Repeat-Dose Toxicity Study Using the AFPL1-Conjugate Nicotine Vaccine in Male Sprague Dawley Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Husbandry
2.2. Vaccine and Vaccination Protocol
2.3. Clinical Observations and Symptoms
2.4. Euthanasia and Blood Collection
2.5. Hematological and Blood Biochemical Evaluation
2.6. Anatomopathological Studies and Organ Weights
2.7. Immunotoxicological Evaluation
2.8. Anti-Nicotine ELISAs
2.9. Statistical Analysis
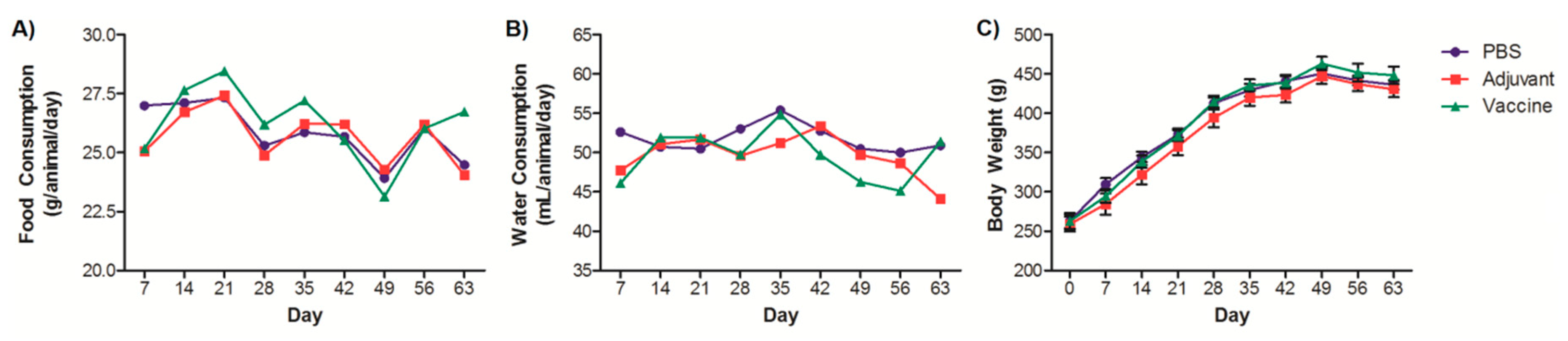
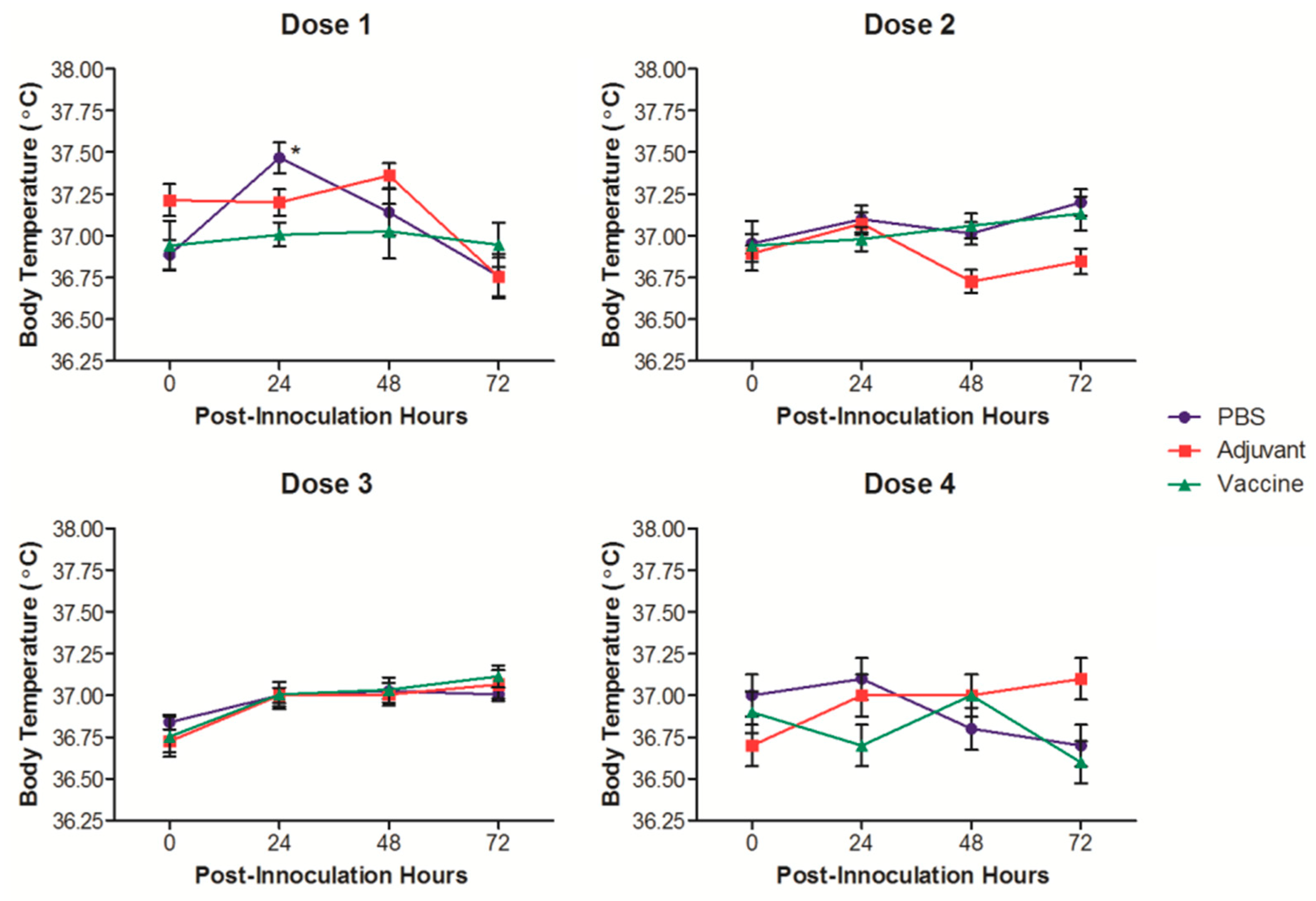
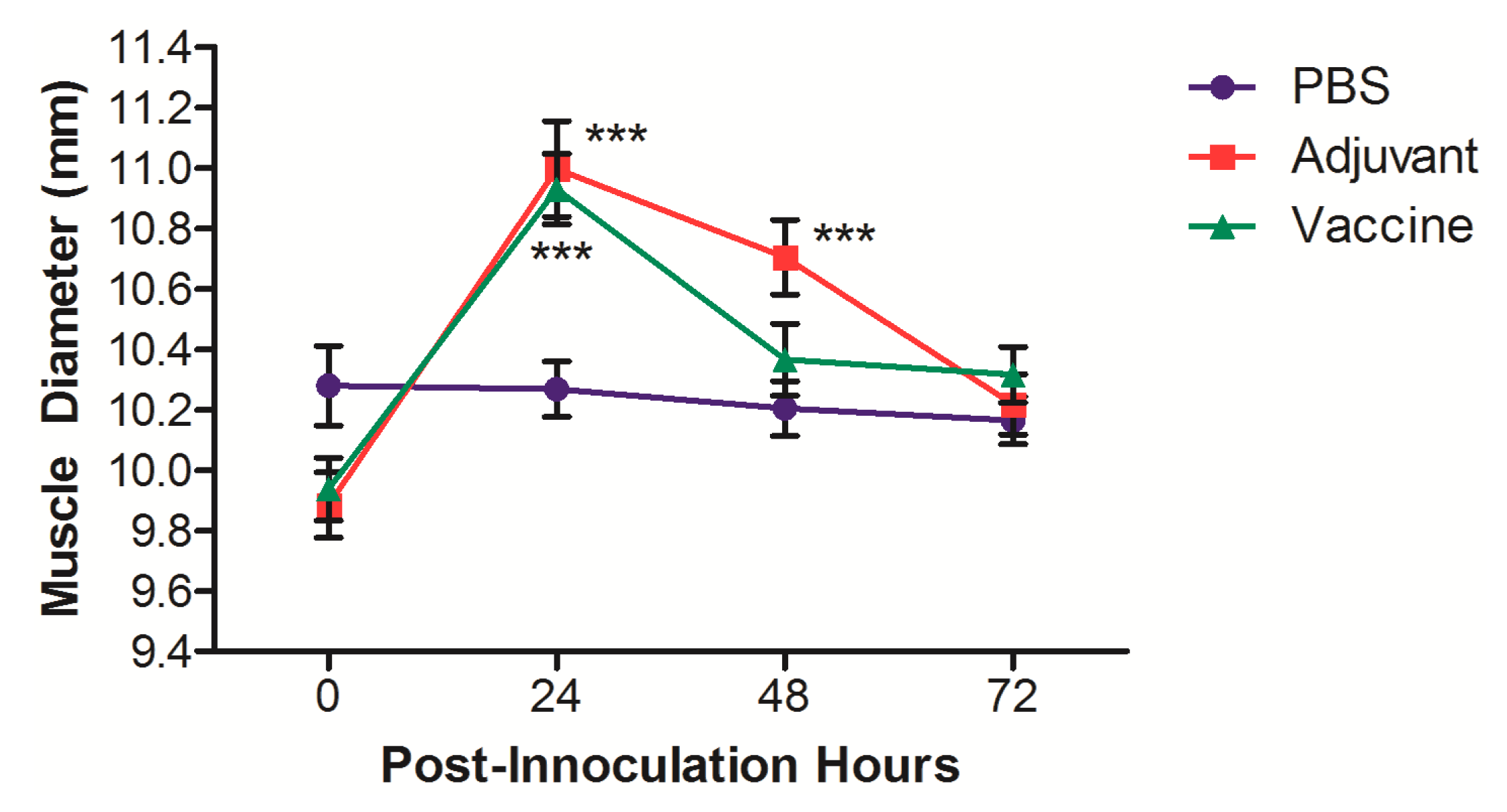
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dobrescu, A.; Bhandari, A.; Sutherland, G.; Dinh, T. The Costs of Tobacco Use in Canada. 2012. Available online: https://www.conferenceboard.ca/press/newsrelease/2017/10/16/smoking-costs-canadian-economy-more-than-$16-billion-in-2012 (accessed on 30 July 2019).
- Statistics Canada Student Tobacco, Alcohol, and Drugs Study 2016–2017. Available online: http://www.canada.ca/en/health-canada/services/canadian-student-tobacco-alcohol-drugs-survey/2016-2017-summary.htm (accessed on 19 July 2019).
- Moreno, A.Y.; Janda, K.D. Immunopharmacotherapy vaccination strategies as a treatment for drug abuse and dependence. Pharmacol. Biochem. Behav. 2009, 92, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Fraleigh, N.L.; Boudreau, J.; Bhardwaj, N.; Eng, N.F.; Murad, Y.; Lafrenie, R.; Acevedo, R.; Oliva, R.; Diaz-Mitoma, F.; Le, H.-T. Evaluating the immunogenicity of an intranasal vaccine against nicotine in mice using the Adjuvant Finlay Proteoliposome (AFPL1). Heliyon 2016, 2, e00147. [Google Scholar] [CrossRef] [PubMed]
- Fraleigh, N.L.; Oliva, R.; Lewicky, J.D.; Martel, A.M.; Acevedo, R.; Dagmar, G.-R.; Le, H.-T. Assessing the immunogenicity and toxicity of the AFPL1-conjugate nicotine vaccine using heterologous and homologous vaccination routes. PLoS ONE 2019, 14, e0221708. [Google Scholar] [CrossRef] [PubMed]
- Forster, F. Study designs for the nonclinical safety testing of new vaccine products. J. Pharmacol. Toxicol. 2012, 66, 1–7. [Google Scholar] [CrossRef]
- Verdier, F. Non-clinical vaccine safety assessment. Toxicology 2002, 174, 37–43. [Google Scholar] [CrossRef]
- Al-Humadi, N. Pre-clinical toxicology considerations for vaccine development. Vaccine 2017, 35, 5762–5767. [Google Scholar] [CrossRef]
- Hu, B.; Wang, J.; Guo, Y.; Chen, T.; Weihua, N.; Yuan, H.; Zhang, N.; Xie, F.; Tai, G. Pre-clinical toxicity and immunogenicity of a MUC1-MBP/BCG anti-tumor vaccine. Int. Immunopharmacol. 2016, 33, 108–118. [Google Scholar] [CrossRef]
- Xian, T.H.; Parasuraman, S.; Sinniah, K.; Ravichandran, M. Repeated dose toxicity evaluation of a cold chain-free, live, attenuated oral cholera vaccine in Sprague Dawley rats. Vaccine 2019, 37, 711–720. [Google Scholar] [CrossRef]
- Park, S.J.; Seo, K.H.; Han, S.I. Toxicity study of streptococcus pneumonia vaccine administered subcutaneously in rats. Toxicol. Res. 2011, 27, 111–118. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Oliva, R.; Fariñas, M.; Infante, J.F.; Hernández, T. Local tolerance study of the VA-MENGOC-BC® antimeningococcal vaccine in Sprague Dawley rats. Evaluation at 24 and 36 months of shelf. VacciMonitor 2019, 28, 9–18. [Google Scholar]
- Taconic. Sprague Dawley Rat Outbred Growth Chart. Available online: https://www.taconic.com/pdfs/sprague-dawley-rat.pdf (accessed on 31 May 2019).
- Brower, M.; Grace, M.; Kotz, C.M.; Koya, V. Comparative analysis of growth characteristics of Sprague Dawley rats obtained from different sources. Lab. Anim. Res. 2015, 31, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Rat/Mouse Default Values. Available online: www.tera.org/Tools/ratmousevalues.pdf (accessed on 31 May 2019).
- Fariñas, M.; Oliva, R.; Infante, J.F.; Valdez, Y.; Nuñez, D.; Valmaceda, T.; Hernández Salazar, T.; Fernández, S. Ensayo piloto de inmunogenicidad y toxicidad preclínica de la vacuna Salmonella typhi conjugada en ratas Sprague Dawley. Retel 2014, 44. Available online: http://www.sertox.com.ar/modules.php?name=Content&pa=showpage&pid=936 (accessed on June 13 2019).
- Baek, Y.-O.; Choi, S.-K.; Shin, S.-H.; Koo, K.-W.; Choi, H.-Y.; Cha, S.-B.; Li, Y.-C.; Yoo, H.-J.; Lee, J.-Y.; Kil, K.-H.; et al. A 6-week oral toxicity study of oral cholera vaccine in Sprague Dawley rats. Toxicol. Res. 2012, 28, 225–233. [Google Scholar] [CrossRef]
- Charles River. Growth Chart SD Rat. Available online: https://www.criver.com/products-services/find-model/sas-sd?region=3611 (accessed on May 31 2019).
- López, Y.; Infante, J.F.; Sifontes, S.; Díaz, D.; Pérez, V.; Año, G.; Hernández, T.; Fernández, S.; Castaño, J.L.; Cedré, B.; et al. Pharmacology and toxicology of an oral tablet whole cells inactivated cholera vaccine in Sprague Dawley rats. Vaccine 2011, 29, 3596–3599. [Google Scholar] [CrossRef]
- López, Y.; Pastor, M.; Infante, J.F.; Díaz, D.; Oliva, R.; Fernández, S.; Cedré, B.; Hernández, T.; Campos, L.; Esquisabel, A.; et al. Repeated dose toxicity study of Vibrio cholerae-loaded gastro-resistant microparticles. J. Microencapsul. 2014, 31, 86–92. [Google Scholar] [CrossRef]
- Núñez, J.F.; Herrera, L.; Infante, J.F.; González, P.; Pérez, V.; Argamasilla, M.; Mayo, J.; Sosa, E.; González, N.; Dupuig, J.; et al. Estudio de toxicidad por dosis única y tolerancia local de una vacuna antimeningocócica tipo B en ratas Sprague Dawley. VacciMonitor 2006, 15, 9–14. [Google Scholar]
- Sosa, E.; Sifontes, S.; Infante, J.F.; Días, D.; López, Y.; Pérez, V.; Hernández, T.; Riverón, L.; Valdés, Y.; García, I.; et al. Estudio de tolerancia local de la vacuna vax-TyVi® en ratas Sprague Dawley. VacciMonitor 2005, 14, 21–27. [Google Scholar]
- Lillie, L.E.; Temple, N.J.; Florence, L.Z. Reference values for young normal Sprague Dawley rats: Weight gain, hematology and clinical chemistry. Hum. Exp. Toxicol. 1996, 15, 612–616. [Google Scholar] [CrossRef]
- Sifontes, S.; Infante, J.F.; Díaz, D.; López, Y.; Pérez, M.; Sosa, E.; Pérez, V.; López, Y.; Álvarez, E.; Martínez, J.C.; et al. Repeated dose toxicity study of a live attenuated oral cholera vaccine in Sprague Dawley rats. Arch. Med. Res. 2009, 40, 527–535. [Google Scholar] [CrossRef]
- World Health Organization Key Facts on Tobacco. Available online: https://www.who.int/news-room/fact-sheets/detail/tobacco (accessed on 18 July 2019).
- Batistia-Duharte, A.; Lindlad, E.B.; Oviedo-Orta, E. Progress in understanding adjuvant immunotoxicity mechanisms. Toxicol. Lett. 2011, 203, 97–105. [Google Scholar] [CrossRef]
- Pérez, O.; Lastre, M.; Cabrera, O.; del Campo, J.; Bracho, G.; Cuello, M.; Balboa, J.; Acevedo, R.; Zayas, C.; Gil, D.; et al. New vaccines require potent adjuvants like AFPL1 and AFCo1. Scand. J. Immunol. 2007, 66, 271–277. [Google Scholar] [CrossRef]
- Romeu, B.; Lastre, M.; Reyes, L.; González, E.; Borrero, Y.; Lescaille, D.; Pérez, D.; Nuñez, D.; Pérez, O. Nasal immunization of mice with AFCo1 or AFPL1 plus capsular polysaccharide Vi from Salmonella typhi induces cellular responses and memory B and T cell responses. Vaccine 2014, 32, 6971–6978. [Google Scholar] [CrossRef] [PubMed]
- del Campo, J.; Zayas, C.; Romeu, B. Mucosal immunization using proteoliposome and cochleate structures from Neisseria meningitidis serogroup B induce mucosal and systemic responses. Methods 2009, 49, 301–308. [Google Scholar] [CrossRef] [PubMed]
- del Campo, J.; Lindqvist, M.; Cuello, M. Intranasal immunization with a proteoliposome-derived cochleate containing recombinant gD protein confers protective immunity against genital herpes in mice. Vaccine 2010, 28, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Infante-Bourzac, J.F.; Sifontes-Rodríguez, S.; Arrencibia-Arrebola, D.F.; Hernández-Salazar, T.; Fariñas-Medina, M.; Pérez, O. Toxicological assessement of the cochleate derived from Neisseria meningitidis proteoliposome in Sprague Dawley rats. N. Am. J. Med. Sci. 2012, 4, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, S.; Herkenham, M. Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J. Neurosci. 2005, 25, 1788–1796. [Google Scholar] [CrossRef]
- Lu, F.; HogenEsch, H. Kinetics of the inflammatory response following intramuscular injection of aluminum adjuvant. Vaccine 2013, 31, 3979–3986. [Google Scholar] [CrossRef]
- Nygaard, U.C.; Løvil, M. Blood and spleen lymphocytes as targets for immunotoxic effects in the rat—A comparison. Toxicology 2002, 174, 153–161. [Google Scholar] [CrossRef]
- Bondy, G.; Armstrong, C.; Coady, L.; Doucet, J.; Robertson, P.; Feeley, M.; Barker, M. Toxicity of chlordane metabolite oxychlordane in female rats: Clinical and histopathological changes. Food Chem. Toxicol. 2003, 41, 291–301. [Google Scholar] [CrossRef]
- Baldrick, P.; Richardson, D.; Elliott, G.; Wheeler, A.W. Safety evaluation of monophosphoryl lipid A (MPL): An immunostimulatory adjuvant. Regul. Toxicol. Pharmacol. 2002, 35, 398–413. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-J.; Bae, E.; Yi, J.; Kim, Y.; Choi, K.; Lee, S.H.; Yoon, J.; Lee, B.C.; Park, K. Repeated-dose toxicity and inflammatory responses in mice by oral administration of silver nanoparticles. Environ. Toxicol. Pharmacol. 2010, 30, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Maurer, P.; Jennings, G.T.; Willers, J.; Rohner, F.; Lindman, Y.; Roubicek, K.; Renner, W.A.; Müller, P.; Bachmann, M.F. A therapeutic vaccine for nicotine dependence: Preclinical efficacy, and phase I safety and immunogenicity. Eur. J. Immunol. 2005, 35, 2031–2040. [Google Scholar] [CrossRef] [PubMed]
- Hatsukami, D.K.; Rennard, S.; Jorenby, D.; Fiore, M.; Koopmeiners, J.; de Vos, A.; Hormith, G.; Pentel, P.R. Safety and immunogenicity of a nicotine conjugate vaccine in current smokers. Clin. Pharmacol. Ther. 2005, 78, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Wagena, E.J.; de Vos, A.; Horwith, G.; van Schayck, C.P. The immunogenicity and safety of a nicotine vaccine in smokers and nonsmokers: Results of a randomized, placebo-controlled phase 1/2 trial. Nicotine Tob. Res. 2008, 10, 213–218. [Google Scholar] [CrossRef] [PubMed]
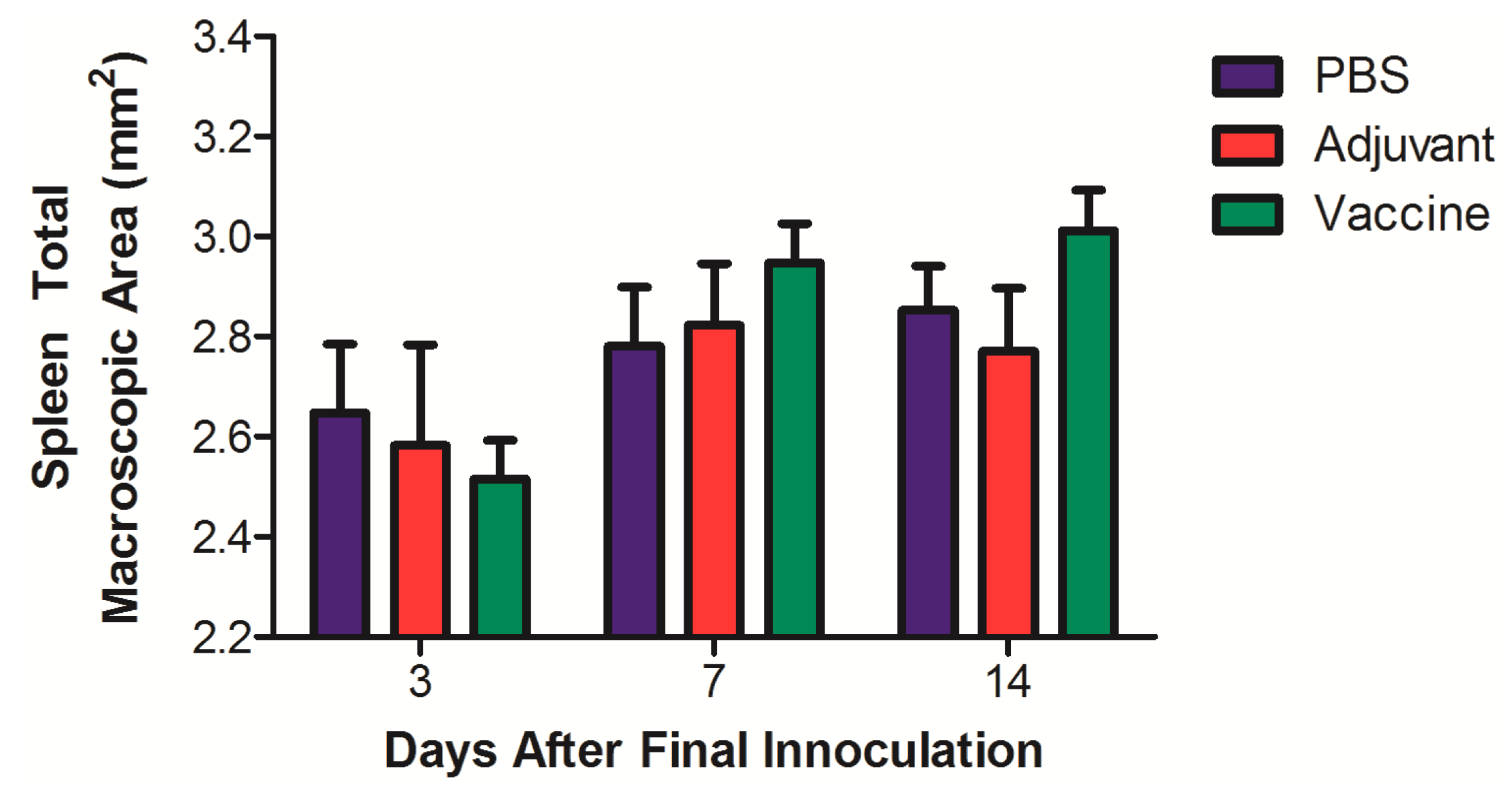
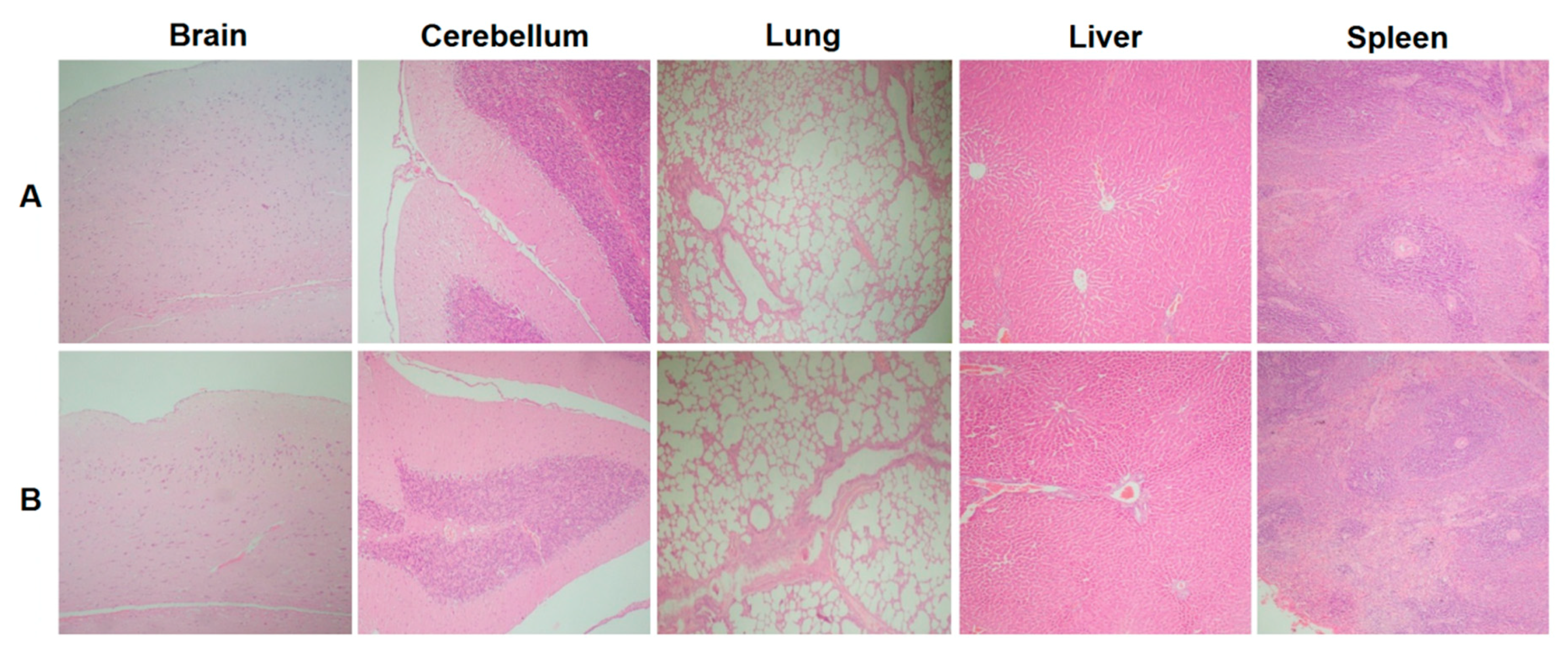
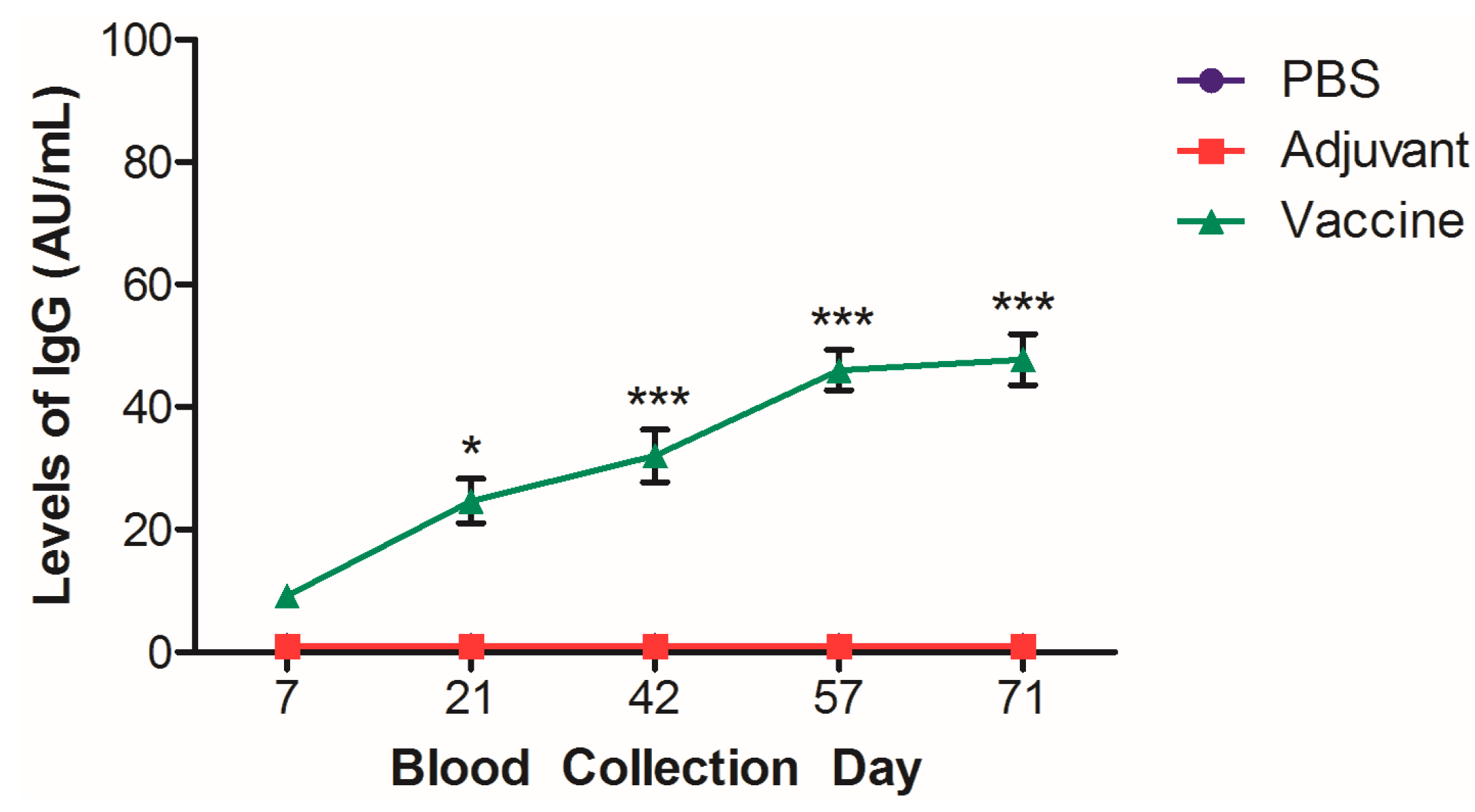
| Group | Animals, n | n Euthanized at x Days After 4th Dose | ||
|---|---|---|---|---|
| x = 3 | x = 7 | x = 14 | ||
| PBS | 15 | 5 | 5 | 5 |
| Adjuvant (AFPL1) | 15 | 5 | 5 | 5 |
| Vaccine | 15 | 5 | 5 | 5 |
| Schedule | Route | Volume | ||
| Day 0 | IM/IN | 200 µL (100 µL per leg)/80 µL (40 µL per nare) | ||
| Day 21 | IN | 80 µL (40 µL per nare) | ||
| Day 42 | IN | 80 µL (40 µL per nare) | ||
| Day 56 | IN | 80 µL (40 µL per nare) | ||
| Group | Brain | Thymus | Heart | Left Lung | Right Lung | Liver | Spleen | Left Kidney | Right Kidney |
|---|---|---|---|---|---|---|---|---|---|
| 3 Days After 4th Dose | |||||||||
| PBS | 0.47 ± 0.01 | 0.10 ±0.01 | 0.32 ± 0.01 | 0.13 ± 0.01 | 0.26 ± 0.01 | 3.01 ± 0.26 | 0.15 ± 0.01 | 0.36 ± 0.01 | 0.36 ± 0.01 |
| Adjuvant | 0.47 ± 0.01 | 0.08 ±0.01 | 0.30 ± 0.01 | 0.14 ± 0.01 | 0.28 ± 0.02 | 2.91 ± 0.14 | 0.16 ± 0.01 | 0.35 ± 0.01 | 0.35 ± 0.01 |
| Vaccine | 0.48 ± 0.01 | 0.11 ±0.01 | 0.30 ± 0.01 | 0.12 ± 0.01 | 0.24 ± 0.01 | 2.79 ± 0.14 | 0.16 ± 0.01 | 0.35 ± 0.01 | 0.37 ± 0.02 |
| 7 Days After 4th Dose | |||||||||
| PBS | 0.47 ± 0.01 | 0.09 ± 0.01 | 0.35 ± 0.01 | 0.13 ± 0.01 | 0.24 ± 0.01 | 2.96 ± 0.09 | 0.16 ± 0.01 | 0.34 ± 0.01 | 0.34 ± 0.01 |
| Adjuvant | 0.49 ± 0.01 | 0.10 ± 0.01 | 0.31 ± 0.01 | 0.13 ± 0.01 | 0.24 ± 0.01 | 2.96 ± 0.20 | 0.17 ± 0.01 | 0.39 ± 0.02 | 0.37 ± 0.02 |
| Vaccine | 0.49 ± 0.02 | 0.12 ± 0.03 | 0.33 ± 0.03 | 0.14 ± 0.02 | 0.26 ± 0.03 | 2.79 ± 0.20 | 0.17 ± 0.01 | 0.34 ± 0.01 | 0.36 ± 0.02 |
| 14 Days After 4th Dose | |||||||||
| PBS | 0.49 ± 0.01 | 0.10 ± 0.01 | 0.31 ± 0.01 | 0.13 ± 0.01 | 0.24 ± 0.01 | 3.16 ± 0.11 | 0.17 ± 0.01 | 0.35 ± 0.01 | 0.36 ± 0.02 |
| Adjuvant | 0.47 ± 0.02 | 0.10 ± 0.01 | 0.28 ± 0.01 | 0.13 ± 0.01 | 0.24 ± 0.01 | 2.80 ± 0.14 | 0.16 ± 0.01 | 0.36 ± 0.01 | 0.35 ± 0.01 |
| Vaccine | 0.44 ± 0.01 | 0.09 ± 0.01 | 0.30 ± 0.01 | 0.12 ± 0.01 | 0.22 ± 0.01 | 3.04 ± 0.10 | 0.17 ± 0.01 | 0.35 ± 0.02 | 0.36 ± 0.02 |
| Group | HBG (g/L) | Hto (mL/100L) | LT (103 mm) | PMN (%) | Lf (%) | E (%) | M (%) | Platelets (× 103 µL) |
|---|---|---|---|---|---|---|---|---|
| 3 Days After 4th Dose | ||||||||
| PBS | 154.2 ± 4.5 | 51.0 ± 1.6 | 5.2 ± 0.2 | 31.3 ± 1.7 | 68.7 ± 1.7 | 0.0 ± 0.0 | 0.0 ± 0.0 | 746.4 ± 33.0 |
| Adjuvant | 154.1 ± 6.3 | 53.3 ± 2.2 | 5.1 ± 0.2 | 33.6 ± 3.8 | 66.1 ± 3.8 | 0.2 ± 0.3 | 0.0 ± 0.0 | 744.2 ± 56.4 |
| Vaccine | 157.1 ± 3.6 | 51.5 ± 1.3 | 5.0 ± 0.1 | 30.4 ± 2.3 | 68.4 ± 1.9 | 0.5 ± 0.6 | 0.0 ± 0.0 | 772.3 ± 51.6 |
| 7 Days After 4th Dose | ||||||||
| PBS | 155.0 ± 1.5 | 51.8 ± 0.5 | 5.2 ± 0.1 | 35.6 ± 3.2 | 63.4 ± 3.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 767.2 ± 43.9 |
| Adjuvant | 154.2 ± 2.9 | 51.3 ± 1.0 | 5.1 ± 0.2 | 35.4 ± 2.6 | 63.5 ± 3.3 | 0.1 ± 0.1 | 0.1 ± 0.1 | 797.0 ± 45.3 |
| Vaccine | 157.1 ± 3.1 | 53.1 ± 1.5 | 5.2 ± 0.2 | 38.8 ± 3.9 | 58.8 ± 3.4 | 0.1 ± 0.2 | 0.1 ± 0.2 | 790.4 ± 44.5 |
| 14 Days After 4th Dose | ||||||||
| PBS | 157.0 ± 4.1 | 52.4 ± 1.3 | 6.4 ± 0.5 | 34.5 ± 5.1 | 65.3 ± 5.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 853.1 ± 29.1 |
| Adjuvant | 158.1 ± 4.6 | 53.5 ± 2.3 | 5.5 ± 0.2 | 29.3 ± 4.1 | 70.0 ± 3.8 | 0.1 ± 0.4 | 0.0 ± 0.0 | 764.0 ± 23.8 |
| Vaccine | 156.0 ± 3.9 | 52.8 ± 1.4 | 6.3 ± 0.5 | 33.7 ± 3.4 | 66.3 ± 3.4 | 0.0 ± 0.0 | 0.0 ± 0.0 | 803.2 ± 30.1 |
| (a) | ||||||
| Group | ALT (UI) | AST (UI) | TP (g/dL) | Bilirubin (mg/dL) | Urates (µM) | Urea (mM) |
| 3 Days After 4th Dose | ||||||
| PBS | 53.67 ± 9.36 | 167.71 ± 15.38 | 5.11 ± 0.55 | 0.07 ± 0.04 | 64.29 ± 18.31 | 8.15 ± 0.61 |
| Adjuvant | 61.02 ± 12.22 | 153.65 ± 12.71 | 5.43 ± 0.42 | 0.16 ± 0.08 | 65.94 ± 17.38 | 8.47 ± 0.91 |
| Vaccine | 59.15 ± 10.34 | 159.43 ± 18.49 | 6.10 ± 0.85 | 0.03 ± 0.04 | 57.86 ± 13.56 | 8.27 ± 0.89 |
| 7 Days After 4th Dose | ||||||
| PBS | 74.38 ± 6.44 | 163.45 ± 16.50 | 5.34 ± 0.17 | 0.01 ± 0.04 | 69.90 ± 10.55 | 9.47 ± 1.19 |
| Adjuvant | 62.71 ± 9.53 | 154.88 ± 14.97 | 5.47 ± 0.40 | 0.06 ± 0.03 | 79.13 ± 18.29 | 8.82 ± 1.23 |
| Vaccine | 70.13 ± 11.19 | 161.26 ± 14.30 | 5.11 ± 0.38 | 0.08 ± 0.04 | 66.31 ± 7.73 | 8.64 ± 0.93 |
| 14 Days After 4th Dose | ||||||
| PBS | 56.45 ± 7.93 | 128.33 ± 11.69 | 5.92 ± 0.22 | 0.01 ± 0.01 | 87.92 ± 17.11 | 8.14 ± 1.50 |
| Adjuvant | 65.51 ± 13.70 | 133.53 ± 13.32 | 5.94 ± 0.41 | 0.10 ± 0.04 | 66.27 ± 11.23 | 8.95 ± 1.25 |
| Vaccine | 42.32 ± 6.37 | 152.44 ± 18.57 | 6.82 ± 0.77 | 0.09 ± 0.04 | 68.32 ± 11.35 | 9.70 ± 1.03 |
| (b) | ||||||
| Group | CPK (UI) | Creatinine (µM) | Glucose (mM) | ALP (UI) | Triglycerides (mM) | Cholesterol (mM) |
| 3 Days After 4th Dose | ||||||
| PBS | 1082.93 ± 201.29 | 65.12 ± 2.87 | 7.45 ± 0.52 | 282.82 ± 17.32 | 0.39 ± 0.12 | 1.25 ± 0.18 |
| Adjuvant | 1229.24 ± 153.11 | 68.69 ± 6.18 | 8.39 ± 0.67 | 288.32 ± 30.09 | 0.76 ± 0.24 | 1.72 ± 0.26 |
| Vaccine | 1189.94 ± 80.87 | 63.16 ± 8.77 | 9.21 ± 0.80 | 287.24 ± 16.14 | 0.94 ± 0.20 | 1.29 ± 0.10 |
| 7 Days After 4th Dose | ||||||
| PBS | 1488.94 ± 173.82 | 60.83 ± 4.61 | 9.70 ± 1.70 | 410.41 ± 38.78 | 0.85 ± 0.19 | 1.18 ± 0.19 |
| Adjuvant | 1362.28 ± 91.59 | 61.32 ± 4.97 | 8.85 ± 1.28 | 452.10 ± 57.35 | 0.59 ± 0.14 | 1.25 ± 0.16 |
| Vaccine | 1228.22 ± 49.77 | 56.60 ± 3.85 | 10.18 ± 1.22 | 434.26 ± 33.24 | 0.63 ± 0.17 | 0.99 ± 0.13 |
| 14 Days After 4th Dose | ||||||
| PBS | 1334.75 ± 150.25 | 75.83 ± 8.27 | 10.47 ± 0.89 | 289.93 ± 20.36 | 1.17 ± 0.22 | 1.27 ± 0.11 |
| Adjuvant | 1302.22 ± 120.83 | 78.14 ± 11.44 | 9.46 ± 1.14 | 264.22 ± 43.21 | 1.07 ± 0.30 | 1.30 ± 0.18 |
| Vaccine | 1422.27 ± 134.45 | 66.49 ± 7.49 | 10.42 ± 1.12 | 213.89 ± 22.83 | 1.51 ± 0.32 | 1.14 ± 0.09 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliva, R.; Fraleigh, N.L.; Lewicky, J.D.; Fariñas, M.; Hernández, T.; Martel, A.L.; Navarro, I.; Dagmar, G.-R.; Acevedo, R.; Le, H.-T. Repeat-Dose Toxicity Study Using the AFPL1-Conjugate Nicotine Vaccine in Male Sprague Dawley Rats. Pharmaceutics 2019, 11, 626. https://doi.org/10.3390/pharmaceutics11120626
Oliva R, Fraleigh NL, Lewicky JD, Fariñas M, Hernández T, Martel AL, Navarro I, Dagmar G-R, Acevedo R, Le H-T. Repeat-Dose Toxicity Study Using the AFPL1-Conjugate Nicotine Vaccine in Male Sprague Dawley Rats. Pharmaceutics. 2019; 11(12):626. https://doi.org/10.3390/pharmaceutics11120626
Chicago/Turabian StyleOliva, Reynaldo, Nya L. Fraleigh, Jordan D. Lewicky, Mildrey Fariñas, Tamara Hernández, Alexandrine L. Martel, Ingrid Navarro, García-Rivera Dagmar, Reinaldo Acevedo, and Hoang-Thanh Le. 2019. "Repeat-Dose Toxicity Study Using the AFPL1-Conjugate Nicotine Vaccine in Male Sprague Dawley Rats" Pharmaceutics 11, no. 12: 626. https://doi.org/10.3390/pharmaceutics11120626
APA StyleOliva, R., Fraleigh, N. L., Lewicky, J. D., Fariñas, M., Hernández, T., Martel, A. L., Navarro, I., Dagmar, G.-R., Acevedo, R., & Le, H.-T. (2019). Repeat-Dose Toxicity Study Using the AFPL1-Conjugate Nicotine Vaccine in Male Sprague Dawley Rats. Pharmaceutics, 11(12), 626. https://doi.org/10.3390/pharmaceutics11120626