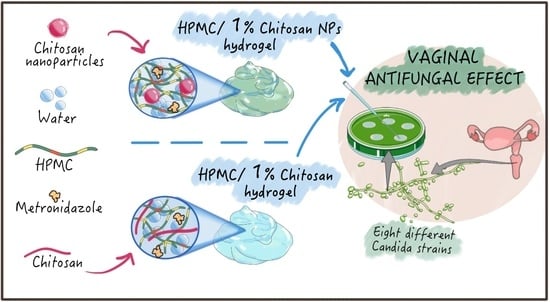
Chitosan Loaded into a Hydrogel Delivery System as a Strategy to Treat Vaginal Co-Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacterial Strains and Culture Conditions
2.3. Methods
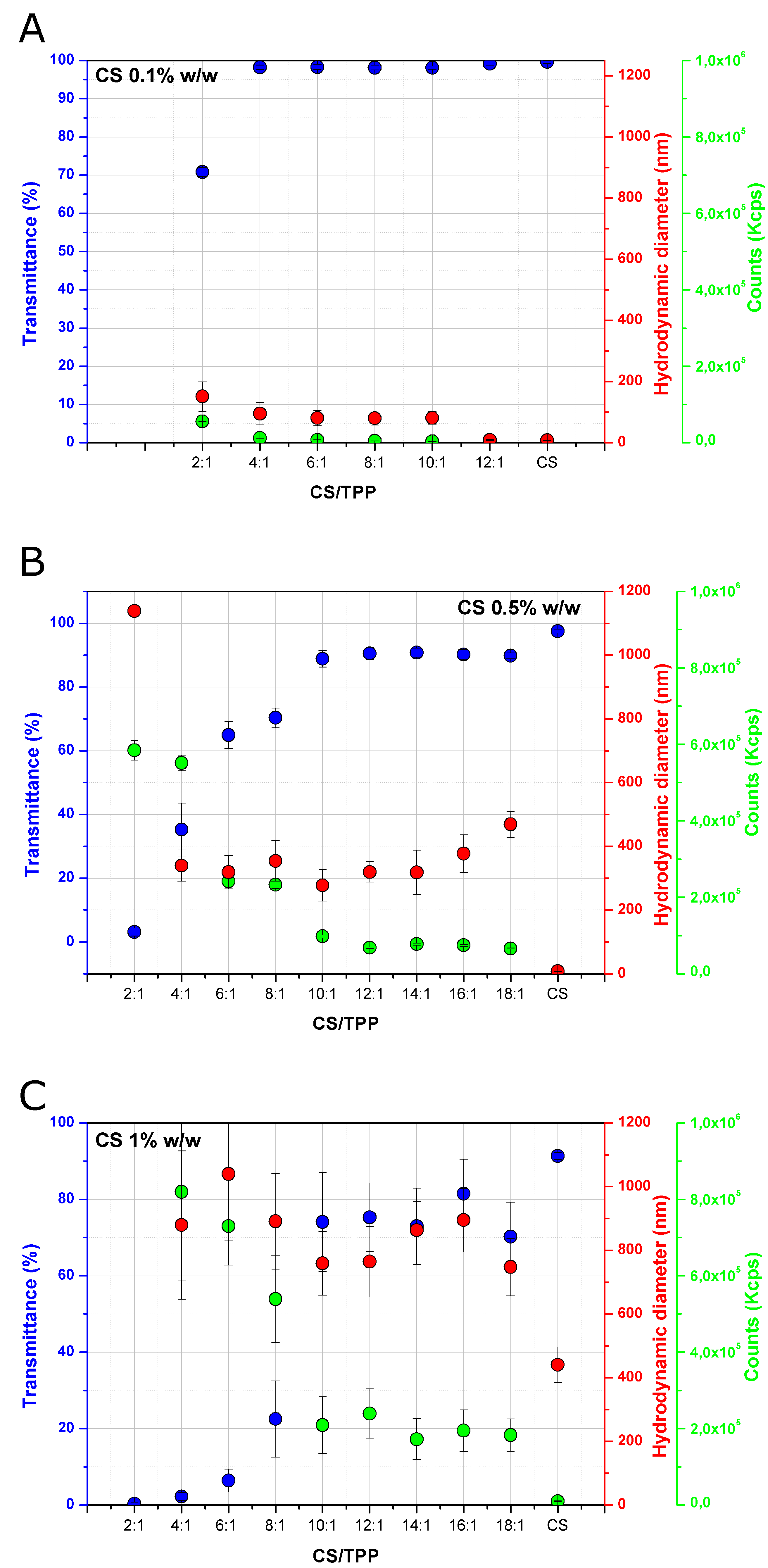
2.3.1. Preparation of Chitosan Nanoparticles (NPs)
2.3.2. Characterization of Chitosan Nanoparticles
2.3.3. Formulation of the Hydrogel Delivery System
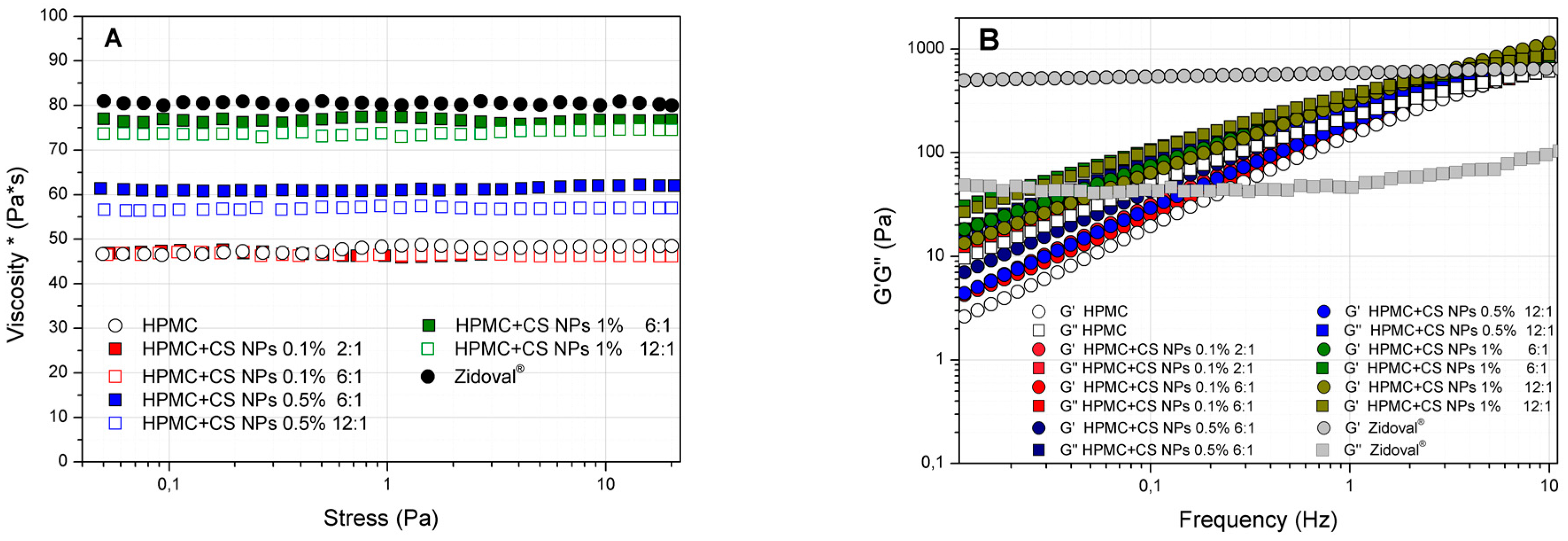
2.3.4. Rheological Analysis
2.3.5. Mucoadhesiveness Test
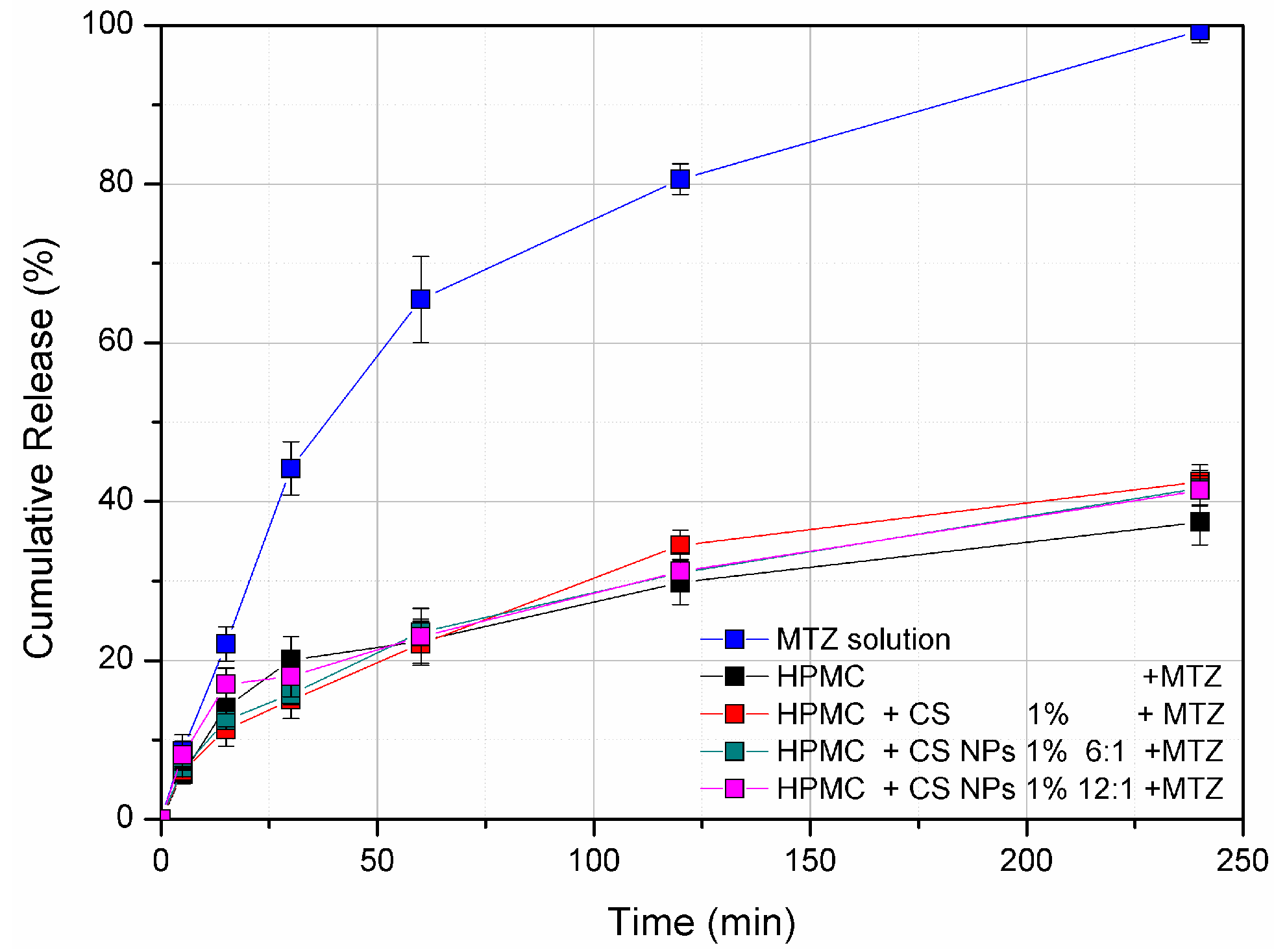
2.3.6. Franz Diffusion Cell Release Study
2.3.7. Antimicrobial Activity of the Hydrogels
2.3.8. Statistical Analyses
3. Results and Discussions
3.1. Characterization of Chitosan Nanoparticles
3.2. Rheological Analysis of HPMC/CS Hydrogels
3.3. Mucoadhesiveness Test
3.4. Franz diffusion Cell In Vitro Release Study
3.5. Antimicrobial Activity of HPMC/CS Hydrogels
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- El-Kamel, A.; Sokar, M.; Naggar, V.; Al Gamal, S. Chitosan and sodium alginate—Based bioadhesive vaginal tablets. AAPS Pharm. Sci. 2002, 4, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Bigucci, F.; Abruzzo, A.; Vitali, B.; Saladini, B.; Cerchiara, T.; Gallucci, M.C.; Luppi, B. Vaginal inserts based on chitosan and carboxymethylcellulose complexes for local delivery of chlorhexidine: Preparation, characterization and antimicrobial activity. Int. J. Pharm. 2015, 478, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Perioli, L.; Ambrogi, V.; Venezia, L.; Pagano, C.; Ricci, M.; Rossi, C. Chitosan and a modified chitosan as agents to improve performances of mucoadhesive vaginal gels. Colloids Surf. B Biointerfaces 2008, 66, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Andersen, T.; Mishchenko, E.; Flaten, E.G.; Sollid, U.J.; Mattsson, S.; Tho, I.; Škalko-Basnet, N. Chitosan-based nanomedicine to fight genital candida infections: Chitosomes. Mar. Drugs 2017, 15. [Google Scholar] [CrossRef] [PubMed]
- Palmeira-de-Oliveira, R.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J. New strategies for local treatment of vaginal infections. Adv. Drug Deliv. Rev. 2015, 92, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Casettari, L.; Cespi, M.; Palmieri, G.F.; Bonacucina, G. Characterization of the interaction between chitosan and inorganic sodium phosphates by means of rheological and optical microscopy studies. Carbohydr. Polym. 2013, 91, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Cespi, M.; Bonacucina, G.; Pucciarelli, S.; Cocci, P.; Perinelli, D.R.; Casettari, L.; Illum, L.; Palmieri, G.F.; Palermo, F.A.; Mosconi, G. Evaluation of thermosensitive poloxamer 407 gel systems for the sustained release of estradiol in a fish model. Eur. J. Pharm. Biopharm. 2014, 88, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Das Neves, J.; Bahia, M.F. Gels as vaginal drug delivery systems. Int. J. Pharm. 2006, 318, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Aka-Any-Grah, A.; Bouchemal, K.; Koffi, A.; Agnely, F.; Zhang, M.; Djabourov, M.; Ponchel, G. Formulation of mucoadhesive vaginal hydrogels insensitive to dilution with vaginal fluids. Eur. J. Pharm. Biopharm. 2010, 76, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Bonferoni, M.C.; Giunchedi, P.; Scalia, S.; Rossi, S.; Sandri, G.; Caramella, C. Chitosan gels for the vaginal delivery of lactic acid: Relevance of formulation parameters to mucoadhesion and release mechanisms. AAPS PharmSciTech 2006, 7, E141–E147. [Google Scholar] [CrossRef]
- Ensign, L.M.; Cone, R.; Hanes, J. Nanoparticle-based drug delivery to the vagina: A review. J. Control. Release 2014, 190, 500–514. [Google Scholar] [CrossRef] [PubMed]
- Vanić, Ž.; Škalko-Basnet, N. Nanopharmaceuticals for improved topical vaginal therapy: Can they deliver? Eur. J. Pharm. Sci. 2013, 50, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.A.; Sandri, G.; D’Autilia, F.; Contri, R.V.; Bonferoni, M.C.; Caramella, C.; Frank, A.G.; Pohlmann, A.R.; Guterres, S.S. Chitosan gel containing polymeric nanocapsules: A new formulation for vaginal drug delivery. Int. J. Nanomed. 2014, 9, 3151–3161. [Google Scholar] [CrossRef]
- Frank, L.A.; Chaves, P.S.; D’Amore, C.M.; Contri, R.V.; Frank, A.G.; Beck, R.C.R.; Pohlmann, A.R.; Buffon, A.; Guterres, S.S. The use of chitosan as cationic coating or gel vehicle for polymeric nanocapsules: Increasing penetration and adhesion of imiquimod in vaginal tissue. Eur. J. Pharm. Biopharm. 2017, 114, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Al-Kassas, R.; Wen, J.; Cheng, A.E.-M.; Kim, A.M.-J.; Liu, S.S.M.; Yu, J. Transdermal delivery of propranolol hydrochloride through chitosan nanoparticles dispersed in mucoadhesive gel. Carbohydr. Polym. 2016, 153, 176–186. [Google Scholar] [CrossRef] [PubMed]
- El-Leithy, E.S.; Shaker, D.S.; Ghorab, M.K.; Abdel-Rashid, R.S. Evaluation of mucoadhesive hydrogels loaded with diclofenac sodium–chitosan microspheres forrectal administration. AAPS PharmSciTech 2010, 11, 1695–1702. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Garrido-Maesto, A.; Jeong, K.C. Application, mode of action, and in vivo activity of chitosan and its micro- and nanoparticles as antimicrobial agents: A review. Carbohydr. Polym. 2017, 176, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Ing, L.J.; Zin, M.N.; Sarwar, A.; Katas, H. Antifungal activity of chitosan nanoparticles and correlation with their physical properties. Int. J. Biomater. 2012, 2112, 9. [Google Scholar] [CrossRef] [PubMed]
- Alburquenque, C.; Bucarey, S.A.; Neira-Carrillo, A.; Urzúa, B.; Hermosilla, G.; Tapia, C.V. Antifungal activity of low molecular weight chitosan against clinical isolates of Candida spp. Med. Mycol. 2010, 48, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Remunan-Lopez, C.; Vila-Jato, J.L.; Alonso, M.J. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Morris, G.A.; Castile, J.; Smith, A.; Adams, G.G.; Harding, S.E. The effect of prolonged storage at different temperatures on the particle size distribution of tripolyphosphate (TPP)—Chitosan nanoparticles. Carbohydr. Polym. 2011, 84, 1430–1434. [Google Scholar] [CrossRef]
- Gratieri, T.; Gelfuso, G.M.; Rocha, E.M.; Sarmento, V.H.; de Freitas, O.; Lopez, R.F.V. A poloxamer/chitosan in situ forming gel with prolonged retention time for ocular delivery. Eur. J. Pharm. Biopharm. 2010, 75, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Campana, R.; Martinelli, V.; Scoglio, S.; Colombo, E.; Benedetti, S.; Baffone, W. Influence of Aphanizomenon flos-aquae and two of its extracts on growth ability and antimicrobial properties of Lactobacillus acidophilus DDS-1. LWT—Food Sci. Technol. 2017, 81, 291–298. [Google Scholar] [CrossRef]
- Gan, Q.; Wang, T.; Cochrane, C.; McCarron, P. Modulation of surface charge, particle size and morphological properties of chitosan-TPP nanoparticles intended for gene delivery. Colloids Surf. B Biointerfaces 2005, 44, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Du, W.-L.; Niu, S.-S.; Xu, Y.-L.; Xu, Z.-R.; Fan, C.-L. Antibacterial activity of chitosan tripolyphosphate nanoparticles loaded with various metal ions. Carbohydr. Polym. 2009, 75, 385–389. [Google Scholar] [CrossRef]
- Yuan, Y.; Cui, Y.; Zhang, L.; Zhu, H.; Guo, Y.-S.; Zhong, B.; Hu, X.; Zhang, L.; Wang, X.; Chen, L. Thermosensitive and mucoadhesive in situ gel based on poloxamer as new carrier for rectal administration of nimesulide. Int. J. Pharm. 2012, 430, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Malli, S.; Bories, C.; Pradines, B.; Loiseau, P.M.; Ponchel, G.; Bouchemal, K. In situ forming Pluronic® F127/chitosan hydrogel limits metronidazole transmucosal absorption. Eur. J. Pharm. Biopharm. 2017, 112, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Allan, C.R.; Hadwiger, L.A. The fungicidal effect of chitosan on fungi of varying cell wall composition. Exp. Mycol. 1979, 3, 285–287. [Google Scholar] [CrossRef]
- Meng, X.; Yang, L.; kennedy, J.F.; Tian, S. Effects of chitosan and oligochitosan on growth of two fungal pathogens and physiological properties in pear fruit. Carbohydr. Polym. 2010, 81, 70–75. [Google Scholar] [CrossRef]
- Palma-Guerrero, J.; Jansson, H.-B.; Salinas, J.; Lopez-Llorca, L.V. Effect of chitosan on hyphal growth and spore germination of plant pathogenic and biocontrol fungi. J. Appl. Microbiol. 2008, 104, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Seyfarth, F.; Schliemann, S.; Elsner, P.; Hipler, U.-C. Antifungal effect of high- and low-molecular-weight chitosan hydrochloride, carboxymethyl chitosan, chitosan oligosaccharide and N-acetyl-d-glucosamine against Candida albicans, Candida krusei and Candida glabrata. Int. J. Pharm. 2008, 353, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Tayel, A.A.; Moussa, S.; El-Tras, W.F.; Knittel, D.; Opwis, K.; Scholmeyer, E. Anticandidal action of fungal chitosan against Candida albicans. Int. J. Biol. Macromol. 2010, 47, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Palmeira-de-Oliveira, A.; Ribeiro, M.P.; Palmeira-de-Oliveira, R.; Gaspar, C.; Costa-de-Oliveira, S.; Correia, I.J.; Pina Vaz, C.; Martinez-de-Oliveira, J.; Queiroza, J.A.; Rodrigues, A.G. Anti-Candida activity of a chitosan hydrogel: Mechanism of action and ctotoxicity profile. Gynecol. Obstet. Investig. 2010, 70, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Achkar, J.M.; Fries, B.C. Candida infections of the genitourinary tract. Clin. Microbiol. Rev. 2010, 23, 253–273. [Google Scholar] [CrossRef] [PubMed]
- Hetticarachchi, N.; Ashbee, H.R.; Wilson, J.D.; Wilson, J.D. Prevalence and management of non-albicans vaginal candidiasis. Sex. Transm. Infect. 2010, 86, 99–100. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D.; Akins, R.A. The Role of Resistance in Candida Infections: Epidemiology and Treatment BT—Antimicrobial Drug Resistance: Clinical and Epidemiological Aspects; Mayers, D.L., Sobel, J.D., Ouellette, M., Kaye, K.S., Marchaim, D., Eds.; Springer International Publishing: Cham, Switzerland, 2017; Volume 2, pp. 1075–1097. ISBN 978-3-319-47266-9. [Google Scholar]
- Kirkpatrick, W.R.; Turner, T.M.; Fothergill, A.W.; McCarthy, D.I.; Redding, S.W.; Rinaldi, M.G.; Patterson, T.F. Fluconazole disk diffusion susceptibility testing of Candida species. J. Clin. Microbiol. 1998, 36, 3429–3432. [Google Scholar] [PubMed]
- Chien, H.F.; Chen, C.P.; Chen, Y.C.; Chang, P.H.; Tsai, T.; Chen, C.T. The Use of Chitosan to Enhance photodynamic inactivation against Candida albicans and its drug-resistant clinical isolates. Int. J. Mol. Sci. 2013, 14, 7445–7456. [Google Scholar] [CrossRef] [PubMed]
- De Groot, P.W.J.; Kraneveld, E.A.; Yin, Q.Y.; Dekker, H.L.; Gross, U.; Crielaard, W.; de Koster, C.G.; Bader, O.; Klis, F.M.; Weig, M. The cell wall of the human pathogen Candida glabrata: Differential incorporation of novel adhesin-like wall proteins. Eukaryot. Cell 2008, 7, 1951–1964. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Hosoya, O.; Taoka, S.; Seki, T.; Kawaguchi, T.; Sigibayashi, K.; Juny, K.; Morimoto, Y. Relationship between solubility of chitosan in alcoholic solution and its gelation. Chem. Pharm. Bull. 1999, 47, 1044–1046. [Google Scholar] [CrossRef]

| Formulations | Fmax (N) | SMax (mm) | Worktot(mJ) | Workin (mJ) |
|---|---|---|---|---|
| HPMC | 0.017 ± 0.001 | 1.615 ± 1.107 | 0.057 ± 0.009 | 0.017 ± 0.003 |
| HPMC + CS 0.5% | 0.018 ± 0.001 | 1.176 ± 0.183 | 0.060 ± 0.020 | 0.019 ± 0.003 |
| HPMC + CS 1% | 0.021 ± 0.002 * | 1.224 ± 0.231 | 0.064 ± 0.025 | 0.020 ± 0.014 |
| HPMC + CS NPs 1% 12:1 | 0.022 ± 0.002 * | 1.056 ± 0.232 | 0.065 ± 0.020 | 0.019 ± 0.004 |
| HPMC + CS NPs 1% 6:1 | 0.023 ± 0.002 * | 0.888 ± 0.267 | 0.060 ± 0.022 | 0.019 ± 0.018 |
| HPMC + CS NPs 0.5% 12:1 | 0.018 ± 0.002 | 1.389 ± 0.182 | 0.058 ± 0.027 | 0.018 ± 0.006 |
| HPMC + CS NPs 0.5% 6:1 | 0.020 ± 0.003 | 0.899 ± 0.250 | 0.051 ± 0.018 | 0.017 ± 0.004 |
| Inhibition Growth Diameter (mm) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Formulations | Albicans Strains | Non-Albicans Strains | ||||||
| C. albicans 11/01 | C. albicans 18/01 | C. albicans 4940 | C. albicans 360923 | C. glabrat 104/1 | C. glabrata 104/22 | C. glabrata 49/55 | C. lusitaniae 360804 | |
| CS 1% | 12 ± 0.6 | 12 ± 0.8 | 13 ± 0.3 | 13 ± 0.2 | 12 ± 0.6 | 13 ± 0.2 | 13 ± 0.3 | 13 ± 0.2 |
| CS NPs 1% 6:1 | 0 | 0 | 0 | 0 | 9 ± 0.2 | 9 ± 0.3 | 9 ± 0.3 | 10 ± 0.1 |
| CS NPs 1% 12:1 | 0 | 0 | 0 | 0 | 12 ± 0.8 | 11 ± 0.6 | 12 ± 0.7 | 13 ± 0.2 |
| HPMC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| HPMC CS 1% | 12 ± 0.5 | 12 ± 0.6 | 12 ± 0.4 | 11 ± 0.3 | 12 ± 0.4 | 11 ± 0.4 | 12 ± 0.3 | 11 ± 0.2 |
| HPMC + CS NPs 1% 6:1 | 9 ± 0.2 | 8 ± 0.3 | 8 ± 0.2 | 11 ± 0.6 | 9 ± 0.3 | 9 ± 0.2 | 10 ± 0.5 | 9 ± 0.1 |
| HPMC + CS NPs 1% 12:1 | 9 ± 0.2 | 11 ± 0.3 | 8 ± 0.2 | 9 ± 0.2 | 10 ± 0.8 | 9 ± 0.2 | 12 ± 0.6 | 15 ± 0.6 |
| MTZ 0.75% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CS 1% MTZ 0.75% | 12 ± 0.6 | 12 ± 0.8 | 13 ± 0.2 | 12 ± 0.8 | 13 ± 0.3 | 14 ± 0.3 | 14 ± 0.3 | 13 ± 0.2 |
| CS NPs 1% 6:1 + MTZ 0.75% | 0 | 0 | 0 | 0 | 9 ± 0.4 | 9 ± 0.4 | 9 ± 0.2 | 11 ± 0.2 |
| CS NPs 1% 12:1 + MTZ 0.75% | 0 | 0 | 0 | 0 | 12 ± 0.6 | 11 ± 0.5 | 12 ± 0.6 | 12 ± 0.7 |
| HPMC + MTZ 0.75% | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| HPMC+CS 1% + MTZ 0.75% | 13 ± 0.5 | 12 ± 0.5 | 11 ± 0.4 | 11 ± 0.2 | 13 ± 0.4 | 10 ± 0.4 | 11 ± 0.2 | 11 ± 0.2 |
| HPMC + CS NPs 1% 6:1 + MTZ 0.75% | 9 ± 0.2 | 9 ± 0.3 | 8 ± 0.2 | 9 ± 0.2 | 9 ± 0.2 | 9 ± 0.2 | 9 ± 0.2 | 11 ± 0.3 |
| HPMC + CS NPs 1% 12:1 + MTZ 0.75% | 12 ± 0.7 | 11 ± 0.6 | 12 ± 1.0 | 10 ± 0.4 | 10 ± 0.6 | 11 ± 0.5 | 12 ± 1.0 | 11 ± 0.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perinelli, D.R.; Campana, R.; Skouras, A.; Bonacucina, G.; Cespi, M.; Mastrotto, F.; Baffone, W.; Casettari, L. Chitosan Loaded into a Hydrogel Delivery System as a Strategy to Treat Vaginal Co-Infection. Pharmaceutics 2018, 10, 23. https://doi.org/10.3390/pharmaceutics10010023
Perinelli DR, Campana R, Skouras A, Bonacucina G, Cespi M, Mastrotto F, Baffone W, Casettari L. Chitosan Loaded into a Hydrogel Delivery System as a Strategy to Treat Vaginal Co-Infection. Pharmaceutics. 2018; 10(1):23. https://doi.org/10.3390/pharmaceutics10010023
Chicago/Turabian StylePerinelli, Diego R., Raffaella Campana, Athanasios Skouras, Giulia Bonacucina, Marco Cespi, Francesca Mastrotto, Wally Baffone, and Luca Casettari. 2018. "Chitosan Loaded into a Hydrogel Delivery System as a Strategy to Treat Vaginal Co-Infection" Pharmaceutics 10, no. 1: 23. https://doi.org/10.3390/pharmaceutics10010023
APA StylePerinelli, D. R., Campana, R., Skouras, A., Bonacucina, G., Cespi, M., Mastrotto, F., Baffone, W., & Casettari, L. (2018). Chitosan Loaded into a Hydrogel Delivery System as a Strategy to Treat Vaginal Co-Infection. Pharmaceutics, 10(1), 23. https://doi.org/10.3390/pharmaceutics10010023