Effective Suckling C57BL/6, Kunming, and BALB/c Mouse Models with Remarkable Neurological Manifestation for Zika Virus Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Virus
2.2. Animals
2.3. Athogenicity Studies
2.4. Real-Time RT-PCR Assay
2.5. Histology
2.6. Statistical Analysis
3. Results
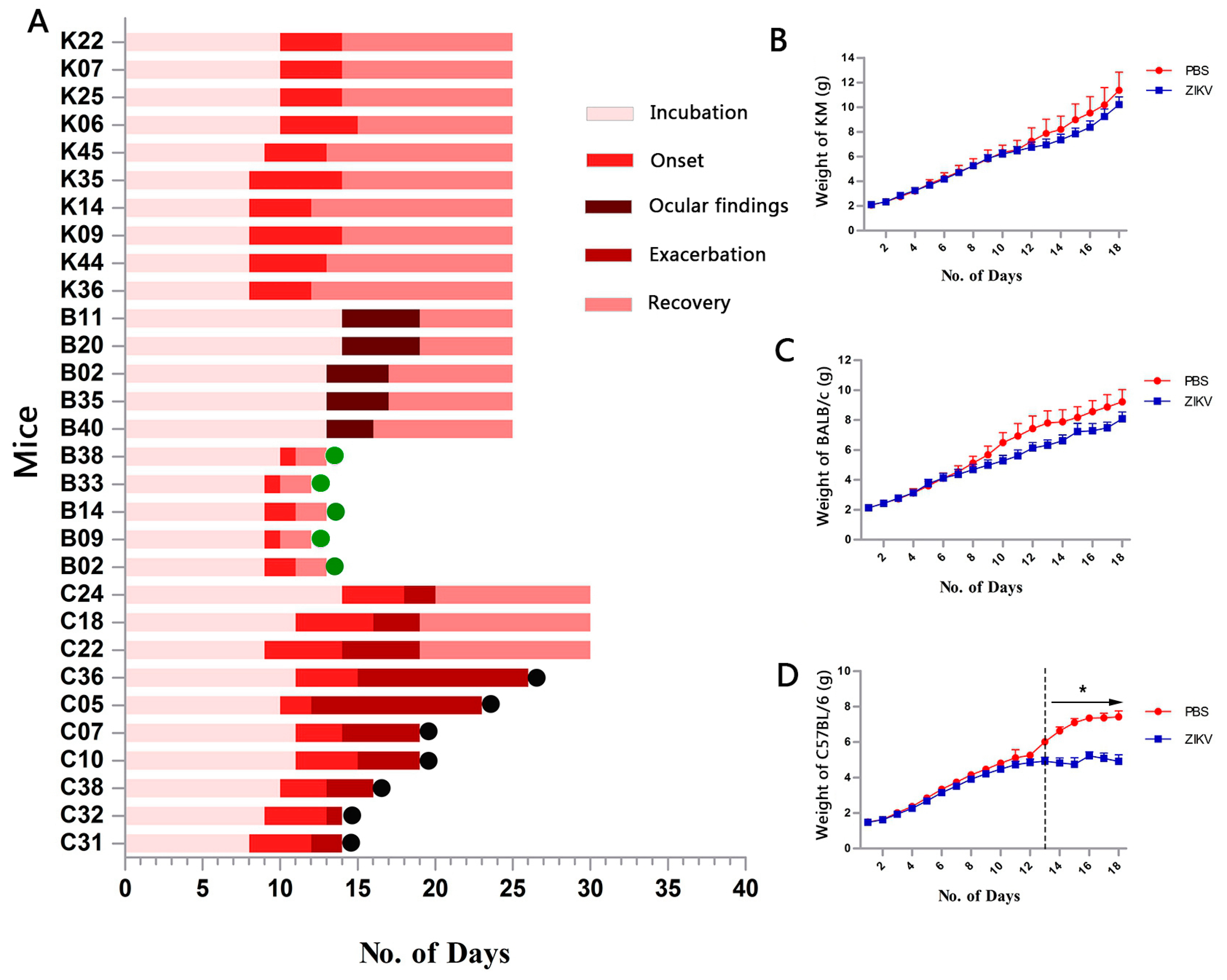
3.1. Infection for ZIKV
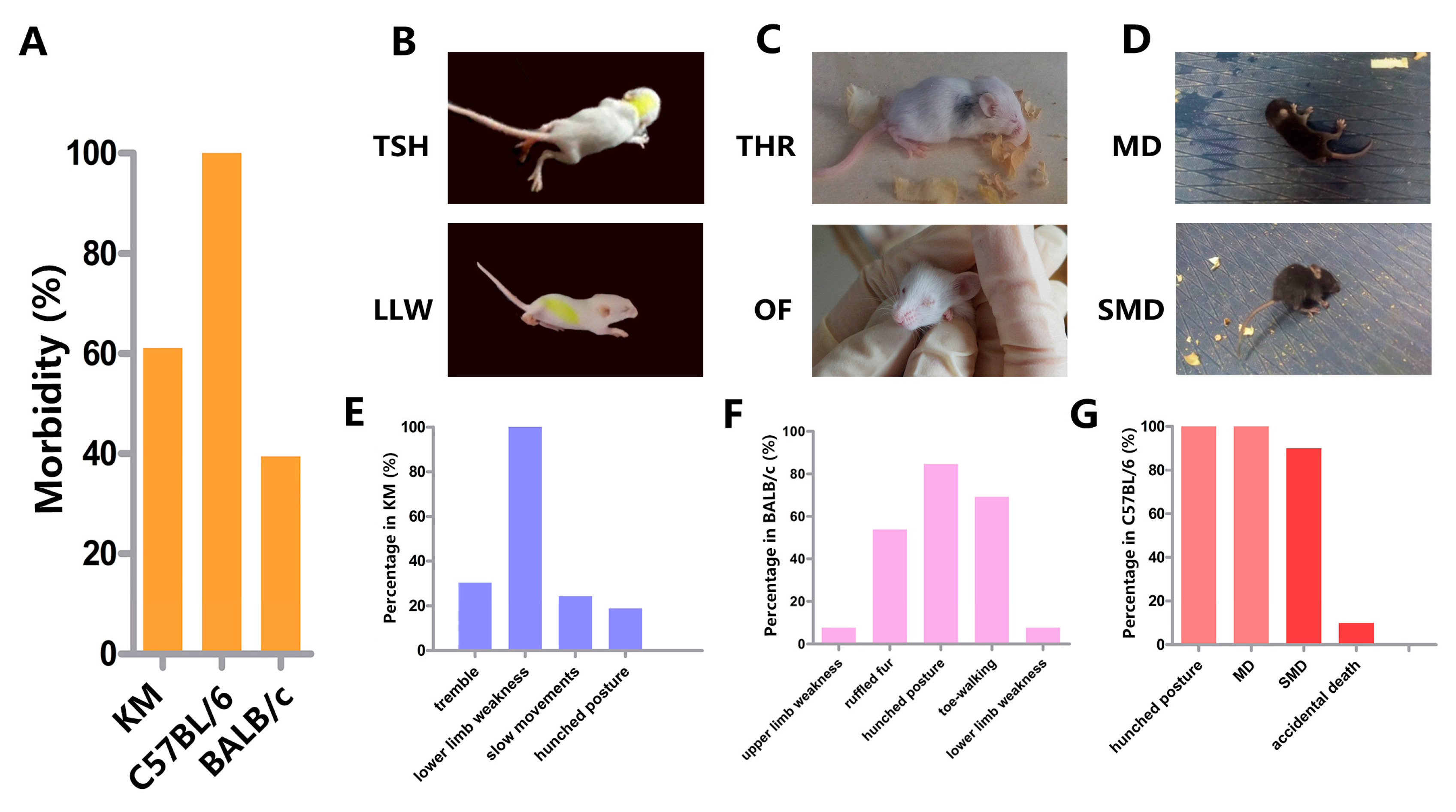
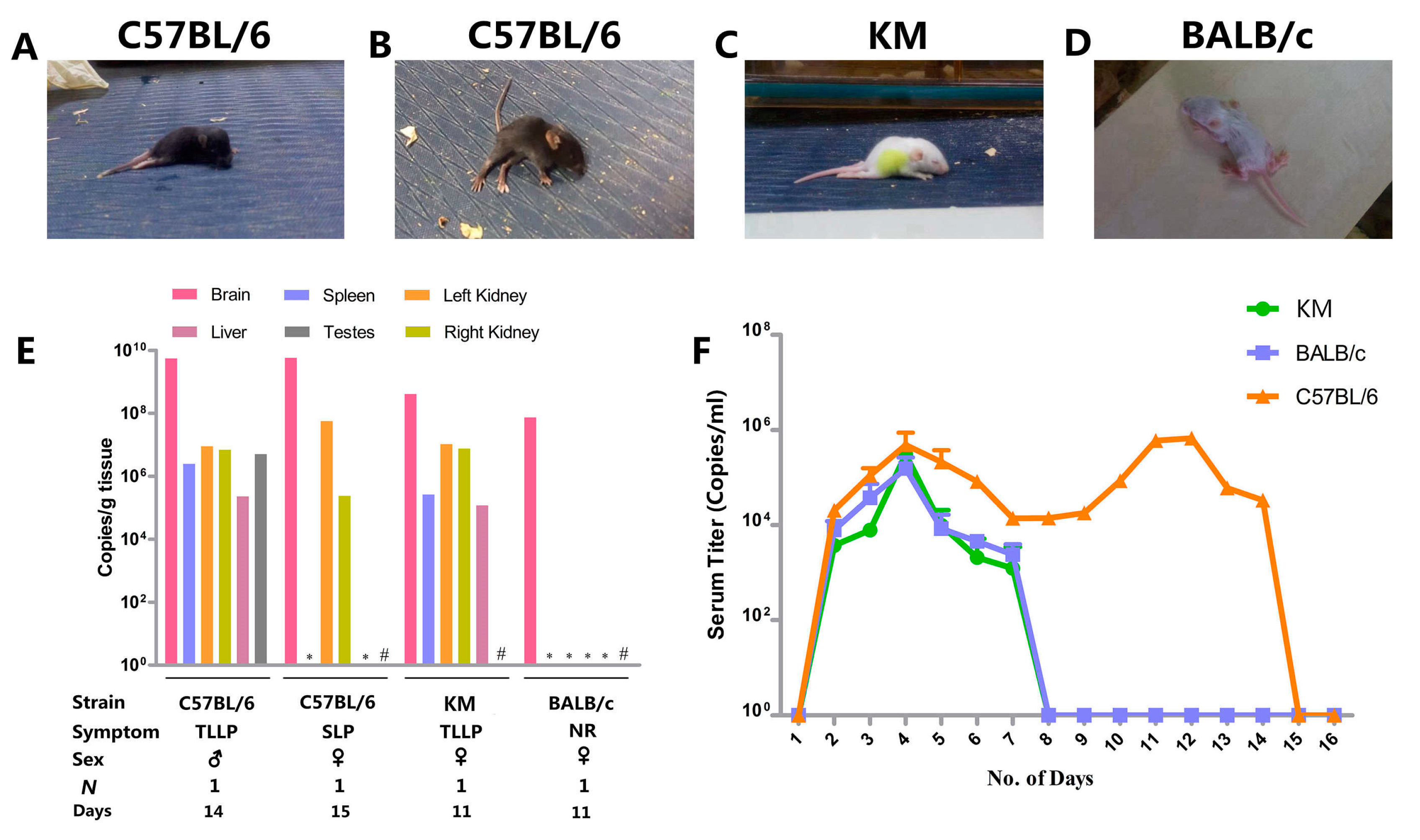
3.2. Clinical Manifestation
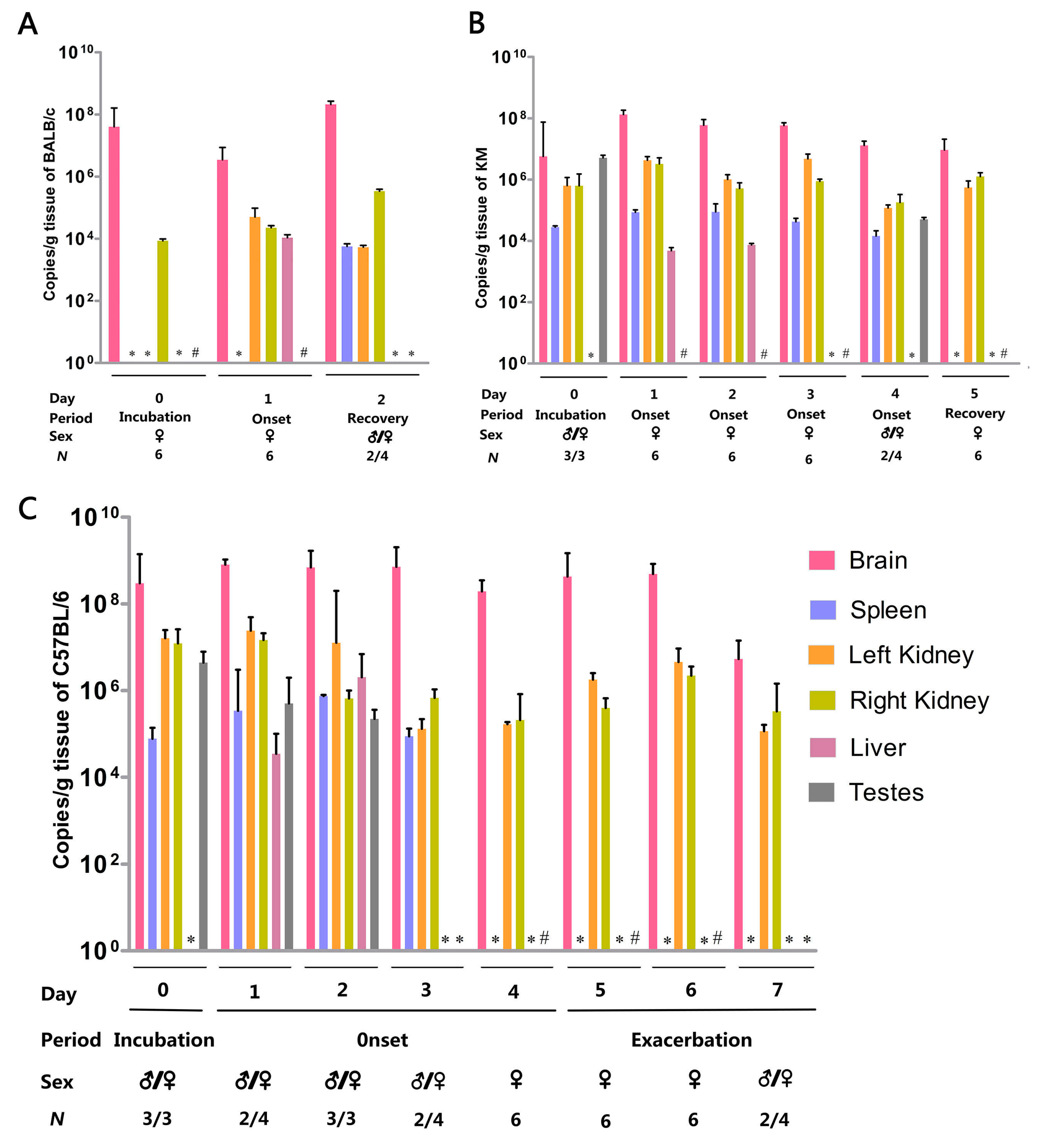
3.3. Organ Viral Loads and Viremia
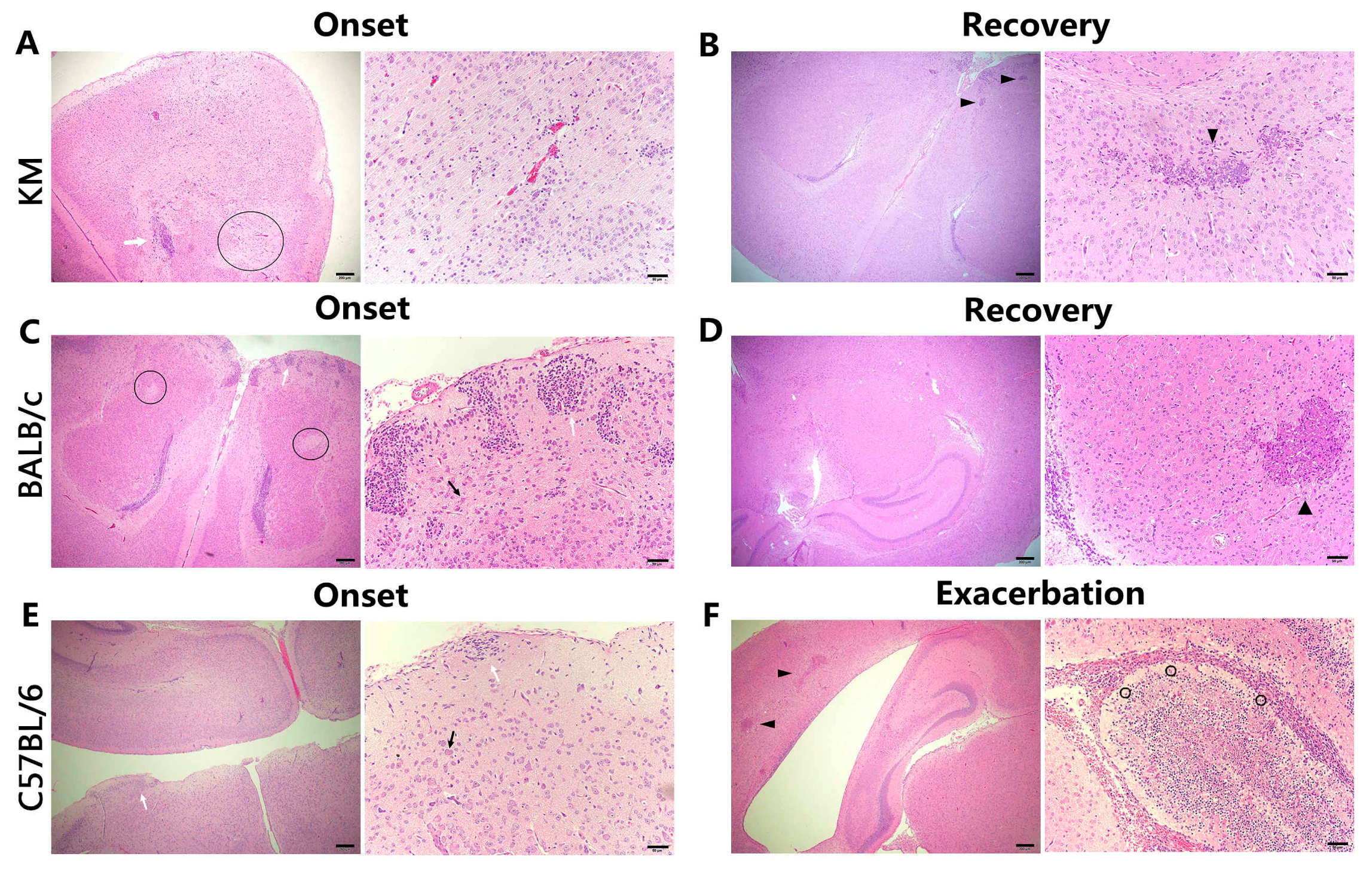
3.4. Histology
4. Discussion
Supplementary Materials
Acknowledgments
Funding Information
Author Contributions
Ethics Statement
Conflicts of Interest
References
- Haddow, A.D.; Schuh, A.J.; Yasuda, C.Y.; Kasper, M.R.; Heang, V.; Huy, R.; Guzman, H.; Tesh, R.B.; Weaver, S.C. Genetic characterization of Zika virus strains: Geographic expansion of the Asian lineage. PLoS Negl. Trop. Dis. 2012, 6, e1477. [Google Scholar] [CrossRef] [PubMed]
- Dick, G.W.; Kitchen, S.F.; Haddow, A.J. Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- Duffy, M.R.; Chen, T.H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and serologic properties of Zika Virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008, 14, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- White, M.K.; Wollebo, H.S.; David Beckham, J.; Tyler, K.L.; Khalili, K. Zika virus: An emergent neuropathological agent. Ann. Neurol. 2016, 80, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Faria, N.R.; Azevedo Rdo, S.; Kraemer, M.U.; Souza, R.; Cunha, M.S.; Hill, S.C.; Thézé, J.; Bonsall, M.B.; Bowden, T.A.; Rissanen, I.; et al. Zika virus in the Americas: Early epidemiological and genetic findings. Science 2016, 352, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Rabaan, A.A.; Bazzi, A.M.; Al-Ahmed, S.H.; Al-Ghaith, M.H.; Al-Tawfiq, J.A. Overview of Zika infection, epidemiology, transmission and control measures. J. Infect. Public Health 2016, 10, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Mlakar, J.; Korva, M.; Tul, N.; Popović, M.; Poljšak-Prijatelj, M.; Mraz, J.; Kolenc, M.; Resman Rus, K.; Vesnaver Vipotnik, T.; Vodušek, V.F.; et al. Zika virus associated with microcephaly. N. Engl. J. Med. 2016, 374, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Cao-Lormeau, V.M.; Blake, A.; Mons, S.; Lastère, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P.; et al. Guillain–Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet 2016, 387, 1531–1539. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Fifth Meeting of the Emergency Committee under the International Health Regulations (2005) Regarding Microcephaly, Other Neurological Disorders and Zika Virus. 2016. Available online: http://www.who.int/mediacentre/news/statements/2016/zika-fifth-ec/en/ (accessed on 18 November 2016).
- WHO (World Health Organization). Zika Virus and Complications. 2016. Available online: http://www.who.int/emergencies/zika-virus/en/ (accessed on 18 November 2016).
- Alam, A.; Ali, S.; Ahamad, S.; Malik, M.Z.; Ishrat, R. From ZikV genome to vaccine: In silico approach for the epitope-based peptide vaccine against Zika virus envelope glycoprotein. Immunology 2016, 149, 386–399. [Google Scholar] [CrossRef] [PubMed]
- Pierson, T.C.; Graham, B.S. Zika Virus: Immunity and vaccine development. Cell 2016, 167, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Xie, X.; Barrett, A.D.; Garcia-Blanco, M.A.; Tesh, R.B.; Vasconcelos, P.F.; Vasilakis, N.; Weaver, S.C.; Shi, P.Y. Zika virus: Diagnosis, therapeutics, and vaccine. ACS Infect. Dis. 2016, 2, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Lazear, H.M.; Govero, J.; Smith, A.M.; Platt, D.J.; Fernandez, E.; Miner, J.J.; Diamond, M.S. A mouse model of Zika virus pathogenesis. Cell Host Microbe 2016, 19, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Dowall, S.D.; Graham, V.A.; Rayner, E.; Atkinson, B.; Hall, G.; Watson, R.J.; Bosworth, A.; Bonney, L.C.; Kitchen, S.; Hewson, R. A susceptible mouse model for Zika virus infection. PLoS Negl. Trop. Dis. 2016, 10, e0004658. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.L.; Tesh, R.B.; Azar, S.R.; Muruato, A.E.; Hanley, K.A.; Auguste, A.J.; Langsjoen, R.M.; Paessler, S.; Vasilakis, N.; Weaver, S.C. Characterization of a novel murine model to study Zika virus. Am. J. Trop. Med. Hyg. 2016, 94, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Aliota, M.T.; Caine, E.A.; Walker, E.C.; Larkin, K.E.; Camacho, E.; Osorio, J.E. Characterization of lethal Zika virus infection in AG129 Mice. PLoS Negl. Trop. Dis. 2016, 10, e0004682. [Google Scholar] [CrossRef] [PubMed]
- Abbink, P.; Larocca, R.A.; De La Barrera, R.A.; Bricault, C.A.; Moseley, E.T.; Boyd, M.; Kirilova, M.; Li, Z.; Ng’ang’a, D.; Nanayakkara, O.; et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science 2016, 353, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Cugola, F.R.; Fernandes, I.R.; Russo, F.B.; Freitas, B.C.; Dias, J.L.; Guimarães, K.P.; Benazzato, C.; Almeida, N.; Pignatari, G.C.; Romero, S.; et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature 2016, 534, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Hickman, H.D.; Pierson, T.C. Zika in the brain: New models shed light on viral infection. Trends Mol. Med. 2016, 22, 639–641. [Google Scholar] [CrossRef] [PubMed]
- Miner, J.J.; Cao, B.; Govero, J.; Smith, A.M.; Fernandez, E.; Cabrera, O.H.; Garber, C.; Noll, M.; Klein, R.S.; Noguchi, K.K.; et al. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell 2016, 165, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, D.; Ye, Q.; Hong, S.; Jiang, Y.; Liu, X.; Zhang, N.; Shi, L.; Qin, C.F.; Xu, Z. Zika virus disrupts neural progenitor development and leads to microcephaly in mice. Cell Stem Cell 2016, 19, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Dudley, D.M.; Aliota, M.T.; Mohr, E.L.; Weiler, A.M.; Lehrer-Brey, G.; Weisgrau, K.L.; Mohns, M.S.; Breitbach, M.E.; Rasheed, M.N.; Newman, C.M.; et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nat. Commun. 2016, 7, 12204. [Google Scholar] [CrossRef] [PubMed]
- Miner, J.J.; Sene, A.; Richner, J.M.; Smith, A.M.; Santeford, A.; Ban, N.; Weger-Lucarelli, J.; Manzella, F.; Rückert, C.; Govero, J.; et al. Zika virus infection in mice causes panuveitis with shedding of virus in tears. Cell Rep. 2016, 16, 3208–3218. [Google Scholar] [CrossRef] [PubMed]
- Govero, J.; Esakky, P.; Scheaffer, S.M.; Fernandez, E.; Drury, A.; Platt, D.J.; Gorman, M.J.; Richner, J.M.; Caine, E.A.; Salazar, V.; et al. Zika virus infection damages the testes in mice. Nature 2016, 540, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.B.; Campos, P.P.; de Jesus Oviedo Socarrás, T.; Pimenta, T.S.; Parreiras, P.M.; Silva, S.S.; Kalapothakis, E.; Andrade, S.P.; Moro, L. Sponge implant in Swiss mice as a model for studying loxoscelism. Toxicon 2012, 59, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.; Bastos, M.F.; Pelizon, A.C.; Peres, C.M.; Cavalcante, D.P.; Sartori, A. Assessment of the neutralizing potency of ovine antivenom in a swiss mice model of Bothrops jararaca envenoming. J. Venom. Anim. Toxins Incl. Trop. Dis. 2005, 11, 336–349. [Google Scholar] [CrossRef]
- Li, T.; Zhao, L.; Feng, F.; Chen, S.; Xia, P. An experimental animal model study of HCMV. Acta Microbiol. Sin. 1996, 36, 292–294. [Google Scholar]
- Yang, H.; Li, X.; Jiang, Q.; Cai, X.; Ma, J. Effects of exercise on memory of mice with dementia and possible mechanisms. Chin. J. Phys. Med. Rehabil. 2012, 34, 17–20. [Google Scholar]
- Sun, S.; Li, Z.; Liu, J.; Zhang, H.; Qiao, M. Correlation between anxiety and depression in animal models: Evidence from light/dark box and tail suspension test in Kunming mice. Chin. Pharmacol. Bull. 2012, 28, 289–293. [Google Scholar]
- Yamamoto, Y.; Tanahashi, T.; Kawai, T.; Chikahisa, S.; Katsuura, S.; Nishida, K.; Teshima-Kondo, S.; Sei, H.; Rokutan, K. Changes in behavior and gene expression induced by caloric restriction in C57BL/6 mice. Physiol. Genom. 2009, 39, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Scarpellini, A.; Funck, M.; Verderio, E.A.; Johnson, T.S. Development of a chronic kidney disease model in C57BL/6 mice with relevance to human pathology. Nephron Extra 2013, 3, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Abraham, R.; Shim, B.S.; Choe, H.; Page, D.T. Zika virus infection during the period of maximal brain growth causes microcephaly and corticospinal neuron apoptosis in wild type mice. Sci. Rep. 2016, 6, 34793. [Google Scholar] [CrossRef] [PubMed]
- Manangeeswaran, M.; Ireland, D.D.; Verthelyi, D. Zika (PRVABC59) Infection Is Associated with T cell Infiltration and neurodegeneration in CNS of immunocompetent neonatal C57Bl/6 mice. PLoS Pathog. 2016, 12, e1006004. [Google Scholar] [CrossRef] [PubMed]
- Arashkia, A.; Roohvand, F.; Memarnejadian, A.; Aghasadeghi, M.R.; Rafati, S. Construction of HCV-polytope vaccine candidates harbouring immune-enhancer sequences and primary evaluation of their immunogenicity in BALB/c. Virus Genes 2010, 40, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhu, Q.; Qin, E.; Yu, M.; Ding, Z.; Shi, H.; Cheng, X.; Wang, C.; Chang, G.; Zhu, Q.; et al. Inactivated SARS-CoV vaccine prepared from whole virus induces a high level of neutralizing antibodies in BALB/c mice. DNA Cell Biol. 2004, 23, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Okada, K.; Kenniston, T.; Raj, V.S.; AlHajri, M.M.; Farag, E.A.; AlHajri, F.; Osterhaus, A.D.; Haagmans, B.L.; Gambotto, A. Immunogenicity of an adenoviral-based Middle East Respiratory Syndrome coronavirus vaccine in BALB/c mice. Vaccine 2014, 32, 5875–5982. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Hellermann, G.R.; Patton, G.; Kumar, M.; Behera, A.; Randall, T.S.; Zhang, J.; Lockey, R.F.; Mohapatra, S.S. An immunocompromised BALB/c mouse model for respiratory syncytial virus infection. Virol. J. 2005, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Saxena, V.; Mathur, A.; Krishnani, N.; Dhole, T.N. Kinetics of cytokine profile during intraperitoneal inoculation of Japanese encephalitis virus in BALB/c mice model. Microbes Infect. 2008, 10, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Li, X.; Tang, C.; Jia, W.; Zhao, Z.; Liu, K.; Gao, X.; Wang, X. Response of BALB/c mice to a monovalent influenza A (H1N1) 2009 split vaccine. Cell Mol. Immunol. 2010, 7, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013, s106–107, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Couderc, T.; Chrétien, F.; Schilte, C.; Disson, O.; Brigitte, M.; Guivel-Benhassine, F.; Touret, Y.; Barau, G.; Cayet, N.; Schuffenecker, I.; et al. A mouse model for Chikungunya: Young age and inefficient type-I interferon signaling are risk factors for severe disease. PLoS Pathog. 2008, 4, e29. [Google Scholar] [CrossRef] [PubMed]
- Pedras-Vasconcelos, J.A.; Puig, M.; Sauder, C.; Wolbert, C.; Ovanesov, M.; Goucher, D.; Verthelyi, D. Immunotherapy with CpG oligonucleotides and antibodies to TNF- rescues neonatal mice from lethal arenavirus-induced meningoencephalitis. J. Immunol. 2008, 15, 8231–8240. [Google Scholar] [CrossRef]
- Pletnikov, M.V.; Rubin, S.A.; Moran, T.H.; Carbone, K.M. Exploring the cerebellum with a new tool: Neonatal Borna disease virus (BDV) infection of the rat’s brain. Cerebellum 2003, 2, 62–70. [Google Scholar] [PubMed]
- Winkelmann, E.R.; Luo, H.; Tian, W. West Nile Virus Infection in the Central Nervous System. F1000Research 2016, 5, 341–370. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Tan, Q.; Sun, J.; Zhou, H.; Guan, D.; Zhang, H.; Ning, D.; Ke, C. First isolation and identification of Zika virus in China. Chin. J. Microbiol. Immunol. 2016, 36, 247–251. [Google Scholar]
- Wu, D.; Zhang, H.; Tan, Q.; Sun, J.; Zhou, H.; Ning, D.; Guan, D. Laboratory test for 18 imported Zika cases in China. Chin. J. Microbiol. Immunol. 2016, 36, 721–726. [Google Scholar]
- Brasil, P.; Calvet, G.A.; Siqueira, A.M.; Wakimoto, M.; de Sequeira, P.C.; Nobre, A.; Quintana Mde, S.; Mendonça, M.C.; Lupi, O.; de Souza, R.V.; et al. Zika virus outbreak in Rio de Janeiro, Brazil: Clinical characterization, epidemiological and virological aspects. PLoS Negl. Trop. Dis. 2016, 10, e4636. [Google Scholar] [CrossRef] [PubMed]
- Dirlikov, E.; Major, C.G.; Mayshack, M.; Medina, N.; Matos, D.; Ryff, K.R.; Torres-Aponte, J.; Alkis, R.; Munoz-Jordan, J.; Colon-Sanchez, C.; et al. Guillain–Barré Syndrome during ongoing Zika virus transmission—Puerto Rico, January 1–July 31, 2016. MMWR. Morb. Mortal. Wkly. Rep. 2016, 65, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Ventura, C.V.; Maia, M.; Bravo-Filho, V.; Góis, A.L.; Belfort, R., Jr. Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet 2016, 387, 228. [Google Scholar] [CrossRef]
- De Paula Freitas, B.; de Oliveira Dias, J.R.; Prazeres, J.; Sacramento, G.A.; Ko, A.I.; Maia, M.; Belfort, R., Jr. Ocular findings in infants with microcephaly associated with presumed Zika virus congenital infection in Salvador, Brazil. JAMA Ophthalmol. 2016, 134, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Miranda, H.A., II.; Costa, M.C.; Frazão, M.A.; Simão, N.; Franchischini, S.; Moshfeghi, D.M. Expanded spectrum of congenital ocular findings in microcephaly with presumed Zika infection. Ophthalmology. 2016, 123, 1788–1794. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A. Autopsy and postmortem studies are concordant: Pathology of Zika virus infection is neurotropic in fetuses and infants with microcephaly following transplacental transmission. Arch. Pathol. Lab. Med. 2016, 141, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Bell, T.M.; Field, E.J.; Narang, H.K. Zika virus infection of the central nervous system of mice. Arch. Gesamte Virusforsch. 1971, 35, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Araujo, A.Q.; Silva, M.T.; Araujo, A.P. Zika virus-associated neurological disorders: A review. Brain 2016, 139, 2122–2130. [Google Scholar] [CrossRef] [PubMed]
- Solomon, I.H.; Milner, D.A.; Folkerth, R.D. Neuropathology of Zika virus infection. J. Neuroinfect. Dis. 2016, 7, 220. [Google Scholar] [CrossRef] [PubMed]
- Gourinat, A.C.; O’Connor, O.; Calvez, E.; Goarant, C.; Dupont-Rouzeyrol, M. Detection of Zika virus in urine. Emerg. Infect. Dis. 2015, 21, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.E.; Meaney-Delman, D.; Neblett-Fanfair, R.; Havers, F.; Oduyebo, T.; Hills, S.L.; Rabe, I.B.; Lambert, A.; Abercrombie, J.; Martin, S.W.; et al. Update: Interim guidance for preconception counseling and prevention of sexual transmission of Zika virus for persons with possible zika virus exposure—United States, September 2016. MMWR. Morb. Mortal. Wkly. Rep. 2016, 65, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.; Ponia, S.S.; Tripathi, S.; Balasubramaniam, V.; Miorin, L.; Sourisseau, M.; Schwarz, M.C.; Sánchez-Seco, M.P.; Evans, M.J.; Best, S.M. Zika virus targets human STAT2 to inhibit type I interferon signaling. Cell Host Microbe 2016, 19, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Costa, F.; Garcia-Blanco, M.A.; Ko, A.I.; Ribeiro, G.S.; Saade, G.; Shi, P.Y.; Vasilakis, N. Zika virus: History, emergence, biology, and prospects for control. Antivir. Res. 2016, 130, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Roche, C.; Nhan, T.X.; Robin, E.; Teissier, A.; Cao-Lormeau, V.M. Detection of Zika virus in saliva. J. Clin. Virol. 2015, 68, 53–55. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Liu, X.; Ke, C.; Wu, Q.; Lu, W.; Qin, Z.; He, X.; Liu, Y.; Deng, J.; Xu, S.; et al. Effective Suckling C57BL/6, Kunming, and BALB/c Mouse Models with Remarkable Neurological Manifestation for Zika Virus Infection. Viruses 2017, 9, 165. https://doi.org/10.3390/v9070165
Yu J, Liu X, Ke C, Wu Q, Lu W, Qin Z, He X, Liu Y, Deng J, Xu S, et al. Effective Suckling C57BL/6, Kunming, and BALB/c Mouse Models with Remarkable Neurological Manifestation for Zika Virus Infection. Viruses. 2017; 9(7):165. https://doi.org/10.3390/v9070165
Chicago/Turabian StyleYu, Jianhai, Xuling Liu, Changwen Ke, Qinghua Wu, Weizhi Lu, Zhiran Qin, Xiaoen He, Yujing Liu, Jieli Deng, Suiqi Xu, and et al. 2017. "Effective Suckling C57BL/6, Kunming, and BALB/c Mouse Models with Remarkable Neurological Manifestation for Zika Virus Infection" Viruses 9, no. 7: 165. https://doi.org/10.3390/v9070165
APA StyleYu, J., Liu, X., Ke, C., Wu, Q., Lu, W., Qin, Z., He, X., Liu, Y., Deng, J., Xu, S., Li, Y., Zhu, L., Wan, C., Zhang, Q., Xiao, W., Xie, Q., Zhang, B., & Zhao, W. (2017). Effective Suckling C57BL/6, Kunming, and BALB/c Mouse Models with Remarkable Neurological Manifestation for Zika Virus Infection. Viruses, 9(7), 165. https://doi.org/10.3390/v9070165




