A Micellar-Hydrogel Nanogrid from a UV Crosslinked Inulin Derivative for the Simultaneous Delivery of Hydrophobic and Hydrophilic Drugs
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
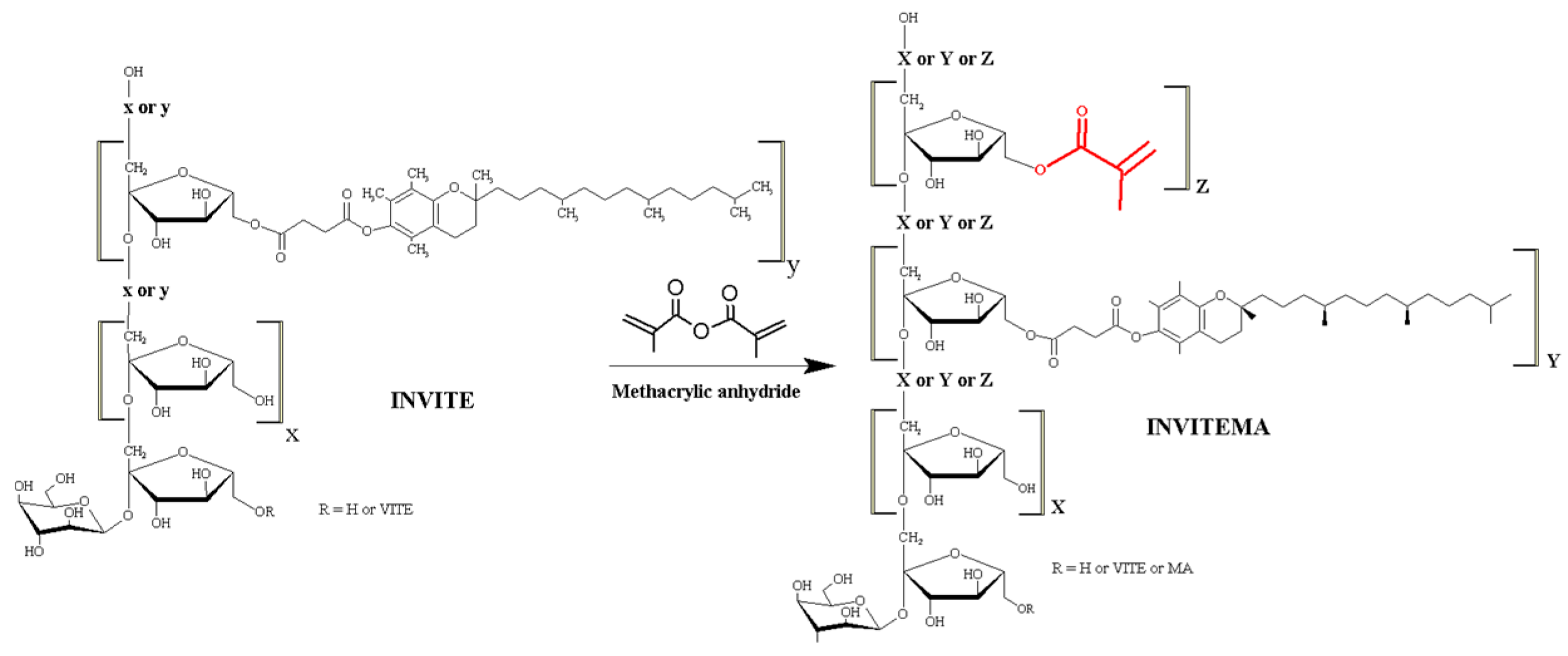
2.3. Synthesis of INVITEMA Conjugates
2.4. CAC Determination
2.5. Preparation and Characterization of Empty or Drug-Loaded INVITEMA Micelles by Direct Dialysis Method
2.6. Differential Scanning Calorimetry Studies
2.7. Size Distribution and Morphology
2.8. Preparation of INVITEMA Nanogrids
2.9. Water Uptake Studies
2.10. Drug Loading Evaluation on INVITEMA3 Nanogrid
2.11. Evaluation of Drug Stability upon UV Irradiation
2.12. Drug Release Studies from INVITEMA Nanogrids
3. Results
3.1. Synthesis and Characterization of INVITEMA Conjugates
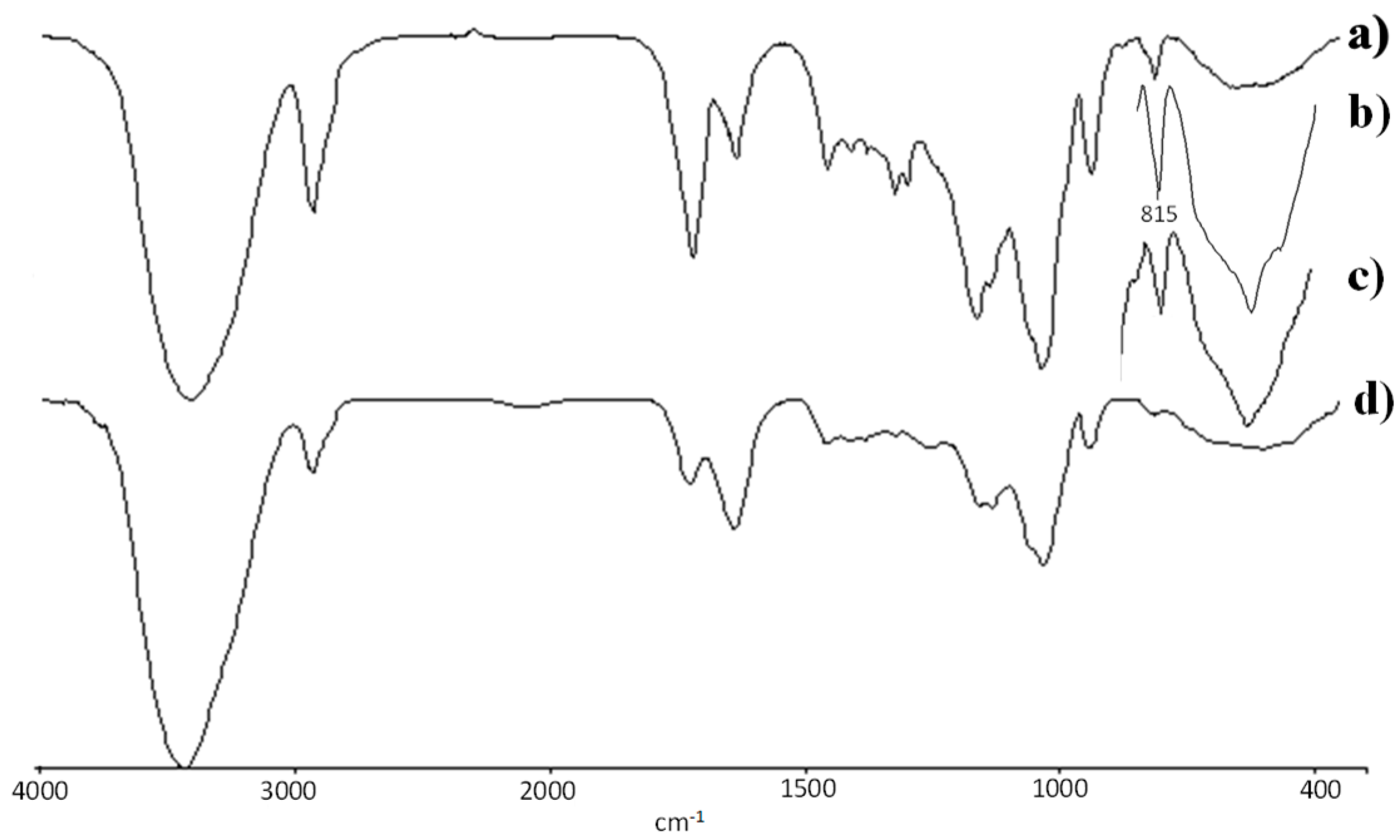
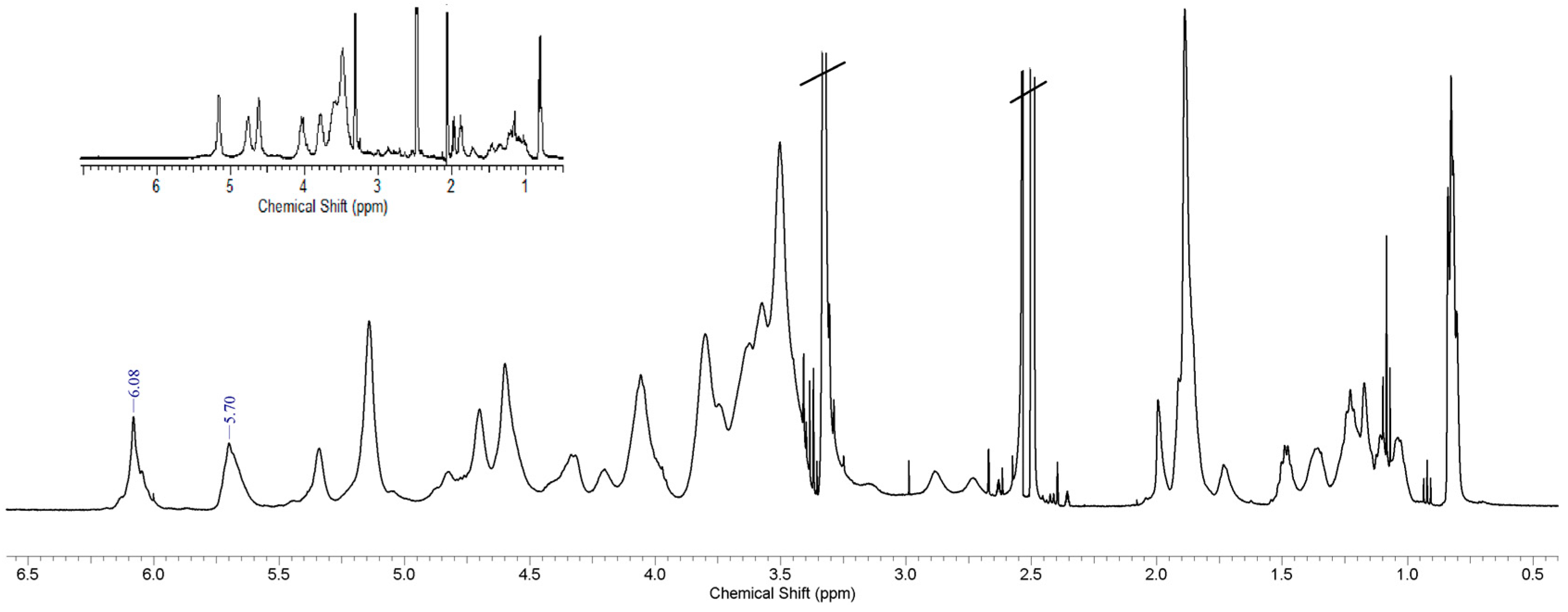
3.2. Spectroscopy Studies, FTIR and 1H-NMR
3.3. Thermal Behaviors of INVITEMA Conjugates
3.4. CAC Determination for INVITEMA Amphiphiles
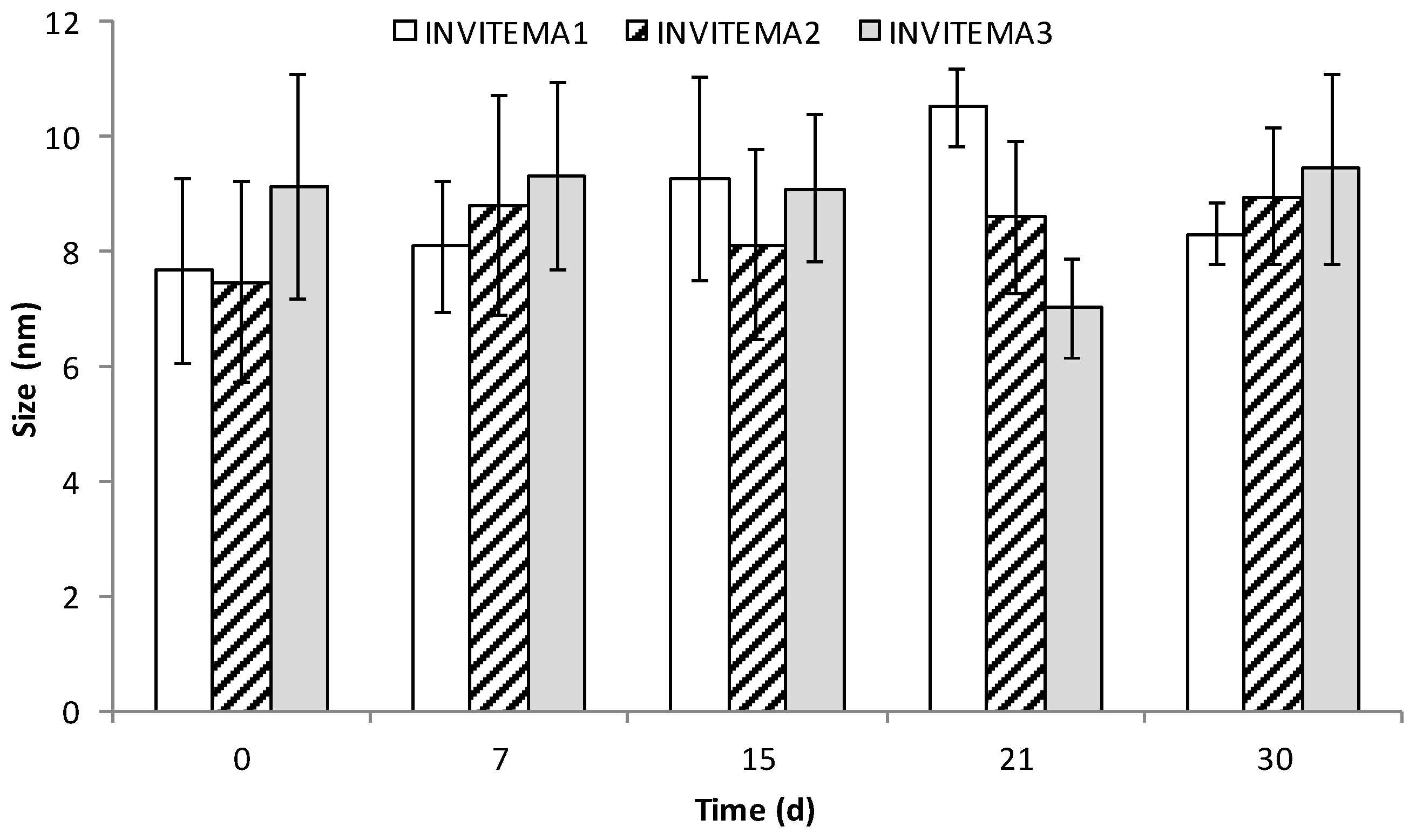
3.5. Size Distribution and Stability of INVITEMA Micelles before Crosslinking
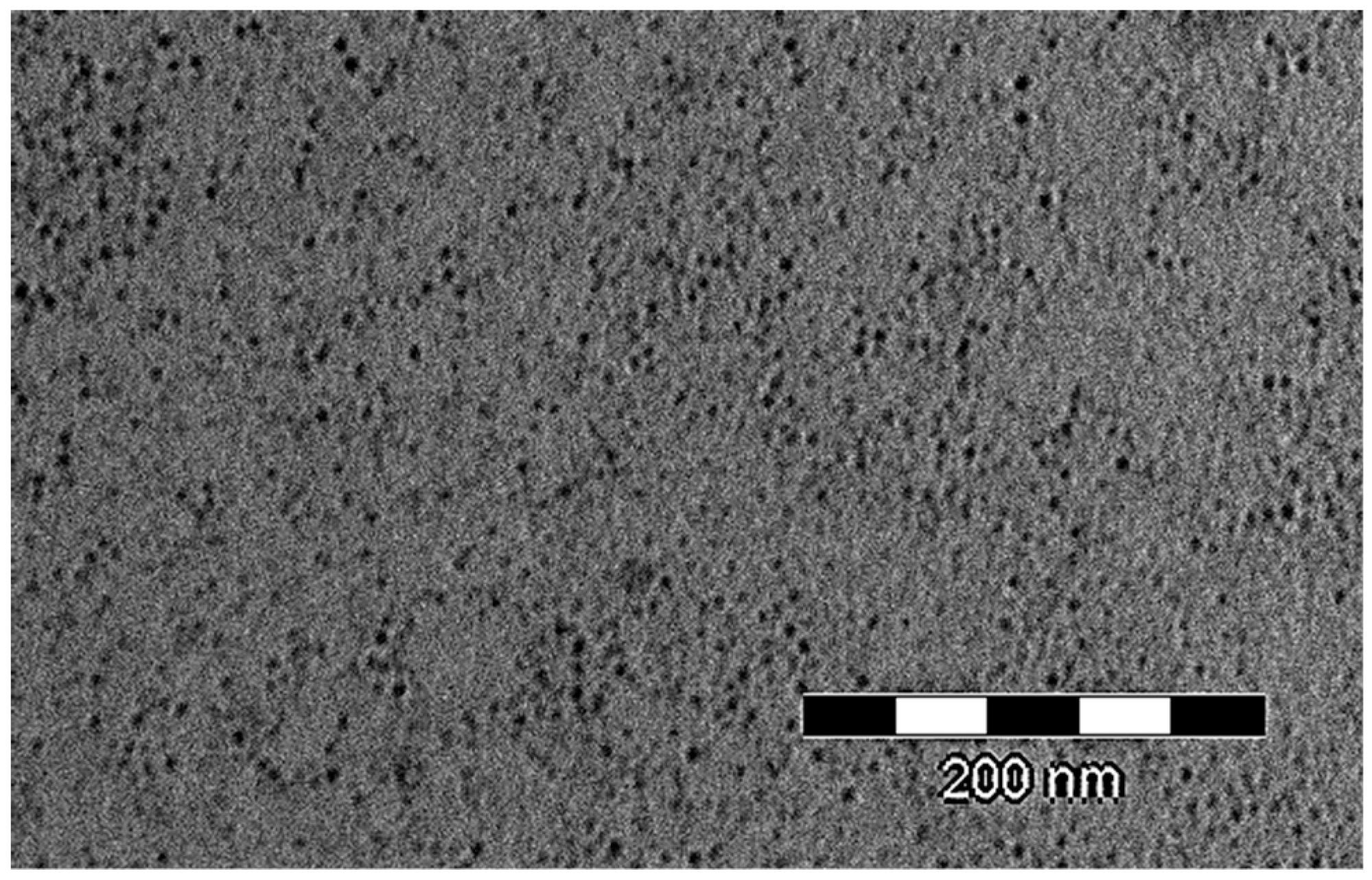
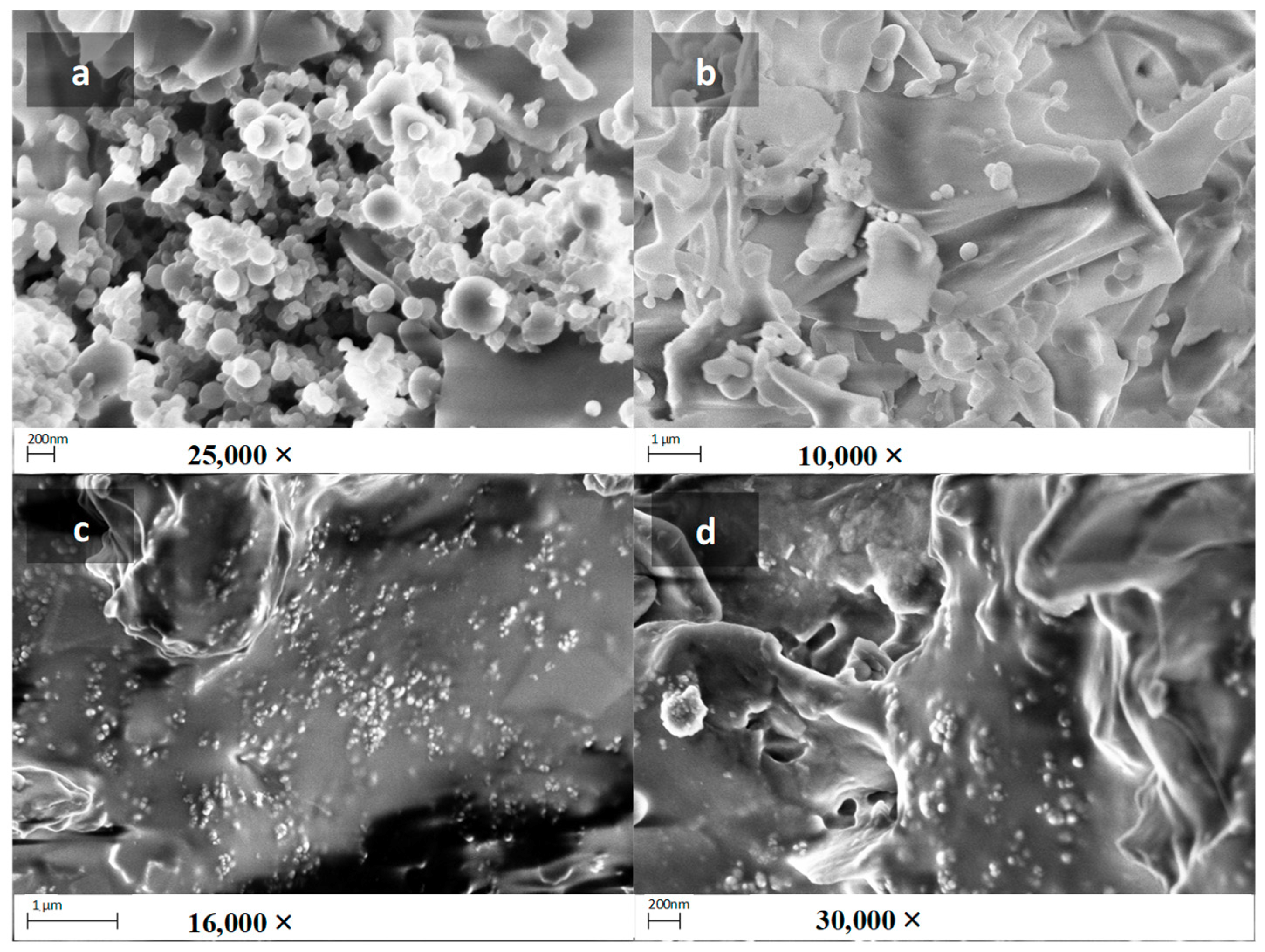
3.6. Nanogrid Formation, SEM and TEM Analysis
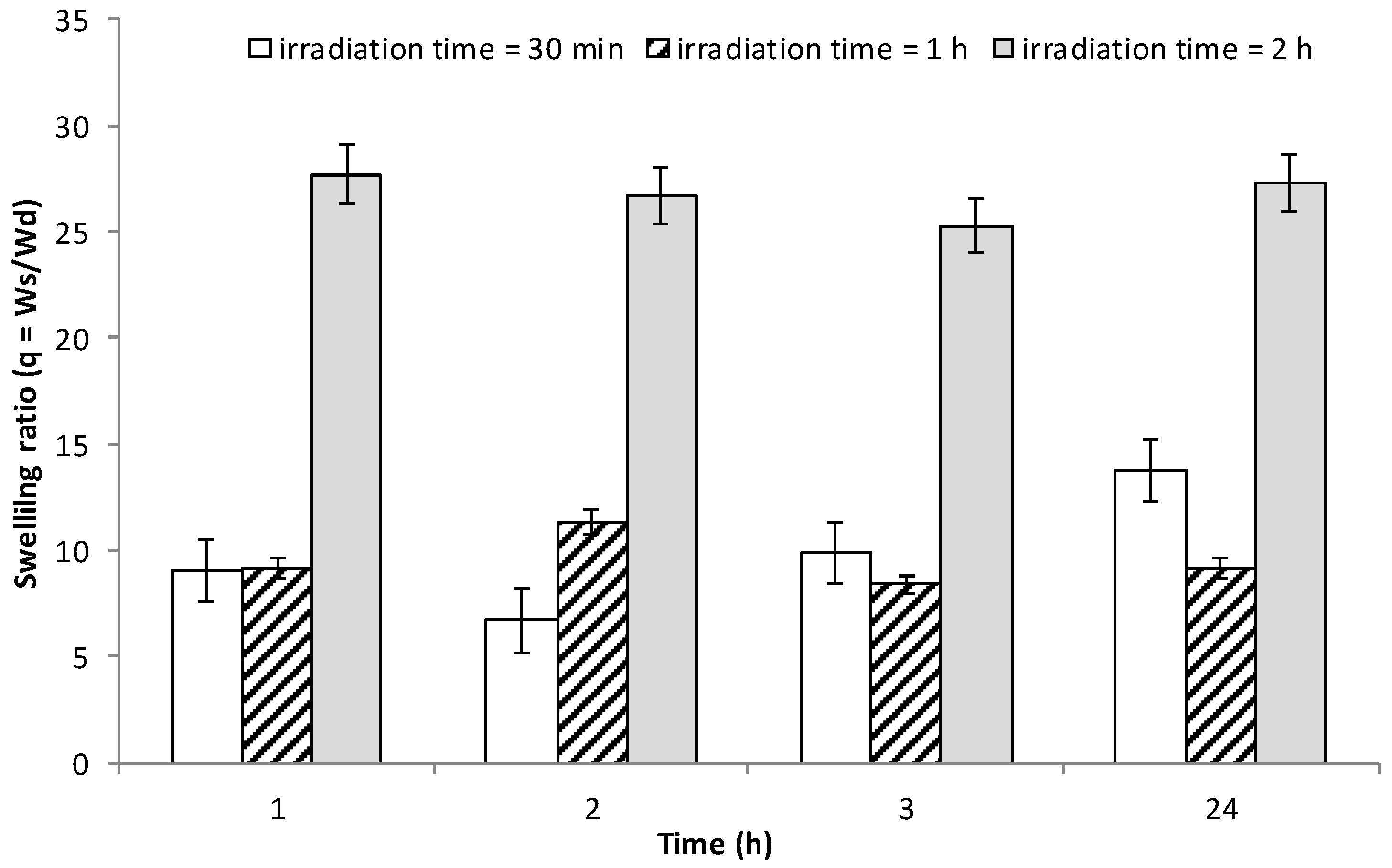
3.7. Water Uptake Studies on INVITEMA3
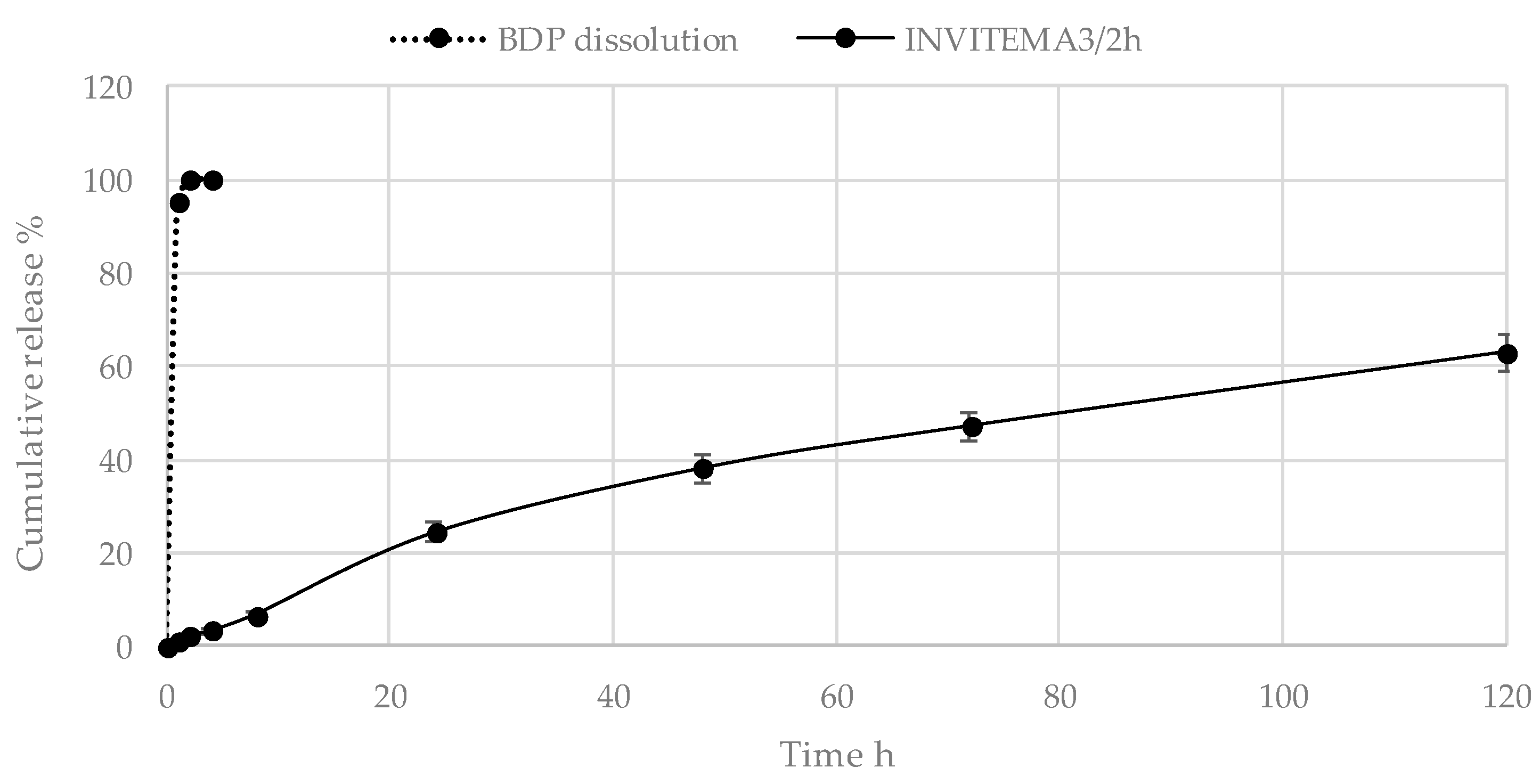
3.8. BDP Release Studies from the INVITEMA Nanogrids
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- De Las Heras Alarcón, C.; Pennadam, S.; Alexander, C. Stimuli responsive polymers for biomedical applications. Chem. Soc. Rev. 2005, 34, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, F.S.; Pitarresi, G.; Fiorica, C.; Matricardi, P.; Albanese, A.; Giammona, G. In situ forming hydrogels of new amino hyaluronic acid/benzoyl-cysteine derivatives as potential scaffolds for cartilage regeneration. Soft Matter 2012, 8, 4918–4927. [Google Scholar] [CrossRef]
- Tripodo, G.; Trapani, A.; Rosato, A.; Di Franco, C.; Tamma, R.; Trapani, G.; Ribatti, D.; Mandracchia, D. Hydrogels for biomedical applications from glycol chitosan and PEG diglycidyl ether exhibit pro-angiogenic and antibacterial activity. Carbohydr. Polym. 2018, 198, 124–130. [Google Scholar] [CrossRef]
- Pitarresi, G.; Casadei, M.A.; Mandracchia, D.; Paolicelli, P.; Palumbo, F.S.; Giammona, G. Photocrosslinking of dextran and polyaspartamide derivatives: A combination suitable for colon-specific drug delivery. J. Control. Release 2007, 119, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Perteghella, S.; Vigani, B.; Mastracci, L.; Grillo, F.; Antonioli, B.; Galuzzi, M.; Tosca, M.C.; Crivelli, B.; Preda, S.; Tripodo, G.; et al. Stromal vascular fraction loaded silk fibroin mats effectively support the survival of diabetic mice after pancreatic islet transplantation. Macromol. Biosci. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Choe, D.; Kim, Y.M.; Nam, J.E.; Nam, K.; Shin, C.S.; Roh, Y.H. Synthesis of high-strength microcrystalline cellulose hydrogel by viscosity adjustment. Carbohydr. Polym. 2018, 180, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Goh, M.; Hwang, Y.; Tae, G. Epidermal growth factor loaded heparin-based hydrogel sheet for skin wound healing. Carbohydr. Polym. 2016, 147, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Pitarresi, G.; Tripodo, G.; Calabrese, R.; Craparo, E.F.; Licciardi, M.; Giarnmona, G. Hydrogels for potential colon drug release by thiol-ene conjugate addition of a new inulin derivative. Macromol. Biosci. 2008, 8, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Jain, A.; Gupta, Y.; Ahirwar, M. Design and development of hydrogel beads for targeted drug delivery to the colon. AAPS PharmSciTech 2007, 8, E1–E8. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, A.R.; Silva, M.B.; Lopes, L.C.; Piai, J.F.; Rubira, A.F.; Muniz, E.C. Hydrogel based on an alginate-Ca2+/chondroitin sulfate matrix as a potential colon-specific drug delivery system. RSC Adv. 2012, 2, 11095–11103. [Google Scholar] [CrossRef]
- Oh, E.J.; Park, K.; Kim, K.S.; Kim, J.; Yang, J.A.; Kong, J.H.; Lee, M.Y.; Hoffman, A.S.; Hahn, S.K. Target specific and long-acting delivery of protein, peptide, and nucleotide therapeutics using hyaluronic acid derivatives. J. Control. Release 2010, 141, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Kedar, U.; Phutane, P.; Shidhaye, S.; Kadam, V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 714–729. [Google Scholar] [CrossRef] [PubMed]
- Balogh, A.; Domokos, A.; Farkas, B.; Farkas, A.; Rapi, Z.; Kiss, D.; Nyiri, Z.; Eke, Z.; Szarka, G.; Örkényi, R.; et al. Continuous end-to-end production of solid drug dosage forms: Coupling flow synthesis and formulation by electrospinning. Chem. Eng. J. 2018, 350, 290–299. [Google Scholar] [CrossRef]
- Cristiano, M.C.; Cosco, D.; Celia, C.; Tudose, A.; Mare, R.; Paolino, D.; Fresta, M. Anticancer activity of all-trans retinoic acid-loaded liposomes on human thyroid carcinoma cells. Colloids Surf. B Biointerfaces 2017, 150, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Targeted polymeric micelles for delivery of poorly soluble drugs. Cell. Mol. Life Sci. 2004, 61, 2549–2559. [Google Scholar] [CrossRef] [PubMed]
- Mandracchia, D.; Tripodo, G.; Latrofa, A.; Dorati, R. Amphiphilic inulin-d-α-tocopherol succinate (INVITE) bioconjugates for biomedical applications. Carbohydr. Polym. 2014, 103, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Tripodo, G.; Pasut, G.; Trapani, A.; Mero, A.; Lasorsa, F.M.; Chlapanidas, T.; Trapani, G.; Mandracchia, D. Inulin-d-α-tocopherol succinate (INVITE) nanomicelles as a platform for effective intravenous administration of curcumin. Biomacromolecules 2015, 16, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Tripodo, G.; Mandracchia, D.; Dorati, R.; Latrofa, A.; Genta, I.; Conti, B. Nanostructured polymeric functional micelles for drug delivery applications. Macromol. Symp. 2013, 334, 17–23. [Google Scholar] [CrossRef]
- Tripodo, G.; Chlapanidas, T.; Perteghella, S.; Vigani, B.; Mandracchia, D.; Trapani, A.; Galuzzi, M.; Tosca, M.C.; Antonioli, B.; Gaetani, P.; et al. Mesenchymal stromal cells loading curcumin-INVITE-micelles: A drug delivery system for neurodegenerative diseases. Colloids Surf. B Biointerfaces 2015, 125, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Tripodo, G.; Pitarresi, G.; Palumbo, F.S.; Craparo, E.F.; Giammona, G. UV-photocrosslinking of inulin derivatives to produce hydrogels for drug delivery application. Macromol. Biosci. 2005, 5, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, L.; Mandracchia, D.; Sorrenti, M.; Colombo, L.; Serra, M.; Tripodo, G. In-solution structural considerations by H-1 NMR and solid-state thermal properties of inulin-d-alpha-tocopherol succinate (INVITE) micelles as drug delivery systems for hydrophobic drugs. Macromol. Chem. Phys. 2014, 215, 2084–2096. [Google Scholar] [CrossRef]
- Mandracchia, D.; Trapani, A.; Perteghella, S.; Sorrenti, M.; Catenacci, L.; Torre, M.L.; Trapani, G.; Tripodo, G. PH-sensitive inulin-based nanomicelles for intestinal site-specific and controlled release of celecoxib. Carbohydr. Polym. 2018, 181, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Mandracchia, D.; Rosato, A.; Trapani, A.; Chlapanidas, T.; Montagner, I.M.; Perteghella, S.; Di Franco, C.; Torre, M.L.; Trapani, G.; Tripodo, G. Design, synthesis and evaluation of biotin decorated inulin-based polymeric micelles as long-circulating nanocarriers for targeted drug delivery. Nanomed. Nanotechnol. Biol. Med.e 2017, 13, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Mandracchia, D.; Tripodo, G.; Trapani, A.; Ruggieri, S.; Annese, T.; Chlapanidas, T.; Trapani, G.; Ribatti, D. Inulin based micelles loaded with curcumin or celecoxib with effective anti-angiogenic activity. Eur. J. Pharm. Sci. 2016, 93, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Rezazadeh, M.; Akbari, V.; Amuaghae, E.; Emami, J. Preparation and characterization of an injectable thermosensitive hydrogel for simultaneous delivery of paclitaxel and doxorubicin. Res. Pharm. Sci. 2018, 13, 181–191. [Google Scholar] [PubMed]
- Yun, Q.; Wang, S.S.; Xu, S.; Yang, J.P.; Fan, J.; Yang, L.L.; Chen, Y.; Fu, S.Z.; Wu, J.B. Use of 5-fluorouracil loaded micelles and cisplatin in thermosensitive chitosan hydrogel as an efficient therapy against colorectal peritoneal carcinomatosis. Macromol. Biosci. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Kaneko, T.; Matsumura, K. Switchable release nano-reservoirs for co-delivery of drugs via a facile micelle-hydrogel composite. J. Mater. Chem. B 2017, 5, 3488–3497. [Google Scholar] [CrossRef]
- Chen, J.; Ao, Y.; Lin, T.; Yang, X.; Peng, J.; Huang, W.; Li, J.; Zhai, M. High-toughness polyacrylamide gel containing hydrophobic crosslinking and its double network gel. Polymer 2016, 87, 73–80. [Google Scholar] [CrossRef]
- Liu, T.; Wu, T.; Liu, H.; Ke, B.; Huang, H.; Jiang, Z.; Xie, M. Ultraviolet-crosslinked hydrogel sustained-release hydrophobic antibiotics with long-term antibacterial activity and limited cytotoxicity. J. Appl. Polym. Sci. 2014, 131, 40438. [Google Scholar] [CrossRef]
- Wen, Y.; Li, F.; Li, C.; Yin, Y.; Li, J. High mechanical strength chitosan-based hydrogels cross-linked with poly(ethylene glycol)/polycaprolactone micelles for the controlled release of drugs/growth factors. J. Mater. Chem. B 2017, 5, 961–971. [Google Scholar] [CrossRef]
| Sample | Molar Amount of Methacrylic Anhydride (MA) | DD % mol/mol | Yield % (w/w) |
|---|---|---|---|
| INVITEMA1 | 0.20 | 18 ± 0.9 | 86 |
| INVITEMA2 | 0.35 | 33 ± 1.4 | 93 |
| INVITEMA3 | 0.50 | 49 ± 1.2 | 88 |
| Sample | Melting Point °C | Glass Transition (Tg) Midpoint °C |
|---|---|---|
| INVITEMA3 micelles | 51.4 ± 0.8 | |
| INVITEMA3 30 min nanogrid | 40.1 ± 0.9 | |
| INVITEMA3 1 h nanogrid | 64.6 ± 1.1 | |
| INVITEMA3 2 h nanogrid | ||
| BDP | 212 ± 0.9 | |
| INVITEMA3 2 h/BDP physical mixture | 200 ± 1.4 (BDP) | |
| INVITEMA3 2 h/BDP drug loaded |
| Sample | CAC (mM) |
|---|---|
| INVITEMA1 | 1.3 × 10−2 |
| INVITEMA2 | 1.2 × 10−2 |
| INVITEMA3 | 1.4 × 10−2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandracchia, D.; Trapani, A.; Perteghella, S.; Di Franco, C.; Torre, M.L.; Calleri, E.; Tripodo, G. A Micellar-Hydrogel Nanogrid from a UV Crosslinked Inulin Derivative for the Simultaneous Delivery of Hydrophobic and Hydrophilic Drugs. Pharmaceutics 2018, 10, 97. https://doi.org/10.3390/pharmaceutics10030097
Mandracchia D, Trapani A, Perteghella S, Di Franco C, Torre ML, Calleri E, Tripodo G. A Micellar-Hydrogel Nanogrid from a UV Crosslinked Inulin Derivative for the Simultaneous Delivery of Hydrophobic and Hydrophilic Drugs. Pharmaceutics. 2018; 10(3):97. https://doi.org/10.3390/pharmaceutics10030097
Chicago/Turabian StyleMandracchia, Delia, Adriana Trapani, Sara Perteghella, Cinzia Di Franco, Maria Luisa Torre, Enrica Calleri, and Giuseppe Tripodo. 2018. "A Micellar-Hydrogel Nanogrid from a UV Crosslinked Inulin Derivative for the Simultaneous Delivery of Hydrophobic and Hydrophilic Drugs" Pharmaceutics 10, no. 3: 97. https://doi.org/10.3390/pharmaceutics10030097
APA StyleMandracchia, D., Trapani, A., Perteghella, S., Di Franco, C., Torre, M. L., Calleri, E., & Tripodo, G. (2018). A Micellar-Hydrogel Nanogrid from a UV Crosslinked Inulin Derivative for the Simultaneous Delivery of Hydrophobic and Hydrophilic Drugs. Pharmaceutics, 10(3), 97. https://doi.org/10.3390/pharmaceutics10030097









