Development of a Region-Specific Physiologically Based Pharmacokinetic Brain Model to Assess Hippocampus and Frontal Cortex Pharmacokinetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Step 1: A Whole-Body Physiologically Based Pharmacokinetic (PBPK) CNS Model
2.2. Step 2: Development of a Rat Regional Brain PBPK Sub-Model
- The CNS is represented by five compartments, namely CSF, intracranial blood, rest of brain tissue, frontal cortex, and hippocampus;
- All compartments are well stirred, with permeability barriers between the intracranial blood and brain;
- There is no rate-limiting diffusion barrier between the ECF and CSF, and the drug equilibration between these two compartments is rapid [29];
- Only an unbound drug, governed by unbound fraction in plasma (fu,plasma), brain tissue (fu,brain) or CSF (fu,CSF), was considered capable of crossing permeability barriers;
- In the absence of published regional fu,brain, the unbound brain fraction was assumed to be equivalent for all brain regions (i.e., hippocampus, rest of brain, and frontal cortex) [47];
- Within the extracellular space of the brain, fluids move either by diffusion or by bulk flow (Qbulk) [48];
- Due to the absence of regional brain in vitro or in vivo permeability data, the regional brain bi-directional passive transport (PS) term was scaled from in vitro Papp and corrected for the regional tissue weight (Table 2, assuming density = 1) using Equations (4) and (5), wherein the term “brain weight” is replaced by “regional brain weight”;
- The temporal concentration profile of the drug in the regional brain ECF would mimic the biophase sampled during microdialysis studies [50];
- Since the liver was considered the only site of clearance for phenytoin based on the literature [51], the prediction for unbound renal clearance (CLR) was excluded from the simulation;
- Active transport from brain tissues (Efflux: CLBout; Influx: CLBin) can be determined as described in our previous CNS PBPK model [28].
2.3. Step 3: Development of a Human Regional Brain PBPK Sub-Model
3. Results
3.1. Step 1: Validation of the PBPK Model
3.2. Step 2: Development of a Rat Regional Brain PBPK Sub-Model
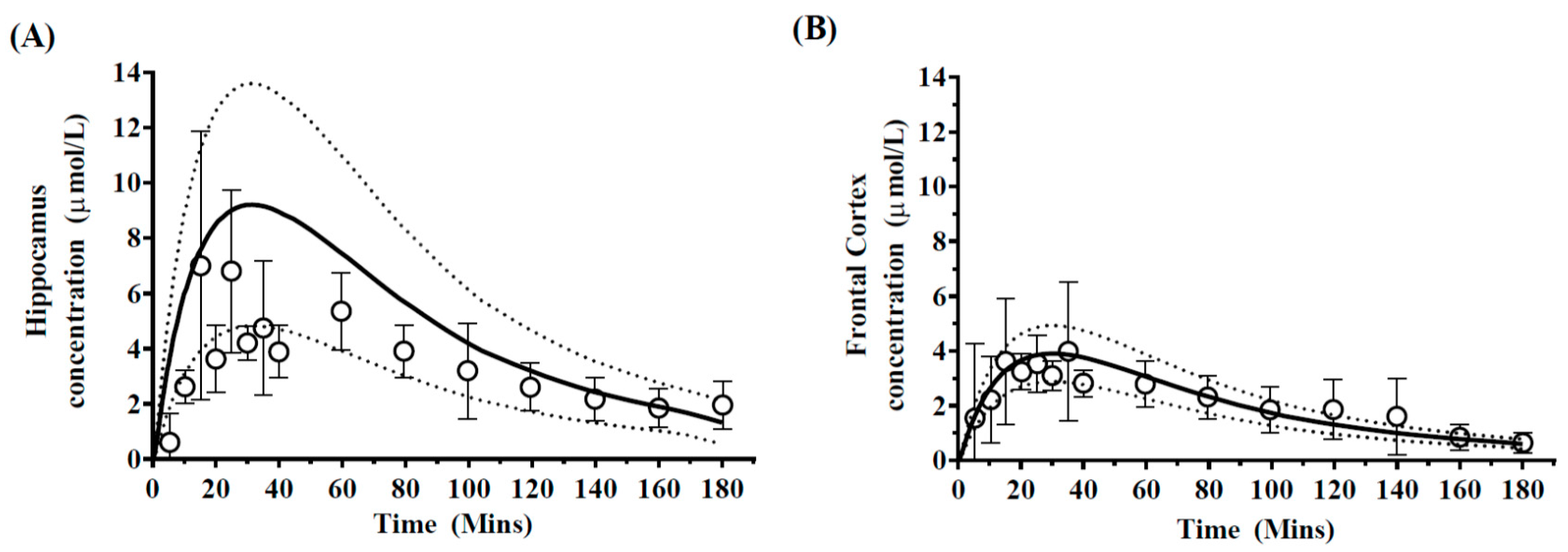
3.2.1. Case 1: Phenyotin
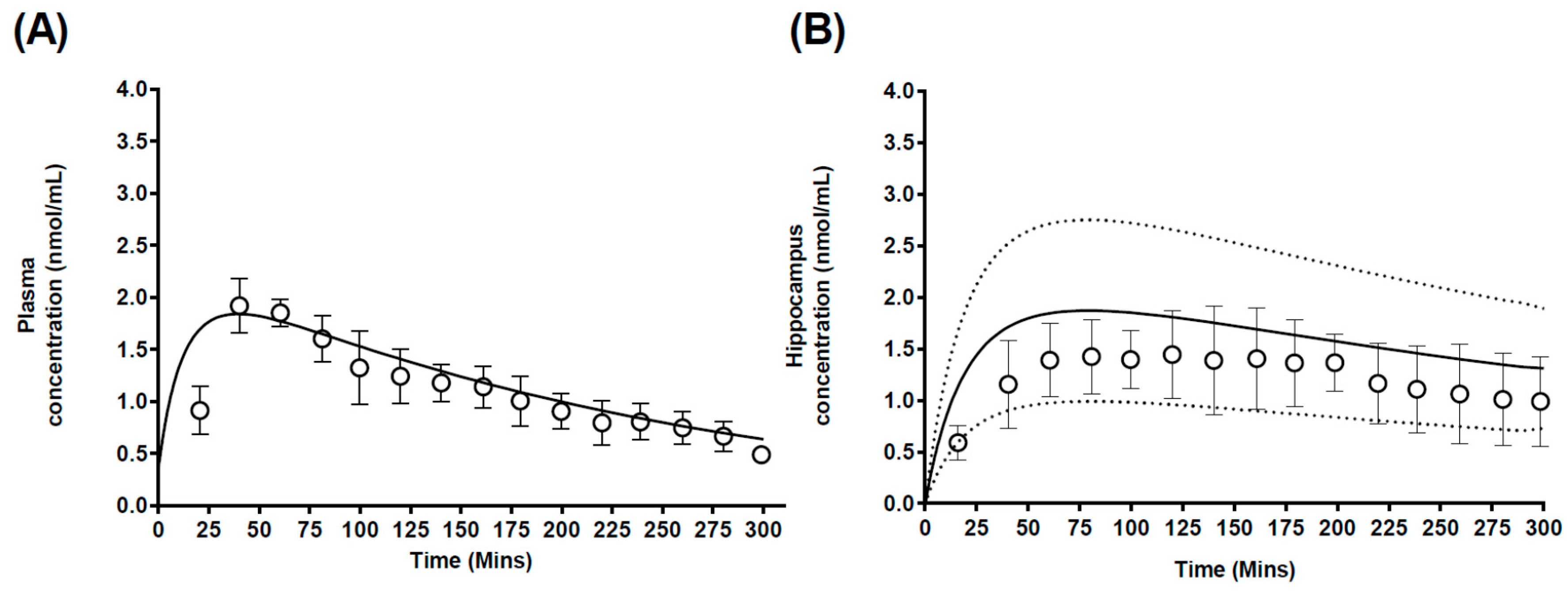
3.2.2. Case 2: Carbamazepine
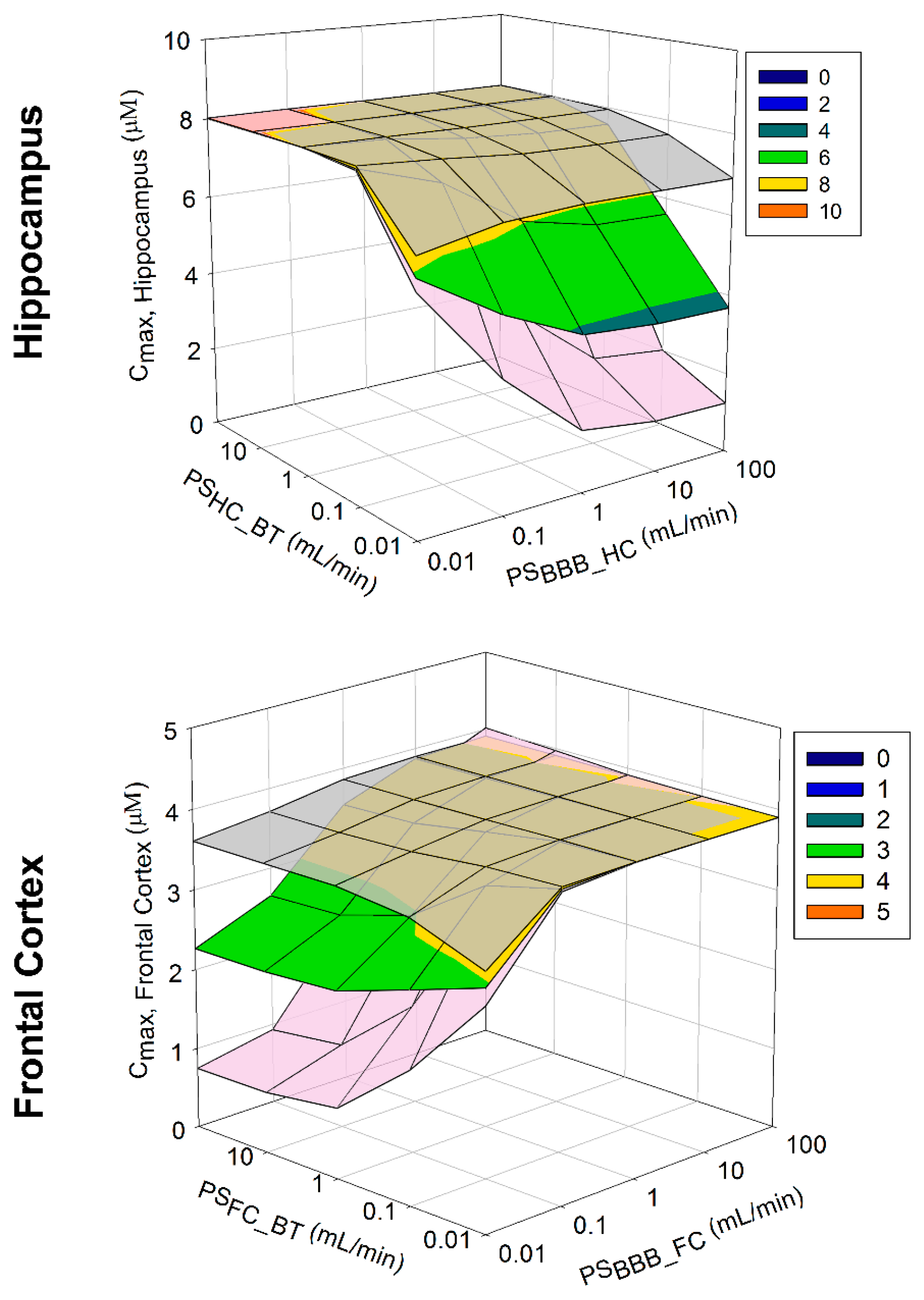
3.2.3. Model Sensitivity Analysis
3.3. Step 3: Development of a Human Regional Brain PBPK Sub-Model
4. Discussion
4.1. Validation of the PBPK Model
4.2. Prediction of Regional Brain Concentrations in Rats
Model Sensitivity Analysis
4.3. Prediction of Regional Brain Concentrations in Humans
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Olesen, J.; Baker, M.G.; Freund, T.; di Luca, M.; Mendlewicz, J.; Ragan, I.; Westphal, M. Consensus document on european brain research. J. Neurol. Neurosurg. Psychiatry 2006, 77 (Suppl. 1), 1–49. [Google Scholar]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Wolburg, H.; Lippoldt, A. Tight junctions of the blood-brain barrier: Development, composition and regulation. Vasc. Pharmacol. 2002, 38, 323–337. [Google Scholar] [CrossRef]
- Alavijeh, M.S.; Chishty, M.; Qaiser, M.Z.; Palmer, A.M. Drug metabolism and pharmacokinetics, the blood-brain barrier, and central nervous system drug discovery. NeuroRX 2005, 2, 554–571. [Google Scholar] [CrossRef] [PubMed]
- Feher, M.; Sourial, E.; Schmidt, J.M. A simple model for the prediction of blood-brain partitioning. Int. J. Pharm. 2000, 201, 239–247. [Google Scholar] [CrossRef]
- Vilar, S.; Chakrabarti, M.; Costanzi, S. Prediction of passive blood-brain partitioning: Straightforward and effective classification models based on in silico derived physicochemical descriptors. J. Mol. Graph. Model. 2010, 28, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, P.; Summerfield, S. Assessment of the blood-brain barrier in CNS drug discovery. Neurobiol. Dis. 2010, 37, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.A.; Di, L.; Kerns, E.H. The effect of plasma protein binding on in vivo efficacy: Misconceptions in drug discovery. Nat. Rev. Drug Discov. 2010, 9, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Elmquist, W.F.; Sawchuk, R.J. Application of microdialysis in pharmacokinetic studies. Pharm. Res. 1997, 14, 267–288. [Google Scholar] [CrossRef] [PubMed]
- Hammarlund-Udenaes, M.; Paalzow, L.K.; de Lange, E.C. Drug equilibration across the blood-brain barrier--pharmacokinetic considerations based on the microdialysis method. Pharm. Res. 1997, 14, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.P.; Delp, M.D.; Lindstedt, S.L.; Rhomberg, L.R.; Beliles, R.P. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol. Ind. Health 1997, 13, 407–484. [Google Scholar] [CrossRef] [PubMed]
- Kalvass, J.C.; Maurer, T.S. Influence of nonspecific brain and plasma binding on CNS exposure: Implications for rational drug discovery. Biopharm. Drug Dispos. 2002, 23, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Doran, A.; Obach, R.S.; Smith, B.J.; Hosea, N.A.; Becker, S.; Callegari, E.; Chen, C.; Chen, X.; Choo, E.; Cianfrogna, J.; et al. The impact of P-glycoprotein on the disposition of drugs targeted for indications of the central nervous system: Evaluation using the mdr1a/1b knockout mouse model. Drug Metab. Dispos. 2005, 33, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Smith, B.J.; Chen, C.; Callegari, E.; Becker, S.L.; Chen, X.; Cianfrogna, J.; Doran, A.C.; Doran, S.D.; Gibbs, J.P.; et al. Evaluation of cerebrospinal fluid concentration and plasma free concentration as a surrogate measurement for brain free concentration. Drug Metab. Dispos. 2006, 34, 1443–1447. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Liu, X.R. Evaluation of the utility of brain slice methods to study brain penetration. Drug Metab. Dispos. 2006, 34, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Summerfield, S.G.; Stevens, A.J.; Cutler, L.; Osuna, M.D.; Hammond, B.; Tang, S.P.; Hersey, A.; Spalding, D.J.; Jeffrey, P. Improving the in vitro prediction of in vivo central nervous system penetration: Integrating permeability, P-glycoprotein efflux, and free fractions in blood and brain. J. Pharmacol. Exp. Ther. 2006, 316, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Summerfield, S.G.; Read, K.; Begley, D.J.; Obradovic, T.; Hidalgo, I.J.; Coggon, S.; Lewis, A.V.; Porter, R.A.; Jeffrey, P. Central nervous system drug disposition: The relationship between in situ brain permeability and brain free fraction. J. Pharmacol. Exp. Ther. 2007, 322, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Summerfield, S.G.; Lucas, A.J.; Porter, R.A.; Jeffrey, P.; Gunn, R.N.; Read, K.R.; Stevens, A.J.; Metcalf, A.C.; Osuna, M.C.; Kilford, P.J.; et al. Toward an improved prediction of human in vivo brain penetration. Xenobiotica 2008, 38, 1518–1535. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Sandberg, J.A.; Slikker, W.; Binienda, Z.; Schlosser, P.M.; Patterson, T.A. Quantitative exposure assessment: Application of physiologically-based pharmacokinetic (PBPK) modeling of low-dose, long-term exposures of organic acid toxicant in the brain. Environ. Toxicol. Pharmacol. 2001, 9, 153–160. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Välitalo, P.A.; Wong, Y.C.; Huntjens, D.R.; Proost, J.H.; Vermeulen, A.; Krauwinkel, W.; Beukers, M.W.; Kokki, H.; Kokki, M.; et al. Prediction of human CNS pharmacokinetics using a physiologically-based pharmacokinetic modeling approach. Eur. J. Pharm. Sci. 2018, 112, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Välitalo, P.A.; van den Berg, D.-J.; Hartman, R.; van den Brink, W.; Wong, Y.C.; Huntjens, D.R.; Proost, J.H.; Vermeulen, A.; Krauwinkel, W.; et al. A generic multi-compartmental CNS distribution model structure for 9 drugs allows prediction of human brain target site concentrations. Pharm. Res. 2017, 34, 333–351. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Välitalo, P.A.; Huntjens, D.R.; Proost, J.H.; Vermeulen, A.; Krauwinkel, W.; Beukers, M.W.; van den Berg, D.-J.; Hartman, R.; Wong, Y.C.; et al. Predicting drug concentration-time profiles in multiple CNS compartments using a comprehensive physiologically-based pharmacokinetic model. CPT Pharmacomet. Syst. Pharmacol. 2017, 6, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Danhof, M.; de Lange, E.C.M. Microdialysis: The key to physiologically based model prediction of human CNS target site concentrations. AAPS J. 2017, 19, 891–909. [Google Scholar] [CrossRef] [PubMed]
- Beal, S.; Sheiner, L.; Boeckmann, A.; Bauer, R. Nonmem User’s Guides (1989–2009); Icon Development Solutions: Ellicott City, MD, USA, 2009. [Google Scholar]
- Campbell, J.; Van Landingham, C.; Crowell, S.; Gentry, R.; Kaden, D.; Fiebelkorn, S.; Loccisano, A.; Clewell, H. A preliminary regional PBPK model of lung metabolism for improving species dependent descriptions of 1,3-butadiene and its metabolites. Chem.-Biol. Interact. 2015, 238, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Neuhoff, S.; Gaohua, L.; Burt, H.; Jamei, M.; Li, L.; Tucker, G.T.; Rostami-Hodjegan, A. Accounting for transporters in renal clearance: Towards a mechanistic kidney model (Mech Kim). In Transporters in Drug Development: Discovery, Optimization, Clinical Study and Regulation; Sugiyama, Y., Steffansen, B., Eds.; Springer: New York, NY, USA, 2013; pp. 155–177. [Google Scholar]
- Jamei, M.; Turner, D.; Yang, J.; Neuhoff, S.; Polak, S.; Rostami-Hodjegan, A.; Tucker, G. Population-based mechanistic prediction of oral drug absorption. AAPS J. 2009, 11, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Badhan, R.K.S.; Chenel, M.; Penny, J.I. Development of a physiologically-based pharmacokinetic model of the rat central nervous system. Pharmaceutics 2014, 6, 97–136. [Google Scholar] [CrossRef] [PubMed]
- Ball, K.; Bouzom, F.; Scherrmann, J.M.; Walther, B.; Decleves, X. A physiologically based modeling strategy during preclinical CNS drug development. Mol. Pharm. 2014, 11, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Ball, K.; Bouzom, F.; Scherrmann, J.-M.; Walther, B.; Declèves, X. Development of a physiologically based pharmacokinetic model for the rat central nervous system and determination of an in vitro–in vivo scaling methodology for the blood–brain barrier permeability of two transporter substrates, morphine and oxycodone. J. Pharm. Sci. 2012, 101, 4277–4292. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.C.; Alavijeh, M.S.; Shorvon, S.D.; Patsalos, P.N. Microdialysis study of the neuropharmacokinetics of phenytoin in rat hippocampus and frontal cortex. Epilepsia 1996, 37, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Sechi, G.P.; Petruzzi, V.; Rosati, G.; Tanca, S.; Monaco, F.; Formato, M.; Rubattu, L.; Deriu, P. Brain interstitial fluid and intracellular-distribution of phenytoin. Epilepsia 1989, 30, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Van Belle, K.; Sarre, S.; Ebinger, G.; Michotte, Y. Brain, liver and blood distribution kinetics of carbamazepine and its metabolic interaction with clomipramine in rats: A quantitative microdialysis study. J. Pharmacol. Exp. Ther. 1995, 272, 1217–1222. [Google Scholar] [PubMed]
- Bouw, R.; Ederoth, P.; Lundberg, J.; Ungerstedt, U.; Nordström, C.H.; Hammarlund-Udenaes, M. Increased blood–brain barrier permeability of morphine in a patient with severe brain lesions as determined by microdialysis. Acta Anaesthesiol. Scand. 2001, 45, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Ederoth, P.; Tunblad, K.; Bouw, R.; Lundberg, C.J.F.; Ungerstedt, U.; Nordström, C.-H.; Hammarlund-Udenaes, M. Blood–brain barrier transport of morphine in patients with severe brain trauma. Br. J. Clin. Pharmacol. 2004, 57, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Matalab 2016b, The MathWorks Inc.: Natick, MA, USA.
- Lin, J.H. Applications and limitations of interspecies scaling and in vitro extrapolation in pharmacokinetics. Drug Metab. Dispos. 1998, 26, 1202–1212. [Google Scholar] [PubMed]
- Houston, J.B. Utility of in-vitro drug-metabolism data in predicting in-vivo metabolic-clearance. Biochem. Pharmacol. 1994, 47, 1469–1479. [Google Scholar] [CrossRef]
- Naritomi, Y.; Terashita, S.; Kimura, S.; Suzuki, A.; Kagayama, A.; Sugiyama, Y. Prediction of human hepatic clearance from in vivo animal experiments and in vitro metabolic studies with liver microsomes from animals and humans. Drug Metab. Dispos. 2001, 29, 1316–1324. [Google Scholar] [PubMed]
- Iwatsubo, T.; Suzuki, H.; Shimada, N.; Chiba, K.; Ishizaki, T.; Green, C.E.; Tyson, C.A.; Yokoi, T.; Kamataki, T.; Sugiyama, Y. Prediction of in vivo hepatic metabolic clearance of ym796 from in vitro data by use of human liver microsomes and recombinant p-450 isozymes. J. Pharmacol. Exp. Ther. 1997, 282, 909–919. [Google Scholar] [PubMed]
- Poulin, P.; Theil, F.P. Prediction of pharmacokinetics prior to in vivo studies. II. Generic physiologically based pharmacokinetic models of drug disposition. J. Pharm. Sci. 2002, 91, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Hammarlund-Udenaes, M.; Friden, M.; Syvanen, S.; Gupta, A. On the rate and extent of drug delivery to the brain. Pharm. Res. 2008, 25, 1737–1750. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Drug and gene delivery to the brain: The vascular route. Neuron 2002, 36, 555–558. [Google Scholar] [CrossRef]
- Davies, B.; Morris, T. Physiological-parameters in laboratory-animals and humans. Pharm. Res. 1993, 10, 1093–1095. [Google Scholar] [CrossRef] [PubMed]
- Spanswick, S.C.; Dyck, R.H. Object/context specific memory deficits following medial frontal cortex damage in mice. PLoS ONE 2012, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ashbrook, D.G.; Williams, R.W.; Lu, L.; Stein, J.L.; Hibar, D.P.; Nichols, T.E.; Medland, S.E.; Thompson, P.M.; Hager, R. Joint genetic analysis of hippocampal size in mouse and human identifies a novel gene linked to neurodegenerative disease. BMC Genom. 2014, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Jiang, W.; Pan, H.; Jiang, Y.; Zeng, S.; Zheng, W. Brain regional pharmacokinetics of P-aminosalicylic acid and its n-acetylated metabolite: Effectiveness in chelating brain manganese. Drug Metab. Dispos. 2011, 39, 1904–1909. [Google Scholar] [CrossRef] [PubMed]
- Syková, E.; Nicholson, C. Diffusion in brain extracellular space. Physiol. Rev. 2008, 88, 1277–1340. [Google Scholar] [CrossRef] [PubMed]
- Murtha, L.A.; Yang, Q.; Parsons, M.W.; Levi, C.R.; Beard, D.J.; Spratt, N.J.; McLeod, D.D. Cerebrospinal fluid is drained primarily via the spinal canal and olfactory route in young and aged spontaneously hypertensive rats. Fluids Barriers CNS 2014, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Hamberger, A.; Jacobson, I.; NystrÖM, B.; Sandberg, M. Microdialysis sampling of the neuronal environment in basic and clinical research. J. Intern. Med. 1991, 230, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Poulin, P.; Theil, F.P. Prediction of pharmacokinetics prior to in vivo studies. 1. Mechanism-based prediction of volume of distribution. J. Pharm. Sci. 2002, 91, 129–156. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J. Evidence for bulk flow of brain interstitial fluid: Significance for physiology and pathology. Neurochem. Int. 2004, 45, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Harnish, P.P.; Samuel, K. Reduced cerebrospinal-fluid production in the rat and rabbit by diatrizoate ventriculocisternal perfusion. Investig. Radiol. 1988, 23, 534–536. [Google Scholar] [CrossRef]
- Brinker, T.; Stopa, E.; Morrison, J.; Klinge, P. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS 2014, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Neuhoff, S.; Johnson, T.N.; Rostami-Hodjegan, A.; Jamei, M. Development of a permeability-limited model of the human brain and cerebrospinal fluid (CSF) to integrate known physiological and biological knowledge: Estimating time varying CSF drug concentrations and their variability using in vitro edata. Drug Metab. Pharmacokinet. 2016, 31, 224–233. [Google Scholar]
- Meno-Tetang, G.M.; Li, H.; Mis, S.; Pyszczynski, N.; Heining, P.; Lowe, P.; Jusko, W.J. Physiologically based pharmacokinetic modeling of fty720 (2-amino-2[2-(-4-octylphenyl)ethyl]propane-1,3-diol hydrochloride) in rats after oral and intravenous doses. Drug Metab. Dispos. 2006, 34, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Jarome, T.; Li, S.-J.; Kim, J.J.; Helmstetter, F.J. Chronic stress selectively reduces hippocampal volume in rats: A longitudinal mri study. Neuroreport 2009, 20, 1554–1558. [Google Scholar] [CrossRef] [PubMed]
- Honeycutt, N.A.; Smith, C.D. Hippocampal volume measurements using magnetic resonance imaging in normal young adults. J. Neuroimaging 1995, 5, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Dexter, B.C.; Rahmouni, K.; Cushman, T.; Hermann, G.M.; Ni, C.; Nopoulos, P.C.; Thedens, D.L.; Roghair, R.D. Neonatal leptin deficiency reduces frontal cortex volumes and programs adult hyperactivity in mice. Behav. Brain Res. 2014, 263, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Semendeferi, K.; Lu, A.; Schenker, N.; Damasio, H. Humans and great apes share a large frontal cortex. Nat. Neurosci. 2002, 5, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Bass, N.H.; Lundborg, P. Postnatal development of bulk flow in the cerebrospinal fluid system of the albino rat: Clearance of carboxyl-[14C]inulin after intrathecal infusion. Brain Res. 1973, 52, 323–332. [Google Scholar] [CrossRef]
- Sakka, L.; Coll, G.; Chazal, J. Anatomy and physiology of cerebrospinal fluid. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2011, 128, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Reichel, A. The role of blood-brain barrier studies in the pharmaceutical industry. Curr. Drug Metab. 2006, 7, 183–203. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, T.; Leahy, D.; Rowland, M. Physiologically based pharmacokinetic modeling 1: Predicting the tissue distribution of moderate-to-strong bases. J. Pharm. Sci. 2005, 94, 1259–1276. [Google Scholar] [CrossRef] [PubMed]
- Tornqvist, E.; Annas, A.; Granath, B.; Jalkesten, E.; Cotgreave, I.; Oberg, M. Strategic focus on 3r principles reveals major reductions in the use of animals in pharmaceutical toxicity testing. PLoS ONE 2014, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Sawada, Y.; Sugiyama, Y.; Iga, T.; Hanano, M. Facilitated transport of benzylpenicillin through the blood-brain barrier in rats. J. Pharmacobio-Dyn. 1989, 12, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Terasaki, T.; Sugiyama, Y. Role of efflux transport across the blood-brain barrier and blood cerebrospinal fluid barrier on the disposition of xenobiotics in the central nervous system. Adv. Drug Deliv. Rev. 1997, 25, 257–285. [Google Scholar] [CrossRef]
- Dahlström, B.E.; Paalzow, L.K. Pharmacokinetics of morphine in plasma and discrete areas of the rat brain. J. Pharmacokinet. Biopharm. 1975, 3, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Yokagawa, K.; Nakashima, E.; Ishizaki, J.; Hasegawa, M.; Kido, H.; Ichimura, F. Brain regional pharmacokinetics of biperiden in rats. Biopharm. Drug Dispos. 1992, 13, 131–140. [Google Scholar] [CrossRef]
- Kamiie, J.; Ohtsuki, S.; Iwase, R.; Ohmine, K.; Katsukura, Y.; Yanai, K.; Sekine, Y.; Uchida, Y.; Ito, S.; Terasaki, T. Quantitative atlas of membrane transporter proteins: Development and application of a highly sensitive simultaneous lc/ms/ms method combined with novel in-silico peptide selection criteria. Pharm. Res. 2008, 25, 1469–1483. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, Y.; Uchida, Y.; Tachikawa, M.; Inoue, T.; Ohtsuki, S.; Terasaki, T. Quantitative atlas of blood-brain barrier transporters, receptors, and tight junction proteins in rats and common marmoset. J. Pharm. Sci. 2013, 102, 3343–3355. [Google Scholar] [CrossRef] [PubMed]
- Adachi, Y.; Suzuki, H.; Sugiyama, Y. Comparative studies on in vitro methods for evaluating in vivo function of mdr1 P-glycoprotein. Pharm. Res. 2001, 18, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Ohtsuki, S.; Kamiie, J.; Terasaki, T. Blood-brain barrier (BBB) pharmacoproteomics: Reconstruction of in vivo brain distribution of 11 P-glycoprotein substrates based on the BBB transporter protein concentration, in vitro intrinsic transport activity, and unbound fraction in plasma and brain in mice. J. Pharmacol. Exp. Ther. 2011, 339, 579–588. [Google Scholar] [PubMed]
- O’Brien, J.S.; Sampson, E.L. Lipid composition of the normal human brain: Gray matter, white matter, and myelin. J. Lipid Res. 1965, 6, 537–544. [Google Scholar] [PubMed]
- O’Brien, J.S.; Sampson, E.L. Fatty acid and fatty aldehyde composition of the major brain lipids in normal human gray matter, white matter, and myelin. J. Lipid Res. 1965, 6, 545–551. [Google Scholar] [PubMed]
- Lohmann, C.; Schachmann, E.; Dandekar, T.; Villmann, C.; Becker, C.M. Developmental profiling by mass spectrometry of phosphocholine containing phospholipids in the rat nervous system reveals temporo-spatial gradients. J. Neurochem. 2010, 114, 1119–1134. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.; Mougan, I. Fatty acid composition of human brain phospholipids during normal development. J. Neurochem. 1998, 71, 2528–2533. [Google Scholar] [CrossRef] [PubMed]
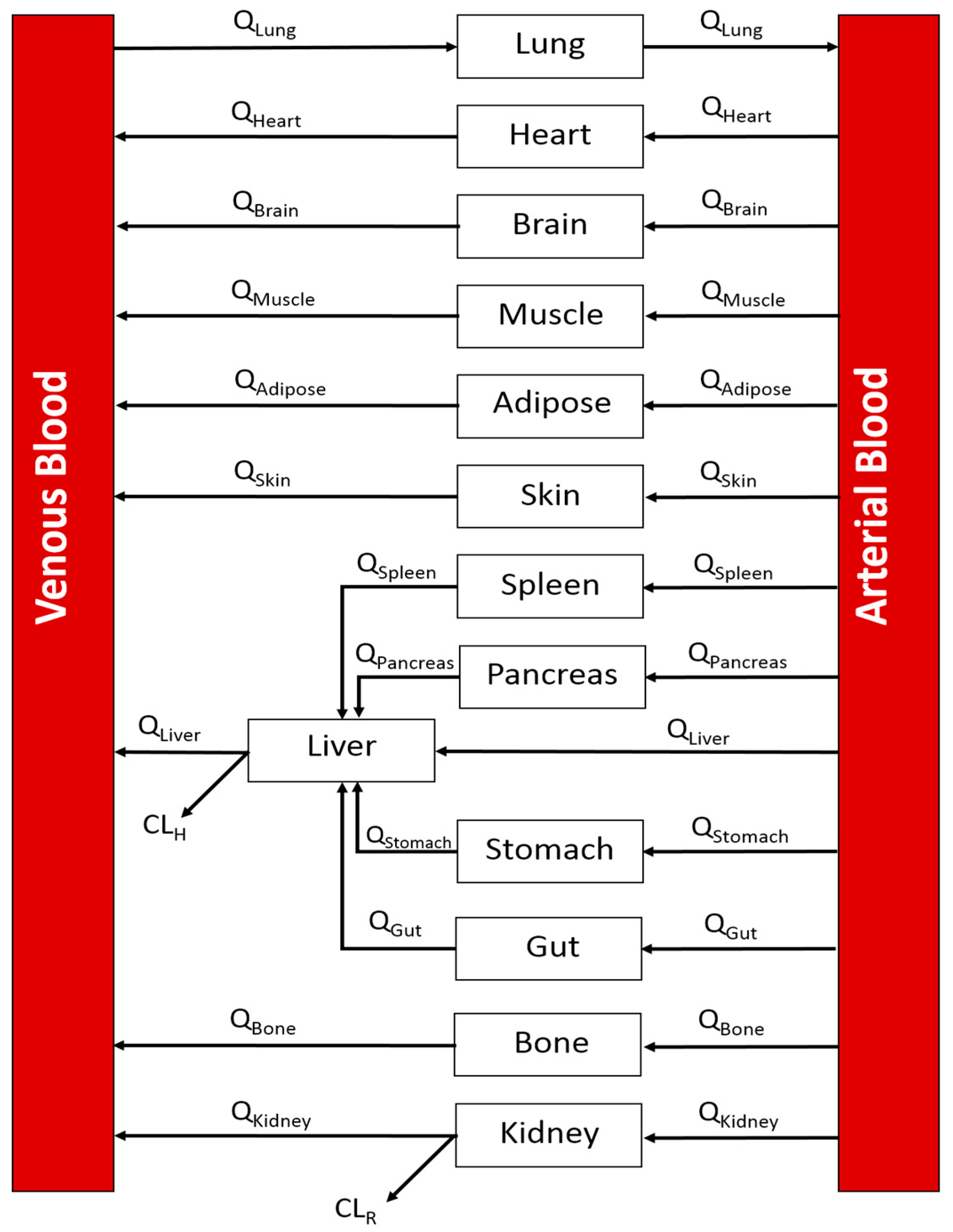
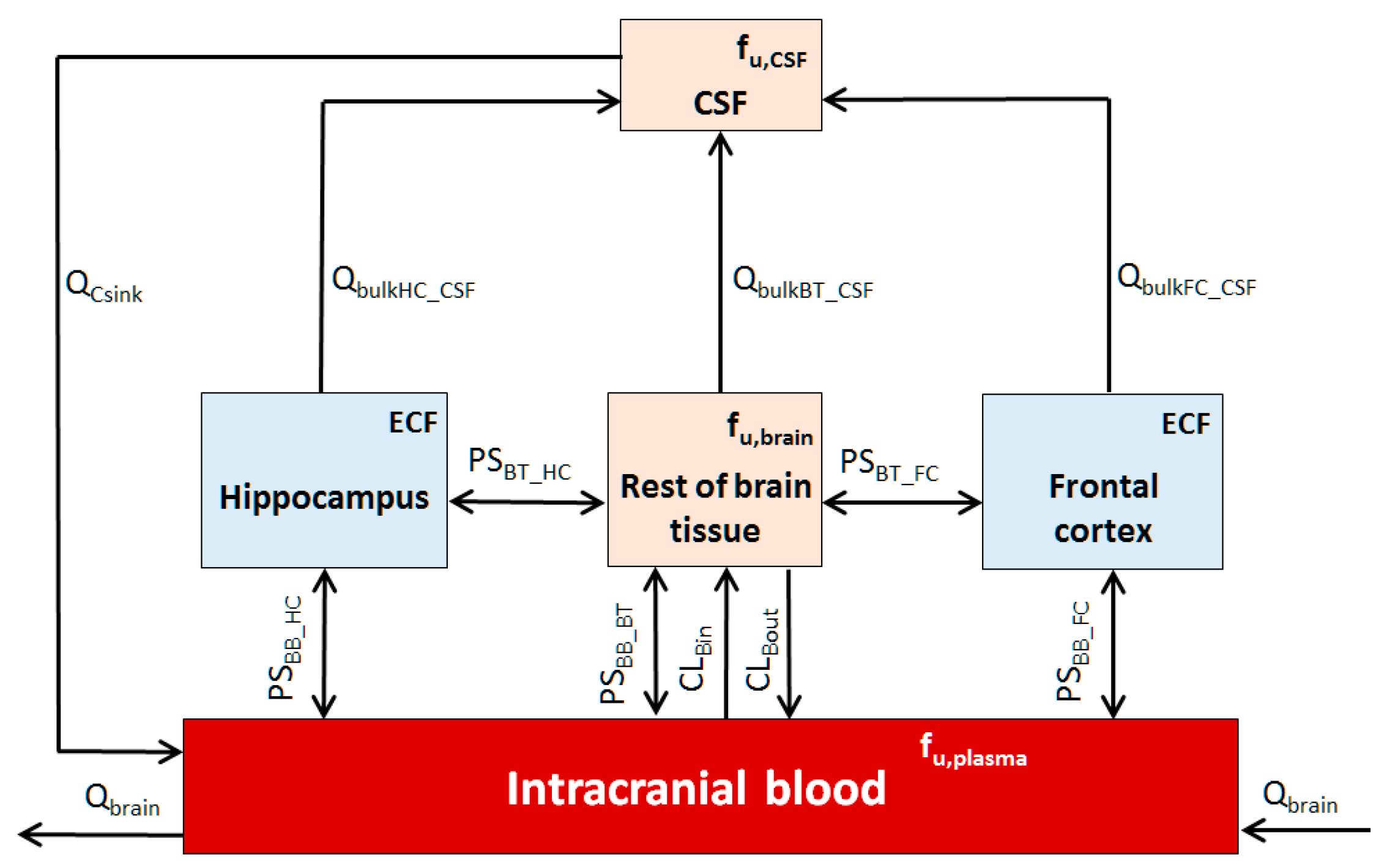
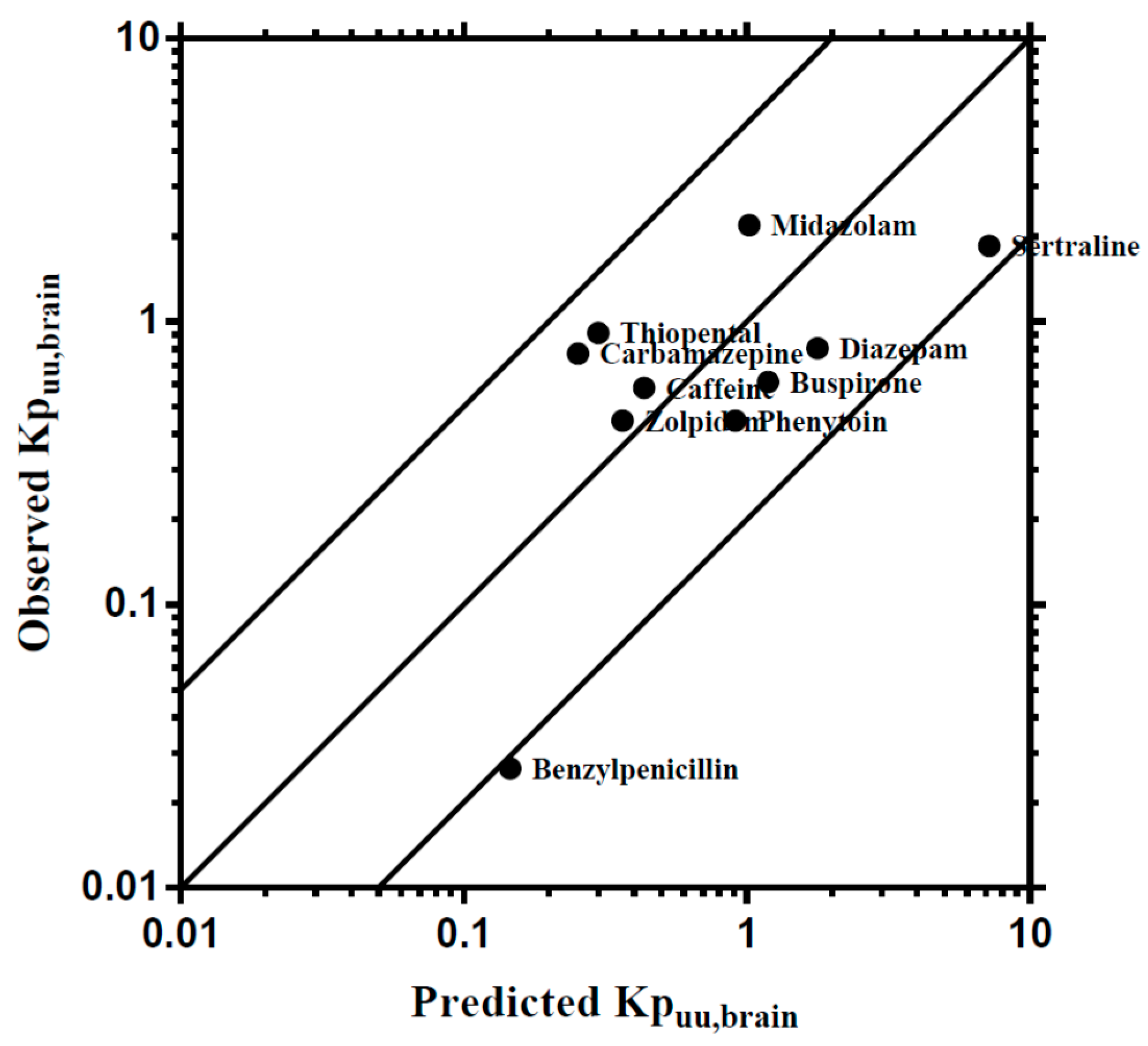
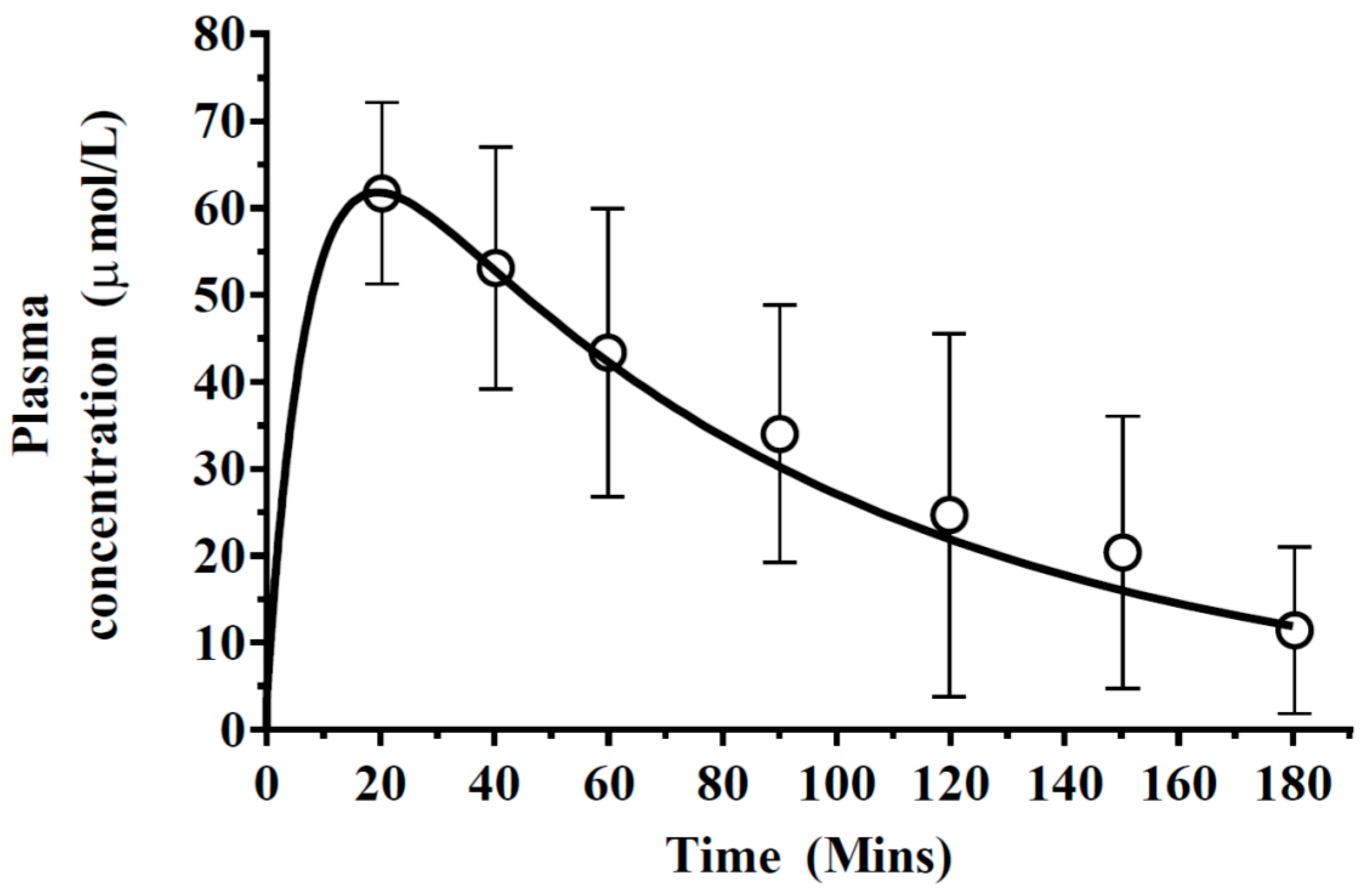

| Tissue | Perfusion | Volume | ||
|---|---|---|---|---|
| Rat | Human | Rat | Human | |
| (mL/min) | (mL/min) | (mL) | (mL) | |
| Adipose | 4.72 | 277.5 | 19.03 | 10,725 |
| Bone | 8.08 | 270 | 10.37 | 9300 |
| Brain | 1.12 | 750 | 1.43 | 1552.5 |
| Gut | 12 | 975 | 6.75 | 1770 |
| Heart | 3.2 | 160.5 | 0.825 | 285 |
| Kidney | 11.6 | 1177.5 | 1.825 | 330 |
| Liver | 20 | 1575 | 10.3 | 1807.5 |
| Lungs | 80 | 5325 | 1.25 | 1252.5 |
| Muscle | 18.96 | 802.5 | 101 | 32,175 |
| Pancreas | 1 | 142.5 | 1.3 | 90 |
| Skin | 4.08 | 322.5 | 47.5 | 8325 |
| Spleen | 0.88 | 82.5 | 0.5 | 202.5 |
| Arterial blood | - | - | 6.8 | 1927.5 |
| Venous blood | - | - | 13.6 | 3855 |
| Rat | Human | |
|---|---|---|
| Flow Rates a | Q (mL/min) | |
| Rest of brain tissue to CSF (bulk flow) | 0.00024 | 0.285 |
| Hippocampus to CSF (bulk flow) | 0.00002 | 0.00114 |
| Frontal cortex to CSF (bulk flow) | 0.00005 | 0.0566 |
| CSF production rate | 0.0037 b | 0.35 c |
| CSF absorption (Qcsink) d | 0.0037 | 0.35 |
| Volume | V (mL) | |
| Intercranial blood e | 0.025 | 75 |
| Rest of brain tissue f | 1.222 | 1211 |
| * Rest of brain tissue ECF e | 0.243 | 267 |
| Hippocampus | 0.093 g | 5.68 h |
| * Hippocampus ECF e | 0.019 | 1.07 |
| Frontal cortex | 0.233 i | 283 j |
| * Frontal cortex ECF e | 0.038 | 53.2 |
| CSF | 0.25 k | 160 l |
| Plasma | Hippocampus | Frontal Cortex | ||||
|---|---|---|---|---|---|---|
| Cmax | AUC | Cmax | AUC | Cmax | AUC | |
| (µmol/L) | (µmol/L·min) | (µmol/L) | (µmol/L·min) | (µmol/L) | (µmol/L·min) | |
| Predicted | 61.79 | 5891.97 | 8.62 ± 3.42 | 718.29 ± 18.31 | 3.87 ± 0.24 | 340.47 ± 11.53 |
| Observed | 61.69 ± 4.7 | 5924.55 ± 340.4 | 7.00 ± 2.2 | 594.74 ± 21.2 | 3.98 ± 1.1 | 370.97 ± 17.1 |
| Plasma | Hippocampus | Frontal Cortex | ||||
|---|---|---|---|---|---|---|
| Cmax | AUC | Cmax | AUC | Cmax | AUC | |
| (µmol/L) | (µmol/L·min) | (µmol/L) | (µmol/L·min) | (µmol/L) | (µmol/L·min) | |
| Predicted | 61.79 | 5891.97 | 8.62 ± 3.42 | 718.29 ± 18.31 | 3.87 ± 0.24 | 340.47 ± 11.53 |
| Observed | 61.69 ± 4.7 | 5924.55 ± 340.4 | 7.00 ± 2.2 | 594.74 ± 21.2 | 3.98 ± 1.1 | 370.97 ± 17.1 |
| Compartment | Cmax | AUC | tmax | |
|---|---|---|---|---|
| (ng/mL) | (ng/mL·min) | (min) | ||
| Plasma | Predicted | 208.2 | 5363 | 7.2 |
| Observed | 178 | 7513 ± 124 | 9.8 | |
| Better Brain | Observed | 10.1 | 941.7 | 31.4 ± 17.1 |
| Worse Brain | Observed | 29.8 | 2732 | 17.8 ± 2.3 |
| Rest of brain | Predicted | 14.5 ± 4.21 | 815 ± 93 | 18.1 |
| Hippocampus | Predicted | 124.4 ± 41.2 | 19,971 ± 3791 | 79.6 |
| Frontal Cortex | Predicted | 38.9 ± 15.7 | 2444 ± 153 | 26.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakaria, Z.; Badhan, R. Development of a Region-Specific Physiologically Based Pharmacokinetic Brain Model to Assess Hippocampus and Frontal Cortex Pharmacokinetics. Pharmaceutics 2018, 10, 14. https://doi.org/10.3390/pharmaceutics10010014
Zakaria Z, Badhan R. Development of a Region-Specific Physiologically Based Pharmacokinetic Brain Model to Assess Hippocampus and Frontal Cortex Pharmacokinetics. Pharmaceutics. 2018; 10(1):14. https://doi.org/10.3390/pharmaceutics10010014
Chicago/Turabian StyleZakaria, Zaril, and Raj Badhan. 2018. "Development of a Region-Specific Physiologically Based Pharmacokinetic Brain Model to Assess Hippocampus and Frontal Cortex Pharmacokinetics" Pharmaceutics 10, no. 1: 14. https://doi.org/10.3390/pharmaceutics10010014
APA StyleZakaria, Z., & Badhan, R. (2018). Development of a Region-Specific Physiologically Based Pharmacokinetic Brain Model to Assess Hippocampus and Frontal Cortex Pharmacokinetics. Pharmaceutics, 10(1), 14. https://doi.org/10.3390/pharmaceutics10010014






