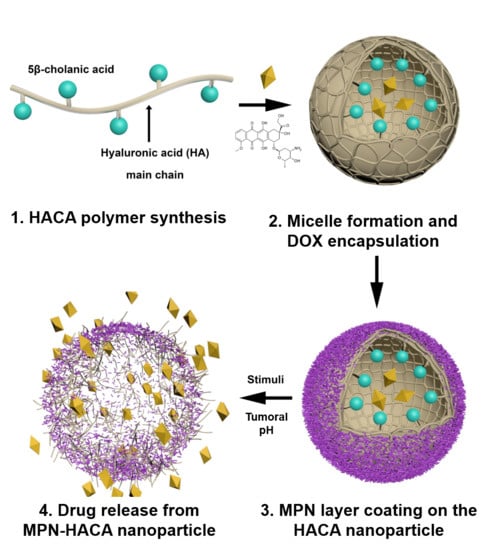
Metal-Phenolic Network-Coated Hyaluronic Acid Nanoparticles for pH-Responsive Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
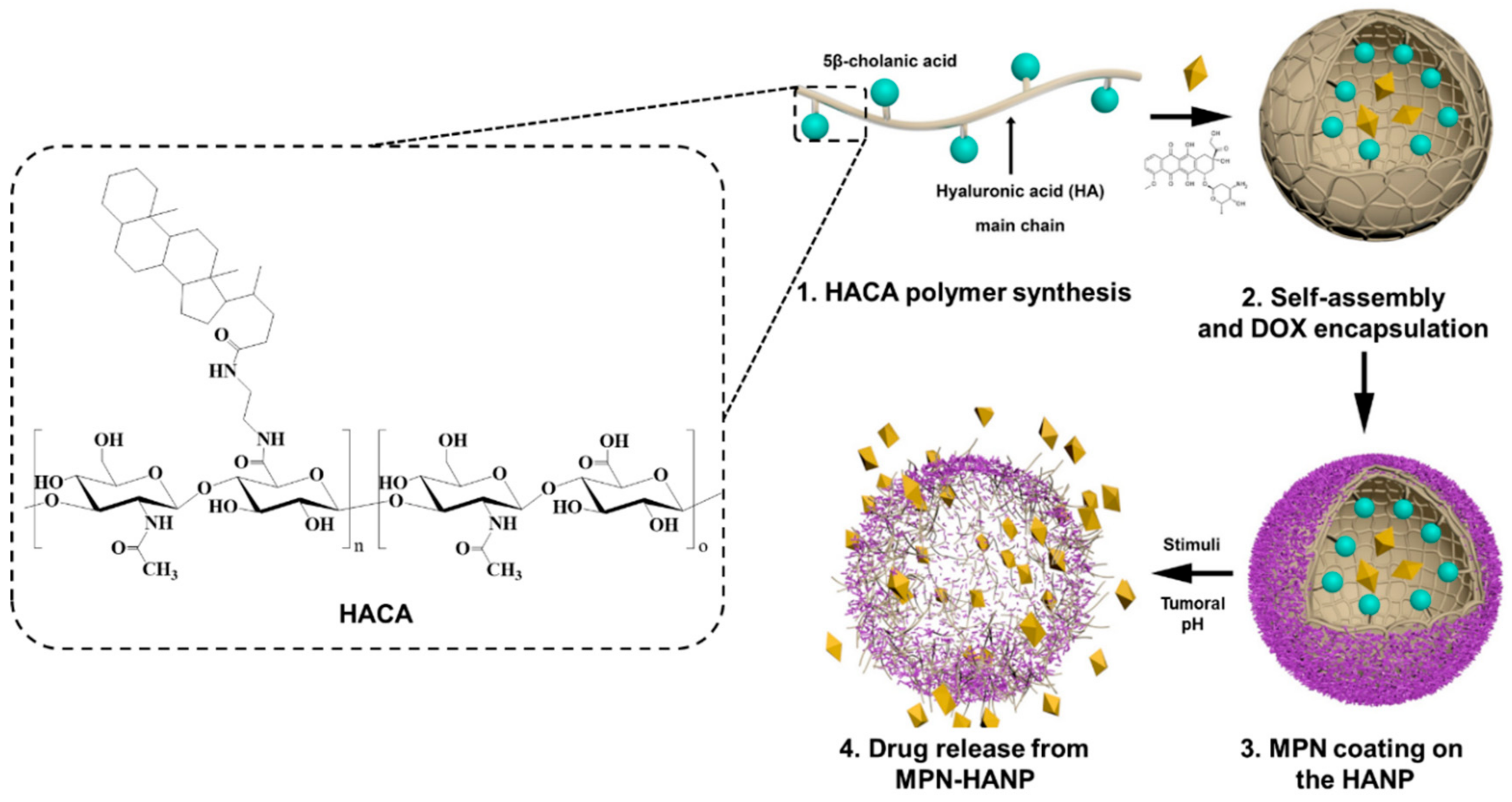
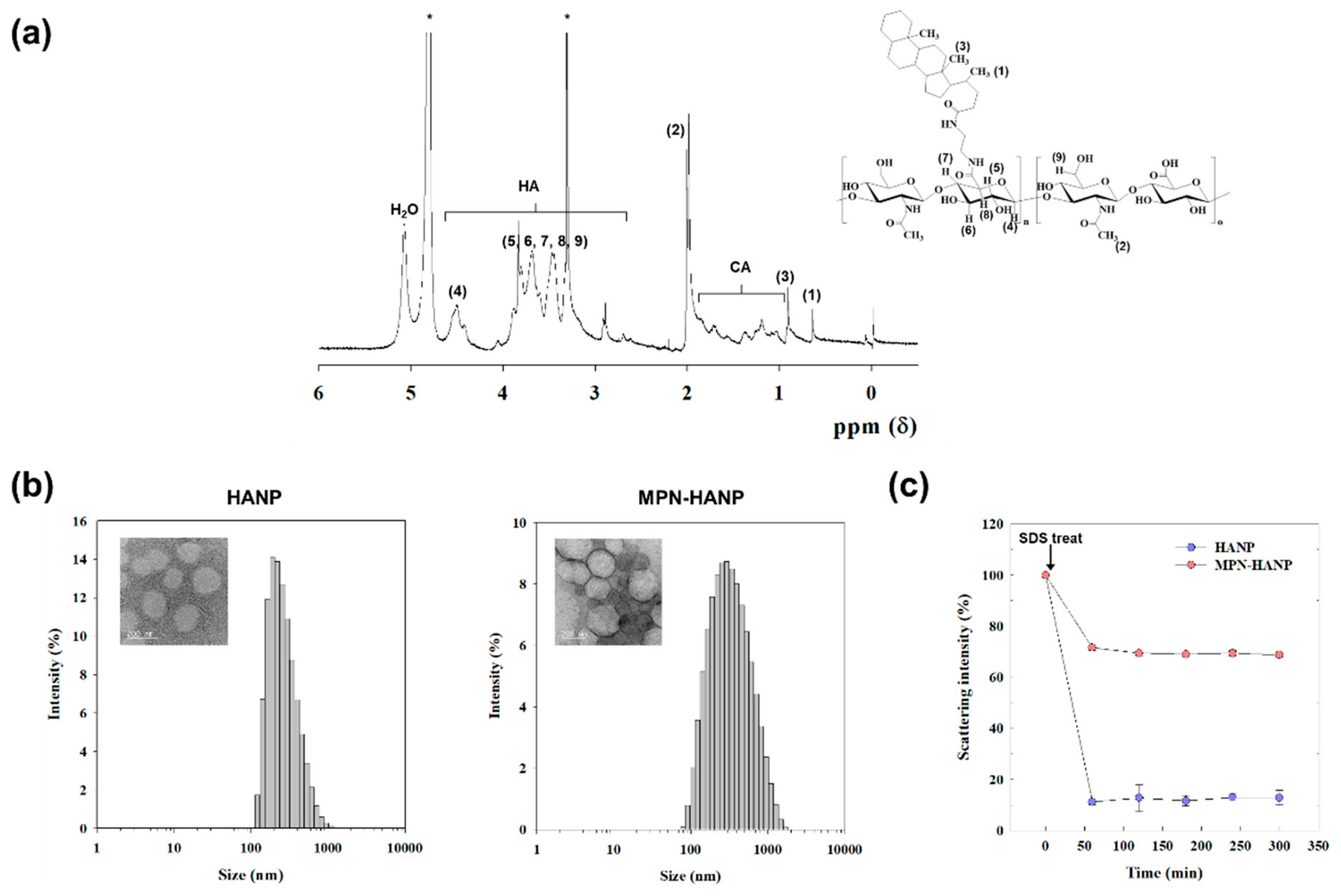
2.2. Synthesis of Amphiphilic HA Derivative (HACA)
2.3. Fabrication of MPN-HANPs
2.4. Characterization of MPN-HANPs
2.5. Preparation of DOX-MPN-HANP
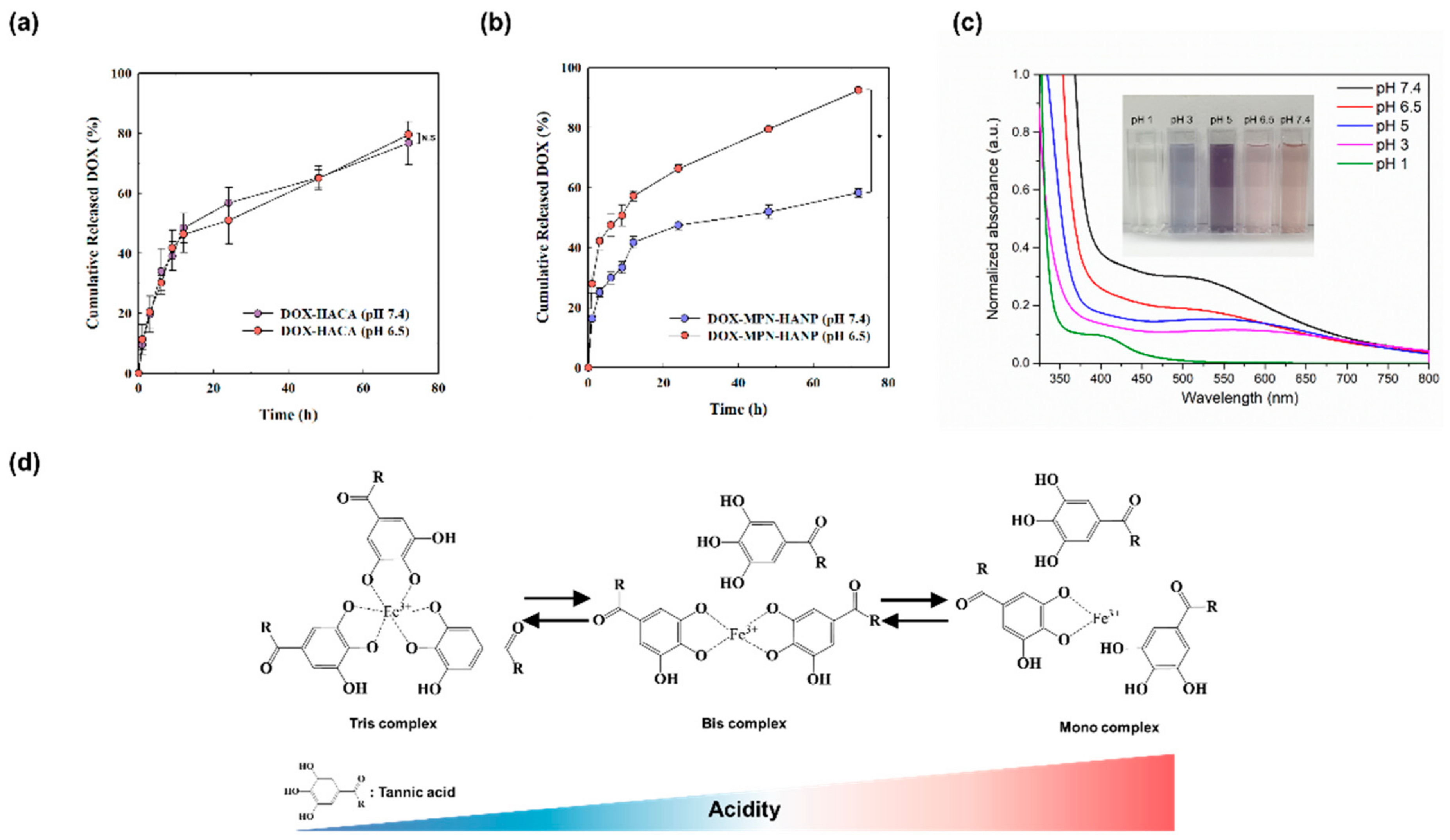
2.6. pH-Sensitive Release Behavior of DOX-MPN-HANP
2.7. In Vitro Cellular Uptake Behavior of DOX-MPN-HANP
2.8. Cytotoxicity of DOX-MPN-HANPs
2.9. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of MPN-HANP
3.2. In Vitro Drug Release Profile
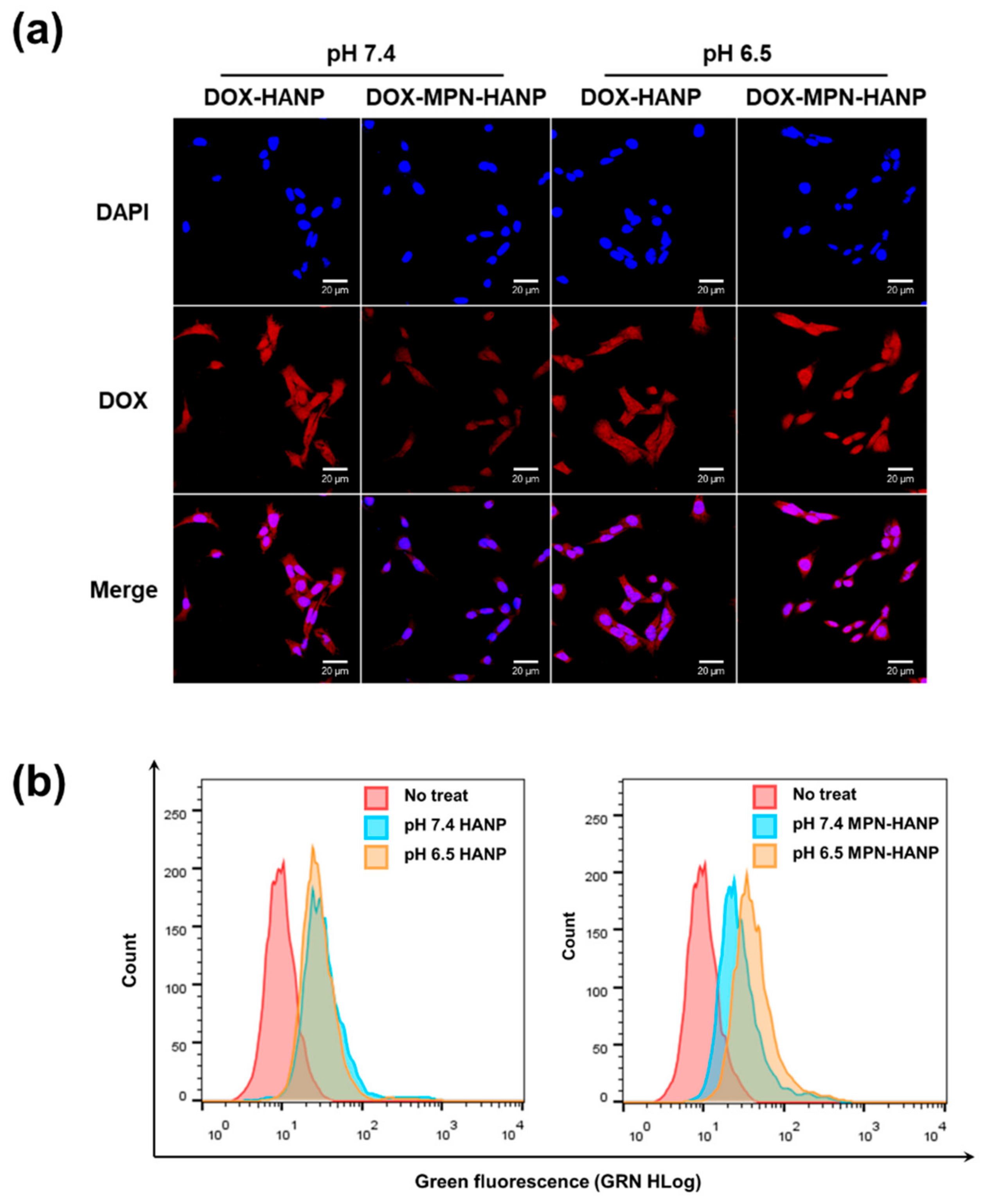
3.3. In Vitro Cellular Uptake Behavior
3.4. In Vitro Cytotoxicity of DOX-MPN-HANP
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2016, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, W.; Zhu, G.; Xie, J.; Chen, X. Rethinking cancer nanotheranostics. Nat. Rev. Mater. 2017, 2, 17024. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Son, S.; Park, K.S.; Zou, W.; Shea, L.D.; Moon, J.J. Cancer nanomedicine for combination cancer immunotherapy. Nat. Rev. Mater. 2019, 4, 398–414. [Google Scholar] [CrossRef]
- Hong, G.; Antaris, A.L.; Dai, H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 2017, 1, 0010. [Google Scholar] [CrossRef]
- Lee, E.S.; Shin, J.M.; Son, S.; Ko, H.; Um, W.; Song, S.H.; Lee, J.A.; Park, J.H. Recent Advances in Polymeric Nanomedicines for Cancer Immunotherapy. Adv. Healthc. Mater. 2019, 8, 1801320. [Google Scholar] [CrossRef] [PubMed]
- Hare, J.I.; Lammers, T.; Ashford, M.B.; Puri, S.; Storm, G.; Barry, S.T. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv. Drug Deliv. Rev. 2017, 108, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-Y.; Kim, D.-W.; Chung, J.-Y.; Shin, S.G.; Kim, S.-C.; Heo, D.S.; Kim, N.K.; Bang, Y.-J. Phase I and Pharmacokinetic Study of Genexol-PM, a Cremophor-Free, Polymeric Micelle-Formulated Paclitaxel, in Patients with Advanced Malignancies. Clin. Cancer Res. 2004, 10, 3708–3716. [Google Scholar] [CrossRef]
- Valle, J.W.; Armstrong, A.; Newman, C.; Alakhov, V.; Pietrzynski, G.; Brewer, J.; Campbell, S.; Corrie, P.; Rowinsky, E.K.; Ranson, M. A phase 2 study of SP1049C, doxorubicin in P-glycoprotein-targeting pluronics, in patients with advanced adenocarcinoma of the esophagus and gastroesophageal junction. Investig. New Drugs 2011, 29, 1029–1037. [Google Scholar] [CrossRef]
- Maeda, H. Macromolecular therapeutics in cancer treatment: The EPR effect and beyond. J. Control. Release 2012, 164, 138–144. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Zhang, M.; Mao, J.; Wu, Y.; Zhang, Y.; Yao, J.; Xu, C.; Guo, W.; Yu, B. Sodium cholate-enhanced polymeric micelle system for tumor-targeting delivery of paclitaxel. Int. J. Nanomed. 2017, 12, 8779. [Google Scholar] [CrossRef]
- Manaspon, C.; Viravaidya-Pasuwat, K.; Pimpha, N. Preparation of folate-conjugated pluronic F127/chitosan core-shell nanoparticles encapsulating doxorubicin for breast cancer treatment. J. Nanomater. 2012, 2012, 22. [Google Scholar] [CrossRef]
- Wang, S.; Huang, P.; Chen, X. Stimuli-Responsive Programmed Specific Targeting in Nanomedicine. ACS Nano 2016, 10, 2991–2994. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Deepagan, V.G.; Shin, S.; Ko, H.; Min, J.; Um, W.; Jeon, J.; Kwon, S.; Lee, E.S.; Suh, M.; et al. Ultrasmall gold nanosatellite-bearing transformable hybrid nanoparticles for deep tumor penetration. Acta Biomater. 2018, 79, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Deepagan, V.G.; Kwon, S.; You, D.G.; Nguyen, V.Q.; Um, W.; Ko, H.; Lee, H.; Jo, D.-G.; Kang, Y.M.; Park, J.H. In situ diselenide-crosslinked polymeric micelles for ROS-mediated anticancer drug delivery. Biomaterials 2016, 103, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Thambi, T.; Park, J.H.; Lee, D.S. Hypoxia-responsive nanocarriers for cancer imaging and therapy: Recent approaches and future perspectives. Chem. Commun. 2016, 52, 8492–8500. [Google Scholar] [CrossRef]
- Xin, Y.; Yuan, J. Schiff’s base as a stimuli-responsive linker in polymer chemistry. Polym. Chem. 2012, 3, 3045–3055. [Google Scholar] [CrossRef]
- Fleige, E.; Quadir, M.A.; Haag, R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug Deliv. Rev. 2012, 64, 866–884. [Google Scholar] [CrossRef]
- Igarashi, E. Factors affecting toxicity and efficacy of polymeric nanomedicines. Toxicol. Appl. Pharm. 2008, 229, 121–134. [Google Scholar] [CrossRef]
- Wolfram, J.; Zhu, M.; Yang, Y.; Shen, J.; Gentile, E.; Paolino, D.; Fresta, M.; Nie, G.; Chen, C.; Shen, H.; et al. Safety of Nanoparticles in Medicine. Curr. Drug Targets 2015, 16, 1671–1681. [Google Scholar] [CrossRef]
- Lee, J.Y.; Spicer, A.P. Hyaluronan: A multifunctional, megaDalton, stealth molecule. Curr. Opin. Cell Biol. 2000, 12, 581–586. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Yang, B.; Yang, B.L.; Savani, R.C.; Turley, E.A. Identification of a common hyaluronan binding motif in the hyaluronan binding proteins RHAMM, CD44 and link protein. EMBO J. 1994, 13, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Hwang, S.R.; Heo, R.; Saravanakumar, G.; Park, J.H. Amphiphilic hyaluronic acid derivative with the bioreducible bond: Synthesis and its implication for intracellular drug delivery. Polym. Degrad. Stabil. 2014, 109, 398–404. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Yoon, H.Y.; Shin, J.M.; Saravanakumar, G.; Noh, K.H.; Song, K.-H.; Jeon, J.-H.; Kim, D.-W.; Lee, K.-M.; Kim, K.; et al. A polymeric conjugate foreignizing tumor cells for targeted immunotherapy in vivo. J. Control. Release 2015, 199, 98–105. [Google Scholar] [CrossRef]
- Ejima, H.; Richardson, J.J.; Liang, K.; Best, J.P.; Van Koeverden, M.P.; Such, G.K.; Cui, J.; Caruso, F. One-Step Assembly of Coordination Complexes for Versatile Film and Particle Engineering. Science 2013, 341, 154–157. [Google Scholar] [CrossRef]
- Ping, Y.; Guo, J.; Ejima, H.; Chen, X.; Richardson, J.J.; Sun, H.; Caruso, F. pH-Responsive Capsules Engineered from Metal–Phenolic Networks for Anticancer Drug Delivery. Small 2015, 11, 2032–2036. [Google Scholar] [CrossRef]
- Liu, Z.; Le, Z.; Lu, L.; Zhu, Y.; Yang, C.; Zhao, P.; Wang, Z.; Shen, J.; Liu, L.; Chen, Y. Scalable fabrication of metal-phenolic nanoparticles by coordination-driven flash nanocomplexation for cancer theranostics. Nanoscale 2019, 11, 9410–9421. [Google Scholar] [CrossRef]
- Shen, G.; Xing, R.; Zhang, N.; Chen, C.; Ma, G.; Yan, X. Interfacial Cohesion and Assembly of Bioadhesive Molecules for Design of Long-Term Stable Hydrophobic Nanodrugs toward Effective Anticancer Therapy. ACS Nano 2016, 10, 5720–5729. [Google Scholar] [CrossRef]
- Ju, Y.; Dai, Q.; Cui, J.; Dai, Y.; Suma, T.; Richardson, J.J.; Caruso, F. Improving Targeting of Metal–Phenolic Capsules by the Presence of Protein Coronas. ACS Appl. Mater. Interfaces 2016, 8, 22914–22922. [Google Scholar] [CrossRef]
- Besford, Q.A.; Ju, Y.; Wang, T.Y.; Yun, G.; Cherepanov, P.; Hagemeyer, C.E.; Cavalieri, F.; Caruso, F. Self-Assembled Metal-Phenolic Networks on Emulsions as Low-Fouling and pH-Responsive Particles. Small 2018, 14, 1802342. [Google Scholar] [CrossRef]
- Choi, K.Y.; Min, K.H.; Na, J.H.; Choi, K.; Kim, K.; Park, J.H.; Kwon, I.C.; Jeong, S.Y. Self-assembled hyaluronic acid nanoparticles as a potential drug carrier for cancer therapy: Synthesis, characterization, and in vivo biodistribution. J. Mater. Chem. 2009, 19, 4102–4107. [Google Scholar] [CrossRef]
- Han, H.S.; Choi, K.Y.; Lee, H.; Lee, M.; An, J.Y.; Shin, S.; Kwon, S.; Lee, D.S.; Park, J.H. Gold-Nanoclustered Hyaluronan Nano-Assemblies for Photothermally Maneuvered Photodynamic Tumor Ablation. ACS Nano 2016, 10, 10858–10868. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Han, H.S.; Sung, S.; Kang, J.H.; Sa, K.H.; Al Faruque, H.; Hong, J.; Nam, E.J.; Kim, I.S.; Park, J.H.; et al. Endogenous inspired biomineral-installed hyaluronan nanoparticles as pH-responsive carrier of methotrexate for rheumatoid arthritis. J. Control. Release 2017, 252, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Hameed, S.; Bhattarai, P.; Liang, X.; Zhang, N.; Xu, Y.; Chen, M.; Dai, Z. Self-assembly of porphyrin-grafted lipid into nanoparticles encapsulating doxorubicin for synergistic chemo-photodynamic therapy and fluorescence imaging. Theranostics 2018, 8, 5501–5518. [Google Scholar] [CrossRef]
- Zhang, Y.; Ni, C.; Shi, G.; Wang, J.; Zhang, M.; Li, W. The polyion complex nano-prodrug of doxorubicin (DOX) with poly (lactic acid-co-malic acid)-block-polyethylene glycol: Preparation and drug controlled release. Med. Chem. Res. 2015, 24, 1189–1195. [Google Scholar] [CrossRef]
- Meghani, N.M.; Amin, H.H.; Park, C.; Park, J.-B.; Cui, J.-H.; Cao, Q.-R.; Lee, B.-J. Design and evaluation of clickable gelatin-oleic nanoparticles using fattigation-platform for cancer therapy. Int. J. Pharm. 2018, 545, 101–112. [Google Scholar] [CrossRef]
- Sungur, Ş.; Uzar, A. Investigation of complexes tannic acid and myricetin with Fe(III). Spectrochim. Acta A 2008, 69, 225–229. [Google Scholar] [CrossRef]

| Sample | Hydrodynamic Size (nm) 1 | Zeta Potential (mV) 2 | DOX Feed Amount (%) | Loading Efficiency (%) 3 | Loading Contents (%) 3 |
|---|---|---|---|---|---|
| HANP | 264.0 ± 13.11 | −42.7 ± 0.38 | - | - | - |
| MPN-HANP | 273.9 ± 18.62 | −24.5 ± 0.11 | - | - | - |
| DOX-HANP | 277.53 ± 5.20 | −36.8 ± 0.47 | 10 | 82.4 | 8.24 |
| DOX-MPN-HANP | 271.3 ± 4.05 | −28.07 ± 0.97 | 10 | 77.1 | 7.71 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.M.; Choi, G.H.; Song, S.H.; Ko, H.; Lee, E.S.; Lee, J.A.; Yoo, P.J.; Park, J.H. Metal-Phenolic Network-Coated Hyaluronic Acid Nanoparticles for pH-Responsive Drug Delivery. Pharmaceutics 2019, 11, 636. https://doi.org/10.3390/pharmaceutics11120636
Shin JM, Choi GH, Song SH, Ko H, Lee ES, Lee JA, Yoo PJ, Park JH. Metal-Phenolic Network-Coated Hyaluronic Acid Nanoparticles for pH-Responsive Drug Delivery. Pharmaceutics. 2019; 11(12):636. https://doi.org/10.3390/pharmaceutics11120636
Chicago/Turabian StyleShin, Jung Min, Gwan Hyun Choi, Seok Ho Song, Hyewon Ko, Eun Sook Lee, Jae Ah Lee, Pil J. Yoo, and Jae Hyung Park. 2019. "Metal-Phenolic Network-Coated Hyaluronic Acid Nanoparticles for pH-Responsive Drug Delivery" Pharmaceutics 11, no. 12: 636. https://doi.org/10.3390/pharmaceutics11120636
APA StyleShin, J. M., Choi, G. H., Song, S. H., Ko, H., Lee, E. S., Lee, J. A., Yoo, P. J., & Park, J. H. (2019). Metal-Phenolic Network-Coated Hyaluronic Acid Nanoparticles for pH-Responsive Drug Delivery. Pharmaceutics, 11(12), 636. https://doi.org/10.3390/pharmaceutics11120636