Syringeable Self-Organizing Gels that Trigger Gold Nanoparticle Formation for Localized Thermal Ablation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Gel Preparation and Nanoparticles Formation
2.3. Nanoparticle Characterization
2.4. Rheology
2.5. Photothermal Measurements
3. Results and Discussion
3.1. Reduction of AuCl4− Ions and Formation of Gold Nanoparticles
3.2. AuNP Morphology: STEM and DLS
3.3. Photothermal Measurements
3.4. Rheological Behavior
3.5. Performance under Irradiation Cycles at 37 °C
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Melancon, M.P.; Zhou, M.; Li, C. Cancer theranostics with near-infrared light-activatable multimodal nanoparticles. Acc. Chem. Res. 2011, 44, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Mieszawska, A.J.; Mulder, W.J.M.; Fayad, Z.A.; Cormode, D.P. Multifunctional gold nanoparticles for diagnosis and therapy of disease. Mol. Pharm. 2013, 10, 831–847. [Google Scholar] [CrossRef]
- Barbosa, S.; Topete, A.; Alatorre-Meda, M.; Villar-Alvarez, E.M.; Alvarez-Lorenzo, C.; Concheiro, A.; Taboada, P.; Mosquera, V. Targeted combinatorial therapy using gold nanostars as theranostic platforms. J. Phys. Chem. C 2014, 118, 26313–26323. [Google Scholar] [CrossRef]
- Liu, T.M.; Yu, J.; Chang, A.; Chiou, A.; Chiang, H.K.; Chuang, Y.C.; Wu, C.H.; Hsu, C.H.; Chen, P.A.; Huang, C.C. One-step shell polymerization of inorganic nanoparticles and their applications in SERS/nonlinear optical imaging, drug delivery, and catalysis. Sci. Rep. 2014, 4, 5593. [Google Scholar] [CrossRef] [PubMed]
- Polo, E.; Poupard, M.F.N.; Guerrini, L.; Taboada, P.; Pelaz, B.; Alvarez-Puebla, R.A.; del Pino, P. Colloidal bioplasmonics. Nano Today 2018, 20, 58–73. [Google Scholar] [CrossRef]
- Adnan, N.N.M.; Cheng, Y.Y.; Ong, N.M.N.; Kamaruddin, T.T.; Rozlan, E.; Schmidt, T.W.; Duong, H.T.T.; Boyer, C. Effect of gold nanoparticle shapes for phototherapy and drug delivery. Polym. Chem. 2016, 7, 2888–2903. [Google Scholar] [CrossRef]
- Choi, C.H.J.; Alabi, C.A.; Webster, P.; Davis, M.E. Mechanism of active targeting in solid tumors with transferrin-containing gold nanoparticles. Proc. Natl. Acad. Sci. USA 2010, 107, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Garcia-Gonzalez, C.A.; Bucio, E.; Concheiro, A. Stimuli-responsive polymers for antimicrobial therapy: Drug targeting, contact-killing surfaces and competitive release. Expert Opin. Drug Deliv. 2016, 13, 1109–1119. [Google Scholar] [CrossRef]
- Cabana, S.; Lecona-Vargas, C.S.; Melendez-Ortiz, H.I.; Contreras-Garcia, A.; Barbosa, S.; Taboada, P.; Magariños, B.; Bucio, E.; Concheiro, A.; Alvarez-Lorenzo, C. Silicone rubber films functionalized with poly(acrylic acid) nanobrushes for immobilization of gold nanoparticles and photothermal therapy. J. Drug Deliv. Sci. Technol. 2017, 42, 245–254. [Google Scholar] [CrossRef]
- Espinosa, A.; Kolosnjaj-Tabi, J.; Abou-Hassan, A.; Sangnier, A.P.; Curcio, A.; Silva, A.K.A.; Di Corato, R.; Neveu, S.; Pellegrino, T.; Liz-Marzan, L.M.; et al. Magnetic (hyper)thermia or photothermia? Progressive comparison of iron oxide and gold nanoparticles heating in water, in cells, and in vivo. Adv. Funct. Mater. 2018, 28, 1803660. [Google Scholar] [CrossRef]
- Zhao, M.X.; Cai, Z.C.; Zhu, B.J.; Zhang, Z.Q. The apoptosis effect on liver cancer cells of gold nanoparticles modified with lithocholic acid. Nanoscale Res. Lett. 2018, 13, 304. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.A.; Salleng, H.J.; Cliffel, D.E.; Feldheim, D.L. In vivo toxicity, biodistribution, and clearance of glutathione-coated gold nanoparticles. Nanomedicine 2013, 9, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Fraga, S.; Brandão, A.; Soares, M.E.; Morais, T.; Duarte, J.A.; Pereira, L.; Soares, L.; Neves, C.; Pereira, E.; Bastos, M.L.; et al. Short- and long-term distribution and toxicity of gold nanoparticles in the rat after a single-dose intravenous administration. Nanomedicine 2014, 10, 1757–1766. [Google Scholar] [CrossRef] [PubMed]
- Yook, S.; Cai1, Z.; Lu, Y.; Winnik, M.A.; Pignol, J.P.; Reilly, R.M. Intratumorally injected 177Lu-labeled gold nanoparticles: Gold nanoseed brachytherapy with application for neoadjuvant treatment of locally advanced breast cancer. J. Nucl. Med. 2016, 57, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.; Lechtman, E.; Mashouf, S.; Pignol, J.P.; Reilly, R.M. Depot system for controlled release of gold nanoparticles with precise intratumoral placement by permanent brachytherapy seed implantation (PSI) techniques. Int. J. Pharm. 2016, 515, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, X.; Huang, N.; Ren, K.; Jin, Q.; Ji, J. Mixed-charge Self-Assembled Monolayers as a facile method to design pH-induced aggregation of large gold nanoparticles for near-infrared photothermal cancer therapy. ACS Appl. Mater. Interfaces 2014, 6, 18930–18937. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Peng, D.; Hao, H.; Hu, J.; Wang, D.; Wang, K.; Liu, J.; Guo, X.; Wei, Y.; Gao, W. Thermally triggered in situ assembly of gold nanoparticles for cancer multimodal imaging and photothermal therapy. ACS Appl. Mater. Interfaces 2017, 9, 10453–10460. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Alexandridis, P. Mechanism of gold metal ion reduction, nanoparticle growth and size control in aqueous amphiphilic block copolymer solutions at ambient conditions. J. Phys. Chem. B 2005, 109, 7766–7777. [Google Scholar] [CrossRef] [PubMed]
- Khullar, P.; Singh, V.; Mahal, A.; Kumar, H.; Kaur, G.; Bakshi, M.S. Block copolymer micelles as nanoreactors for self-assembled morphologies of gold nanoparticles. J. Phys. Chem. B 2013, 117, 3028–3039. [Google Scholar] [CrossRef]
- Alexandridis, P.; Tsianou, M. Block copolymer-directed metal nanoparticle morphogenesis and organization. Eur. Polym. J. 2011, 47, 569–583. [Google Scholar] [CrossRef]
- Goy-Lopez, S.; Taboada, P.; Cambon, A.; Juarez, J.; Alvarez-Lorenzo, C.; Concheiro, A.; Mosquera, V. Modulation of size and shape of Au nanoparticles using amino-X-shaped poly(ethylene oxide)-poly(propylene oxide) block copolymers. J. Phys. Chem. B 2010, 114, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Rey-Rico, A.; Frisch, J.; Venkatesan, J.K.; Schmitt, G.; Rial-Hermida, I.; Taboada, P.; Concheiro, A.; Madry, H.; Alvarez-Lorenzo, C.; Cucchiarini, M. PEO–PPO–PEO carriers for rAAV-mediated transduction of human articular chondrocytes in vitro and in a human osteochondral defect model. ACS Appl. Mater. Interfaces 2016, 8, 20600–20613. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Sosnik, A.; Chiappetta, D.A.; Veiga, F.; Concheiro, A.; Alvarez-Lorenzo, C. Single and mixed poloxamine micelles as nanocarriers for solubilization and sustained release of ethoxzolamide for topical glaucoma therapy. J. R. Soc. Interfaces 2012, 9, 2059–2069. [Google Scholar] [CrossRef]
- Gonzalez-Lopez, J.; Alvarez-Lorenzo, C.; Taboada, P.; Sosnik, A.; Sández-Macho, I.; Concheiro, A. Self-associative behavior and drug-solubilizing ability of poloxamine (Tetronic) block copolymers. Langmuir 2008, 24, 10688–10697. [Google Scholar] [CrossRef] [PubMed]
- Rey-Rico, A.; Silva, M.; Couceiro, J.; Concheiro, A.; Alvarez-Lorenzo, C. Osteogenic efficiency of in situ gelling poloxamine systems with and without bone morphogenetic protein-2. Eur. Cells Mater. 2011, 21, 317–340. [Google Scholar] [CrossRef]
- Vyas, B.; Pillai, S.A.; Bahadur, A.; Bahadur, P. A comparative study on micellar and solubilizing behavior of three EO-PO based star block copolymers varying in hydrophobicity and their application for the in vitro release of anticancer drugs. Polymers 2018, 10, 76. [Google Scholar] [CrossRef]
- Puig-Rigall, J.; Obregon-Gomez, I.; Monreal-Perez, P.; Radulescu, A.; Blanco-Prieto, M.J.; Dreiss, C.A.; Gonzalez-Gaitano, G. Phase behaviour, micellar structure and linear rheology of tetrablock copolymer Tetronic 908. J. Colloid Interfaces Sci. 2018, 524, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Evora, M.; Reyes, R.; Alvarez-Lorenzo, C.; Concheiro, A.; Delgado, A.; Evora, C. Bone regeneration induced by an in situ gel-forming poloxamine, bone morphogenetic protein-2 system. J. Biomed. Nanotechnol. 2014, 10, 959–969. [Google Scholar] [CrossRef]
- Sandez-Macho, I.; Casas, M.; Lage, E.V.; Rial-Hermida, M.I.; Concheiro, A.; Alvarez-Lorenzo, C. Interaction of poloxamine block copolymers with lipid membranes: Role of copolymer structure and membrane cholesterol content. Colloids Surf. B Biointerfaces 2015, 133, 270–277. [Google Scholar] [CrossRef]
- Wang, W. Tolerability of hypertonic injectables. Int. J. Pharm. 2015, 490, 308–315. [Google Scholar] [CrossRef]
- Bahadur, A.; Cabana-Montenegro, S.; Aswal, V.K.; Lage, E.V.; Sandez-Macho, I.; Concheiro, A.; Alvarez-Lorenzo, C.; Bahadur, P. NaCl-triggered self-assembly of hydrophilic poloxamine block copolymers. Int. J. Pharm. 2015, 494, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Pandit, N.; Trygstad, T.; Croy, S.; Bohorquez, M.; Koch, C. Effect of salts on the micellization, clouding, and solubilization behavior of Pluronic F127 solutions. J. Colloid Interface Sci. 2000, 222, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Enomoto, H.; Torigoe, K.; Sakai, H.; Abe, M. Surfactant- and reducer-free synthesis of gold nanoparticles in aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp. 2009, 347, 18–26. [Google Scholar] [CrossRef]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Rivera, B.; Price, R.E.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar] [CrossRef] [PubMed]
- Jaque, D.; Martínez Maestro, L.; del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.L.; Martín Rodríguez, E.; Solé, J.G. Nanoparticles for photothermal therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Yang, S.; Guo, L.; Li, G.; Ge, J.; Huang, Y.; Bielawski, C.W.; Geng, J. The enhanced photothermal effect of graphene/conjugated polymer composites: Photoinduced energy transfer and applications in photocontrolled switches. Chem. Commun. 2014, 50, 14345–14348. [Google Scholar] [CrossRef] [PubMed]
- Rahoui, N.; Jiang, B.; Hegazy, M.; Taloub, N.; Wang, Y.; Yu, M.; Huang, Y.D. Gold modified polydopamine coated mesoporous silica nano-structures for synergetic chemo-photothermal effect. Colloid Surf. B Biointerfaces 2018, 171, 176–185. [Google Scholar] [CrossRef]
- Klekotko, M.; Olesiak-Banska, J.; Matczyszyn, K. Photothermal stability of biologically and chemically synthesized gold nanoprisms. J. Nanopart. Res. 2017, 19, 327. [Google Scholar] [CrossRef]

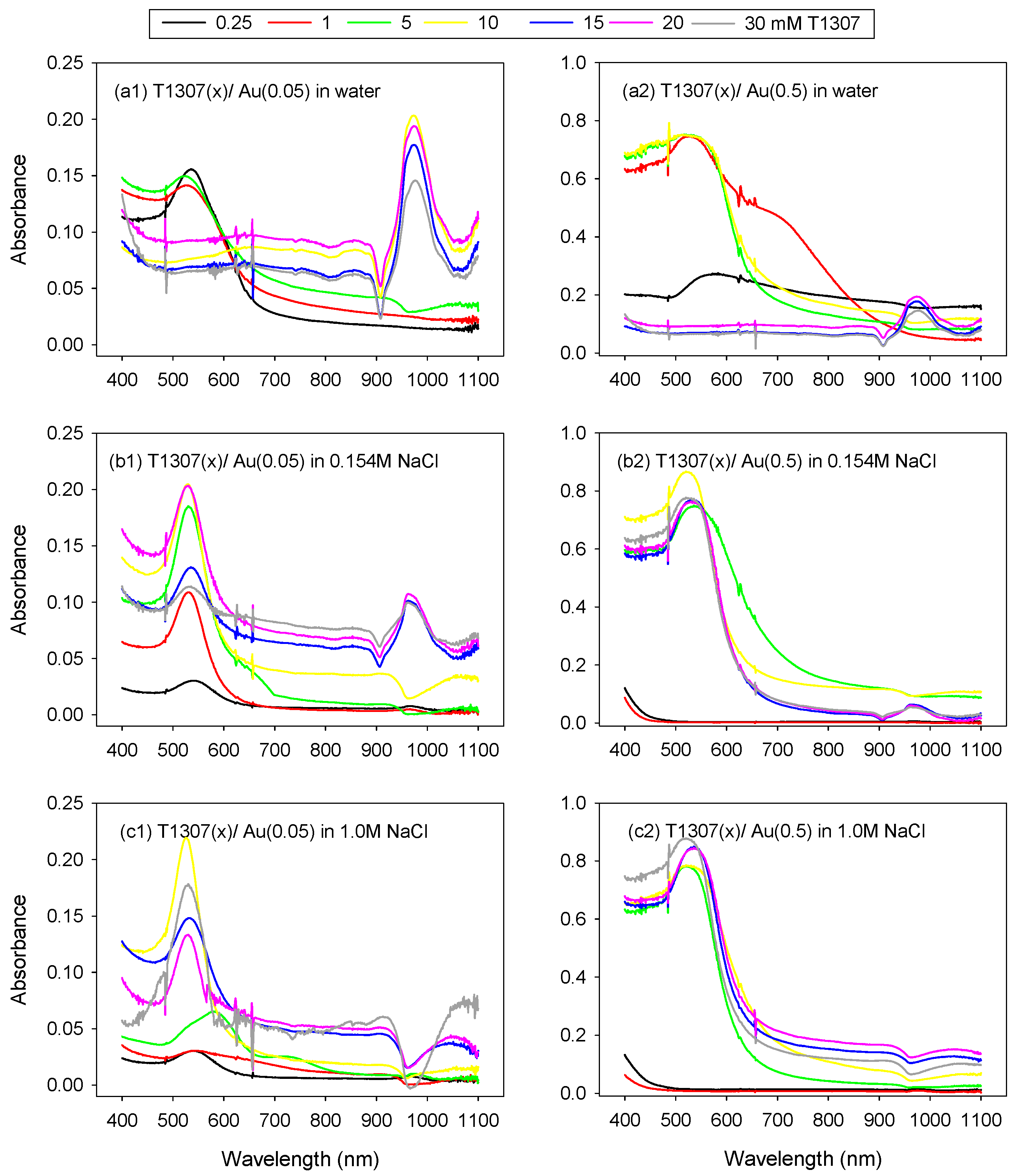

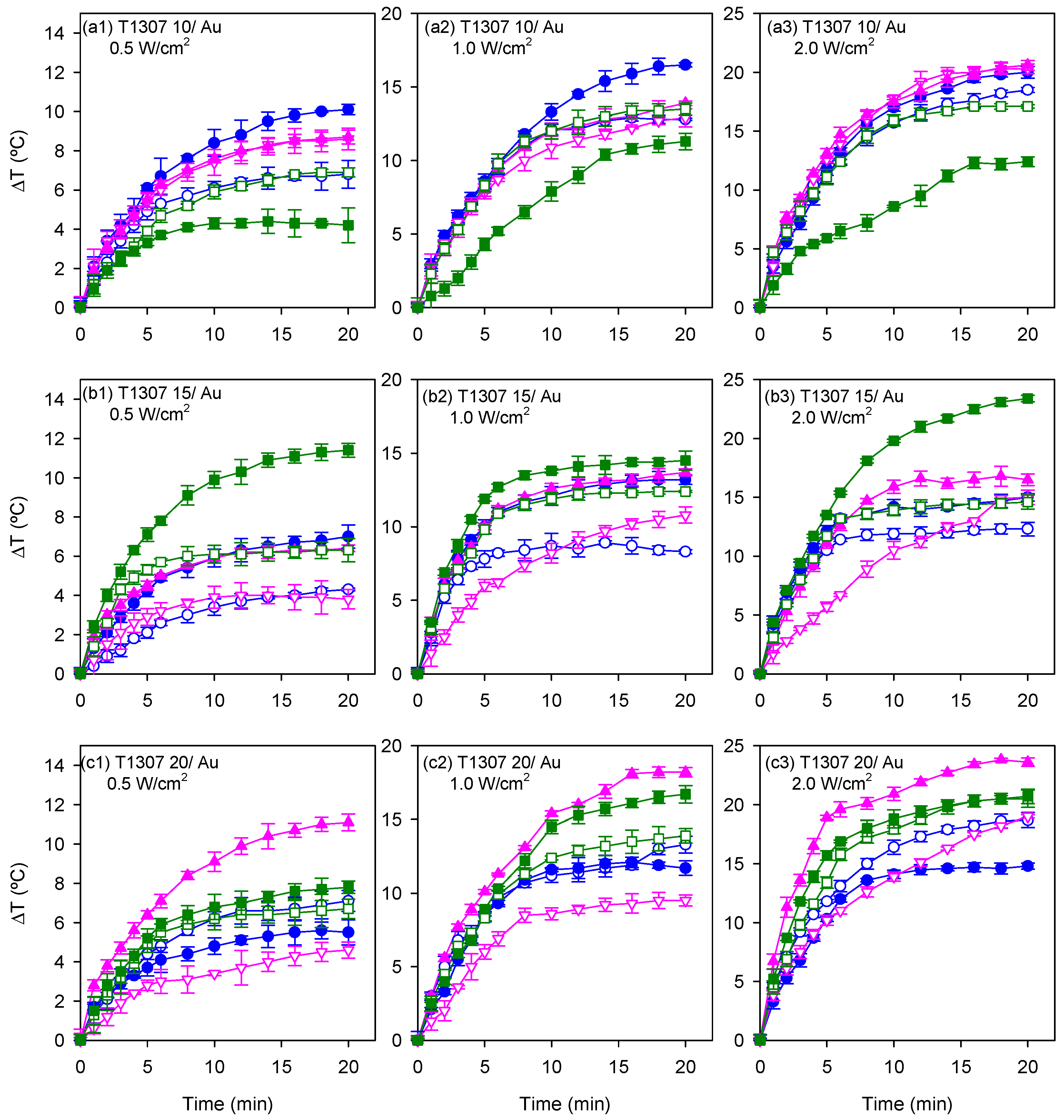
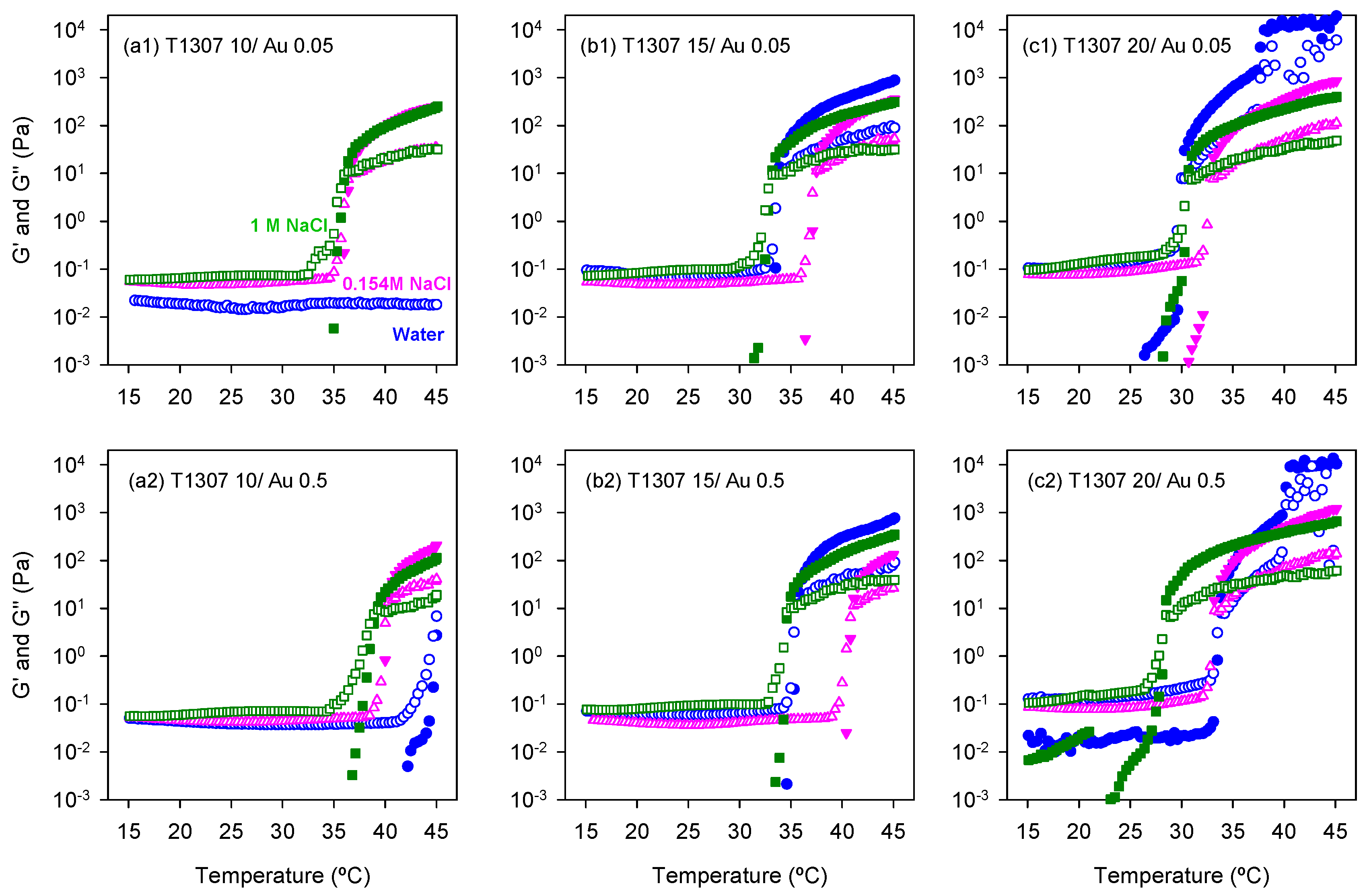
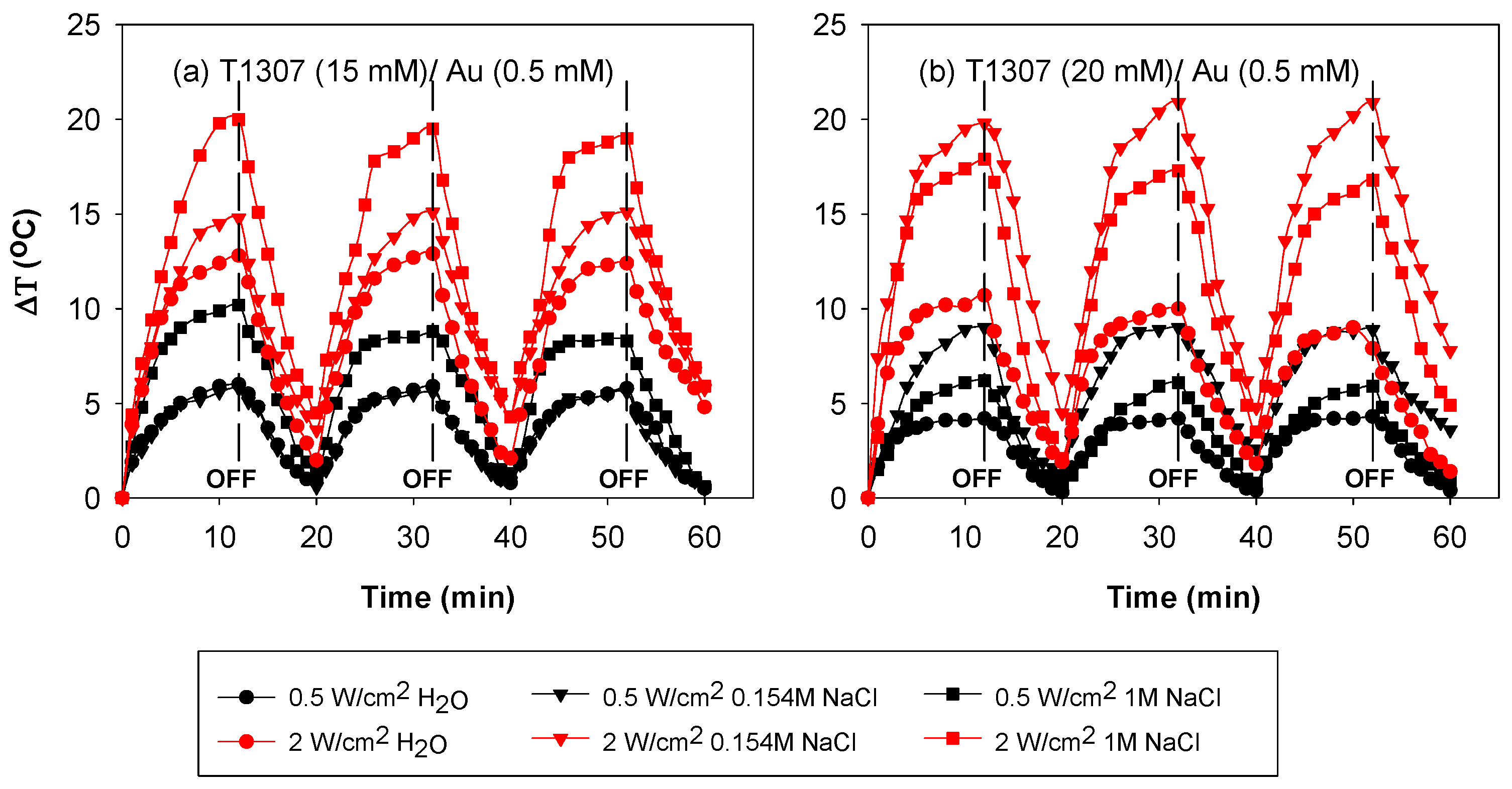
| [AuCl4] mM | [T1307] mM | Ø (nm) in H2O | Ø (nm) in 0.154 M NaCl | Ø (nm) in 1 M NaCl |
|---|---|---|---|---|
| 0.05 | 0.25 | 2.34 (0.24) | 75.8 (21.2) | 12.95 (1.63) |
| 1 | 35.99 (2.47) | 83.6 (21.5) | 113.6 (31.2) | |
| 5 | 68.43 (7.01) | 125.9 (23.9) | 238.6 (60.5) | |
| 10 | 116.5 (15.57) | 325.8 (65.0) | 742.1 (81.9) | |
| 0.5 | 0.25 | 14.28 (1.01) | 102.0 (19.3) | 94.5 (20.0) |
| 1 | 36.07 (7.93) | 194.1 (44.5) | 268.5 (58.1) | |
| 5 | 94.46 (20.02) | 284.0 (61.7) | 157.3 (62.0) | |
| 10 | 187.6 (19.89) | 466.6 (72.3) | 725.8 (98.1) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabana-Montenegro, S.; Barbosa, S.; Taboada, P.; Concheiro, A.; Alvarez-Lorenzo, C. Syringeable Self-Organizing Gels that Trigger Gold Nanoparticle Formation for Localized Thermal Ablation. Pharmaceutics 2019, 11, 52. https://doi.org/10.3390/pharmaceutics11020052
Cabana-Montenegro S, Barbosa S, Taboada P, Concheiro A, Alvarez-Lorenzo C. Syringeable Self-Organizing Gels that Trigger Gold Nanoparticle Formation for Localized Thermal Ablation. Pharmaceutics. 2019; 11(2):52. https://doi.org/10.3390/pharmaceutics11020052
Chicago/Turabian StyleCabana-Montenegro, Sonia, Silvia Barbosa, Pablo Taboada, Angel Concheiro, and Carmen Alvarez-Lorenzo. 2019. "Syringeable Self-Organizing Gels that Trigger Gold Nanoparticle Formation for Localized Thermal Ablation" Pharmaceutics 11, no. 2: 52. https://doi.org/10.3390/pharmaceutics11020052
APA StyleCabana-Montenegro, S., Barbosa, S., Taboada, P., Concheiro, A., & Alvarez-Lorenzo, C. (2019). Syringeable Self-Organizing Gels that Trigger Gold Nanoparticle Formation for Localized Thermal Ablation. Pharmaceutics, 11(2), 52. https://doi.org/10.3390/pharmaceutics11020052








