EphA2-Receptor Targeted PEGylated Nanoliposomes for the Treatment of BRAFV600E Mutated Parent- and Vemurafenib-Resistant Melanoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Method
2.3. Cell Culture
2.4. Preparation of Liposomes
2.5. Characterization of Liposomes
2.6. Stability Study
2.7. Freeze Drying of Liposome
2.8. Differential Scanning Calorimetry (DSC) Thermograms of TMB and TPL
2.9. In Vitro Release Study
2.10. In Vitro Hemolysis Study
2.11. Plasma-to-Blood Ratio
2.12. Cellular Uptake of Liposomes
2.13. In Vitro Cytotoxicity Test
2.14. Western Blot Assay
2.15. Statistical Analysis
3. Results
3.1. Preparation of TMB-Loaded PEGylated Liposomes
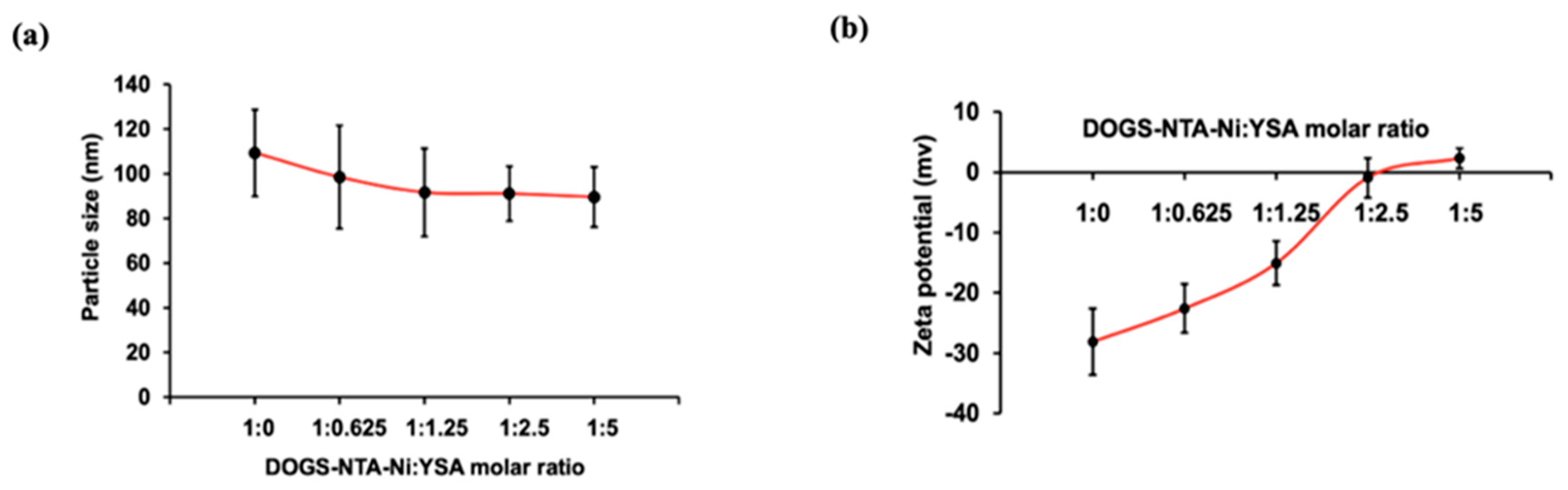
3.2. Particle Size and Zeta Potential
3.3. Stability of Liposomes
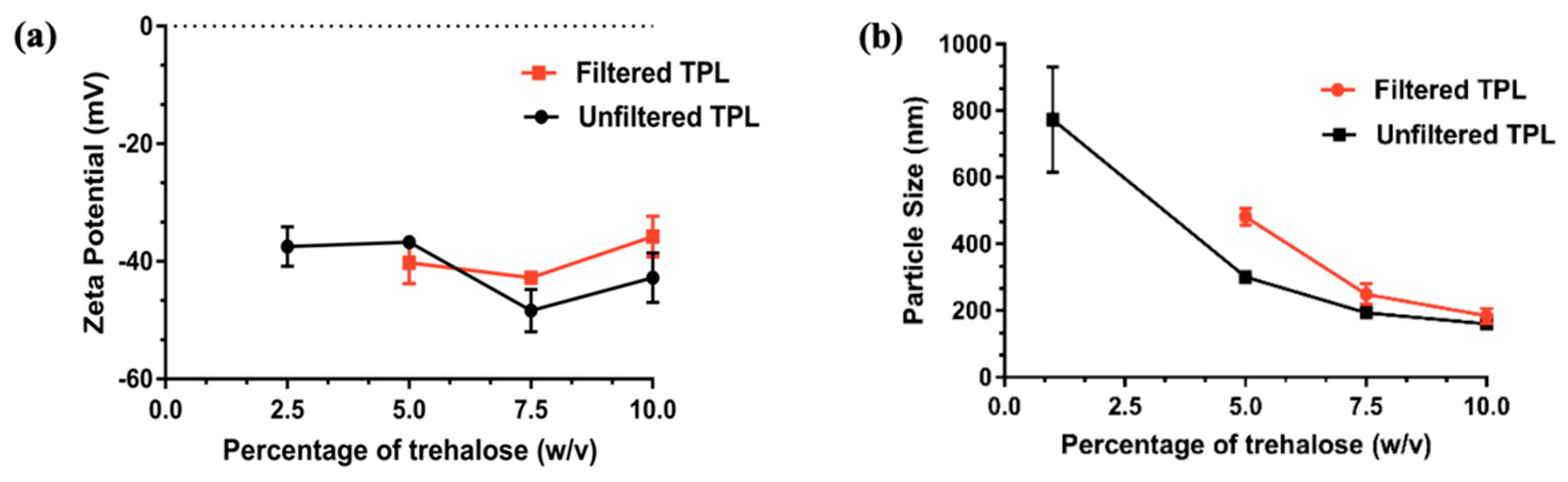
3.4. Freeze Drying of Liposomes
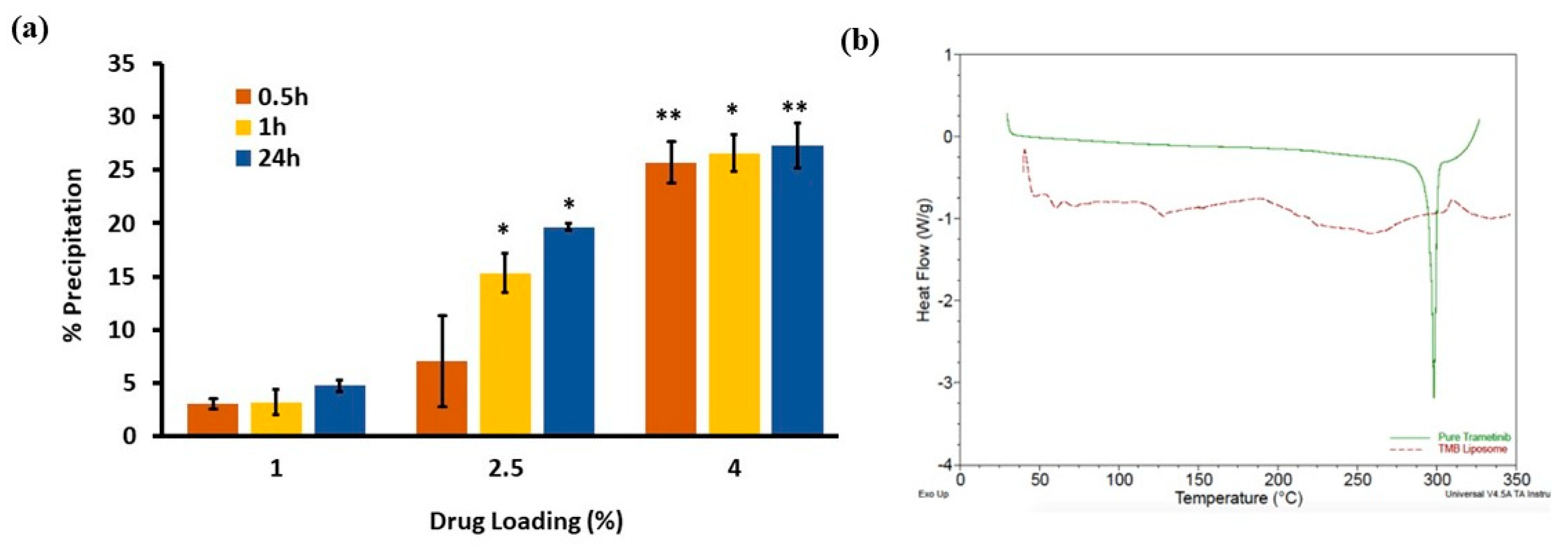
3.5. DSC Thermograms of TMB and TPL
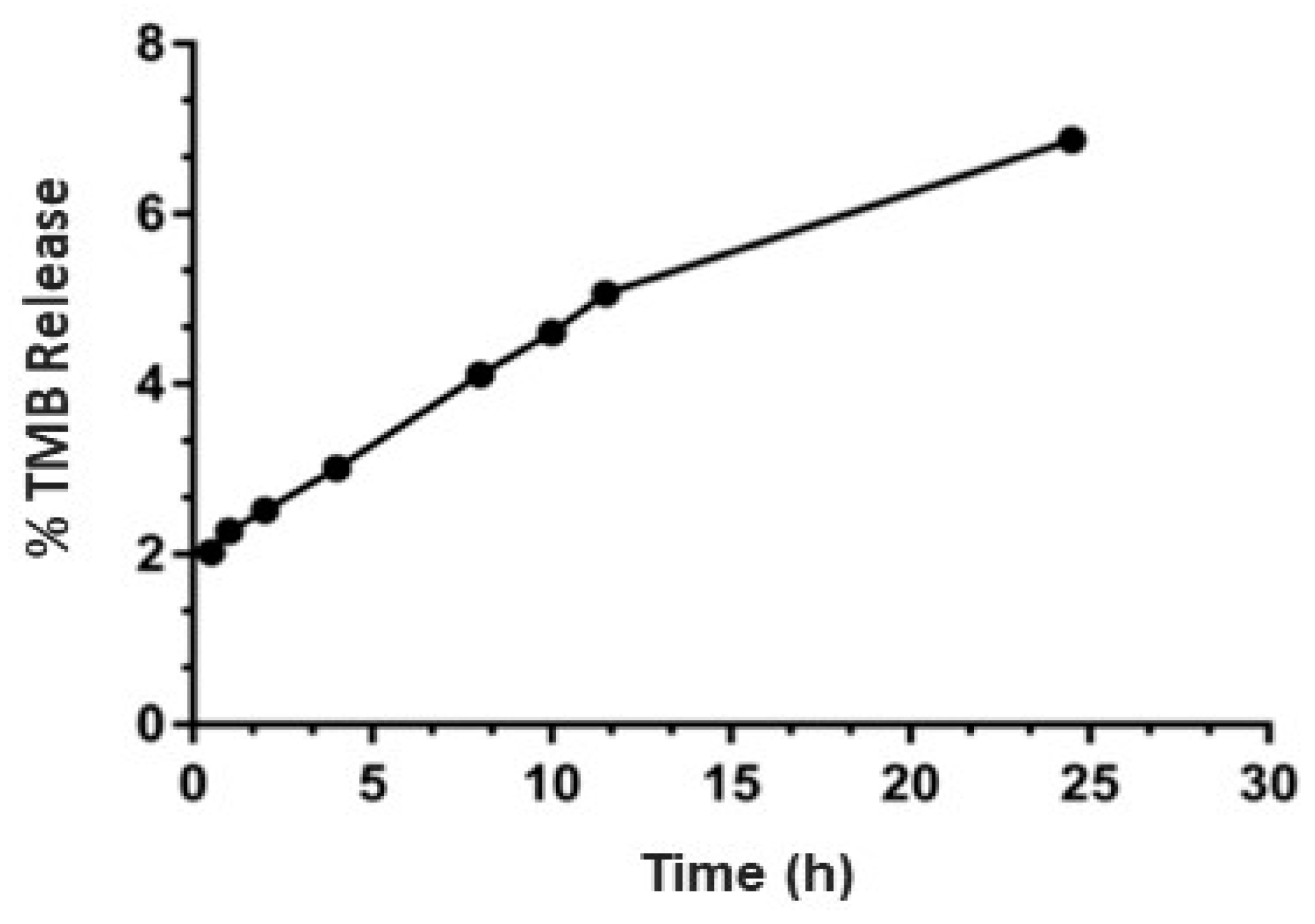
3.6. Drug Release Study
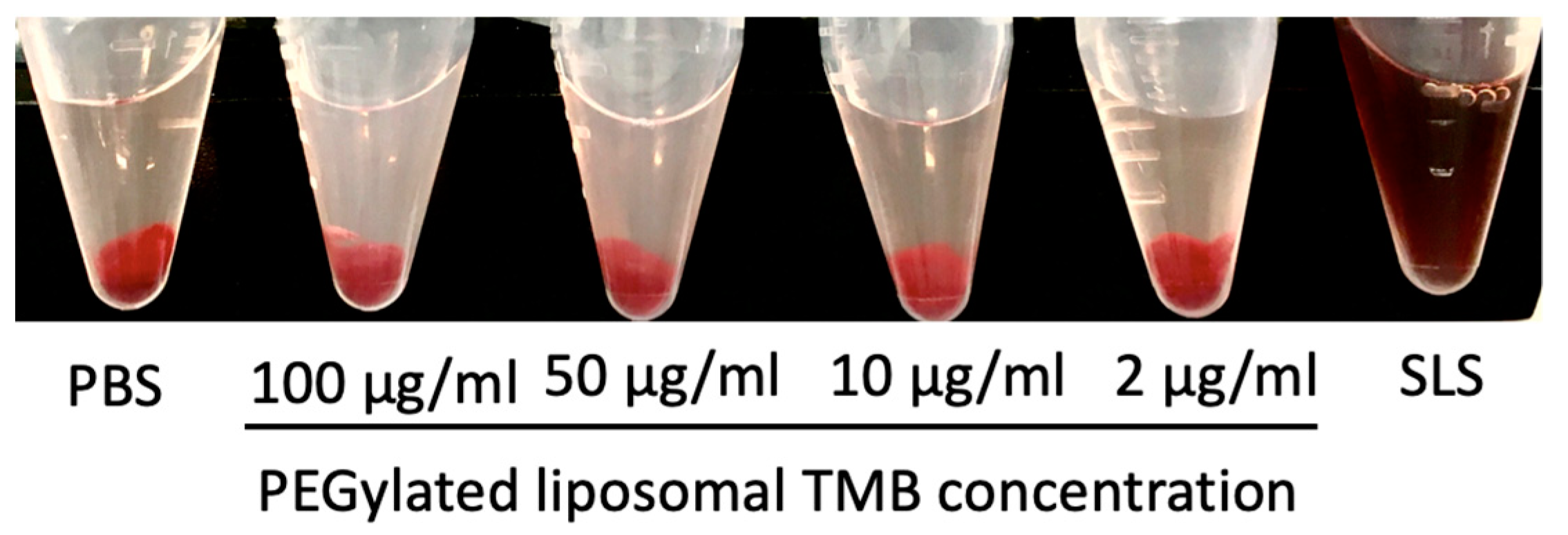
3.7. In Vitro Hemolysis Study and Plasma to Blood Ratio
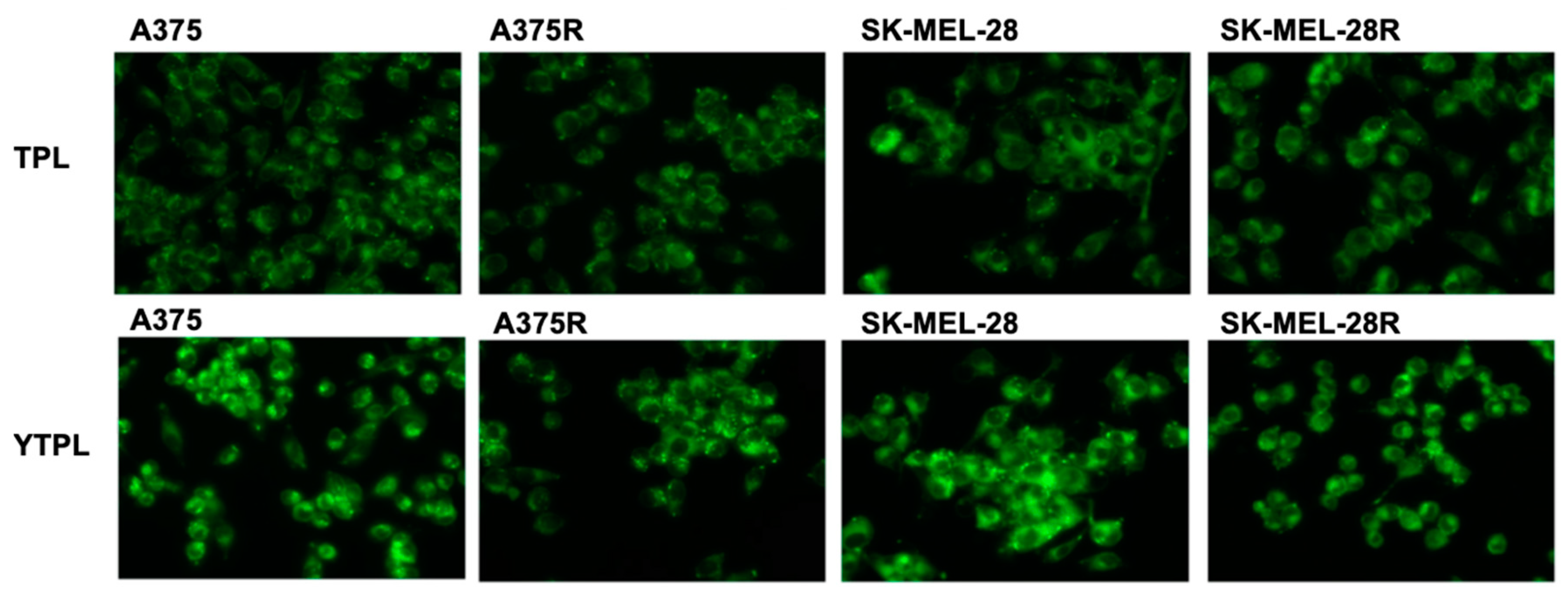
3.8. Uptake Study
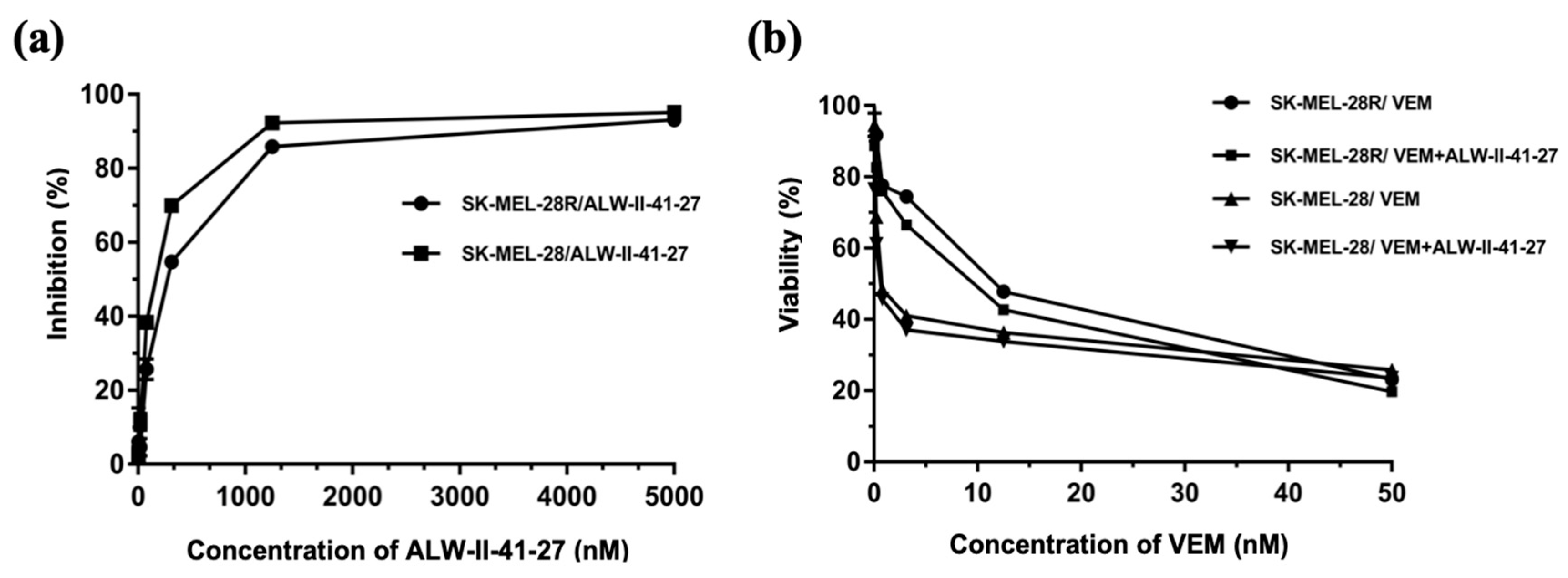
3.9. In Vitro Cytotoxicity Test
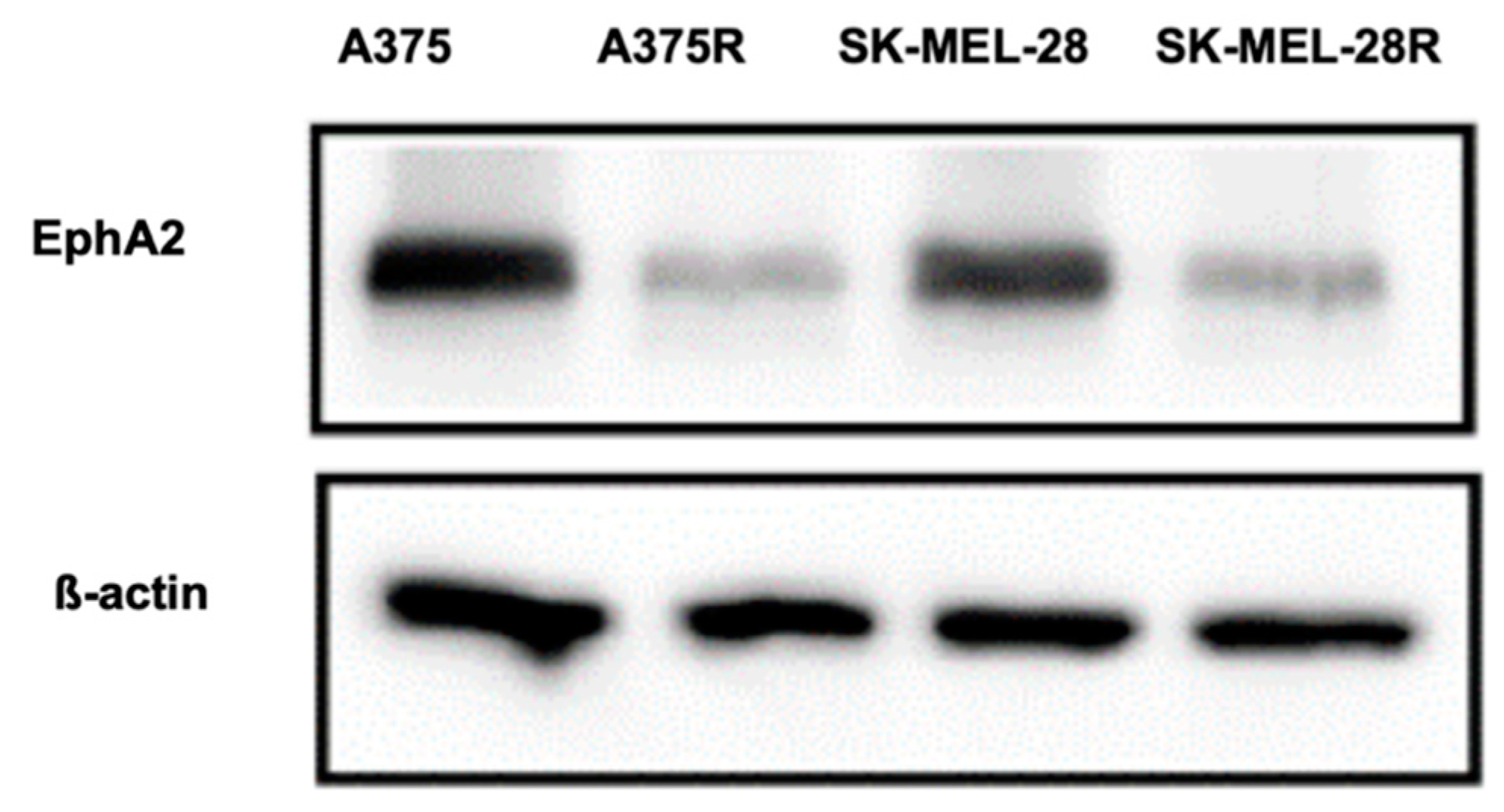
3.10. Western Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Murray, T.; Thun, M.J. Cancer statistics, 2008. CA: A cancer J. Clin. 2008, 58, 71–96. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Tykodi, S.S.; Thompson, J.A. Treatment of metastatic melanoma: an overview. Oncology 2009, 23, 488. [Google Scholar] [PubMed]
- Flaherty, K.T.; Infante, J.R.; Daud, A.; Gonzalez, R.; Kefford, R.F.; Sosman, J.; Hamid, O.; Schuchter, L.; Cebon, J.; Ibrahim, N. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. New Engl. J. Med. 2012, 367, 1694–1703. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, K.T.; Robert, C.; Hersey, P.; Nathan, P.; Garbe, C.; Milhem, M.; Demidov, L.V.; Hassel, J.C.; Rutkowski, P.; Mohr, P. Improved survival with MEK inhibition in BRAF-mutated melanoma. New Engl. J. Med. 2012, 367, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; de Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; Grob, J.J. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. New Engl. J. Med. 2014, 371, 1877–1888. [Google Scholar] [CrossRef] [PubMed]
- Lugowska, I.; Koseła-Paterczyk, H.; Kozak, K.; Rutkowski, P. Trametinib: a MEK inhibitor for management of metastatic melanoma. Onco Targets Ther. 2015, 8, 2251. [Google Scholar] [PubMed]
- Duncan, K.; Chang, L.; Patronas, M. MEK inhibitors: a new class of chemotherapeutic agents with ocular toxicity. Eye 2015, 29, 1003. [Google Scholar] [CrossRef]
- Welsh, S.J.; Corrie, P.G. Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma. Ther. Adv. Med Oncol. 2015, 7, 122–136. [Google Scholar] [CrossRef]
- Singh, R.; Lillard Jr, J.W. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Control. release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Greish, K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. In Cancer Nanotechnology; Humana Press: Totowa, NJ, USA, 2010; pp. 25–37. [Google Scholar]
- Kobayashi, H.; Watanabe, R.; Choyke, P.L. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics 2014, 4, 81. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.; Henriksen, J.R.; Jørgensen, J.T.; Amitay, Y.; Shmeeda, H.; Gabizon, A.A.; Kjær, A.; Andresen, T.L.; Hansen, A.E. Folate receptor targeting of radiolabeled liposomes reduces intratumoral liposome accumulation in human KB carcinoma xenografts. Int. J. Nanomed. 2018, 13, 7647. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Ji, Y. RGD-modified polymer and liposome nanovehicles: Recent research progress for drug delivery in cancer therapeutics. Eur. J. Pharm. Sci. 2018, 128, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Malaekeh-Nikouei, B.; Golmohammadzadeh, S.; Salmani-Chamanabad, S.; Mosallaei, N.; Jamialahmadi, K. Preparation, characterization, and moisturizing effect of liposomes containing glucosamine and N-acetyl glucosamine. J. Cosmet. Dermatol. 2013, 12, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New developments in liposomal drug delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef] [PubMed]
- D’souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): a versatile polymer for pharmaceutical applications. Expert Opin. drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Hendrix, M.J.; Seftor, E.A.; Seftor, R.E.; Chao, J.-T.; Chien, D.-S.; Chu, Y.-W. Tumor cell vascular mimicry: Novel targeting opportunity in melanoma. Pharmacol. Ther. 2016, 159, 83–92. [Google Scholar] [CrossRef]
- Udayakumar, D.; Zhang, G.; Ji, Z.; Njauw, C.-N.; Mroz, P.; Tsao, H. EphA2 is a critical oncogene in melanoma. Oncogene 2011, 30, 4921. [Google Scholar] [CrossRef]
- Neill, T.; Buraschi, S.; Goyal, A.; Sharpe, C.; Natkanski, E.; Schaefer, L.; Morrione, A.; Iozzo, R.V. EphA2 is a functional receptor for the growth factor progranulin. J. Cell Biol. 2016, 215, 687–703. [Google Scholar] [CrossRef]
- Tandon, M.; Vemula, S.V.; Mittal, S.K. Emerging strategies for EphA2 receptor targeting for cancer therapeutics. Expert Opin. Ther. Targets 2011, 15, 31–51. [Google Scholar] [CrossRef]
- Miao, B.; Ji, Z.; Tan, L.; Taylor, M.; Zhang, J.; Choi, H.G.; Frederick, D.T.; Kumar, R.; Wargo, J.A.; Flaherty, K.T. EPHA2 is a mediator of vemurafenib resistance and a novel therapeutic target in melanoma. Cancer Discov. 2015, 5, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wang, S.; De, S.K.; Barile, E.; Quinn, B.A.; Zharkikh, I.; Purves, A.; Stebbins, J.L.; Oshima, R.G.; Fisher, P.B. Design and characterization of novel EphA2 agonists for targeted delivery of chemotherapy to cancer cells. Chem. Biol. 2015, 22, 876–887. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Doddapaneni, R.; Sekar, V.; Chowdhury, N.; Singh, M. Combination approach of YSA peptide anchored docetaxel stealth liposomes with oral antifibrotic agent for the treatment of lung cancer. Mol. Pharm. 2016, 13, 2049–2058. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.R.; Pasquale, E.B.; Hristova, K. A small peptide promotes EphA2 kinase-dependent signaling by stabilizing EphA2 dimers. Biochim. et Biophys. Acta (BBA)-Gen. Subj. 2016, 1860, 1922–1928. [Google Scholar] [CrossRef][Green Version]
- Wang, S.; Placzek, W.J.; Stebbins, J.L.; Mitra, S.; Noberini, R.; Koolpe, M.; Zhang, Z.; Dahl, R.; Pasquale, E.B.; Pellecchia, M. Novel targeted system to deliver chemotherapeutic drugs to EphA2-expressing cancer cells. J. Med. Chem. 2012, 55, 2427–2436. [Google Scholar] [CrossRef] [PubMed]
- Koolpe, M.; Dail, M.; Pasquale, E.B. An ephrin mimetic peptide that selectively targets the EphA2 receptor. J. Biol. Chem. 2002, 277, 46974–46979. [Google Scholar] [CrossRef]
- Huynh, N.T.; Roger, E.; Lautram, N.; Benoît, J.-P.; Passirani, C. The rise and rise of stealth nanocarriers for cancer therapy: passive versus active targeting. Nanomedicine 2010, 5, 1415–1433. [Google Scholar] [CrossRef]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv. drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef]
- Bharali, D.J.; Khalil, M.; Gurbuz, M.; Simone, T.M.; Mousa, S.A. Nanoparticles and cancer therapy: a concise review with emphasis on dendrimers. Int. J. Nanomed. 2009, 4, 1–7. [Google Scholar]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751. [Google Scholar] [CrossRef]
- Zou, L.; Ding, W.; Zhang, Y.; Cheng, S.; Li, F.; Ruan, R.; Wei, P.; Qiu, B. Peptide-modified vemurafenib-loaded liposomes for targeted inhibition of melanoma via the skin. Biomaterials 2018, 182, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yap, M.L.; McFadyen, J.D.; Wang, X.; Zia, N.A.; Hohmann, J.D.; Ziegler, M.; Yao, Y.; Pham, A.; Harris, M.; Donnelly, P.S. Targeting activated platelets: a unique and potentially universal approach for cancer imaging. Theranostics 2017, 7, 2565. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Gowda, R.; Newswanger, R.K.; Leibich, P.; Fell, B.; Rosenberg, G.; Robertson, G.P. Targeting cholesterol transport in circulating melanoma cells to inhibit metastasis. Pigment. Cell Melanoma Res. 2017, 30, 541–552. [Google Scholar] [CrossRef]
- Nogueira, E.; Gomes, A.C.; Preto, A.; Cavaco-Paulo, A. Design of liposomal formulations for cell targeting. Colloids Surf. B Biointerfaces 2015, 136, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Marques-Gallego, P.; de Kroon, A.I. Ligation strategies for targeting liposomal nanocarriers. Biomed. Res. Int. 2014, 2014, 129458. [Google Scholar] [CrossRef]
- Riaz, M.K.; Riaz, M.A.; Zhang, X.; Lin, C.; Wong, K.H.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface Functionalization and Targeting Strategies of Liposomes in Solid Tumor Therapy: A Review. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Wagner, A.; Vorauer-Uhl, K. Liposome technology for industrial purposes. J. drug Deliv. 2011, 2011. [Google Scholar] [CrossRef]
- Guo, Z.; Li, Y.; Fu, Y.; Guo, T.; Li, X.; Yang, S.; Xie, J. Enhanced antisense oligonucleotide delivery using cationic liposomes incorporating fatty acid-modified polyethylenimine. Curr. Pharm. Biotechnol. 2014, 15, 800–805. [Google Scholar] [CrossRef]
- Patel, K.; Doddapaneni, R.; Chowdhury, N.; Boakye, C.H.; Behl, G.; Singh, M. Tumor stromal disrupting agent enhances the anticancer efficacy of docetaxel loaded PEGylated liposomes in lung cancer. Nanomedicine 2016, 11, 1377–1392. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975. [Google Scholar] [CrossRef] [PubMed]
- Tai, K.; Liu, F.; He, X.; Ma, P.; Mao, L.; Gao, Y.; Yuan, F. The effect of sterol derivatives on properties of soybean and egg yolk lecithin liposomes: Stability, structure and membrane characteristics. Food Res. Int. 2018, 109, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Weissig, V.; Pettinger, T.K.; Murdock, N. Nanopharmaceuticals (part 1): products on the market. Int. J. Nanomed. 2014, 9, 4357–4373. [Google Scholar] [CrossRef] [PubMed]
- Ingvarsson, P.T.; Yang, M.; Nielsen, H.M.; Rantanen, J.; Foged, C. Stabilization of liposomes during drying. Expert Opin. Drug Deliv. 2011, 8, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Wang, W. Lyophilization and development of solid protein pharmaceuticals. Int. J. Pharm. 2000, 203, 1–60. [Google Scholar] [CrossRef]
- Hedoux, A.; Paccou, L.; Achir, S.; Guinet, Y. Mechanism of protein stabilization by trehalose during freeze-drying analyzed by in situ micro-raman spectroscopy. J. Pharm. Sci. 2013, 102, 2484–2494. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Han, D.; Cai, C.; Tang, X. An overview of liposome lyophilization and its future potential. J. Control. Release 2010, 142, 299–311. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, L.; Qian, Y.; Chen, Y. The effects of cryoprotectants on the freeze-drying of ibuprofen-loaded solid lipid microparticles (SLM). Eur. J. Pharm. Biopharm. 2008, 69, 750–759. [Google Scholar] [CrossRef]
- Kumar, K.N.; Mallik, S.; Sarkar, K. Role of freeze-drying in the presence of mannitol on the echogenicity of echogenic liposomes. J. Acoust. Soc. Am. 2017, 142, 3670. [Google Scholar] [CrossRef]
- Wei, X.; Patil, Y.; Ohana, P.; Amitay, Y.; Shmeeda, H.; Gabizon, A.; Barenholz, Y. Characterization of Pegylated Liposomal Mitomycin C Lipid-Based Prodrug (Promitil) by High Sensitivity Differential Scanning Calorimetry and Cryogenic Transmission Electron Microscopy. Mol. Pharm. 2017, 14, 4339–4345. [Google Scholar] [CrossRef]
| Method | Solubility of TMB | Entrapment Efficiency of TPL (1% w/w TMB Loading) |
|---|---|---|
| Thin film hydration | Soluble | 51.6% |
| Modified hydration | Soluble | 96.2% |
| Particle Size (nm) | Zeta Potential (mV) | |||
|---|---|---|---|---|
| Before Freeze Drying | After Freeze Drying | Before Freeze Drying | After Freeze Drying | |
| YTPL (10% trehalose) | 91.20 ± 12.16 | 159.10 ± 7.50 | −0.92 ± 3.27 | −4.44 ± 0.49 |
| Unfiltered TPL | 109.45 ± 9.40 | 128.40 ± 1.84 | −35.55 ± 9.60 | −47.30 ± 1.61 |
| TMB Concentration (μg/mL) | ||||
|---|---|---|---|---|
| 100 | 50 | 10 | 2 | |
| % Hemolysis | 5.14 | 3.70 | 1.03 | 0 |
| IC50 (nM) | A375 | SK-MEL-28 |
|---|---|---|
| TMB | 0.83 ± 0.36 | 0.74 ± 0.38 |
| TPL | 0.68 ± 0.15 | 0.60 ± 0.13 |
| YTPL | 0.69 ± 0.40 | 0.77 ± 0.15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Rathod, D.; Abo-Ali, E.M.; Dukhande, V.V.; Patel, K. EphA2-Receptor Targeted PEGylated Nanoliposomes for the Treatment of BRAFV600E Mutated Parent- and Vemurafenib-Resistant Melanoma. Pharmaceutics 2019, 11, 504. https://doi.org/10.3390/pharmaceutics11100504
Fu Y, Rathod D, Abo-Ali EM, Dukhande VV, Patel K. EphA2-Receptor Targeted PEGylated Nanoliposomes for the Treatment of BRAFV600E Mutated Parent- and Vemurafenib-Resistant Melanoma. Pharmaceutics. 2019; 11(10):504. https://doi.org/10.3390/pharmaceutics11100504
Chicago/Turabian StyleFu, Yige, Drishti Rathod, Ehab M. Abo-Ali, Vikas V. Dukhande, and Ketan Patel. 2019. "EphA2-Receptor Targeted PEGylated Nanoliposomes for the Treatment of BRAFV600E Mutated Parent- and Vemurafenib-Resistant Melanoma" Pharmaceutics 11, no. 10: 504. https://doi.org/10.3390/pharmaceutics11100504
APA StyleFu, Y., Rathod, D., Abo-Ali, E. M., Dukhande, V. V., & Patel, K. (2019). EphA2-Receptor Targeted PEGylated Nanoliposomes for the Treatment of BRAFV600E Mutated Parent- and Vemurafenib-Resistant Melanoma. Pharmaceutics, 11(10), 504. https://doi.org/10.3390/pharmaceutics11100504





