A Functional Wound Dressing as a Potential Treatment for Cutaneous Leishmaniasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Factorial Design for Development of AmB-Loaded Hydrogels
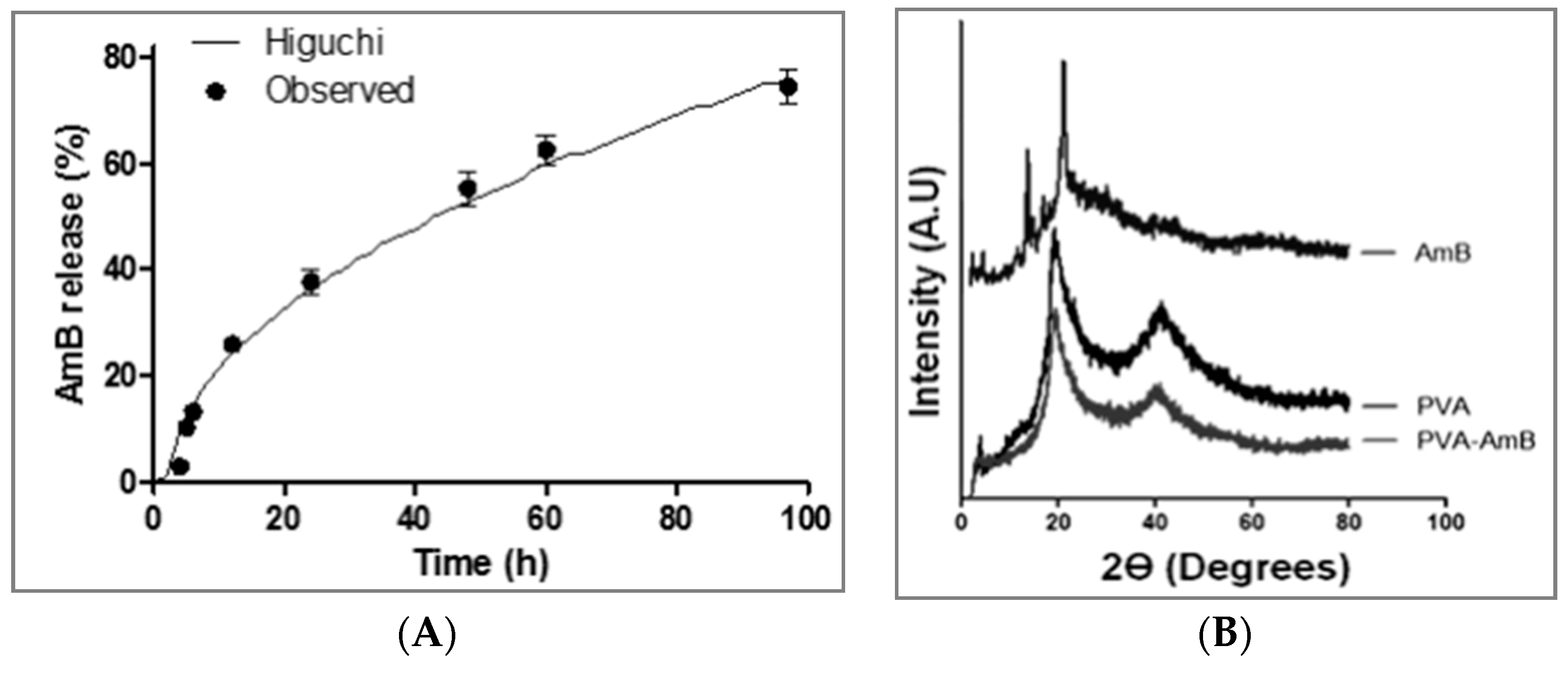
2.3. X-ray Diffraction Study
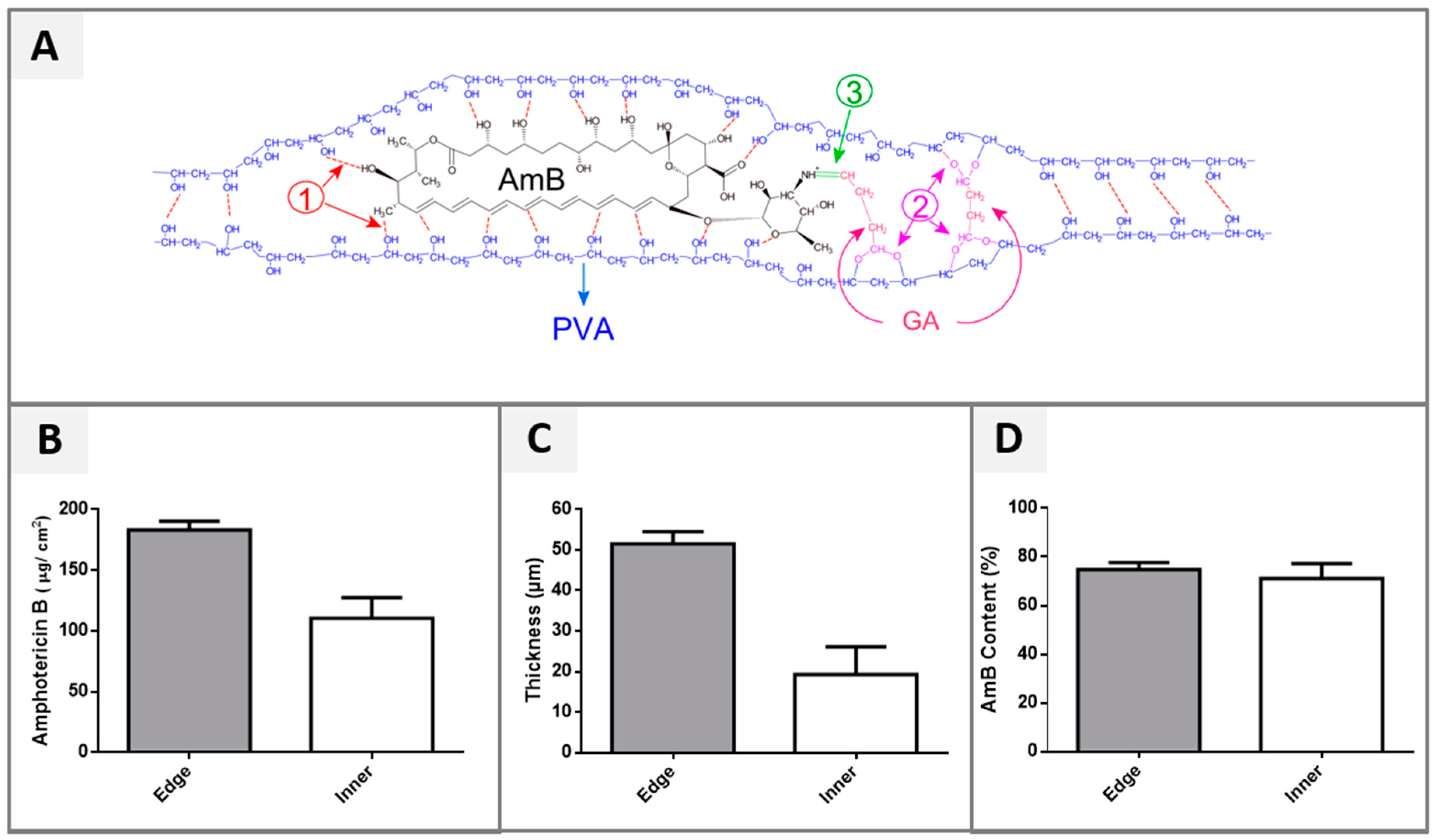
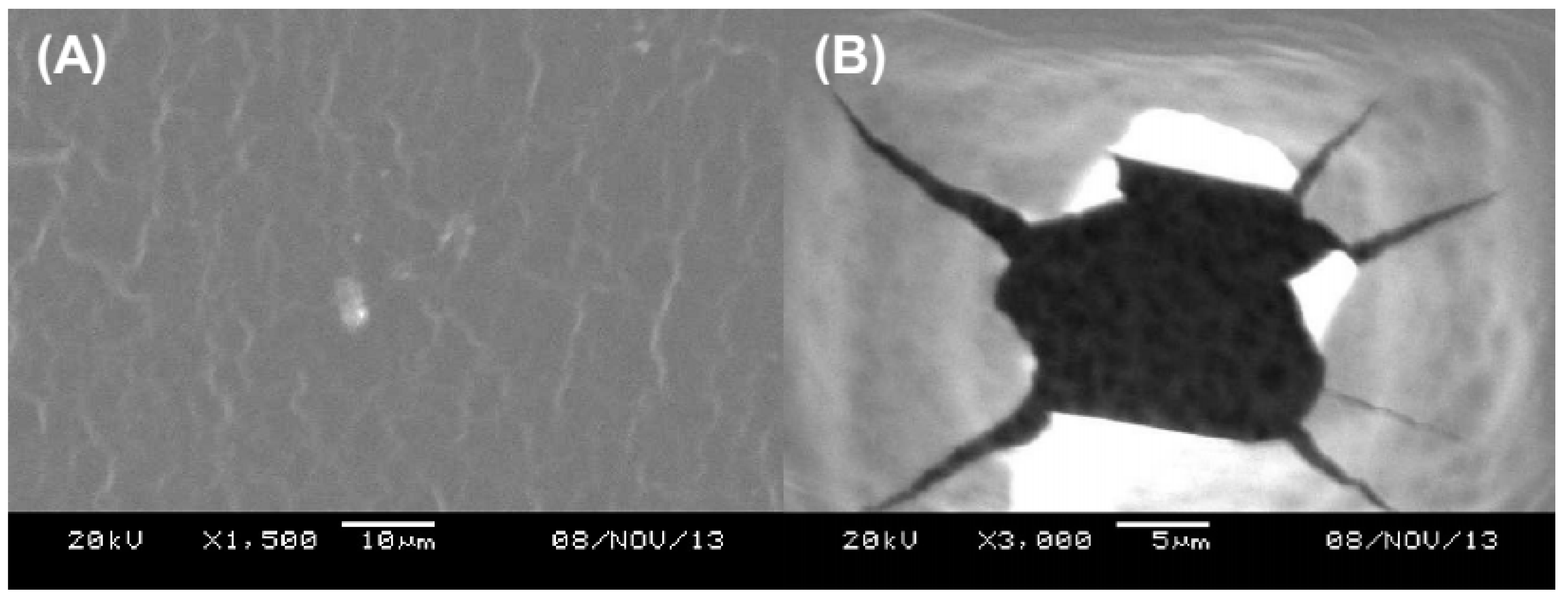
2.4. Hydrogel Thickness and Morphology
2.5. Drug Loading Efficiency (%)
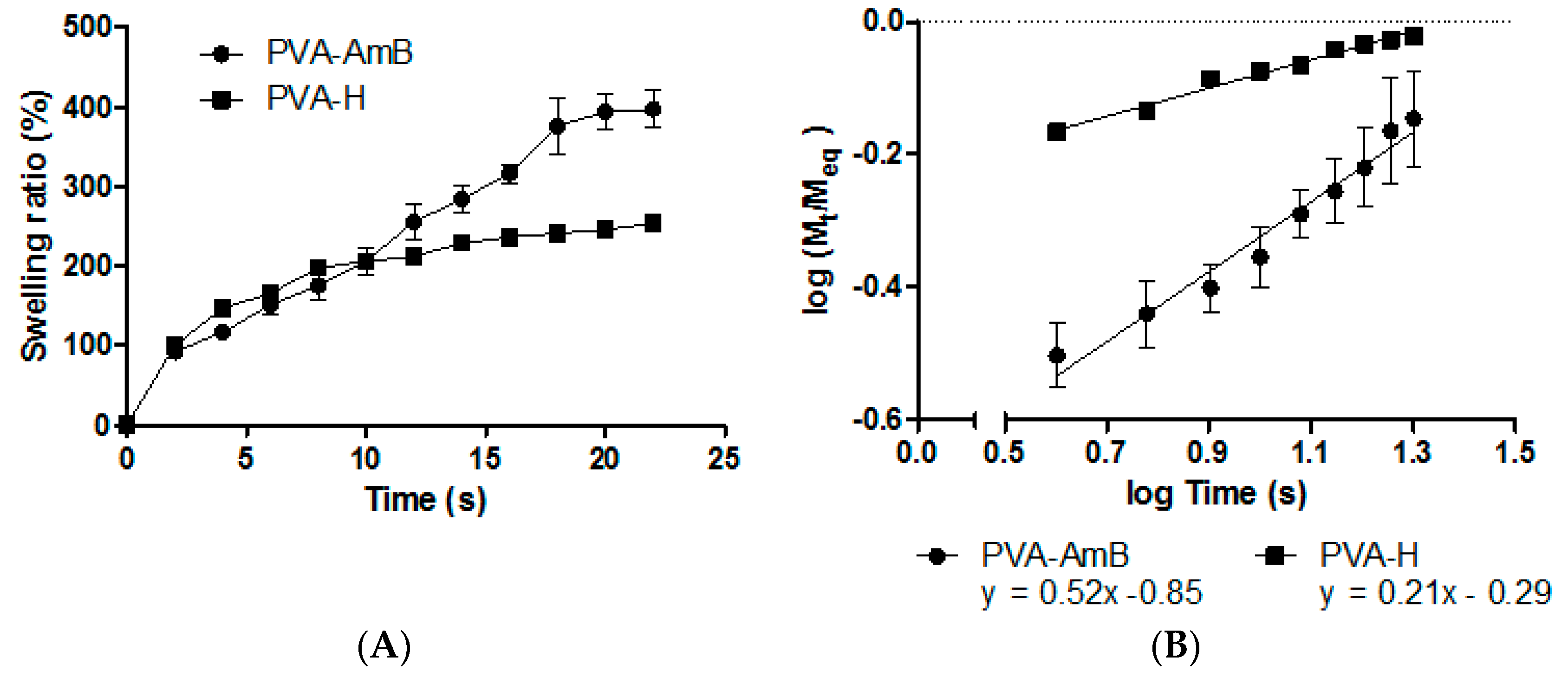
2.6. Determination of Swelling Behavior
2.7. In Vitro Drug Release
2.8. Mathematical Analysis of the In Vitro Release Kinetics
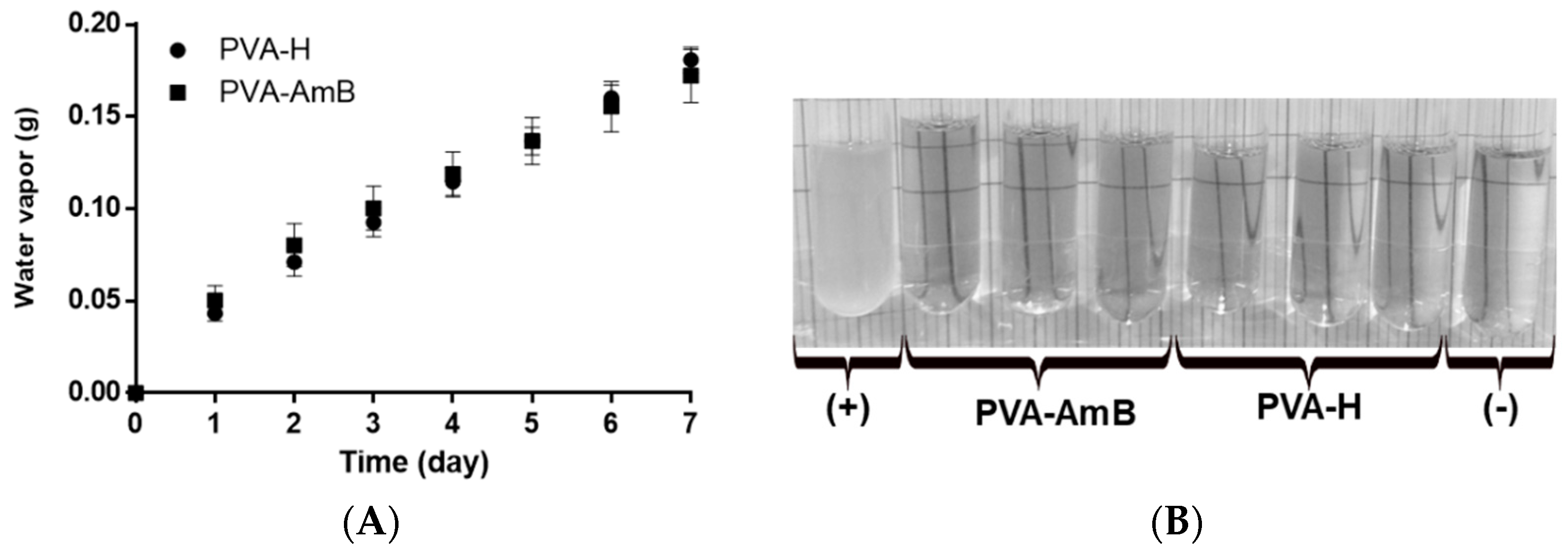
2.9. Water Vapor Transmission
2.10. Microbial Permeability Assay
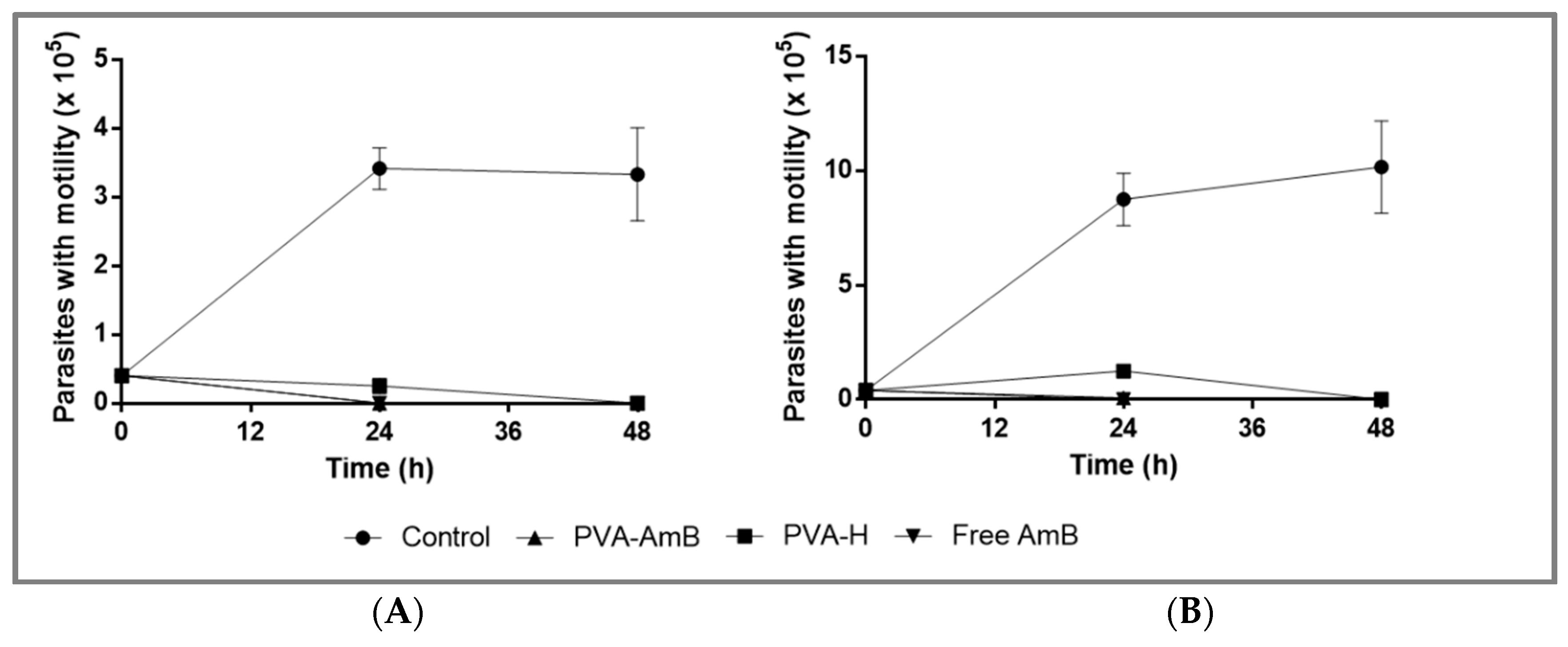
2.11. Leishmanicidal Activity Assay
2.12. Antifungal Activity
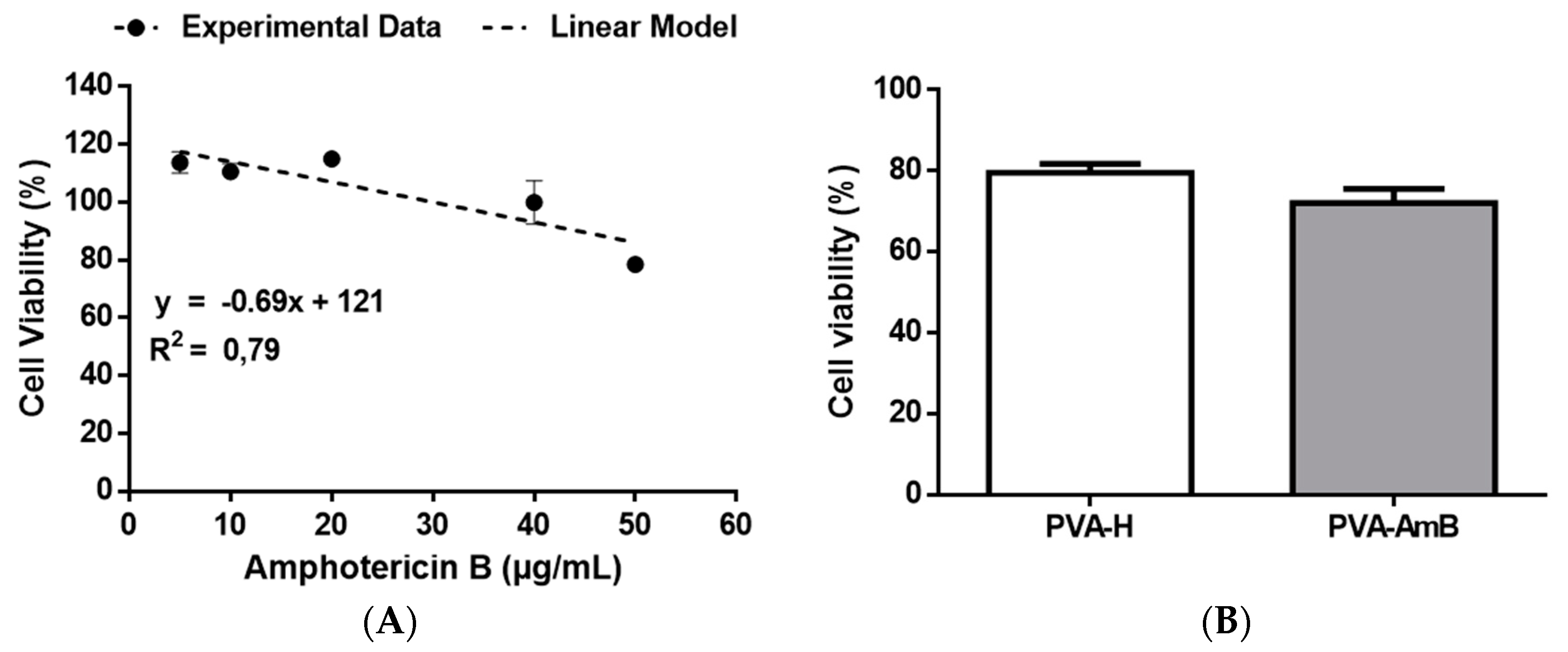
2.13. Cytotoxicity Assay
2.14. Statistical Analysis
3. Results and Discussion
3.1. Factorial Design
3.2. Drug Loading Efficiency (%)
3.3. Swelling Behavior
3.4. In Vitro Drug Release
3.5. Water Vapor Permeability
3.6. Microbial Permeability Assay
3.7. Biological Activity Assays
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alvar, J.; Arana, B.I. Appraisal of Leishmaniasis Chemotherapy, Current Status and Pipeline StrategiesChapter 1 Leishmaniasis, Impact and Therapeutic Needs. In Drug Discovery for Leishmaniasis; The Royal Society of Chemistry: London, UK, 2018; pp. 1–13. [Google Scholar]
- WHO. Sustaining the Drive to Overcome the Global Impact of Neglected Tropical Diseases: Second WHO Report on Neglected Tropical Diseases; WHO Library: Paris, France, 2013; pp. 67–71. [Google Scholar]
- WHO. Leishmaniasis—Fact sheet N°375; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Okwor, I.; Uzonna, J. Vaccines and vaccination strategies against human cutaneous leishmaniasis. Hum. Vaccines 2009, 5, 291–301. [Google Scholar] [CrossRef][Green Version]
- Bacon, K.M.; Hotez, P.J.; Kruchten, S.D.; Kamhawi, S.; Bottazzi, M.E.; Valenzuela, J.G.; Lee, B.Y. The potential economic value of a cutaneous leishmaniasis vaccine in seven endemic countries in the Americas. Vaccine 2013, 31, 480–486. [Google Scholar] [CrossRef]
- WHO. Control of the Leishmaniasis. In Proceedings of the Meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, Switzerland, 22–26 March 2010. [Google Scholar]
- Neves, L.O.; Talhari, A.C.; Gadelha, E.P.N.; da Silva Júnior, R.M.; Guerra, J.A.O.; Ferreira, L.C.L.; Talhari, S. Estudo clínico randomizado comparando antimoniato de meglumina, pentamidina e anfotericina B para o tratamento da leishmaniose cutânea ocasionada por Leishmania guyanensis. An. Bras. Dermatol. 2011, 86, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Hamill, R.J.; Sobel, J.D.; El-Sadr, W.; Johnson, P.C.; Graybill, J.R.; Javaly, K.; Barker, D.E.; Baker, C.J.; AmBisome Cryptococcal Meningitis Study Group. Comparison of 2 Doses of Liposomal Amphotericin B and Conventional Amphotericin B Deoxycholate for Treatment of AIDS-Associated Acute Cryptococcal Meningitis: A Randomized, Double-Blind Clinical Trial of Efficacy and Safety. Clin. Infect. Dis. 2010, 51, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Solomon, M.; Pavlotzky, F.; Barzilai, A.; Schwartz, E. Liposomal amphotericin B in comparison to sodium stibogluconate for Leishmania braziliensis cutaneous leishmaniasis in travelers. J. Am. Acad. Dermatol. 2013, 68, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Mehta, J.; Blake, J.; Craddock, C. Comparative efficacy of amphotericin B lipid complex and liposomal amphotericin B for the treatment of invasive fungal infections in HSCT recipients and other immunocompromised patient populations with hematologic malignancies: A critical review. Open Transplant. J. 2011, 5, 23–29. [Google Scholar] [CrossRef]
- El-Sayed, M.; Anwar, A.E. Intralesional sodium stibogluconate alone or its combination with either intramuscular sodium stibogluconate or oral ketoconazole in the treatment of localized cutaneous leishmaniasis: A comparative study. J. Eur. Acad. Dermatol. Venereol. 2009, 24, 335–340. [Google Scholar] [CrossRef]
- Asilian, A.; Sadeghinia, A.; Faghihi, G.; Momeni, A. Comparative study of the efficacy of combined cryotherapy and intralesional meglumine antimoniate (Glucantime) vs. cryotherapy and intralesional meglumine antimoniate (Glucantime) alone for the treatment of cutaneous leishmaniasis. Int. J. Pharm. 2004, 43, 281–283. [Google Scholar] [CrossRef]
- Safi, N. Evaluation of thermotherapy for the treatment of cutaneous Leishmaniasis in Kabul, Afghanistan: A randomized controlled trial. Int. J. Infect. Dis. 2005, 16 (Suppl. 1), 1148–1155. [Google Scholar] [CrossRef][Green Version]
- Kim, D.H.; Chung, H.J.; Bleys, J.; Ghohestani, R.F. Is paromomycin an effective and safe treatment against cutaneous leishmaniasis? A aeta-analysis of 14 randomized controlled trials. PLoS Negl. Trop. Dis. 2009, 3, e381. [Google Scholar] [CrossRef]
- Frankenburg, S.; Glick, D.; Klaus, S.; Barenholz, Y. Efficacious topical treatment for murine cutaneous leishmaniasis with ethanolic formulations of amphotericin B. Antimicrob. Agents Chemother. 1998, 42, 3092–3096. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kaya, A.Z.; Turani, N.; Akyuz, M. The effectiveness of a hydrogel dressing compared with standard management of pressure ulcers. J. Wound Care 2005, 14, 42–44. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Zoellner, P.; Kapp, H.; Smola, H. Clinical performance of a hydrogel dressing in chronic wounds: A prospective observational study. J. Wound Care 2007, 16, 133–136. [Google Scholar] [CrossRef]
- Singh, B.; Pal, L. Sterculia crosslinked PVA and PVA-poly(AAm) hydrogel wound dressings for slow drug delivery: Mechanical, mucoadhesive, biocompatible and permeability properties. J. Mech. Behav. Biomed. 2012, 9, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Chen, X.; Yu, S.; Zhou, H. Comparison of different in vitro mucoadhesion testing methods for hydrogels. J. Drug Deliv. Sci. Technol. 2017, 40, 157–163. [Google Scholar] [CrossRef]
- Han, F.; Dong, Y.; Song, A.; Yin, R.; Li, S. Alginate/chitosan based bi-layer composite membrane as potential sustained-release wound dressing containing ciprofloxacin hydrochloride. Appl. Surf. Sci. 2014, 311, 626–634. [Google Scholar] [CrossRef]
- Ci, L.-Q.; Huang, Z.-G.; Lv, F.-M.; Wang, J.; Feng, L.-L.; Sun, F.; Cao, S.-J.; Liu, Z.-P.; Liu, Y.; Wei, G.; et al. Enhanced Delivery of Imatinib into Vaginal Mucosa via a New Positively Charged Nanocrystal-Loaded in Situ Hydrogel Formulation for Treatment of Cervical Cancer. Pharmaceutics 2019, 11, 15. [Google Scholar] [CrossRef]
- Jøraholmen, M.W.; Basnet, P.; Tostrup, M.J.; Moueffaq, S.; Škalko-Basnet, N. Localized Therapy of Vaginal Infections and Inflammation: Liposomes-In-Hydrogel Delivery System for Polyphenols. Pharmaceutics 2019, 11, 53. [Google Scholar] [CrossRef]
- Mateus, D.; Marto, J.; Trindade, P.; Gonçalves, H.; Salgado, A.; Machado, P.; Melo-Gouveia, A.; Ribeiro, H.M.; Almeida, A.J. Improved Morphine-Loaded Hydrogels for Wound-Related Pain Relief. Pharmaceutics 2019, 11, 76. [Google Scholar] [CrossRef]
- Wichterle, O.; LÍM, D. Hydrophilic gels for biological use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, S.; Feng, W.H. PVA hydrogel properties for biomedical application. J. Mech. Behav. Biomed. Mater. 2011, 4, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Li, P.; Schonherr, H. Three-Dimensional Microstructured Poly(vinyl alcohol) Hydrogel Platform for the Controlled Formation of Multicellular Cell Spheroids. Biomacromolecules 2018, 19, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Chang, Y.S.; Oka, M. A two year in vivo study of polyvinyl alcohol-hydrogel (PVA-H) artificial meniscus. Biomaterials 2005, 26, 3243–3248. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.K.; Campbell, G.; Zhang, Z.F.; Hui, A.J.; Boughner, D.R. Optimizing the tensile properties of polyvinyl alcohol hydrogel for the construction of a bioprosthetic heart valve stent. J. Biomed. Mater. Res. 2002, 63, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Pillai, M.M.; Gopinathan, J.; Kumar, R.S.; Kumar, G.S.; Shanthakumari, S.; Sahanand, K.S.; Bhattacharyya, A.; Selvakumar, R. Tissue engineering of human knee meniscus using functionalized and reinforced silk-polyvinyl alcohol composite three-dimensional scaffolds: Understanding the in vitro and in vivo behavior. J. Biomed. Mater. Res. Part A 2018, 106, 1722–1731. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, K.; Hayami, T.; Hyon, S.H.; Tsutsumi, S. Control of proliferation and differentiation of osteoblasts on apatite-coated poly(vinyl alcohol) hydrogel as an artificial articular cartilage material. J.Biomed. Mater. Res. Part A 2010, 92, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.Q.; Zhou, X.Q.; Gao, Z.H.; Shi, X.C.; Wang, Z.L.; Wang, Y.; Zhang, P.B. Preparation and Characterization of Silver Sulfadiazine-Loaded Polyvinyl Alcohol Hydrogels as an Antibacterial Wound Dressing. J. Pharm. Sci. 2018, 107, 2377–2384. [Google Scholar] [CrossRef]
- Gao, T.L.; Jiang, M.H.; Liu, X.Q.; You, G.J.; Wang, W.Y.; Sun, Z.H.; Ma, A.G.; Chen, J. Patterned Polyvinyl Alcohol Hydrogel Dressings with Stem Cells Seeded for Wound Healing. Polymers 2019, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- Hyon, S.H.; Cha, W.I.; Ikada, Y.; Kita, M.; Ogura, Y.; Honda, Y. Poly(vinyl alcohol) hydrogels as soft contact-lens material. J. Biomater. Sci. Polym. Ed. 1994, 5, 397–406. [Google Scholar] [CrossRef]
- Freire, M.C.L.C.; Alexandrino, F.; Marcelino, H.R.; Picciani, P.H.d.S.; Silva, K.G.d.H.e.; Genre, J.; Oliveira, A.G.d.; Egito, E.S.T.d. Understanding drug release data through thermodynamic analysis. Materials 2017, 10, 651. [Google Scholar] [CrossRef]
- Hickey, A.S.; Peppas, N.A. Mesh size and diffusive characteristics of semicrystalline poly(vinyl alcohol) membranes prepared by freezing/thawing techniques. J. Membr. Sci. 1995, 107, 229–237. [Google Scholar] [CrossRef]
- Li, T.; Lei, Y.; Guo, M.; Yan, H. Crosslinked poly(vinyl alcohol) hydrogel microspheres containing dispersed fenofibrate nanocrystals as an oral sustained delivery system. Eur. Polym. J. 2018, 101, 77–82. [Google Scholar] [CrossRef]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Qi, M.; Gu, Y.; Sakata, N.; Kim, D.; Shirouzu, Y.; Yamamoto, C.; Hiura, A.; Sumi, S.; Inoue, K. Pva hydrogel sheet macroencapsulation for the bioartificial pancreas. Biomaterials 2004, 25, 5885–5892. [Google Scholar] [CrossRef]
- Nakano, K.; Tozuka, Y.; Takeuchi, H. Effect of surface properties of liposomes coated with a modified polyvinyl alcohol (PVA-R) on the interaction with macrophage cells. Int. J. Pharm. 2008, 354, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Suktham, K.; Koobkokkruad, T.; Saesoo, S.; Saengkrit, N.; Surassmo, S. Physical and biological characterization of sericin-loaded copolymer liposomes stabilized by polyvinyl alcohol. Colloids Surf. B Biointerfaces 2016, 148, 487–495. [Google Scholar] [CrossRef]
- Alves, M.H.; Young, C.J.; Bozzetto, K.; Poole-Warren, L.A.; Martens, P.J. Degradable, click poly(vinyl alcohol) hydrogels: Characterization of degradation and cellular compatibility. Biomed. Mater. 2012, 7, 024106. [Google Scholar] [CrossRef] [PubMed]
- DeMerlis, C.C.; Schoneker, D.R. Review of the oral toxicity of polyvinyl alcohol (PVA). Food Chem. Toxicol. 2003, 41, 319–326. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Engi. C Biomim. Supramol. Syst. 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Sittiwong, J.; Niamlang, S.; Paradee, N.; Sirivat, A. Electric field-controlled benzoic acid and sulphanilamide delivery from poly(vinyl alcohol) hydrogel. AAPS PharmSciTech 2012, 13, 1407–1415. [Google Scholar] [CrossRef]
- Tripathi, R.; Mishra, B. Development and evaluation of sodium alginate-polyacrylamide graft-co-polymer-based stomach targeted hydrogels of famotidine. AAPS PharmSciTech 2012, 13, 1091–1102. [Google Scholar] [CrossRef]
- Farmacopeia Brasileira; ANVISA, Ed.; FioCruz: Brasília, Brazil, 2010; Volume 1, p. 510. [Google Scholar]
- Veeruraj, A.; Liu, L.; Zheng, J.; Wu, J.; Arumugam, M. Evaluation of astaxanthin incorporated collagen film developed from the outer skin waste of squid Doryteuthis singhalensis for wound healing and tissue regenerative applications. Mater. Sci. Eng. C 2019, 95, 29–42. [Google Scholar] [CrossRef]
- Lemke, A.; Kiderlen, A.F.; Kayser, O. Amphotericin B. Appl. Microbiol. Biotechnol. 2005, 68, 151–162. [Google Scholar] [CrossRef]
- Santos, C.M.; Oliveira, R.B.; Arantes, V.T.; Caldeira, L.R.; Oliveira, M.C.; Egito, E.S.T.; Ferreira, L.A.M. Amphotericin B-Loaded Nanocarriers for Topical Treatment of Cutaneous Leishmaniasis: Development, Characterization, and In Vitro Skin Permeation Studies. J. Biomed. Nanotechnol. 2012, 8, 322–329. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef]
- ASTM. Standard Test Methods for Water Vapor Transmission of Materials. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 1996. [Google Scholar]
- Vargas, E.A.T.; do Vale Baracho, N.C.; de Brito, J.; de Queiroz, A.A.A. Hyperbranched polyglycerol electrospun nanofibers for wound dressing applications. Acta Biomater. 2010, 6, 1069–1078. [Google Scholar] [CrossRef]
- Perez, A.P.; Altube, M.J.; Schilrreff, P.; Apezteguia, G.; Celes, F.S.; Zacchino, S.; de Oliveira, C.I.; Romero, E.L.; Morilla, M.J. Topical amphotericin B in ultradeformable liposomes: Formulation, skin penetration study, antifungal and antileishmanial activity in vitro. Colloids Surf. B Biointerfaces 2016, 139, 190–198. [Google Scholar] [CrossRef]
- Ruiz, H.K.; Serrano, D.R.; Dea-Ayuela, M.A.; Bilbao-Ramos, P.E.; Bolás-Fernández, F.; Torrado, J.J.; Molero, G. New amphotericin B-gamma cyclodextrin formulation for topical use with synergistic activity against diverse fungal species and Leishmania spp. Int. J. Pharm. 2014, 473, 148–157. [Google Scholar] [CrossRef]
- Zauli-Nascimento, R.C.; Miguel, D.C.; Yokoyama-Yasunaka, J.K.; Pereira, L.I.; Pelli de Oliveira, M.A.; Ribeiro-Dias, F.; Dorta, M.L.; Uliana, S.R. In vitro sensitivity of Leishmania (Viannia) braziliensis and Leishmania (Leishmania) amazonensis Brazilian isolates to meglumine antimoniate and amphotericin B. Trop. Med. Int. Health 2010, 15, 68–76. [Google Scholar] [CrossRef]
- Falk, R.; Domb, A.J.; Polacheck, I. A novel injectable water-soluble amphotericin B-arabinogalactan conjugate. Antimicrob. Agents Chemother. 1999, 43, 1975–1981. [Google Scholar] [CrossRef]
- Reis, E.F.; Campos, F.S.; Lage, A.p.; Leite, R.C.; Heneine, L.G.; Vasconcelos, W.L.; Lobato, Z.I.P.; Mansur, H.S. Synthesis and characterization of poly (vinyl alcohol) hydrogels and hybrids for rMPB70 protein adsorption. Mater. Res. 2006, 9, 185–191. [Google Scholar] [CrossRef]
- Clarke, E.G.C. Amphotericin. In Clarke’s Isolation and Identification of Drugs in Pharmaceuticals, Body Fluids and Post-Mortem Material, 2nd ed.; Moffat, A.C., Ed.; Pharmaceutical Press: London, 1986; p. 1200. [Google Scholar]
- Chang, M.; Liu, X.; Meng, L.; Wang, X.; Ren, J. Xylan-Based Hydrogels as a Potential Carrier for Drug Delivery: Effect of Pore-Forming Agents. Pharmaceutics 2018, 10, 261. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, D.C.; Quilty, F.P.; Corrigan, O.I. Effect of drug physicochemical properties on swelling/deswelling kinetics and pulsatile drug release from thermoresponsive poly(N-isopropylacrylamide) hydrogels. J. Control. Release 2004, 98, 97–114. [Google Scholar] [CrossRef]
- Ruiz, J.; Mantecón, A.; Cádiz, V. Synthesis and properties of hydrogels from poly (vinyl alcohol) and ethylenediaminetetraacetic dianhydride. Polymer 2001, 42, 6347–6354. [Google Scholar] [CrossRef]
- Vera, L.A.; Macedo, J.L.; Ciuffo, I.A.; Santos, C.G.; Santos, J.B. Antimicrobial susceptibility of aerobic bacteria isolated from leishmaniotic ulcers in Corte de Pedra, BA. Rev. Soc. Bras. Med. Trop. 2006, 39, 47–50. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Gu, B.; Sun, X.; Papadimitrakopoulos, F.; Burgess, D.J. Seeing is believing, PLGA microsphere degradation revealed in PLGA microsphere/PVA hydrogel composites. J. Control. Release 2016, 228, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Sibbald, R.G.; Orsted, H.; Schultz, G.S.; Coutts, P.; Keast, D.; Board, I.W.B.P.A.; Board, C.C.W.A. Preparing the wound bed 2003: Focus on infection and inflammation. Ostomy Wound Manag. 2003, 49, 24–51. [Google Scholar]
- Gu, S.Y.; Wang, Z.M.; Ren, J.; Zhang, C.Y. Electrospinning of gelatin and gelatin/poly(l-lactide) blend and its characteristics for wound dressing. Mater. Sci. Eng. C 2009, 29, 1822–1828. [Google Scholar] [CrossRef]
- Mi, F.L.; Shyu, S.S.; Wu, Y.B.; Lee, S.T.; Shyong, J.Y.; Huang, R.N. Fabrication and characterization of a sponge-like asymmetric chitosan membrane as a wound dressing. Biomaterials 2001, 22, 165–173. [Google Scholar] [CrossRef]
- Ngouateu, O.B.; Kollo, P.; Ravel, C.; Dereure, J.; Kamtchouing, P.; Same-Ekobo, A.; von Stebut, E.; Maurer, M.; Dondji, B. Clinical features and epidemiology of cutaneous leishmaniasis and Leishmania major/HIV co-infection in Cameroon: Results of a large cross-sectional study. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 137–142. [Google Scholar] [CrossRef]
- Carranza-Tamayo, C.O.; de Assis, T.S.; Neri, A.T.; Cupolillo, E.; Rabello, A.; Romero, G.A. Prevalence of Leishmania infection in adult HIV/AIDS patients treated in a tertiary-level care center in Brasilia, Federal District, Brazil. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 743–748. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.-H.; Jeon, O.; Kwon, I.C.; Park, K. Engineered polymers for advanced drug delivery. Eur. J. Pharm. Biopharm. 2009, 71, 420–430. [Google Scholar] [CrossRef]
- Garg, T.; Singh, S.; Goyal, A.K. Stimuli-Sensitive Hydrogels: An Excellent Carrier for Drug and Cell Delivery. Crit. Rev. 2013, 30, 369–409. [Google Scholar] [CrossRef]
- Baranoski, S.; Ayello, E.A. Wound Treatment Options. In Wound Care Essentials: Practice Principles, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012; p. 183. [Google Scholar]
- Escobar, P.; Matu, S.; Marques, C.; Croft, S.L. Sensitivities of Leishmania species to hexadecylphosphocholine (miltefosine), ET-18-OCH(3) (edelfosine) and amphotericin B. Acta Trop. 2002, 81, 151–157. [Google Scholar] [CrossRef]
- ISO. Biological Evaluation of Medical Devices—Part 5: Tests for in Vitrocytotoxicity, 3rd ed.; International Organization for Standardization: Geneva, Switzerland, 2009; Volume 10993. [Google Scholar]
- Davy, K.P.; Seals, D.R. Total blood volume in healthy young and older men. J. Appl. Physiol. 1994, 76, 2059–2062. [Google Scholar] [CrossRef]
- Bekersky, I.; Fielding, R.M.; Dressler, D.E.; Lee, J.W.; Buell, D.N.; Walsh, T.J. Plasma protein binding of amphotericin B and pharmacokinetics of bound versus unbound amphotericin B after administration of intravenous liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate. Antimicrob. Agents Chemother. 2002, 46, 834–840. [Google Scholar] [CrossRef]
- da Silva-Filho, M.A.; da Silva Siqueira, S.D.V.; Freire, L.B.; de Araujo, I.B.; e Silva, K.G.D.H.; da Cunha Medeiros, A.; Araujo-Filho, I.; de Oliveira, A.G.; do Egito, E.S.T. How can micelle systems be rebuilt by a heating process? Int. J. Nanomed. 2012, 7, 141–150. [Google Scholar] [CrossRef]
- Vertut-Croquin, A.; Bolard, J.; Chabbert, M.; Gary-Bobo, C. Differences in the interaction of the polyene antibiotic amphotericin B with cholesterol- or ergosterol-containing phospholipid vesicles. A circular dichroism and permeability study. Biochemistry 1983, 22, 2939–2944. [Google Scholar] [CrossRef]
- Barwicz, J.; Christian, S.; Gruda, I. Effects of the aggregation state of amphotericin B on its toxicity to mice. Antimicrob. Agents Chemother. 1992, 36, 2310–2315. [Google Scholar] [CrossRef] [PubMed]
- Mazerski, J.; Bolard, J.; Borowski, E. Self-association of some polyene macrolide antibiotics in aqueous media. Biochim. Biophys. Acta (BBA) Gen. Subj. 1982, 719, 11–17. [Google Scholar] [CrossRef]
- Tancréde, P.; Barwicz, J.; Jutras, S.; Gruda, I. The effect of surfactants on the aggregation state of amphotericin B. Biochim. Biophys. Acta (BBA) Biomembr. 1990, 1030, 289–295. [Google Scholar] [CrossRef]
| a Model | Equation | R2_adj | RMSE |
|---|---|---|---|
| b Zero-order | 0.87 | 9.8 | |
| c First-order | ) | 0.97 | 4.7 |
| d Quadratic | 0.98 | 4.1 | |
| e Higuchi | 0.93 | 7.2 | |
| *e Higuchi with Tlag | 0.99 | 3.1 | |
| f Baker–Lonsdale | 0.87 | 9.9 | |
| *f Baker–Lonsdale with Tlag | 0.99 | 2.3 | |
| g Korsmeyer–Peppas | 0.5 | 16.5 | |
| *g Korsmeyer–Peppas with Tlag | 0.48 | 17.7 | |
| h Hopfenberg | 0.94 | 6.47 | |
| *h Hopfenberg with Tlag | 0.94 | 6.85 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexandrino-Junior, F.; Silva, K.G.d.H.e.; Freire, M.C.L.C.; Lione, V.d.O.F.; Cardoso, E.A.; Marcelino, H.R.; Genre, J.; Oliveira, A.G.d.; Egito, E.S.T.d. A Functional Wound Dressing as a Potential Treatment for Cutaneous Leishmaniasis. Pharmaceutics 2019, 11, 200. https://doi.org/10.3390/pharmaceutics11050200
Alexandrino-Junior F, Silva KGdHe, Freire MCLC, Lione VdOF, Cardoso EA, Marcelino HR, Genre J, Oliveira AGd, Egito ESTd. A Functional Wound Dressing as a Potential Treatment for Cutaneous Leishmaniasis. Pharmaceutics. 2019; 11(5):200. https://doi.org/10.3390/pharmaceutics11050200
Chicago/Turabian StyleAlexandrino-Junior, Francisco, Kattya Gyselle de Holanda e Silva, Marjorie Caroline Liberato Cavalcanti Freire, Viviane de Oliveira Freitas Lione, Elisama Azevedo Cardoso, Henrique Rodrigues Marcelino, Julieta Genre, Anselmo Gomes de Oliveira, and Eryvaldo Sócrates Tabosa do Egito. 2019. "A Functional Wound Dressing as a Potential Treatment for Cutaneous Leishmaniasis" Pharmaceutics 11, no. 5: 200. https://doi.org/10.3390/pharmaceutics11050200
APA StyleAlexandrino-Junior, F., Silva, K. G. d. H. e., Freire, M. C. L. C., Lione, V. d. O. F., Cardoso, E. A., Marcelino, H. R., Genre, J., Oliveira, A. G. d., & Egito, E. S. T. d. (2019). A Functional Wound Dressing as a Potential Treatment for Cutaneous Leishmaniasis. Pharmaceutics, 11(5), 200. https://doi.org/10.3390/pharmaceutics11050200






