infection Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Beer, Z.W.; Duong, T.A.; Wingfield, M.J. The divorce of Sporothrix and Ophiostoma: Solution to a problematic relationship. Stud. Mycol. 2016, 83, 165–191. [Google Scholar] [CrossRef]
- Gremião, I.D.; Miranda, L.H.; Reis, E.G.; Rodrigues, A.M.; Pereira, S.A. Zoonotic Epidemic of Sporotrichosis: Cat to Human Transmission. PLoS Pathog. 2017, 13, e1006077. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.B.; de Almeida Paes, R.; Schubach, A.O. Sporothrix schenckii and Sporotrichosis. Clin. Microbiol. Rev. 2011, 24, 633–654. [Google Scholar] [CrossRef] [PubMed]
- Téllez, M.D.; Batista-Duharte, A.; Portuondo, D.; Quinello, C.; Bonne-Hernández, R.; Carlos, I.Z. Sporothrix schenckii complex biology: Environment and fungal pathogenicity. Microbiology 2014, 160 Pt 11, 2352–2365. [Google Scholar] [CrossRef]
- Ramírez-Soto, M.C.; Aguilar-Ancori, E.G.; Tirado-Sánchez, A.; Bonifaz, A. Ecological Determinants of Sporotrichosis Etiological Agents. J. Fungi 2018, 4, 95. [Google Scholar] [CrossRef] [PubMed]
- Batista-Duharte, A.; Téllez-Martínez, D.; Aparecida Jellmayer, J.; Leandro Portuondo Fuentes, D.; Campos Polesi, M.; Martins Baviera, A.; Zeppone Carlos, I. Repeated Exposition to Mercury (II) Chloride Enhances Susceptibility to S. schenckii sensu stricto Infection in Mice. J. Fungi 2018, 4, 64. [Google Scholar] [CrossRef]
- Tellez-Martinez, D.; Batista-Duharte, A.; Silva, V.P.; Portuondo, D.F.; Ferreira, L.S.; Marisa Polesi, C.; Costa, C.; Carlos, I.Z. Adaptive stress response induced by toluene increases Sporothrix schenckii virulence and host immune response. bioRxiv 2019. [Google Scholar] [CrossRef]
- Kauffman, C.A.; Bustamante, B.; Chapman, S.W.; Pappas, P.G. Clinical practice guidelines for the management of sporotrichosis: 2007 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2007, 45, 1255–1265. [Google Scholar] [CrossRef]
- De Almeida, J.R.F.; Kaihami, G.H.; Jannuzzi, G.P.; de Almeida, S.R. Therapeutic vaccine using a monoclonal antibody against a 70-kDa glycoprotein in mice infected with highly virulent Sporothrix schenckii and Sporothrix brasiliensis. Med. Mycol. 2015, 53, 42–50. [Google Scholar] [CrossRef]
- Batista-Duharte, A.; Pereira, S.A.; Freitas, D.F.S.; Gutierrez-Galhardo, M.C.; Fuentes, D.P.; Carlos, I.Z. Therapeutic and Prophylactic Tools for Sporotrichosis: Current Strategies and Future Tendencies. In Sporotrichosis: New Developments and Future Prospects; Carlos, I.Z., Ed.; Springer: Cham, Switzerland, 2015; pp. 147–177. [Google Scholar]
- Edwards, J.E. Fungal cell wall vaccines: An update. J. Med. Microbiol. 2012, 6, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Portuondo, D.L.; Ferreira, L.S.; Urbaczek, A.C.; Batista-Duharte, A.; Carlos, I.Z. Adjuvants and delivery systems for antifungal vaccines: Current state and future developments. Med. Mycol. 2015, 53, 69–89. [Google Scholar] [CrossRef]
- Di Pasquale, A.; Preiss, S.; Tavares Da Silva, F.; Garçon, N. Vaccine Adjuvants: From 1920 to 2015 and beyond. Vaccines 2015, 3, 320–343. [Google Scholar] [CrossRef] [PubMed]
- Pérez, O.; Batista-Duharte, A.; González, E.; Zayas, C.; Balboa, J.; Cuello, M.; Cabrera, O.; Lastre, M.; Schijns, V.E. Human prophylactic vaccine adjuvants and their determinant role in new vaccine formulations. Braz. J. Med. Biol. Res. 2012, 45, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Hendrick, M.J.; Goldschmidt, M.H.; Shofer, F.S.; Wang, Y.Y.; Somlyo, A.P. Postvaccinal sarcomas in the cat: Epidemiology and electron probe microanalytical identification of aluminum. Cancer Res. 1992, 52, 5391–5394. [Google Scholar] [PubMed]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine adjuvants: Putting innate immunity to work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Batista-Duharte, A.; Téllez-Martínez, D.; Fuentes, D.L.P.; Carlos, I.Z. Molecular adjuvants that modulate regulatory T cell function in vaccination: A critical appraisal. Pharmacol. Res. 2018, 129, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhao, D.; Li, D.; Wang, X.; Jin, Z.; Zhao, K. Polymer-based nanomaterials and applications for vaccines and drugs. Polymers 2018, 10, 31. [Google Scholar] [CrossRef]
- Deville, S.; Carneaux, E.; Bertrand, F.; Cauchard, S.; Cauchard, J.; Dupuis, L. Adjuvant formulation for companion animals vaccines. Procedia Vaccinol. 2011, 4, 104–112. [Google Scholar] [CrossRef]
- Portuondo, D.L.; Batista-Duharte, A.; Ferreira, L.S.; Martínez, D.T.; Polesi, M.C.; Duarte, R.A.; de Paula, E.; Silva, A.C.; Marcos, C.M.; Almeida, A.M.; et al. A cell wall protein-based vaccine candidate induce protective immune response against Sporothrix schenckii infection. Immunobiology 2016, 221, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Portuondo, D.L.; Batista-Duharte, A.; Ferreira, L.S.; de Andrade, C.R.; Quinello, C.; Téllez-Martínez, D.; de Aguiar Loesch, M.L.; Carlos, I.Z. Comparative efficacy and toxicity of two vaccine candidates against Sporothrix schenckii using either Montanide™ Pet Gel A or aluminum hydroxide adjuvants in mice. Vaccine 2017, 35, 4430–4436. [Google Scholar] [CrossRef] [PubMed]
- Portuondo, D.L.; Dores-Silva, P.R.; Ferreira, L.S.; Téllez-Martínez, D.; Marcos, C.M.; de Aguiar Loesch, M.L.; Guzman, Q.F.; Borges, J.C.; Batista-Duharte, A.; Carlos, I.Z. Immunization with recombinant enolase of Sporothrix spp (rSsEno) confers effective protection against sporotrichosis in mice. bioRxiv 2019. [Google Scholar] [CrossRef]
- Taborda, C.P.; Nosanchuk, J.D. Vaccines, Immunotherapy and New Antifungal Therapy against Fungi: Updates in the New Frontier. Front. Microbiol. 2017, 8, 1743. [Google Scholar] [CrossRef]
- Nami, S.; Mohammadi, R.; Vakili, M.; Khezripour, K.; Mirzaei, H.; Morovati, H. Fungal vaccines, mechanism of actions and immunology: A comprehensive review. Biomed. Pharmacother. 2019, 109, 333–344. [Google Scholar] [CrossRef]
- Spellberg, B. Vaccines for invasive fungal infections. F1000 Med. Rep. 2011, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Di Pasquale, A.; Bonanni, P.; Garçon, N.; Stanberry, L.R.; El-Hodhod, M.; Tavares Da Silva, F. Vaccine safety evaluation: Practical aspects in assessing benefits and risks. Vaccine 2016, 34, 6672–6680. [Google Scholar] [CrossRef] [PubMed]
- Moyer, T.J.; Zmolek, A.C.; Irvine, D.J. Beyond antigens and adjuvants: Formulating future vaccines. J. Clin. Investig. 2016, 126, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Batista-Duharte, A.; Martínez, D.T.; Carlos, I.Z. Efficacy and safety of immunological adjuvants. Where is the cut-off? Biomed. Pharmacother. 2018, 105, 616–624. [Google Scholar] [CrossRef]
- Adams, J.R.; Haughney, S.L.; Mallapragada, S.K. Effective polymer adjuvants for sustained delivery of protein subunit vaccines. Acta Biomater. 2015, 14, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Shakya, A.K.; Nandakumar, K.S. Polymers as immunological adjuvants: An update on recent developments. J. BioSci. Biotechnol. 2012, 1, 99–210. [Google Scholar]
- Shakya, A.K.; Nandakumar, K.S. Applications of polymeric adjuvants in studying autoimmune responses and vaccination againstinfectious diseases. J. R. Soc. Interface 2013, 10, 20120536. [Google Scholar] [CrossRef]
- Uenotsuchi, T.; Takeuchi, S.; Matsuda, T.; Urabe, K.; Koga, T.; Uchi, H.; Nakahara, T.; Fukagawa, S.; Kawasaki, M.; Kajiwara, H.; et al. Differential induction of Th1-prone immunity by human dendritic cells activated with Sporothrix schenckii of cutaneous and visceral origins to determine their different virulence. Int. Immunol. 2006, 18, 1637–1646. [Google Scholar] [CrossRef]
- Maia, D.C.; Sassá, M.F.; Placeres, M.C.; Carlos, I.Z. Influence of Th1/Th2 cytokines and nitric oxide in murine systemic infection induced by Sporothrix schenckii. Mycopathologia 2006, 161, 11–19. [Google Scholar] [CrossRef]
- Quinello, C.; Ferreira, L.S.; Picolli, I.; Loesch, M.L.; Portuondo, D.L.; Batista-Duharte, A.; Carlos, I.Z. Sporothrix schenckii Cell Wall Proteins-Stimulated BMDCs Are Able to Induce a Th1-Prone Cytokine Profile In Vitro. J. Fungi 2018, 4, 106. [Google Scholar] [CrossRef] [PubMed]
- Batista-Duharte, A.; Téllez-Martínez, D.; Roberto de Andrade, C.; Portuondo, D.L.; Jellmayer, J.A.; Polesi, M.C.; Carlos, I.Z. Sporothrix brasiliensis induces a more severe disease associated with sustained Th17 and regulatory T cells responses than Sporothrix schenckii sensu stricto in mice. Fungal Biol. 2018, 122, 1163–1170. [Google Scholar] [CrossRef]
- Flores-García, A.; Velarde-Félix, J.S.; Garibaldi-Becerra, V.; Rangel-Villalobos, H.; Torres-Bugarín, O.; Zepeda-Carrillo, E.A.; Ruíz-Bernés, S.; Ochoa-Ramírez, L.A. Recombinant murine IL-12 promotes a protective Th1/cellular response in Mongolian gerbils infected with Sporothrix schenckii. J. Chemother. 2015, 27, 87–93. [Google Scholar] [CrossRef]
- Batista-Duharte, A.; Lastre, M.; Romeu, B.; Portuondo, D.L.; Téllez-Martínez, D.; Manente, F.A.; Pérez, O.; Carlos, I.Z. Antifungal and immunomodulatory activity of a novel cochleate for amphotericin B delivery against Sporothrix schenckii. Int. Immunopharmacol. 2016, 40, 277–287. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, J.R.F.; Jannuzzi, G.P.; Kaihami, G.H.; Breda, L.C.D.; Ferreira, K.S.; de Almeida, S.R. An immunoproteomic approach revealing peptides from Sporothrix brasiliensis that induce a cellular immune response in subcutaneous sporotrichosis. Sci. Rep. 2018, 8, 4192. [Google Scholar] [CrossRef]
- Chen, F.; Jiang, R.; Wang, Y.; Zhu, M.; Zhang, X.; Dong, S.; Shi, H.; Wang, L. Recombinant Phage Elicits Protective Immune Response against Systemic S. globosa Infection in Mouse Model. Sci. Rep. 2017, 7, 42024. [Google Scholar] [CrossRef] [PubMed]
- García-Lozano, A.; Toriello, C.; Antonio-Herrera, L.; Bonifaz, L.C. Sporothrix schenckii Immunization, but Not Infection, Induces Protective Th17 Responses Mediated by Circulating Memory CD4+ T Cells. Front. Microbiol. 2018, 9, 1275. [Google Scholar] [CrossRef]
- Li Causi, E.; Parikh, S.C.; Chudley, L.; Layfield, D.M.; Ottensmeier, C.H.; Stevenson, F.K.; Di Genova, G. Vaccination expands antigen-specific CD4+ memory T cells and mobilizes bystander central-memory T cells. PLoS ONE 2015, 10, e0136717. [Google Scholar] [CrossRef] [PubMed]
- Read, A.F.; Baigent, S.J.; Powers, C.; Kgosana, L.B.; Blackwell, L.; Smith, L.P.; Kennedy, D.A.; Walkden-Brown, S.W.; Nair, V.K. Imperfect Vaccination Can Enhance the Transmission of Highly Virulent Pathogens. PLoS Biol. 2015, 13, e1002198. [Google Scholar] [CrossRef] [PubMed]
- Alba-Fierro, C.A.; Pérez-Torres, A.; Toriello, C.; Pulido-Camarillo, E.; López-Romero, E.; Romo-Lozano, Y.; Gutiérrez-Sánchez, G.; Ruiz-Baca, E. Immune Response Induced by an Immunodominant 60 kDa Glycoprotein of the Cell Wall of Sporothrix schenckii in Two Mice Strains with Experimental Sporotrichosis. J. Immunol. Res. 2016, 2016, 6525831. [Google Scholar] [CrossRef] [PubMed]
- Batista-Duharte, A.; Portuondo, D.; Pérez, O.; Carlos, I.Z. Systemic immunotoxicity reactions induced by adjuvanted vaccines. Int. Immunopharmacol. 2014, 20, 170–180. [Google Scholar] [CrossRef] [PubMed]


| Description | |
|---|---|
| Composition | Gel particles of sodium polyacrylate in water. |
| Particle size | 90% of the particles are smaller than 1.2 μm in diameter. |
| Stability | Highly stable at room temperature. |
| Mechanisms of action | Depot effect with slow release of antigens, due to polymer adsorption properties. Improves the recruitment and activation of the innate immune system cells and inducement of specific immune response. |
| Vaccine preparation | Montanide™ GEL adjuvants are ready-to-use adjuvants that can be combined with a wide range of antigens by gentle mixing. |
| Routes of administration | Parenteral and mucosal administration. |
| Uses | Montanide™ GEL adjuvants are recommended for a wide variety of livestock species and for pets and horses. They can be formulated with a wide range of antigens. |
| Safety | Montanide™ adjuvants and their components have been considered as safe by the Committee for Veterinary Medical Products (CVMP) for use in immunological products. They are included in Part I of the Annex of the European Council Regulation n° 37/2010/EU as substances needing no further MRL studies, in the Out of Scope list (EMACVMP-519714-2009), and included in already registered veterinary commercial products. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Téllez-Martínez, D.; Leandro Portuondo, D.; Loesch, M.L.; Batista-Duharte, A.; Zeppone Carlos, I. A Recombinant Enolase-Montanide™ PetGel A Vaccine Promotes a Protective Th1 Immune Response against a Highly Virulent Sporothrix schenckii by Toluene Exposure. Pharmaceutics 2019, 11, 144. https://doi.org/10.3390/pharmaceutics11030144
Téllez-Martínez D, Leandro Portuondo D, Loesch ML, Batista-Duharte A, Zeppone Carlos I. A Recombinant Enolase-Montanide™ PetGel A Vaccine Promotes a Protective Th1 Immune Response against a Highly Virulent Sporothrix schenckii by Toluene Exposure. Pharmaceutics. 2019; 11(3):144. https://doi.org/10.3390/pharmaceutics11030144
Chicago/Turabian StyleTéllez-Martínez, Damiana, Deivys Leandro Portuondo, Maria Luiza Loesch, Alexander Batista-Duharte, and Iracilda Zeppone Carlos. 2019. "A Recombinant Enolase-Montanide™ PetGel A Vaccine Promotes a Protective Th1 Immune Response against a Highly Virulent Sporothrix schenckii by Toluene Exposure" Pharmaceutics 11, no. 3: 144. https://doi.org/10.3390/pharmaceutics11030144
APA StyleTéllez-Martínez, D., Leandro Portuondo, D., Loesch, M. L., Batista-Duharte, A., & Zeppone Carlos, I. (2019). A Recombinant Enolase-Montanide™ PetGel A Vaccine Promotes a Protective Th1 Immune Response against a Highly Virulent Sporothrix schenckii by Toluene Exposure. Pharmaceutics, 11(3), 144. https://doi.org/10.3390/pharmaceutics11030144








