Preclinical Evaluation of UDCA-Containing Oral Formulation in Mice for the Treatment of Wet Age-Related Macular Degeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animal Care
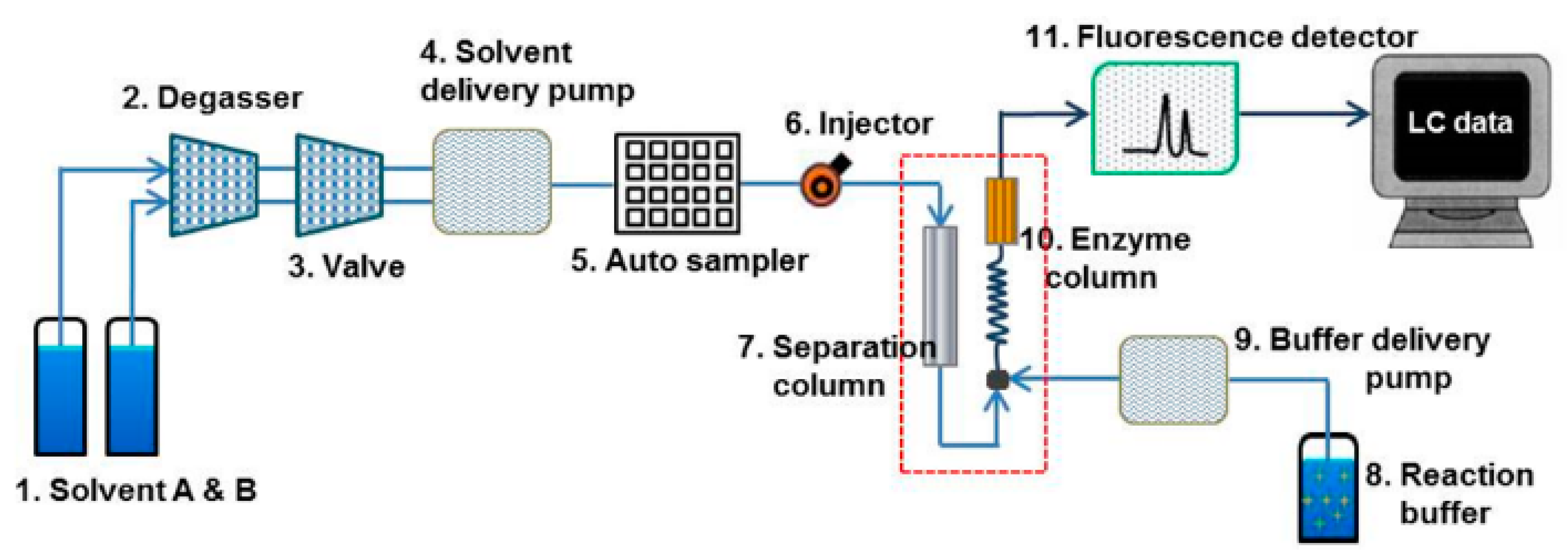
2.3. Establishment of High-Performance Liquid Chromatography (HPLC) Setup
2.4. Preparation of Bile Standard Solutions for Calibration Curves
2.5. Biomatrix Sample Extraction
2.6. Fluorescent HPLC Method Validation
2.7. In Vivo Pharmacokinetic Studies
2.7.1. Drug Administration and Sample Collection
2.7.2. Pharmacokinetic Analysis
2.8. In Vivo Ocular Efficacy Study in Mice
2.8.1. Drug Administration and Sample Collection
2.8.2. Laser Induced Choroidal Neovascularization
2.8.3. Western Blot Analysis
2.8.4. Electroretinography Measurement
2.9. Data Analysis
3. Results
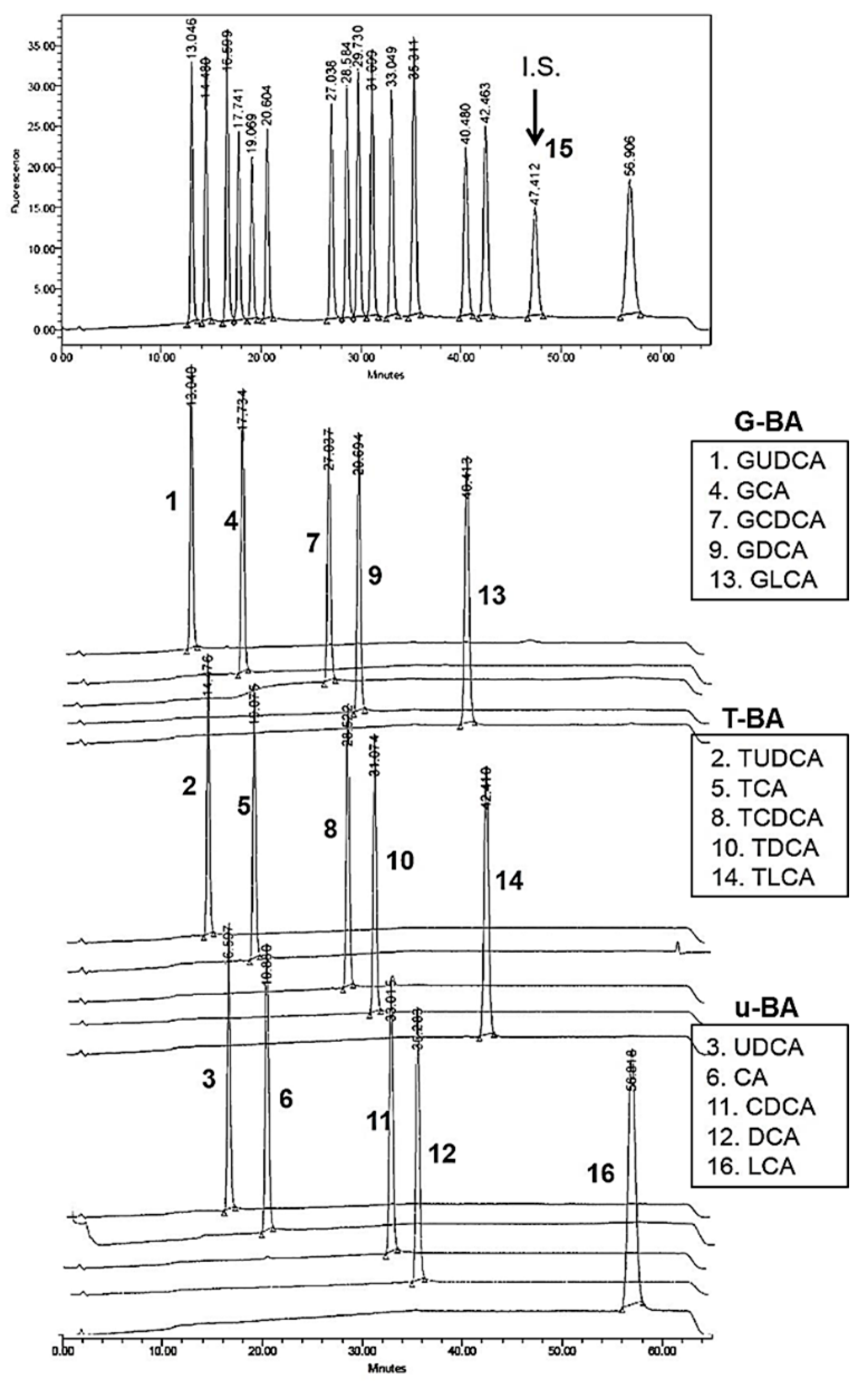
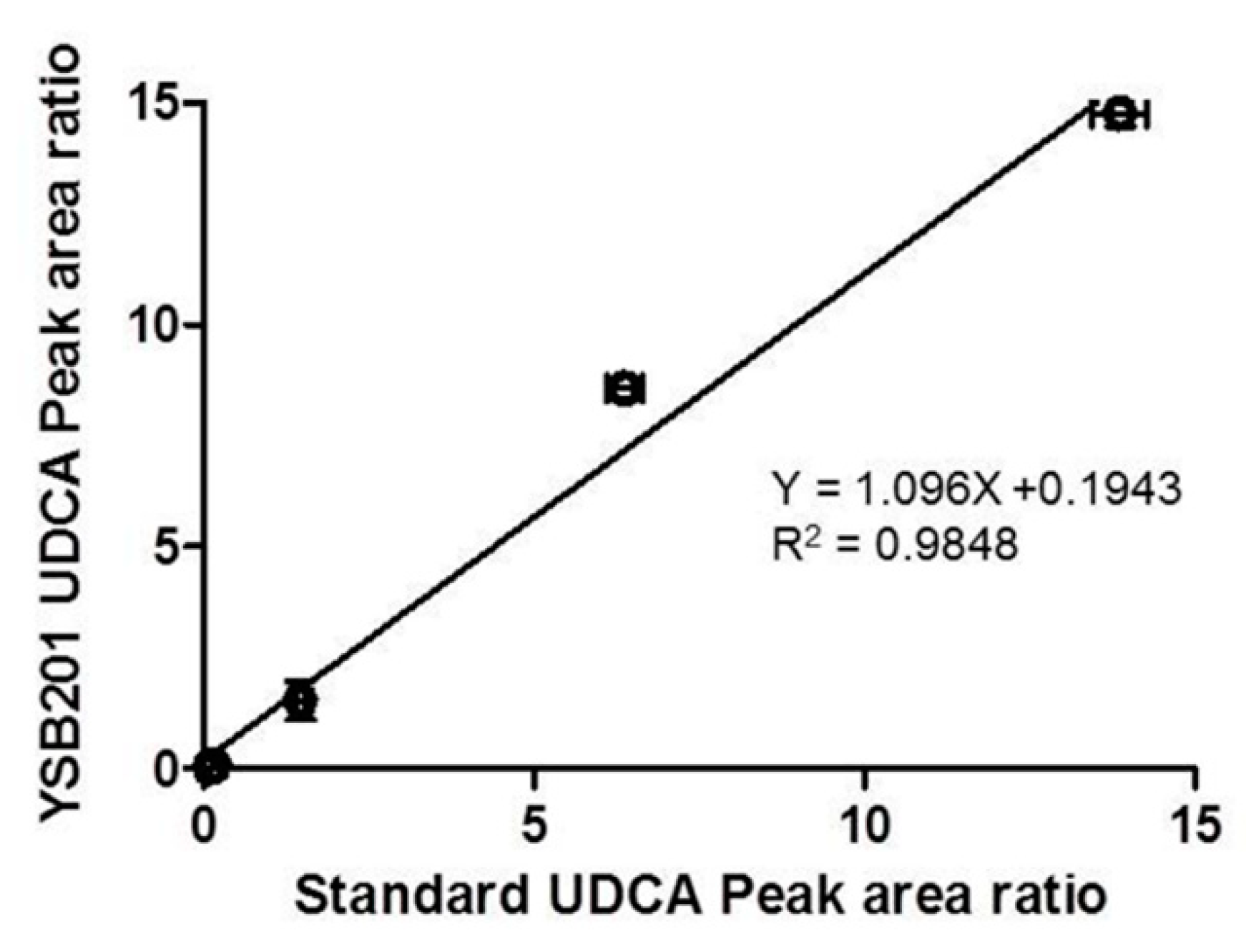
3.1. Assessment of Fluorescent HPLC Method Validation
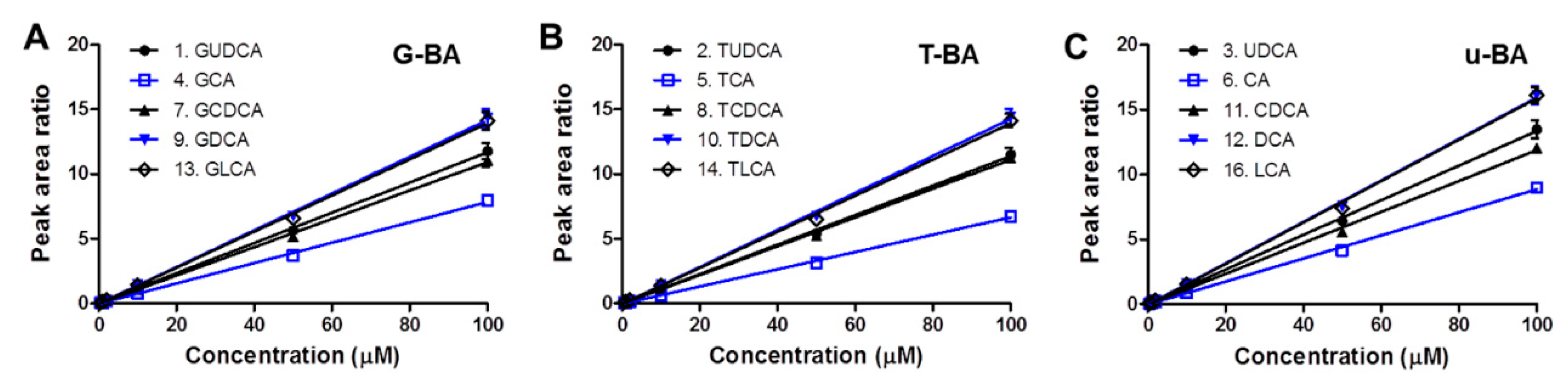
3.1.1. Specificity and Calibration Linearity
3.1.2. Validation Results with Quality Control Analysis in Biomatrices
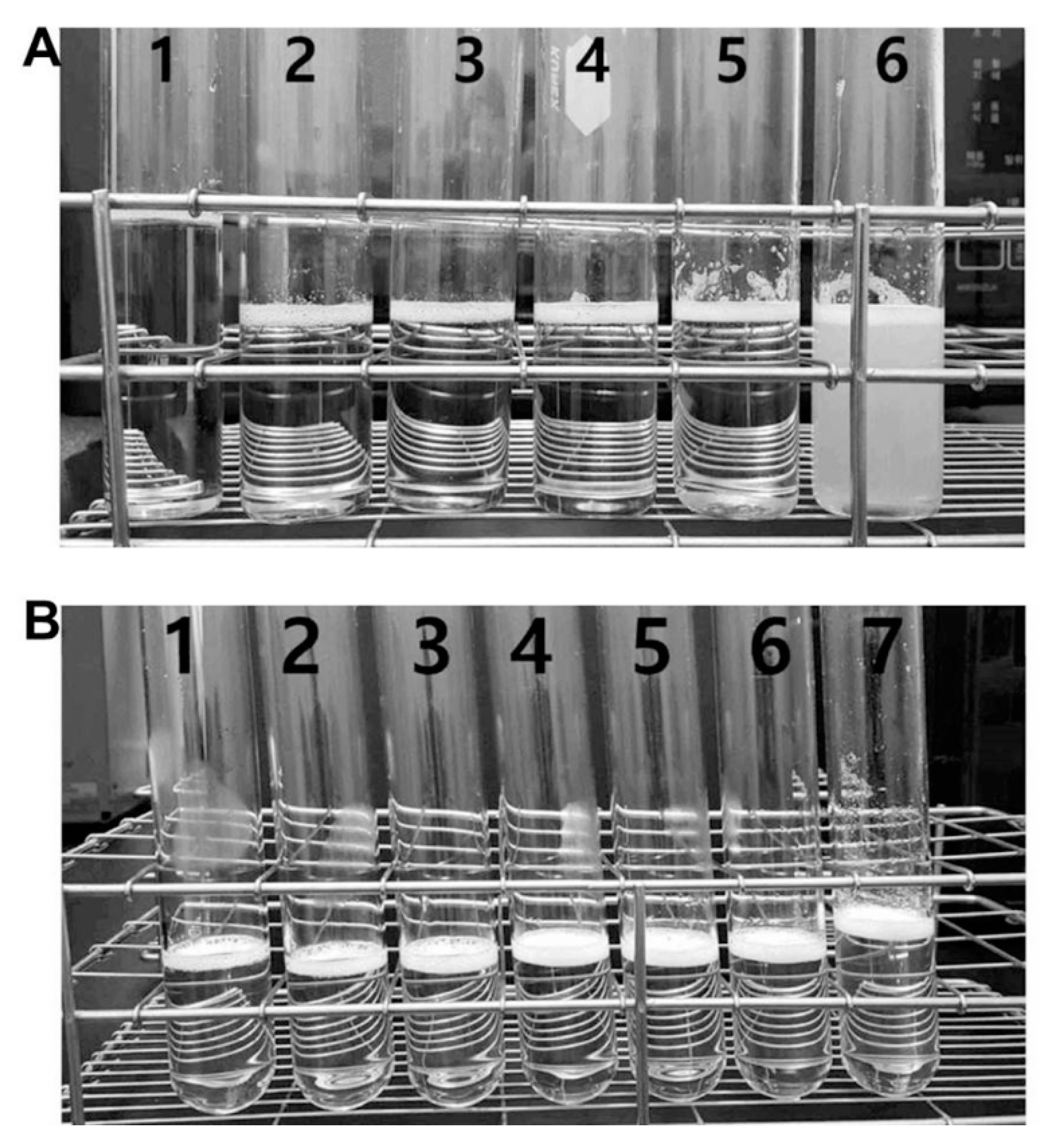
3.2. Aqueous Solubility and Drug Content of Oral UDCA Formulation
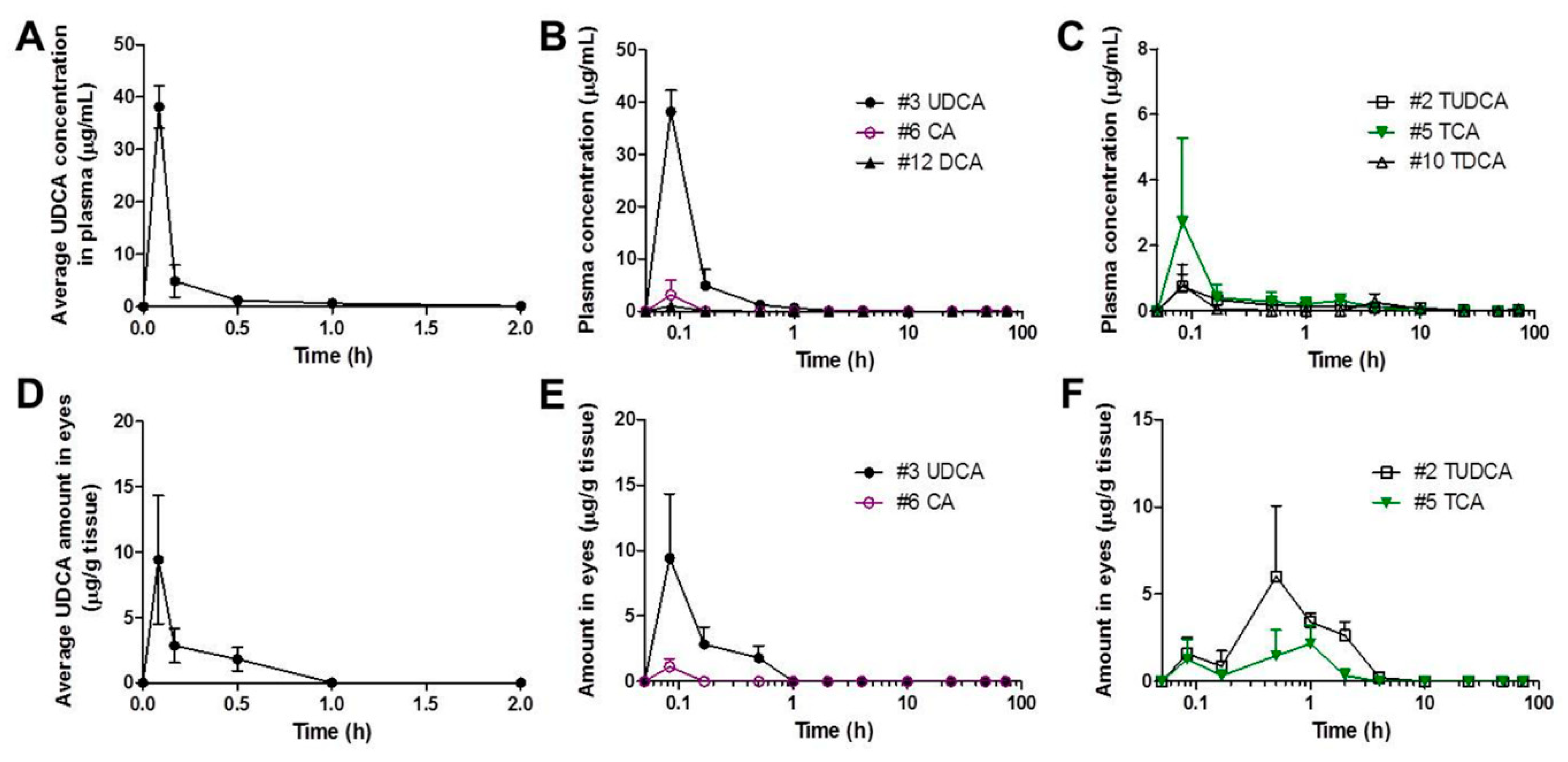
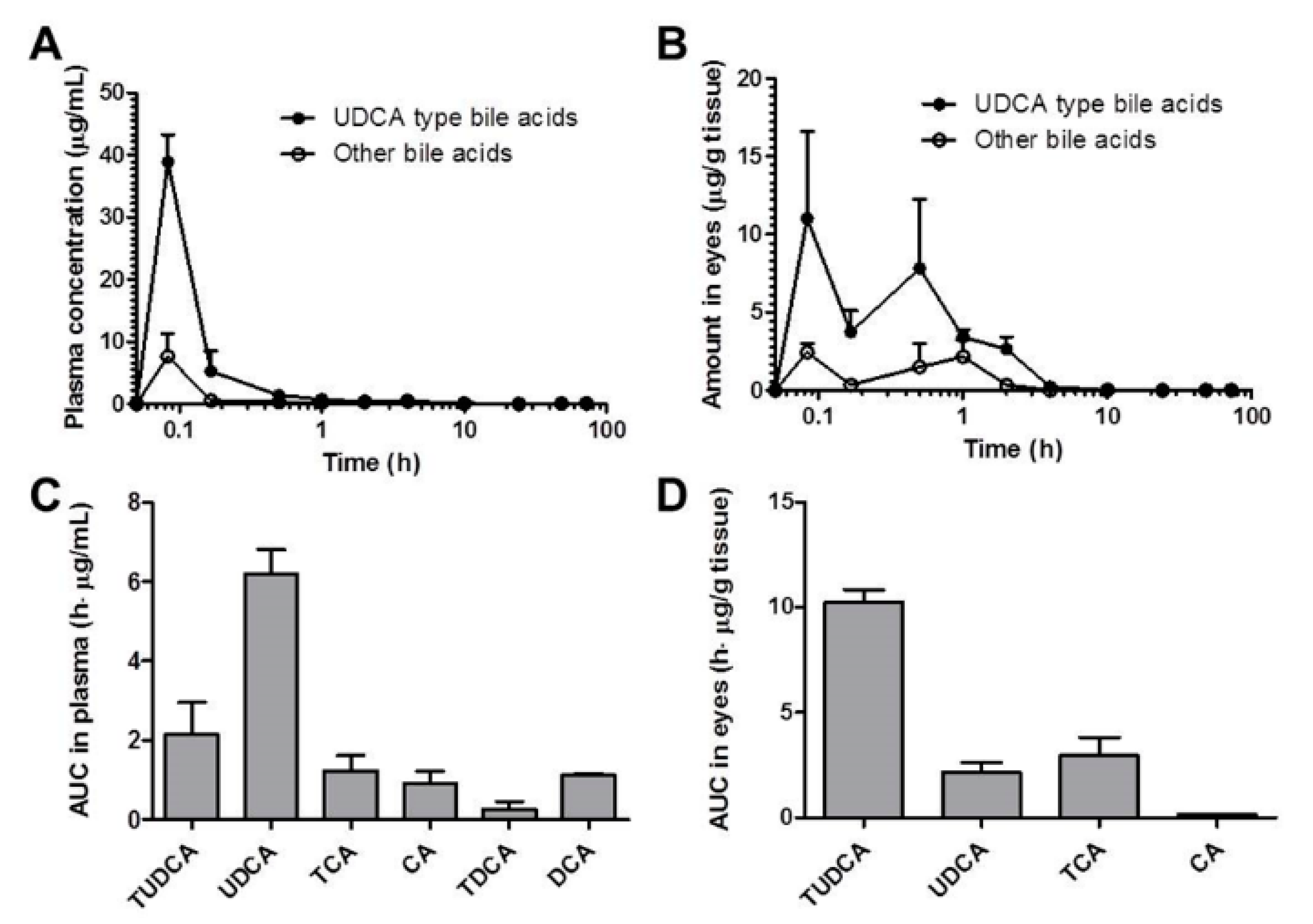
3.3. Pharmacokinetics of UDCA and Metabolites in Plasma and Eyes
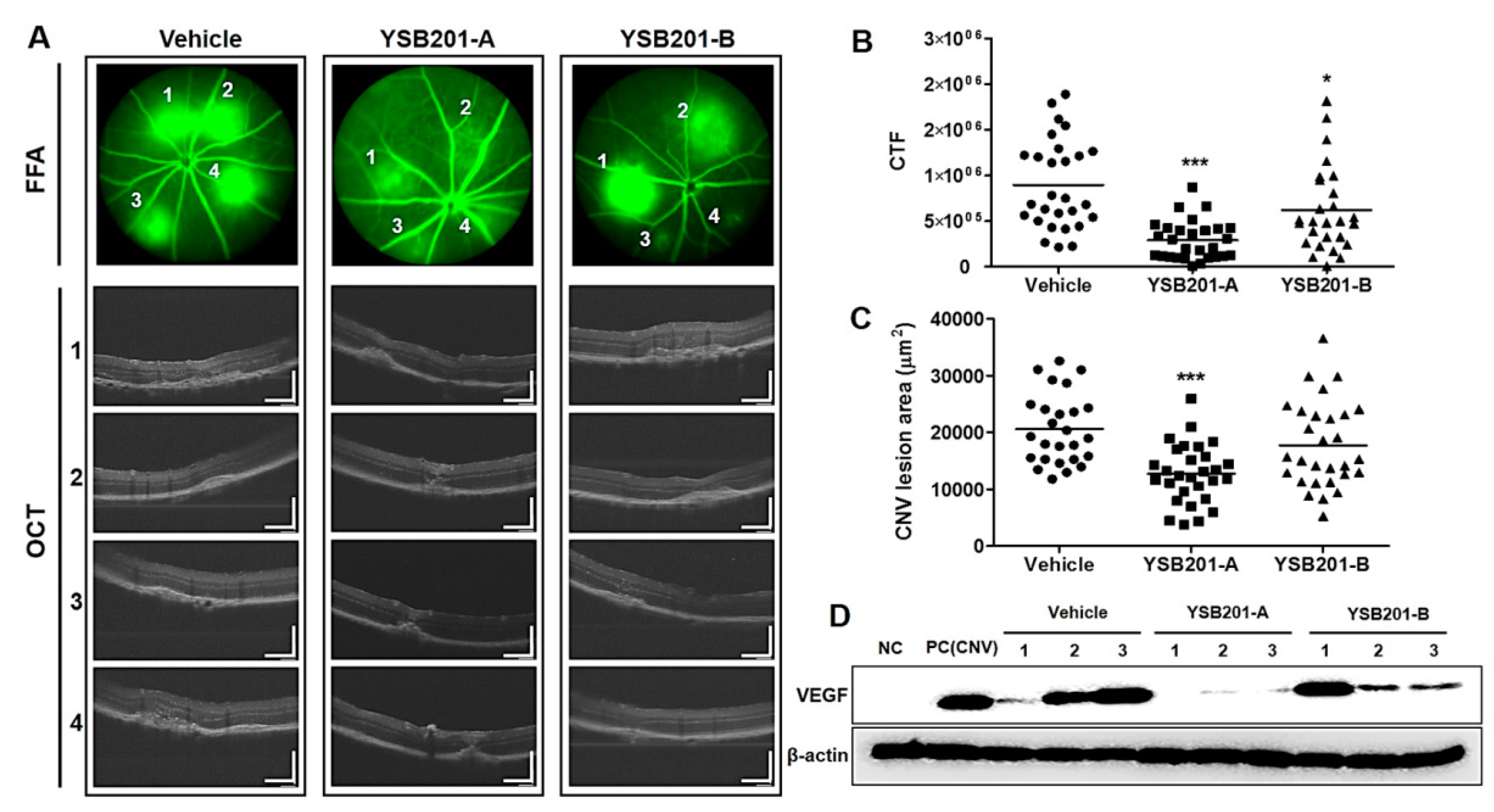
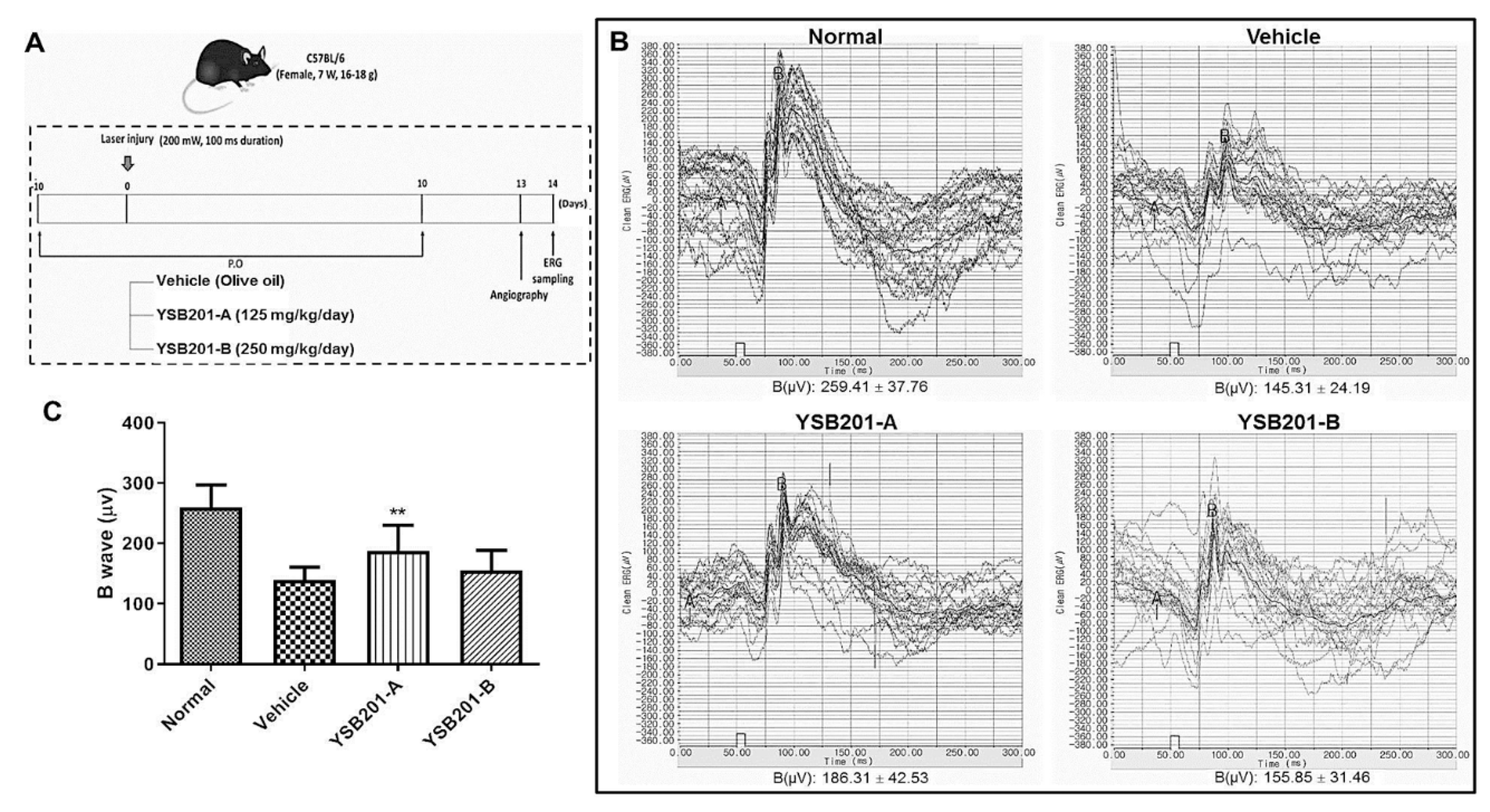
3.4. Inhibitory Effects of Choroidal Neovascularization by Oral UDCA Formulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kulkarni, A.D.; Kuppermann, B.D. Wet age-related macular degeneration. Adv. Drug Deliv. Rev. 2005, 57, 1994–2009. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Hui, P. Emerging pharmacologic therapies for wet age-related macular degeneration. Ophthalmologica 2009, 223, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef]
- Woo, S.J.; Kim, J.H.; Yu, H.G. Ursodeoxycholic acid and tauroursodeoxycholic acid suppress choroidal neovascularization in a laser-treated rat model. J. Ocul Pharmacol. Ther. 2010, 26, 223–229. [Google Scholar] [CrossRef]
- Bhutto, I.; Lutty, G. Understanding age-related macular degeneration (AMD): Relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol. Asp. Med. 2012, 33, 295–317. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, P.K. Emerging therapies for neovascular age-related macular degeneration: Drugs in the pipeline. Ophthalmology 2013, 120, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Laud, K.; Fine, H.F.; Klancnik, J.M.; Meyerle, C.B.; Yannuzzi, L.A.; Sorenson, J.; Slakter, J.; Fisher, Y.L.; Cooney, M.J. Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age-related macular degeneration. Retina 2006, 26, 383–390. [Google Scholar]
- Avery, R.L.; Pieramici, D.J.; Rabena, M.D.; Castellarin, A.A.; Ma’an, A.N.; Giust, M.J. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology 2006, 113, 363–372. [Google Scholar] [CrossRef]
- Hofmann, A.F.; Hagey, L. Bile acids: Chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell. Mol. Life Sci. 2008, 65, 2461–2483. [Google Scholar] [CrossRef]
- Beuers, U. Drug insight: Mechanisms and sites of action of ursodeoxycholic acid in cholestasis. Nat. Rev. Gastroenterol. Hepatol. 2006, 3, 318. [Google Scholar] [CrossRef]
- Ackerman, H.D.; Gerhard, G.S. Bile Acids in Neurodegenerative Disorders. Front. Aging Neurosci. 2016, 8, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boatright, J.H.; Nickerson, J.M.; Moring, A.G.; Pardue, M.T. Bile acids in treatment of ocular disease. J. Ocul Biol. Dis. Inform. 2009, 2, 149–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Moya, P.; Romero-Calvo, I.; Requena, P.; Hernández-Chirlaque, C.; Aranda, C.J.; González, R.; Zarzuelo, A.; Suárez, M.D.; Olga, M.A.; José, J.G.; et al. Dose-dependent antiinflammatory effect of ursodeoxycholic acid in experimental colitis. Int. Immunopharmacol. 2013, 15, 372–380. [Google Scholar] [CrossRef]
- Rodrigues, C.M.; Steer, C.J. The therapeutic effects of ursodeoxycholic acid as an anti-apoptotic agent. Expert Opin. Investig. Drugs 2001, 10, 1243–1253. [Google Scholar] [CrossRef]
- Ramalho, R.M.; Ribeiro, P.S.; Sola, S.; Castro, R.E.; Steer, C.J.; Rodrigues, C.M. Inhibition of the E2F-1/p53/Bax pathway by tauroursodeoxycholic acid in amyloid beta-peptide-induced apoptosis of PC12 cells. J. Neurochem. 2004, 90, 567–575. [Google Scholar] [CrossRef]
- Maharjan, P.; Cho, K.H.; Maharjan, A.; Shin, M.C.; Moon, C.; Min, K.A. Pharmaceutical challenges and perspectives in developing ophthalmic drug formulations. J. Pharm. Investig. 2019, 49, 215–228. [Google Scholar] [CrossRef]
- Carey, M.C. Bile acids and bile salts: Ionization and solubility properties. Hepatology 1984, 4, 66–71. [Google Scholar] [CrossRef]
- Šarenac, T.M.; Mikov, M. Bile Acid Synthesis: From Nature to the Chemical Modification and Synthesis and Their Applications as Drugs and Nutrients. Front. Pharmacol. 2018, 9, 939. [Google Scholar] [CrossRef]
- Van de Meeberg, P.; Houwen, R.; Sinaasappel, M.; Heijerman, H.; Bijleveld, C.M.; Vanberge-Henegouwen, G. Low-Dose versus High-Dose Ursodeoxycholic Acid in Cystic Fibrosis-Related Cholestatic Liver Disease Results of a Randomized Study with 1-Year Follow-up. Scand. J. Gastroenterol. 1997, 32, 369–373. [Google Scholar] [CrossRef]
- Verma, A.; Jazrawi, R.P.; Ahmed, H.A.; Davis, T.; Bland, J.M.; Benson, M.; Orchard, R.T.; Theodossi, A.; Maxwell, J.D.; Northfield, T.C. Optimum dose of ursodeoxycholic acid in primary biliary cirrhosis. Eur. J. Gastroenterol. Hepatol. 1999, 11, 1069–1076. [Google Scholar] [CrossRef]
- Toda, T.; Ohi, K.; Kudo, T.; Yoshida, T.; Ikarashi, N.; Ito, K.; Sugiyama, K. Ciprofloxacin suppresses Cyp3a in mouse liver by reducing lithocholic acid-producing intestinal flora. Drug Metab. Pharmacokinet. 2009, 24, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Alnouti, Y.; Csanaky, I.L.; Klaassen, C.D. Quantitative-profiling of bile acids and their conjugates in mouse liver, bile, plasma, and urine using LC–MS/MS. J. Chromatogr. B 2008, 873, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-B.; Kim, K.-S.; Ryu, H.-M.; Yoon, I.-S.; Cho, H.-J.; Chung, S.-J.; Chong, S.; Kim, D.-D. Comparison of saline vs. blood replenishment after blood sampling in a rat pharmacokinetic study. J. Pharm. Investig. 2019, 49, 543–551. [Google Scholar] [CrossRef]
- Sauer, P.; Benz, C.; Rudolph, G.; Kloters-Plachky, P.; Stremmel, W.; Stiehl, A. Influence of cholestasis on absorption of ursodeoxycholic acid. Dig. Dis. Sci. 1999, 44, 817–822. [Google Scholar] [CrossRef]
- Poupon, R. Ursodeoxycholic acid and bile-acid mimetics as therapeutic agents for cholestatic liver diseases: An overview of their mechanisms of action. Clin. Res. Hepatol. Gastroenterol. 2012, 36, 3–12. [Google Scholar] [CrossRef]
- Park, I.H.; Kim, M.K.; Kim, S.U. Ursodeoxycholic acid prevents apoptosis of mouse sensory neurons induced by cisplatin by reducing P53 accumulation. Biochem. Biophys. Res. Commun. 2008, 377, 1025–1030. [Google Scholar] [CrossRef]
- Chun, H.S.; Low, W.C. Ursodeoxycholic acid suppresses mitochondria-dependent programmed cell death induced by sodium nitroprusside in SH-SY5Y cells. Toxicology 2012, 292, 105–112. [Google Scholar] [CrossRef]
- Thao, T.D.H.; Ryu, H.-C.; Yoo, S.-H.; Rhee, D.-K. Antibacterial and anti-atrophic effects of a highly soluble, acid stable UDCA formula in Helicobacter pylori-induced gastritis. Biochem. Pharmacol. 2008, 75, 2135–2146. [Google Scholar] [CrossRef]
- Singh, D.; Tiwary, A.K.; Bedi, N. Canagliflozin loaded SMEDDS: Formulation optimization for improved solubility, permeability and pharmacokinetic performance. J. Pharm. Investig. 2019, 49, 67–85. [Google Scholar] [CrossRef]
- Chamulitrat, W.; Gan-Schreier, H.; Wang, J.; Xu, W.; Katava, N.; Tuma, S.; Pathil-Warth, A.; Stremmel, W. Evaluation for oral use of Ursodeoxycholyl Lysophosphatidylethanolamide (UDCA-LPE): Effective resorption of sodium salt UDCA-LPE. Z. Gastroenterol. 2013, 51, P_2_05. [Google Scholar] [CrossRef]
- Pei, Y.; Chen, J.; Li, W.-L. Progress in research and application of silymarin. Med. Aromat. Plant Sci. Biotechnol. 2009, 3, 1–8. [Google Scholar]
- Yu, K.S.; Oh, J.Y.; Kim, M.C.; Kang, S.H.; Lee, N.S.; Han, S.-Y.; Ryu, K.H.; Jeong, Y.G.; Kim, D.K. Controlled Release of Ursodeoxycholic Acid from Pullulan Acetate Nanoparticles to Modulate Glutamate-Induced Excitotoxicity in PC-12 Cells. J. Nanomater. 2018, 2018, 7130450. [Google Scholar] [CrossRef]
- Kendall, C.; Premji, S.; Stewart, R.; Stewart, R.; Boatright, J. Ocular delivery of TUDCA provides neuroprotection in a mouse model of retinal degeneration. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4932. [Google Scholar]
- Kim, D.; Maharjan, P.; Jin, M.; Park, T.; Maharjan, A.; Amatya, R.; Yang, J.; Min, K.A.; Shin, M.C. Potential Albumin-Based Antioxidant Nanoformulations for Ocular Protection against Oxidative Stress. Pharmaceutics 2019, 11, 297. [Google Scholar] [CrossRef] [PubMed]
| Bile Acid (Peak No.) | Plasma | Eyes | ||||
|---|---|---|---|---|---|---|
| Conc. (μM) | Precision (%) | Accuracy (%) | Conc. (μM) | Precision (%) | Accuracy (%) | |
| G-BA | 0.2 | 10.07 | 98 (9.32) | 0.2 | 7.08 | 113 (3.60) |
| 0.5 | 4.18 | 101 (5.34) | 0.5 | 9.43 | 100 (5.96) | |
| GUDCA (1) | 1 | 9.07 | 95 (8.32) | 1 | 1.07 | 100 (1.30) |
| 10 | 4.66 | 107 (3.37) | 10 | 6.28 | 113 (3.04) | |
| 100 | 5.25 | 101 (1.33) | 100 | 3.47 | 99 (0.97) | |
| GCA (4) | 0.2 | 9.54 | 107 (10.56) | 0.2 | 7.15 | 109 (2.30) |
| 0.5 | 4.62 | 98 (5.57) | 0.5 | 13.02 | 99 (9.73) | |
| 1 | 11.36 | 97 (8.70) | 1 | 2.53 | 100 (2.19) | |
| 10 | 5.50 | 104 (2.60) | 10 | 7.21 | 110 (3.62) | |
| 100 | 3.85 | 101 (1.34) | 100 | 2.90 | 99 (0.77) | |
| GCDCA (7) | 0.2 | 12.09 | 100 (11.12) | 0.2 | 26.02 | 117 (4.47) |
| 0.5 | 4.34 | 98 (3.85) | 0.5 | 19.75 | 101 (6.13) | |
| 1 | 9.51 | 95 (9.50) | 1 | 8.70 | 99 (1.59) | |
| 10 | 4.72 | 104 (3.46) | 10 | 10.66 | 108 (6.35) | |
| 100 | 4.24 | 101 (1.34) | 100 | 5.65 | 100 (3.34) | |
| GDCA (9) | 0.2 | 11.48 | 103 (9.64) | 0.2 | 28.89 | 122 (12.38) |
| 0.5 | 5.76 | 99 (7.20) | 0.5 | 19.63 | 102 (7.14) | |
| 1 | 8.91 | 97 (8.60) | 1 | 7.14 | 99 (1.78) | |
| 10 | 4.48 | 104 (3.26) | 10 | 11.01 | 108 (7.30) | |
| 100 | 4.98 | 101 (1.41) | 100 | 5.70 | 99 (1.45) | |
| GLCA (13) | 0.2 | 13.18 | 107 (5.31) | 0.1 | 27.41 | 103 (16.17) |
| 0.5 | 7.00 | 99 (5.70) | 0.2 | 9.12 | 101 (7.66) | |
| 1 | 8.03 | 94 (8.29) | 1 | 9.36 | 101 (1.04) | |
| 10 | 4.79 | 104 (3.03) | 10 | 9.12 | 109 (5.75) | |
| 100 | 5.01 | 101 (1.36) | 100 | 2.82 | 100 (0.24) | |
| Bile Acid (Peak No.) | Plasma | Eyes | ||||
|---|---|---|---|---|---|---|
| Conc. (μM) | Precision (%) | Accuracy (%) | Conc. (μM) | Precision (%) | Accuracy (%) | |
| T-BA | 0.2 | 6.48 | 99 (10.06) | 0.2 | 13.64 | 110 (1.91) |
| 0.5 | 3.41 | 100 (4.58) | 0.5 | 5.05 | 100 (1.39) | |
| TUDCA (2) | 1 | 8.58 | 96 (7.57) | 1 | 0.91 | 100 (0.42) |
| 10 | 3.76 | 104 (2.47) | 10 | 6.31 | 113 (3.08) | |
| 100 | 4.61 | 101 (1.15) | 100 | 2.89 | 99 (0.90) | |
| TCA (5) | 0.2 | 12.47 | 103 (9.08) | 0.2 | 3.77 | 110 (7.98) |
| 0.5 | 3.50 | 99 (6.50) | 0.5 | 5.70 | 101 (6.39) | |
| 1 | 10.72 | 97 (8.16) | 1 | 3.56 | 99 (1.50) | |
| 10 | 5.28 | 101 (2.96) | 10 | 7.33 | 102 (14.04) | |
| 100 | 2.47 | 101 (1.07) | 100 | 2.81 | 99 (0.54) | |
| TCDCA (8) | 0.2 | 9.40 | 104 (10.79) | 0.05 | 25.51 | 79 (52.07) |
| 0.5 | 4.06 | 99 (6.19) | 0.1 | 16.11 | 89 (31.35) | |
| 1 | 10.64 | 97 (8.42) | 1 | 7.06 | 100 (1.76) | |
| 10 | 5.08 | 105 (4.04) | 10 | 10.91 | 109 (6.73) | |
| 100 | 3.12 | 103 (5.52) | 100 | 6.09 | 99 (1.50) | |
| TDCA (10) | 0.2 | 8.95 | 105 (10.59) | 0.1 | 46.04 | 93 (8.18) |
| 0.5 | 3.34 | 102 (3.96) | 0.5 | 17.85 | 101 (9.19) | |
| 1 | 8.10 | 97 (7.04) | 1 | 7.42 | 99 (2.41) | |
| 10 | 4.44 | 103 (3.44) | 10 | 11.23 | 109 (7.43) | |
| 100 | 4.22 | 101 (1.32) | 100 | 5.73 | 99 (1.48) | |
| TLCA (14) | 0.2 | 15.54 | 98 (10.56) | 0.2 | 14.01 | 101 (9.39) |
| 0.5 | 7.80 | 99 (2.88) | - | - | - | |
| 1 | 13.40 | 100 (7.57) | 1 | 3.52 | 100 (0.46) | |
| 10 | 4.38 | 103 (3.28) | 10 | 7.07 | 107 (3.29) | |
| 100 | 3.64 | 102 (1.14) | 100 | 2.04 | 100 (0.15) | |
| Bile Acid (Peak No.) | Plasma | Eyes | ||||
|---|---|---|---|---|---|---|
| Conc. (μM) | Precision (%) | Accuracy (%) | Conc. (μM) | Precision (%) | Accuracy (%) | |
| u-BA | 0.2 | 9.01 | 102 (10.82) | 0.2 | 36.77 | 104 (20.36) |
| 0.5 | 4.55 | 99 (4.57) | 0.5 | 7.24 | 99 (4.74) | |
| UDCA (3) | 1 | 8.58 | 96 (7.31) | 2 | 14.56 | 108 (19.22) |
| 10 | 4.26 | 106 (2.80) | 10 | 6.67 | 114 (2.77) | |
| 100 | 5.08 | 101 (1.37) | 100 | 2.70 | 99 (0.91) | |
| CA (6) | 0.2 | 7.19 | 96 (13.40) | 0.1 | 22.42 | 89 (13.90) |
| 0.5 | 4.89 | 102 (3.64) | 0.5 | 15.18 | 104 (12.17) | |
| 1 | 8.67 | 98 (7.43) | 1 | 4.62 | 99 (2.99) | |
| 10 | 5.32 | 103 (2.90) | 10 | 7.73 | 108 (4.22) | |
| 100 | 2.27 | 101 (1.20) | 100 | 3.14 | 99 (0.79) | |
| CDCA (11) | 0.2 | 23.81 | 101 (10.02) | 0.1 | 31.96 | 90 (6.72) |
| 0.5 | 5.66 | 101 (7.62) | 0.5 | 16.60 | 96 (7.28) | |
| 1 | 9.77 | 95 (7.66) | 1 | 7.46 | 101 (1.25) | |
| 10 | 5.39 | 103 (2.86) | 10 | 12.03 | 109 (8.10) | |
| 100 | 3.05 | 101 (1.25) | 100 | 4.93 | 99 (1.27) | |
| DCA (12) | 0.2 | 5.73 | 104 (10.53) | 0.1 | 27.87 | 102 (13.76) |
| 0.5 | 4.25 | 100 (5.18) | 0.5 | 19.08 | 96 (5.35) | |
| 1 | 8.22 | 96 (8.06) | 1 | 5.91 | 101 (1.07) | |
| 10 | 4.74 | 103 (3.07) | 10 | 11.26 | 111 (7.34) | |
| 100 | 4.28 | 101 (1.23) | 100 | 4.51 | 99 (1.01) | |
| LCA (16) | 0.2 | 17.75 | 99 (9.72) | 0.2 | 21.92 | 86 (8.31) |
| 0.5 | 12.08 | 102 (11.57) | 0.5 | 22.05 | 101 (12.31) | |
| 1 | 12.07 | 98 (12.86) | 1 | 3.35 | 100 (2.75) | |
| 10 | 4.66 | 102 (2.89) | 10 | 9.60 | 105 (6.72) | |
| 100 | 4.02 | 101 (1.17) | 100 | 4.81 | 100 (1.51) | |
| PK Parameter (units) | Plasma | Eyes |
|---|---|---|
| Cmax (μg/mL or μg/g of tissues) | 38.18 ± 7.05 | 10.15 ± 7.34 |
| Tmax (h) | 0.08 ± 0.0 | 0.11 ± 0.05 |
| AUC(0–48h) (h·μg/mL or h·μg/g of tissues) | 6.19 ± 1.08 | 2.14 ± 0.82 |
| k (h−1) | 0.63 ± 0.09 | 18.14 ± 0.54 |
| t1/2 (h) | 1.12 ± 0.15 | 0.04 ± 0.001 |
| MRT (h) | 1.29 ± 0.18 | 0.27 ± 0.12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maharjan, P.; Kim, D.; Jin, M.; Ko, H.J.; Song, Y.H.; Lee, Y.; Ahn, B.-N.; Kim, S.-K.; Lee, Y.; Shin, M.C.; et al. Preclinical Evaluation of UDCA-Containing Oral Formulation in Mice for the Treatment of Wet Age-Related Macular Degeneration. Pharmaceutics 2019, 11, 561. https://doi.org/10.3390/pharmaceutics11110561
Maharjan P, Kim D, Jin M, Ko HJ, Song YH, Lee Y, Ahn B-N, Kim S-K, Lee Y, Shin MC, et al. Preclinical Evaluation of UDCA-Containing Oral Formulation in Mice for the Treatment of Wet Age-Related Macular Degeneration. Pharmaceutics. 2019; 11(11):561. https://doi.org/10.3390/pharmaceutics11110561
Chicago/Turabian StyleMaharjan, Pooja, Daseul Kim, Minki Jin, Hwi Jin Ko, Yeong Ho Song, Yoonjin Lee, Byul-Nim Ahn, Si-Kyung Kim, Yujin Lee, Meong Cheol Shin, and et al. 2019. "Preclinical Evaluation of UDCA-Containing Oral Formulation in Mice for the Treatment of Wet Age-Related Macular Degeneration" Pharmaceutics 11, no. 11: 561. https://doi.org/10.3390/pharmaceutics11110561
APA StyleMaharjan, P., Kim, D., Jin, M., Ko, H. J., Song, Y. H., Lee, Y., Ahn, B.-N., Kim, S.-K., Lee, Y., Shin, M. C., Min, K. A., & Yang, J. (2019). Preclinical Evaluation of UDCA-Containing Oral Formulation in Mice for the Treatment of Wet Age-Related Macular Degeneration. Pharmaceutics, 11(11), 561. https://doi.org/10.3390/pharmaceutics11110561






