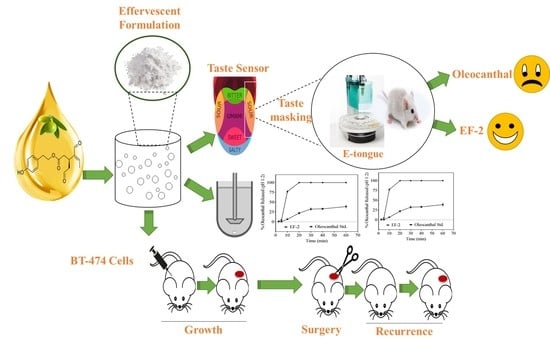
Optimization of Taste-Masked (–)-Oleocanthal Effervescent Formulation with Potent Breast Cancer Progression and Recurrence Suppressive Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Antibodies
2.2. (–)-Oleocanthal Isolation from Extra-Virgin Olive Oil Samples
2.3. Determination of Effervescent Components
2.4. Preparation of Effervescent Powder
2.5. Determination of Solution pH
2.6. Determination of Effervescence Time
2.7. Determination of CO2 Content
2.8. Flow Properties of Powder
2.8.1. Angle of Repose (θ)
2.8.2. Compressibility Index
2.8.3. Hausner’s Ratio
2.9. Fourier Transform Infrared Spectroscopy
2.10. Thermal Analysis of the Effervescent Powder by Differential Scanning Calorimetry (DSC)
2.11. Scanning Electron Microscopy (SEM) and Confocal Laser Scanning Microscopy (CLSM)
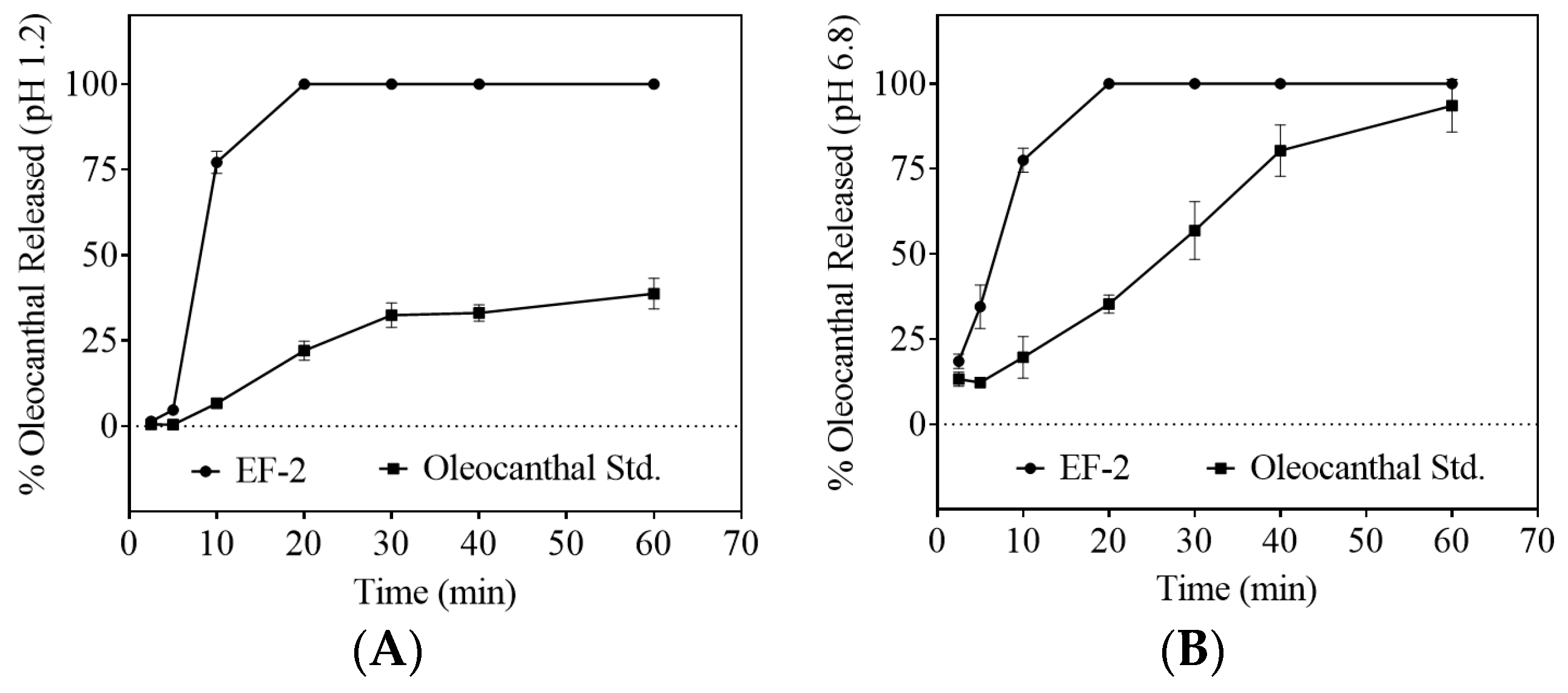
2.12. In-Vitro Dissolution Study
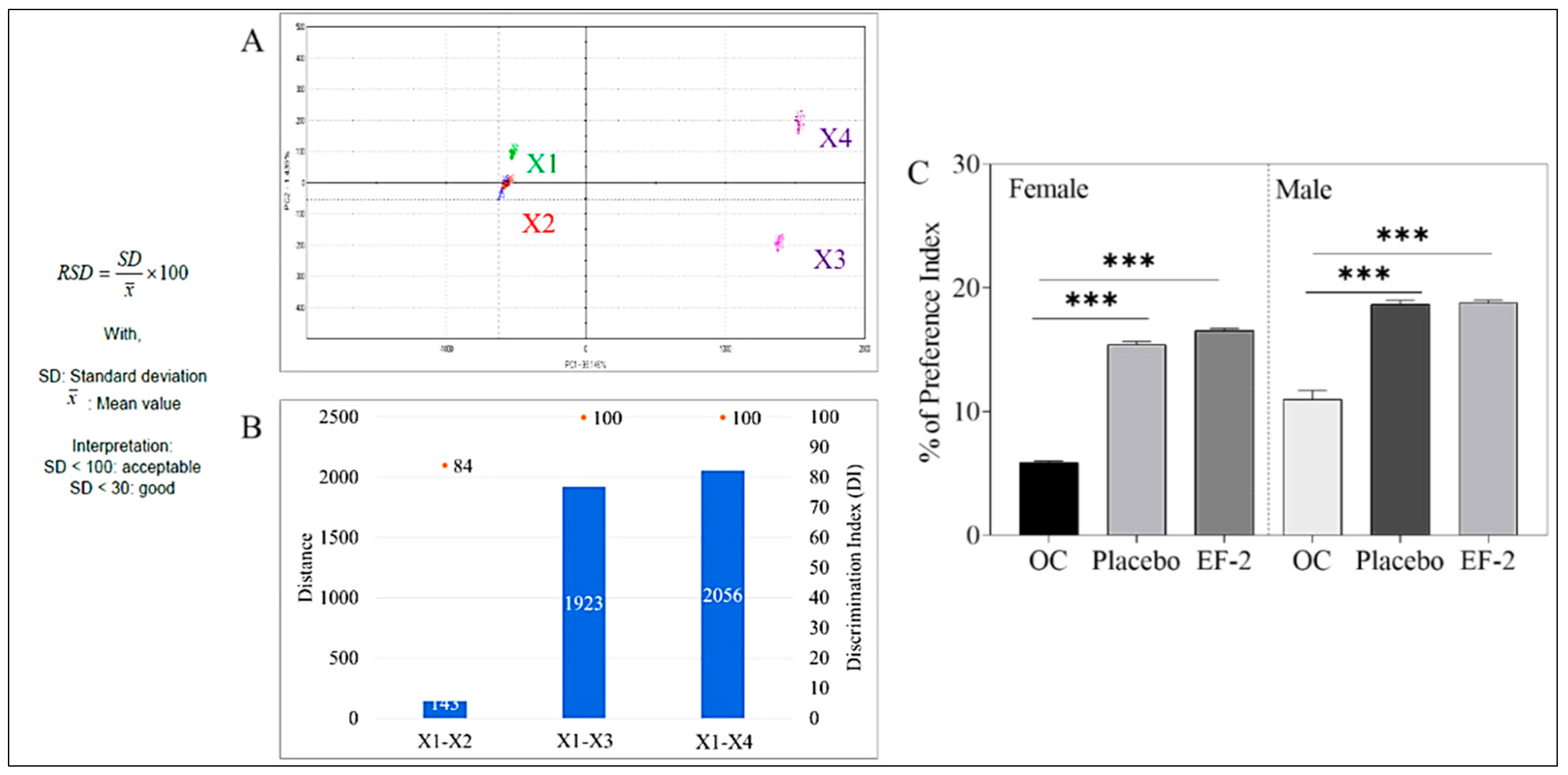
2.13. Taste Assessment Using an Electronic Tongue
2.14. Animal Preference Experiment
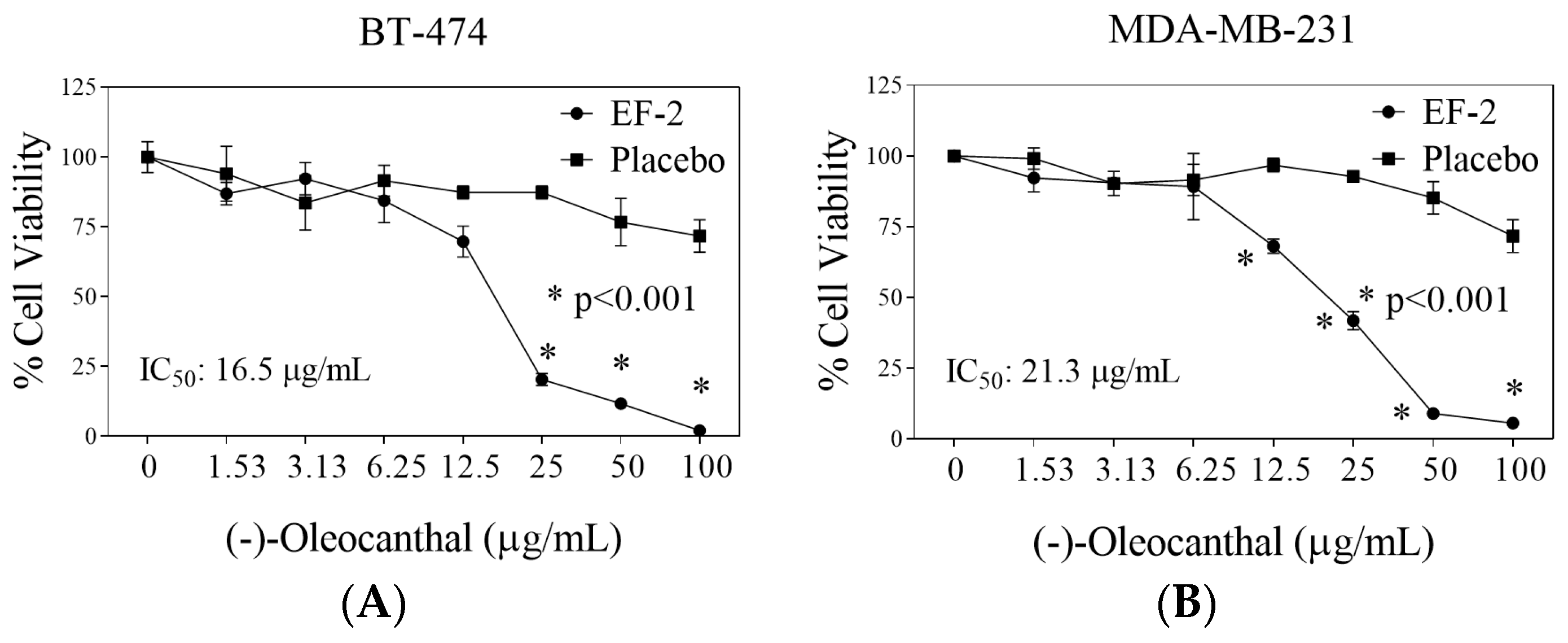
2.15. Cell Lines and Culture Conditions
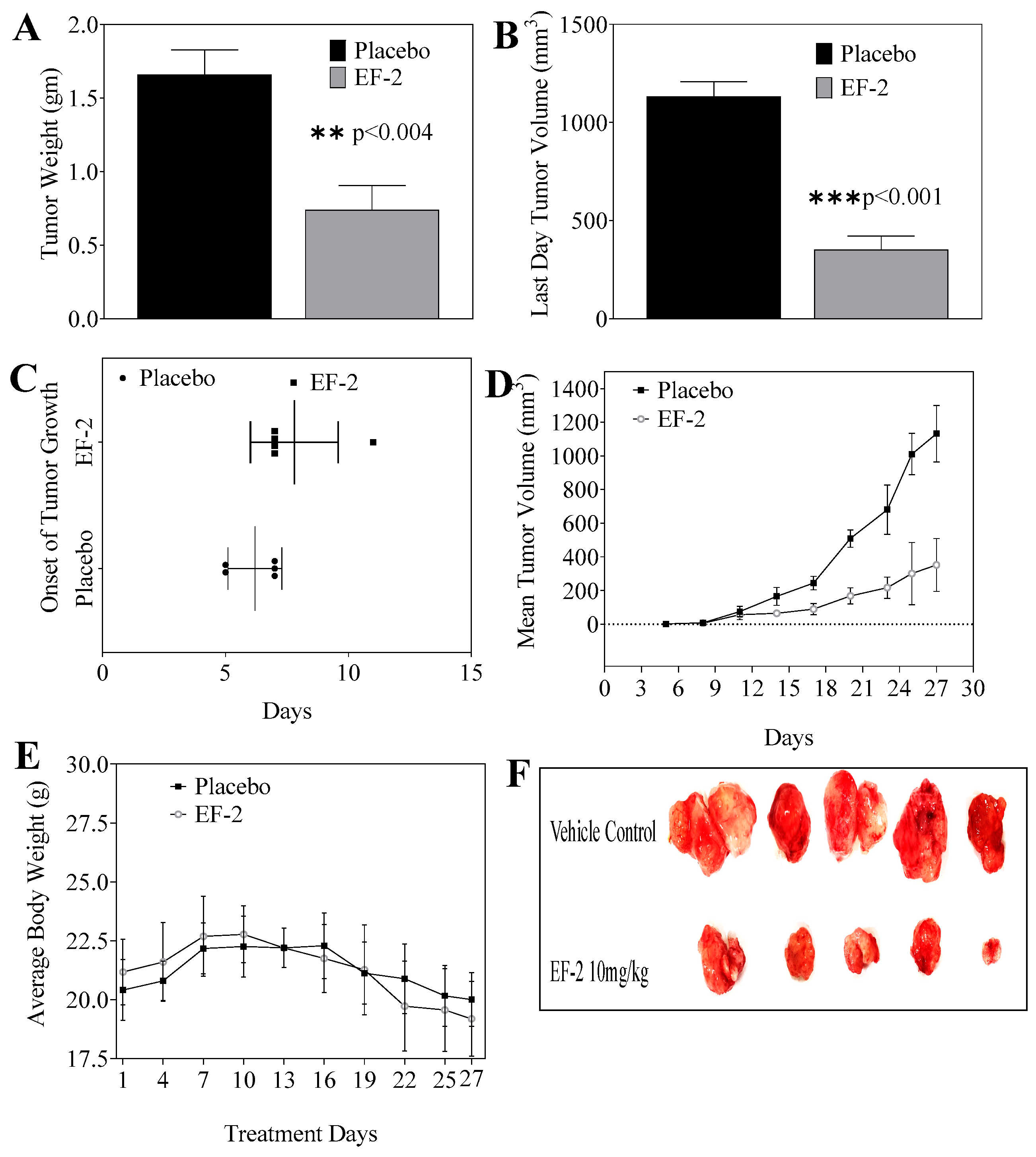
2.16. Effects of EF-2 on BT-474 Nude Mice Tumor Xenograft Progression and Recurrence Models
2.16.1. Tumor Growth Inhibition
2.16.2. Tumor Recurrence Inhibition
2.17. Western Blot Analysis
2.18. Evaluation of EF-2 Treatment on the Human Serum CA 15-3 Biomarker Level
2.19. Statistical Analysis
3. Results
3.1. Determination of Ph, Effervescence Time, and CO2 Content of OC Effervescent Formulations
3.2. Flow Properties of Effervescent Powder
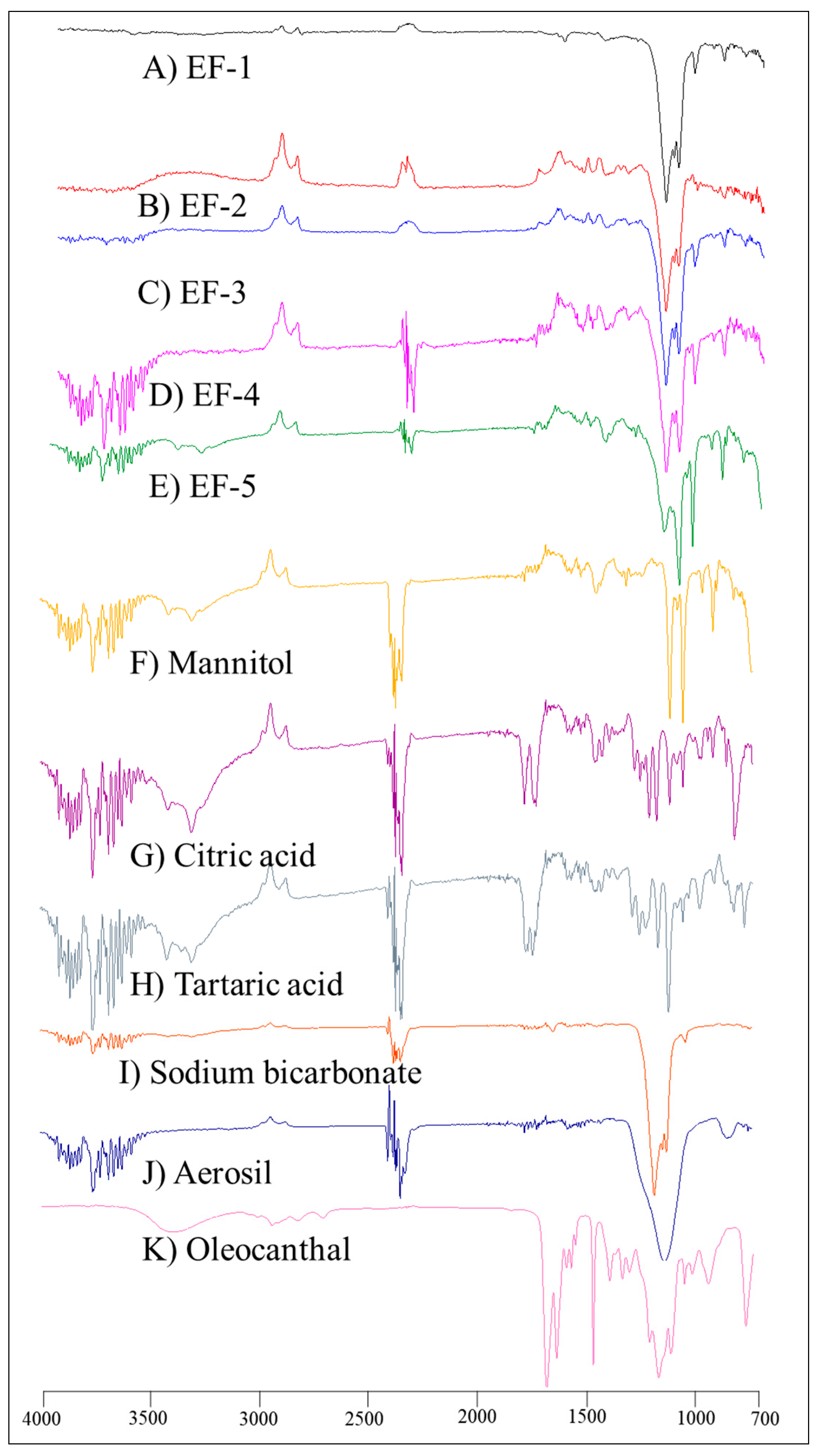
3.3. FT-IR Spectroscopy
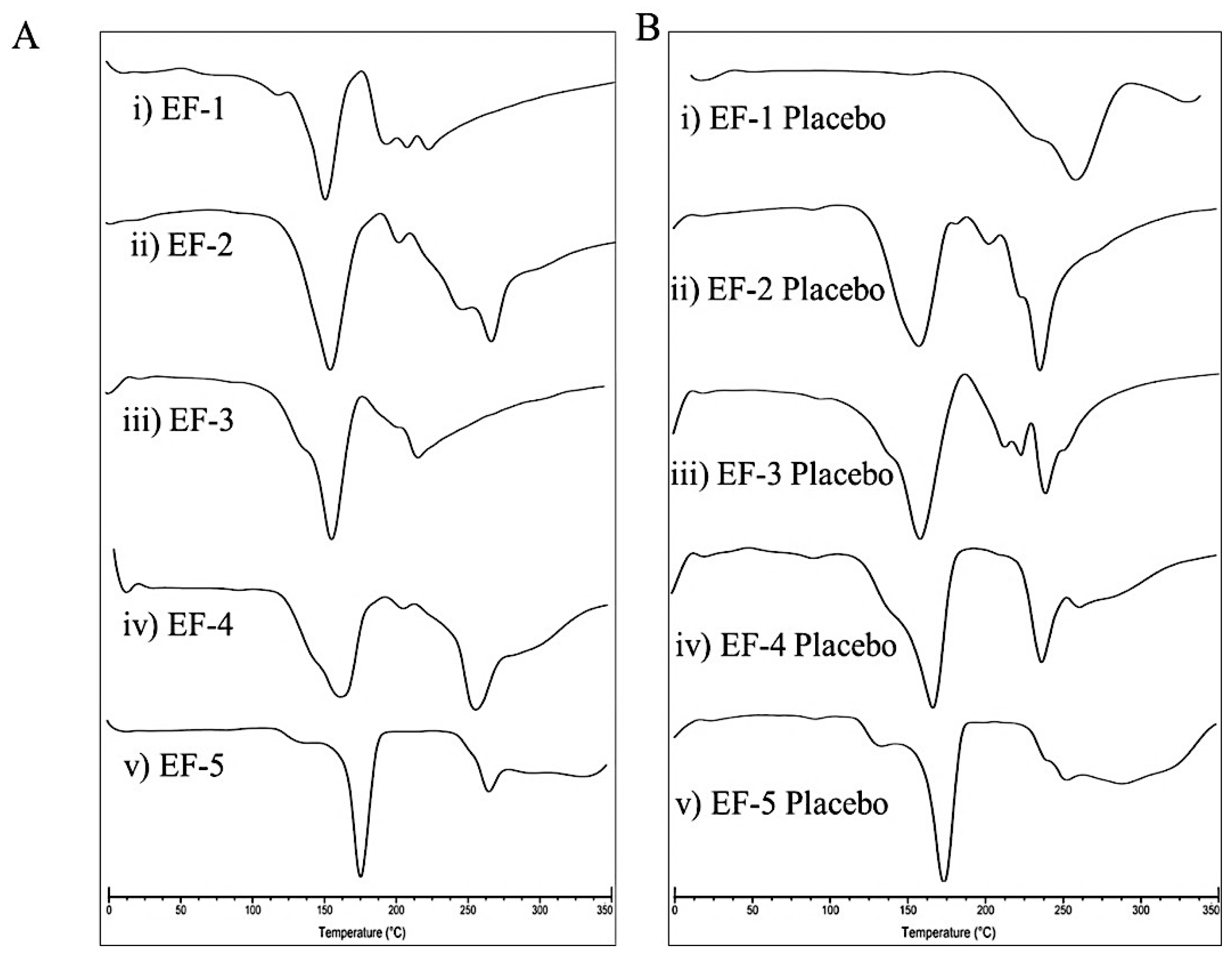
3.4. Differential Scanning Calorimetry (DSC)
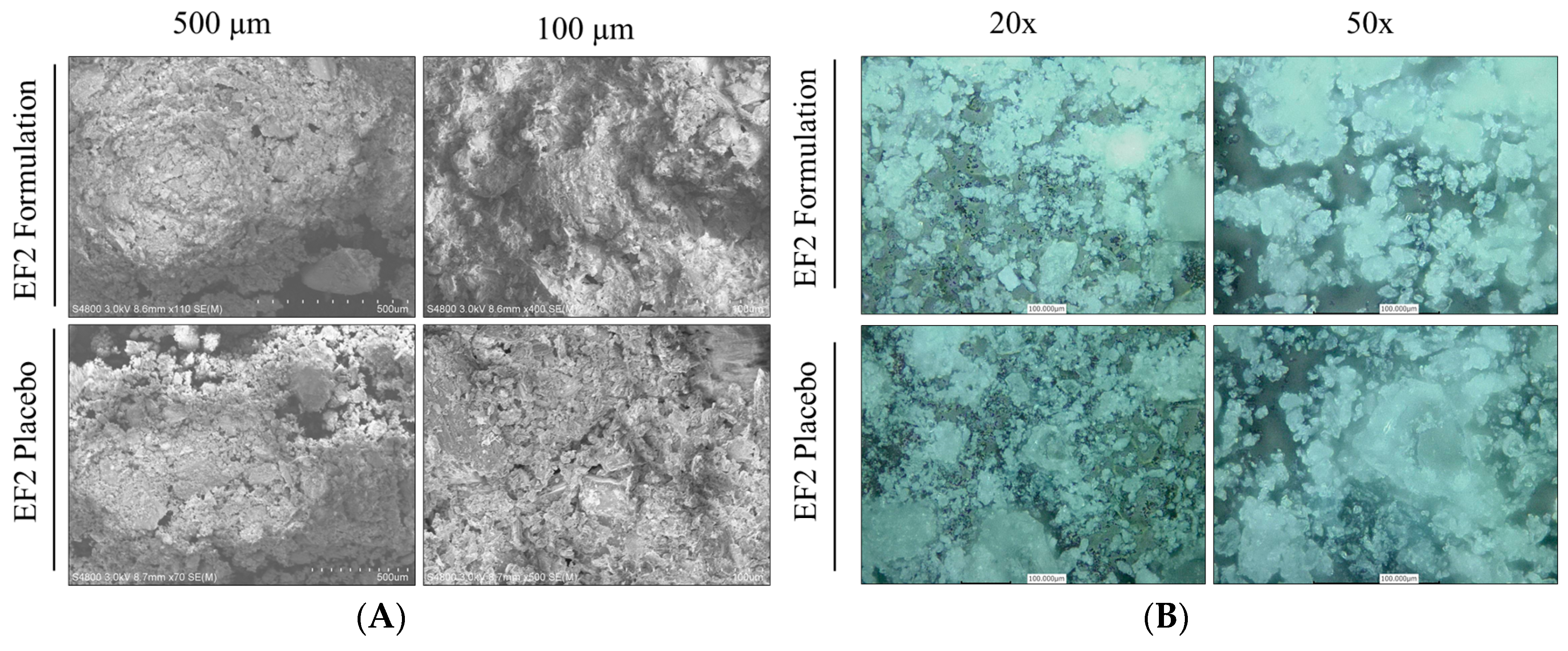
3.5. Characterization of EF-2 Surface Morphology Using SEM and CLSM
3.6. Dissolution Study
3.7. Taste Masking Evaluation of EF-2 Using the E-Tongue Taste Map and Taste Comparison
3.8. Animal Preference Test
3.9. Evaluation of EF-2 Formulation Effects In Vitro Against BC Cells
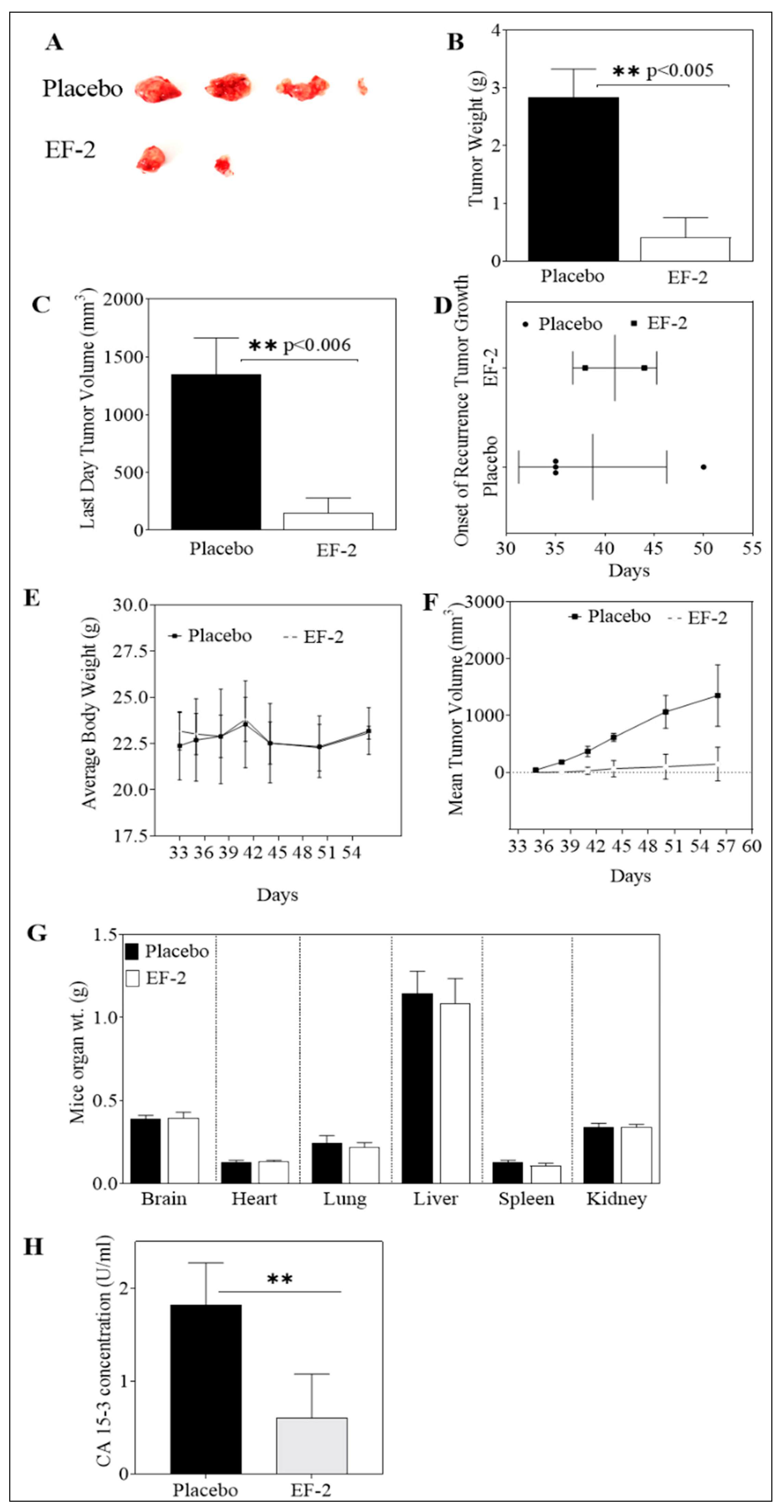
3.10. Evaluation of In Vivo Oral Early EF-2 Treatment Activity Against the Growth of ER+/HER2+ BC in Nude Mouse Xenograft Model
3.11. Evaluation of In Vivo Oral EF-2 Ability to Inhibit the ER+/HER2+ BC Locoregional Recurrence in Nude Mouse Xenograft Model After Primary Tumor Surgical Excision
3.12. In Vivo Effects of EF-2 Treatment on the Level of the Human Serum CA 15-3 Biomarker in HER2+/ER+ BC Recurrence Model
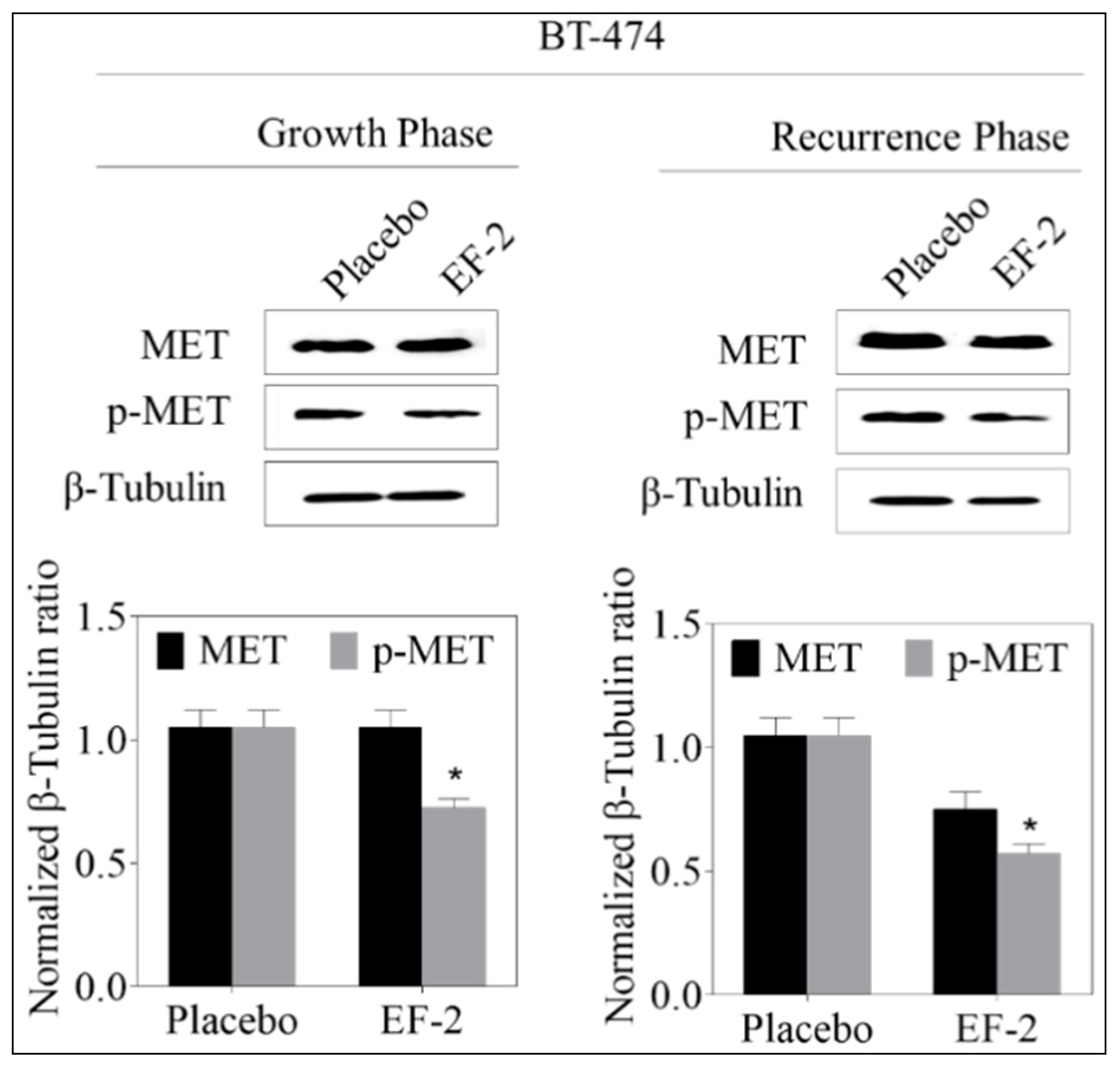
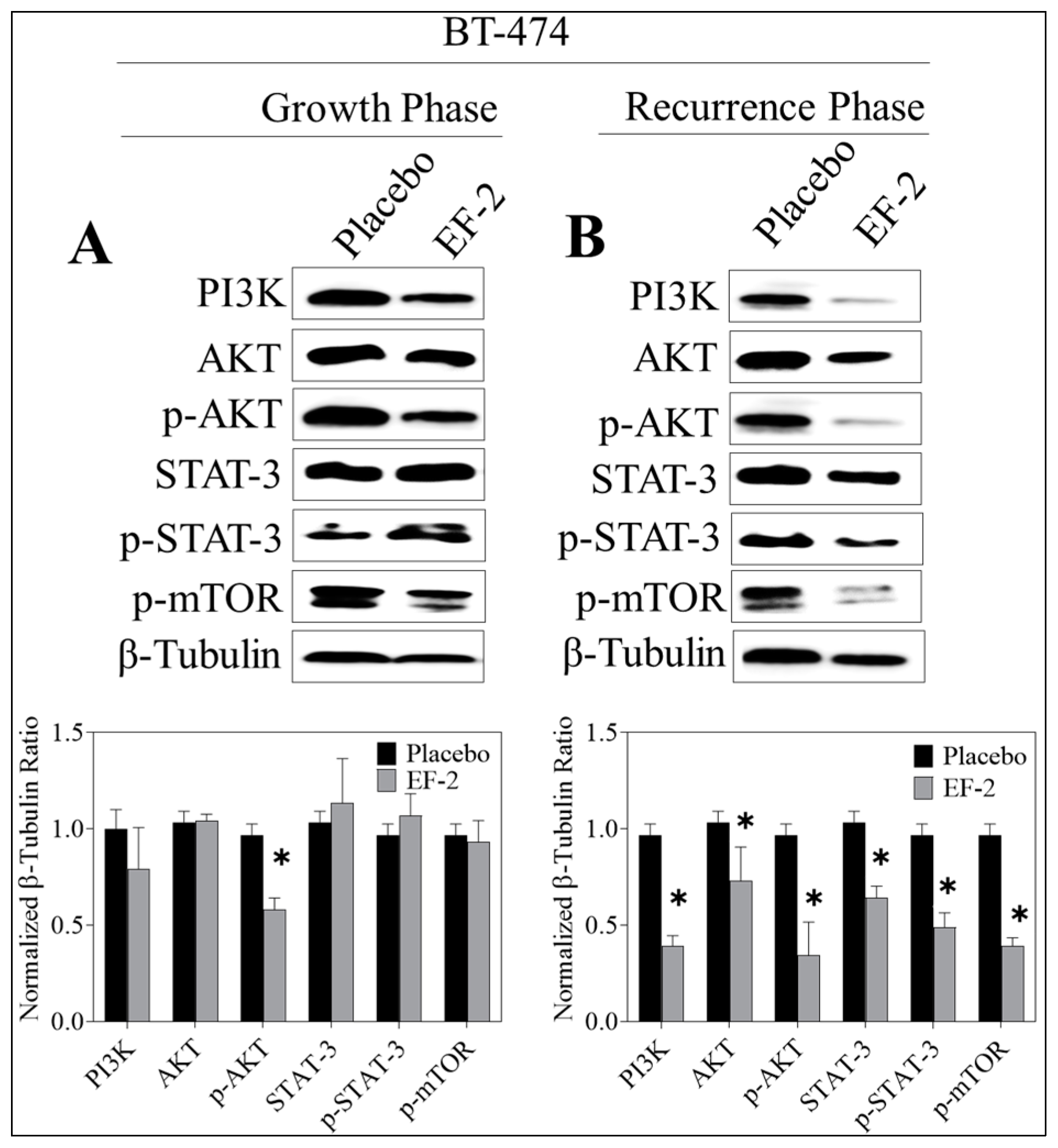
3.13. Western Blotting Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pang, K.L.; Chin, K.Y. The biological activities of oleocanthal from a molecular perspective. Nutrients 2018, 10, 570. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, L.; Keast, R. Oleocanthal, a phenolic derived from virgin olive oil: A review of the beneficial effects on inflammatory disease. Int. J. Mol. Sci. 2014, 15, 12323–12334. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.; Lucas, L.; Keast, R. Biological activities of phenolic compounds present in virgin olive oil. Int. J. Mol. Sci. 2010, 11, 458–479. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.; Lucas, L.J.; Keast, R.S. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr. Opin. Biotechnol. 2012, 23, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Segura-Carretero, A.; Curiel, J.A. Current disease-targets for oleocanthal as promising natural therapeutic agent. Int. J. Mol. Sci. 2018, 19, 2899. [Google Scholar] [CrossRef]
- Andrewes, P.; Busch, J.L.; de Joode, T.; Groenewegen, A.; Alexandre, H. Sensory properties of virgin olive oil polyphenols: Identification of deacetoxy-ligstroside aglycon as a key contributor to pungency. J. Agric Food Chem. 2003, 51, 1415–1420. [Google Scholar] [CrossRef]
- Akl, M.R.; Ayoub, N.M.; Mohyeldin, M.M.; Busnena, B.A.; Foudah, A.I.; Liu, Y.Y.; Sayed, K.A. Olive phenolics as c-Met inhibitors: (-)-Oleocanthal attenuates cell proliferation, invasiveness, and tumor growth in breast cancer models. PLoS ONE 2014, 9, e97622. [Google Scholar] [CrossRef]
- Montedoro, G.; Maurizio, M.; Baldioli, M.; Miniati, E. Simple and hydrolyzable phenolic compounds in virgin olive oil. 1. Their extraction, separation, and quantitative and semiquantitative evaluation by HPLC. J. Agric. Food Chem. 1992, 40, 1571–1576. [Google Scholar] [CrossRef]
- Montedoro, G.; Servili, M.; Baldioli, M.; Selvaggini, R.; Miniati, E.; Macchioni, A. Simple and hydrolyzable compounds in virgin olive oil. 3. Spectroscopic characterizations of the secoiridoid derivatives. J. Agric. Food Chem. 1993, 41, 2228–2234. [Google Scholar] [CrossRef]
- Beauchamp, G.K.; Keast, R.S.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.H.; Smith, A.B.; Breslin, P.A. Phytochemistry: Ibuprofen-like activity in extra-virgin olive oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef]
- Scotece, M.; Gómez, R.; Conde, J.; Lopez, V.; Gómez-Reino, J.J.; Lago, F.; Smith, A.B.; Gualillo, O. Further evidence for the anti-inflammatory activity of oleocanthal: Inhibition of MIP-1α and IL-6 in J774 macrophages and in ATDC5 chondrocytes. Life Sci. 2012, 91, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Peyrot des Gachons, C.; Uchida, K.; Bryant, B.; Shima, A.; Sperry, J.B.; Dankulich-Nagrudny, L.; Tominaga, M.; Smith, A.B.; Beauchamp, G.K.; Breslin, P.A. Unusual pungency from extra-virgin olive oil is attributable to restricted spatial expression of the receptor of oleocanthal. J. Neurosci. 2011, 31, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.; Breslin, P.A.S.; Beauchamp, G.K.; Keast, R.S.J. Sensory characterization of the irritant properties of oleocanthal, a natural anti-inflammatory agent in extra-virgin olive oils. Chem. Senses 2009, 34, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Batarseh, Y.S.; Mohamed, L.A.; Al Rihani, S.B.; Mousa, Y.M.; Siddique, A.B.; El Sayed, K.A.; Kaddoumi, A. Oleocanthal ameliorates amyloid-beta oligomers’ toxicity on astrocytes and neuronal cells: In vitro studies. Neuroscience 2017, 352, 204–215. [Google Scholar] [CrossRef]
- Qosa, H.; Batarseh, Y.S.; Mohyeldin, M.M.; El Sayed, K.A.; Keller, J.N.; Kaddoumi, A. Oleocanthal enhances amyloid-beta clearance from the brains of TgSwDI mice and in vitro across a human blood-brain barrier model. ACS Chem. Neurosci. 2015, 6, 1849–1859. [Google Scholar] [CrossRef]
- Monti, M.C.; Margarucci, L.; Riccio, R.; Casapullo, A. Modulation of tau protein fibrillization by oleocanthal. J. Nat. Prod. 2012, 75, 1584–1588. [Google Scholar] [CrossRef]
- Pitt, J.; Roth, W.; Lacor, P.; Smith, A.B., III; Blankenship, M.; Velasco, P.; De Felice, F.; Breslin, P.; Klein, W.L. Alzheimer’s-associated Abeta oligomers show altered structure; immunoreactivity and synaptotoxicity with low doses of oleocanthal. Toxicol. Appl. Pharmacol. 2009, 240, 189–197. [Google Scholar] [CrossRef]
- Pei, T.; Meng, Q.; Han, J.; Sun, H.; Li, L.; Song, R.; Sun, B.; Pan, S.; Liang, D.; Liu, L. (-)-Oleocanthal inhibits growth and metastasis by blocking activation of STAT3 in human hepatocellular carcinoma. Oncotarget 2016, 7, 43475–43491. [Google Scholar] [CrossRef]
- Elnagar, A.Y.; Sylvester, P.W.; El Sayed, K.A. (-)-Oleocanthal as a c-Met inhibitor for the control of metastatic breast and prostate cancers. Planta Med. 2011, 77, 1013–1019. [Google Scholar] [CrossRef]
- Subramaniam, A.; Shanmugam, M.K.; Perumal, E.; Li, F.; Nachiyappan, A.; Dai, X.; Swamy, S.N.; Ahn, K.S.; Kumar, A.P.; Tan, B.K.; et al. Potential role of signal transducer and activator of transcription 3 (STAT3) signaling pathway in inflammation, survival, proliferation and invasion of hepatocellular carcinoma. Biochim Biophys. Acta. 2013, 1835, 46–60. [Google Scholar] [CrossRef]
- Polini, B.; Digiacomo, M.; Carpi, S.; Bertini, S.; Gado, F.; Saccomanni, G.; Macchia, M.; Nieri, P.; Manera, C.; Fogli, S. Oleocanthal and oleacein contribute to the in vitro therapeutic potential of extra virgin oil-derived extracts in non-melanoma skin cancer. Toxicol. In Vitro. 2018, 52, 243–250. [Google Scholar] [CrossRef] [PubMed]
- LeGendre, O.; Breslin, P.A.; Foster, D.A. (-)-Oleocanthal rapidly and selectively induces cancer cell death via lysosomal membrane permeabilization. Mol. Cell Oncol. 2015, 2, e1006077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayoub, N.M.; Siddique, A.B.; Ebrahim, H.Y.; Mohyeldin, M.M.; El Sayed, K.A. The olive oil phenolic (-)-oleocanthal modulates estrogen receptor expression in luminal breast cancer in vitro and in vivo and synergizes with tamoxifen treatment. Eur. J. Pharmacol. 2017, 810, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, B.; Shetty, R.; Kulkarni, V.; Sen, H.; Bhushan, I. Effervescent Composition and Method of Making it. WIPO (PCT) WO2016042372A1, 17 September 2014. Available online: https://patents.google.com/patent/WO2016042372A1 (accessed on 28 March 2019).
- Parikh, M.D. (Ed.) Effervescent powder. In Hand Book of Pharmaceutical Granulation Technology, 2nd ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2005; pp. 365–384. Available online: http://www.gmpua.com/Process/Tablet/Granulation/GranulationTechnology.pdf. (accessed on 16 April 2019).
- Siddique, A.B.; Ebrahim, H.E.; Qusa, M.; Btarsah, Y.; Fayaad, A.; Tajmim, A.; Nazzal, S.; Kaddoumi, A.; El Sayed, K.A. Novel liquid-liquid extraction and self-emulsion methods for simplified isolation of extra-virgin olive oil phenolics with emphasis on (-)-oleocanthal and its oral anti-breast cancer activity. PLoS ONE 2019, 14, e0214798. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Shirwaikar, A.; Nair, A. Preparation and evaluation of fast-disintegrating effervescent tablets of glibenclamide. Drug Dev. Ind. Pharm. 2009, 35, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Train, D. Some aspects of the property of angle of repose of powders. J. Pharm. Pharmacol. 1958, 10, 127–135. [Google Scholar] [CrossRef]
- Wei, Y.; Nedley, M.P.; Bhaduri, S.B.; Bredzinski, X.; Boddu, S.H.S. Masking the bitter taste of injectable lidocaine HCl formulation for dental procedures. AAPS PharmSciTech 2015, 16, 455–465. [Google Scholar] [CrossRef]
- Podrazka, M.; Baczynska, E.; Kundys, M.; Jelen, P.S.; Nery, E.W. Electronic tongue-A tool for all tastes? Biosensors 2018, 8, 3. [Google Scholar] [CrossRef]
- Nesil, T.; Kanit, L.; Pogun, S. Bitter taste and nicotine preference: Evidence for sex differences in rats. Am. J. Drug Alcohol Ab. 2005, 41, 57–67. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell viability assays. In Assay Guidance Manual; Weidner, J., Ed.; 2004. Available online: https://www.ncbi.nlm.nih.gov/books/NBK144065/ (accessed on 1 May 2013).
- Siddique, A.B.; Ayoub, N.M.; Tajmim, A.; Meyer, S.A.; Hill, R.A.; El Sayed, K.A. (-)-Oleocanthal prevents breast cancer locoregional recurrence after primary tumor surgical excision and neoadjuvant targeted therapy in orthotopic nude mouse models. Cancers 2019, 11, 637. [Google Scholar] [CrossRef]
- Aslani, A.; Fattahi, F. Formulation, characterization and physicochemical evaluation of potassium citrate effervescent tablets. Adv. Pharm. Bull. 2013, 3, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wan, J.; Wu, L.; Yi, T.; Liu, W.; Xu, H.; Yang, X. A new strategy for enhancing the oral bioavailability of drugs with poor water-solubility and low liposolubility based on phospholipid complex and supersaturated SEDDS. PLoS ONE 2013, 8, e84530. [Google Scholar] [CrossRef] [PubMed]
- Dyminski, D.; Paterno, L.; Takeda, H.; Bolini, H.; Mattoso, L.; Cândido, L. Correlation between human panel and electronic tongue responses on the analysis of commercial sweeteners. Sens. Lett. 2006, 4, 403–408. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Meng, Q.; Li, N.; Ren, L. Evaluation of beef by electronic tongue system TS-5000Z: Flavor assessment, recognition and chemical compositions according to its correlation with flavor. PLoS ONE 2015, 10, e0137807. [Google Scholar] [CrossRef] [PubMed]
- Holliday, D.L.; Speirs, V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011, 13, 215. [Google Scholar] [CrossRef]
- Siddique, A.; Ibrahim, H.Y.; Akl, M.R.; Ayoub, N.M.; Goda, A.A.; Mohyeldin, M.M.; Nagumalli, S.K.; Hananeh, W.M.; Liu, Y.Y.; Meyer, S.A.; et al. (-)-Oleocanthal combined with lapatinib treatment synergized against HER-2 positive breast cancer in vitro and in vivo. Nutrients 2019, 11, 412. [Google Scholar] [CrossRef]
- Ahmad, A. Pathways to breast cancer recurrence. ISRN Oncol. 2013, 2013. [Google Scholar] [CrossRef]
- Shim, H.J.; Kim, S.H.; Kang, B.J.; Choi, B.G.; Kim, H.S.; Cha, E.S.; Song, B.J. Breast cancer recurrence according to molecular subtype. Asian Pac. J. Cancer Prev. 2014, 15, 5539–5544. [Google Scholar] [CrossRef]
- Ho-Yen, C.M.; Jones, J.L.; Kermorgant, S. The clinical and functional significance of c-Met in breast cancer: A review. Breast Cancer Res. 2015, 17, 52. [Google Scholar] [CrossRef]
- Ho-Yen, C.M.; Green, A.R.; Rakha, E.A.; Brentnall, A.R.; Ellis, I.O.; Kermorgant, S.; Jones, J.L. c-Met in invasive breast cancer. Is there a relationship with the basal-like subtype? Cancer 2014, 120, 163–171. [Google Scholar] [CrossRef]
- Samiee, S.; Berardi, P.; Bouganim, N.; Vandermeer, L.; Arnaout, A.; Dent, S.; Mirsky, D.; Chasen, M.; Caudrelier, J.M.; Clemons, M. Excision of the primary tumor in patients with metastatic breast cancer: A clinical dilemma. Curr. Oncol. 2012, 19, e270–e279. [Google Scholar] [CrossRef] [PubMed]
- Pienta, K.J.; Robertson, B.A.; Coffey, D.S.; Taichman, R.S. The cancer diaspora: Metastasis beyond the seed and soil hypothesis. Clin. Cancer Res. 2013, 19, 5849–5855. [Google Scholar] [CrossRef] [PubMed]
- Comen, E.; Norton, L.; Massagu, E.J. Clinical implications of cancer self-seeding. Nat. Rev. Clin. Oncol. 2011, 8, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef] [PubMed]
| Code | Citric Acid (mg) | Tartaric Acid (mg) | Sodium Bicarbonate (mg) | Effervescence Time (sec) | pH |
|---|---|---|---|---|---|
| P1 | 84.0 | 166.0 | 250.0 | 54.00 ± 0.31 | 5.63 ± 0.23 |
| P2 | 80.0 | 160.0 | 240.0 | 55.00 ± 0.46 | 5.61 ± 0.11 |
| P3 | 74.0 | 146.0 | 220.0 | 46.00 ± 0.38 | 5.60 ± 0.21 |
| P4 | 74.0 | 160.0 | 220.0 | 42.00 ± 0.21 | 5.67 ± 0.35 |
| P5 | 40.0 | 80.0 | 120.0 | 51.00 ± 0.51 | 5.23 ± 0.24 |
| P6 | 120.0 | 0.0 | 80.0 | 43.00 ± 0.53 | 4.48 ± 0.32 |
| P7 | 120.0 | 0.0 | 90.0 | 45.00 ± 0.42 | 4.43 ± 0.21 |
| P8 | 120.0 | 0.0 | 120.0 | 46.00 ± 0.34 | 4.53 ± 0.23 |
| P9 | 0.0 | 120.0 | 120.0 | 30.00 ± 0.22 | 4.42 ± 0.43 |
| P10 | 0.0 | 250.0 | 250.0 | 28.00 ± 0.37 | 4.27 ± 0.26 |
| P11 | 84.0 | 84.0 | 250.0 | 54.00 ± 0.71 | 4.42 ± 0.27 |
| P12 | 84.0 | 126.0 | 250.0 | 50.00 ± 0.11 | 4.39 ± 0.31 |
| P13 | 84.0 | 252.0 | 250.0 | 37.00 ± 0.41 | 4.27 ± 0.26 |
| P14 | 84.0 | 336.0 | 250.0 | 24.00 ± 0.45 | 4.25 ± 0.35 |
| P15 | 166.0 | 166.0 | 250.0 | 28.00 ± 0.36 | 4.31 ± 0.21 |
| P16 | 42.0 | 84.0 | 250.0 | 55.00 ± 0.43 | 4.58 ± 0.41 |
| P17 | 63.2 | 84.0 | 250.0 | 43.00 ± 0.53 | 4.52 ± 0.32 |
| P18 | 126.0 | 84.0 | 250.0 | 31.00 ± 0.45 | 4.40 ± 0.49 |
| P19 | 168.0 | 84.0 | 250.0 | 54.00 ± 0.32 | 4.32 ± 0.25 |
| P20 | 0.0 | 84.0 | 250.0 | 54.00 ± 0.41 | 4.52 ± 0.27 |
| P21 | 84.0 | 0.0 | 250.0 | 42.00 ± 0.44 | 4.75 ± 0.32 |
| P22 | 168.0 | 0.0 | 250. | 36.00 ± 0.42 | 4.49 ± 0.30 |
| P23 | 126.0 | 0.0 | 250.0 | 58.00 ± 0.18 | 4.59 ± 0.28 |
| P24 | 126.0 | 0.0 | 125.0 | 78.00 ± 0.39 | 4.58 ± 0.41 |
| P25 | 126.0 | 0.0 | 187.5 | 48.00 ± 0.52 | 4.54 ± 0.29 |
| P26 | 126.0 | 0.0 | 375.0 | 55.00 ± 0.22 | 4.55 ± 0.22 |
| P27 | 126.0 | 0.0 | 500.0 | 39.00 ± 0.37 | 4.52 ± 0.31 |
| Ingredients (mg) | Formulation Code | ||||
|---|---|---|---|---|---|
| EF-1 | EF-2 | EF-3 | EF-4 | EF-5 | |
| Oleocanthal | 10 | 10 | 10 | 10 | 10 |
| Citric acid | 84 | 80 | 74 | 40 | 120 |
| Tartaric acid | 166 | 160 | 146 | 80 | - |
| Sodium bicarbonate | 250 | 240 | 220 | 160 | 80 |
| Aerosil-200 | 20 | 20 | 20 | 20 | 20 |
| Mannitol | 460 | 480 | 440 | 680 | 760 |
| Flavor | 10 | 10 | 10 | 10 | 10 |
| Formulation Code | CO2 Content (mg) | pH | Effervescence Time (sec) |
|---|---|---|---|
| EF-1 | 44.33 ± 7.77 | 3.86 ± 0.32 | 42.67 ± 1.41 |
| EF-2 | 63.00 ± 2.00 | 4.36 ± 0.11 | 68.00 ± 3.78 |
| EF-3 | 37.67 ± 9.87 | 3.21 ± 0.20 | 47.00 ± 2.12 |
| EF-4 | 59.67 ± 4.04 | 3.68 ± 0.41 | 40.00 ± 5.66 |
| EF-5 | 59.67 ± 18.56 | 3.95 ± 0.17 | 36.67 ± 5.66 |
| Formulation Code | Bulk Density | Tapped Density | Hausner’s Ratio | Angle of Repose (θ) (Degrees) | Carr’s Index |
|---|---|---|---|---|---|
| EF-1 | 0.50 ± 0.11 | 0.60 ± 0.02 | 1.20 ± 0.58 | 33.82 ± 2.87 | 16.67 ± 4.32% |
| EF-2 | 0.58 ± 0.15 | 0.60 ± 0.23 | 1.02 ± 0.87 | 28.68 ± 3.14 | 5.00 ± 3.21% |
| EF-3 | 0.46 ± 0.21 | 0.58 ± 0.21 | 1.25 ± 0.92 | 25.64 ± 1.87 | 19.97 ± 4.54% |
| EF-4 | 0.54 ± 0.17 | 0.46 ± 0.31 | 1.17 ± 0.59 | 33.82 ± 3.01 | 14.81 ± 3.43% |
| EF-5 | 0.39 ± 0.13 | 0.48 ± 0.22 | 1.23 ± 0.62 | 20.80 ± 2.43 | 18.75 ± 3.33% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tajmim, A.; Siddique, A.B.; El Sayed, K. Optimization of Taste-Masked (–)-Oleocanthal Effervescent Formulation with Potent Breast Cancer Progression and Recurrence Suppressive Activities. Pharmaceutics 2019, 11, 515. https://doi.org/10.3390/pharmaceutics11100515
Tajmim A, Siddique AB, El Sayed K. Optimization of Taste-Masked (–)-Oleocanthal Effervescent Formulation with Potent Breast Cancer Progression and Recurrence Suppressive Activities. Pharmaceutics. 2019; 11(10):515. https://doi.org/10.3390/pharmaceutics11100515
Chicago/Turabian StyleTajmim, Afsana, Abu Bakar Siddique, and Khalid El Sayed. 2019. "Optimization of Taste-Masked (–)-Oleocanthal Effervescent Formulation with Potent Breast Cancer Progression and Recurrence Suppressive Activities" Pharmaceutics 11, no. 10: 515. https://doi.org/10.3390/pharmaceutics11100515
APA StyleTajmim, A., Siddique, A. B., & El Sayed, K. (2019). Optimization of Taste-Masked (–)-Oleocanthal Effervescent Formulation with Potent Breast Cancer Progression and Recurrence Suppressive Activities. Pharmaceutics, 11(10), 515. https://doi.org/10.3390/pharmaceutics11100515