New Paradigms for the Study of Ocular Alphaherpesvirus Infections: Insights into the Use of Non-Traditional Host Model Systems
Abstract
1. Introduction
2. In Vitro 2D Cell Culture Systems
2.1. Immortalized Cell Lines
2.2. Primary Corneal Epithelial Cells
2.3. Limitations of 2D Cell Culture Systems
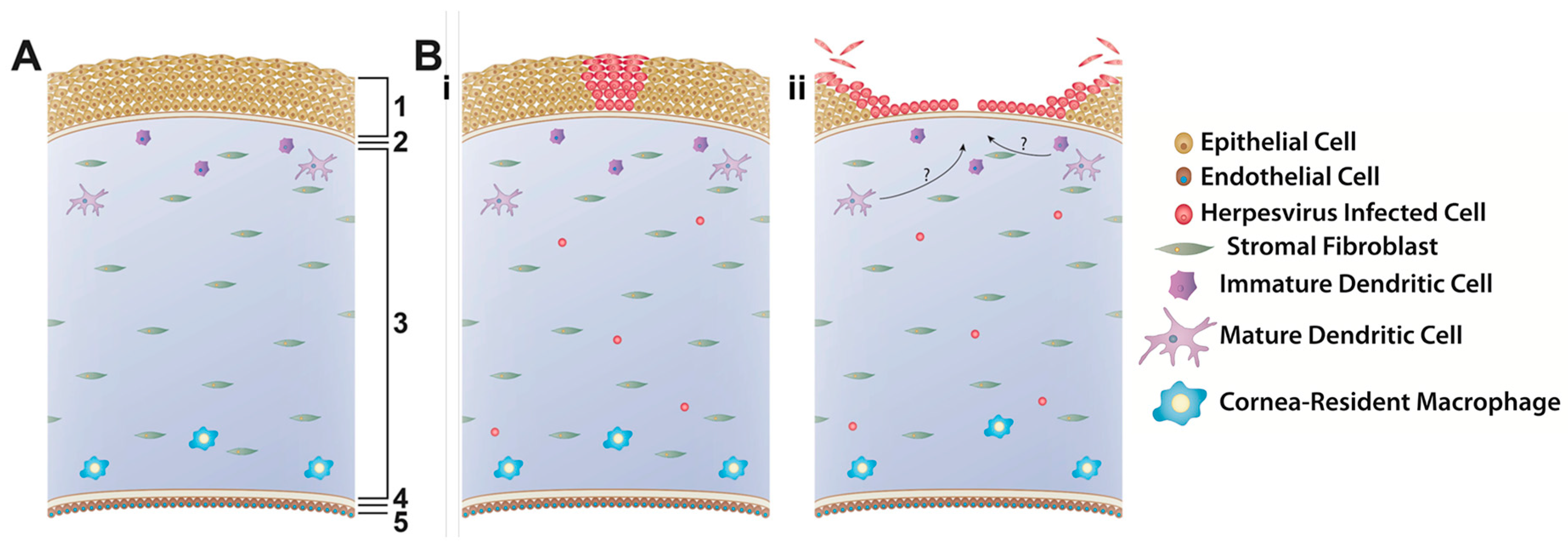
3. In Vitro 3D Cell Culture/Explant Systems
3.1. Corneal Facsimile
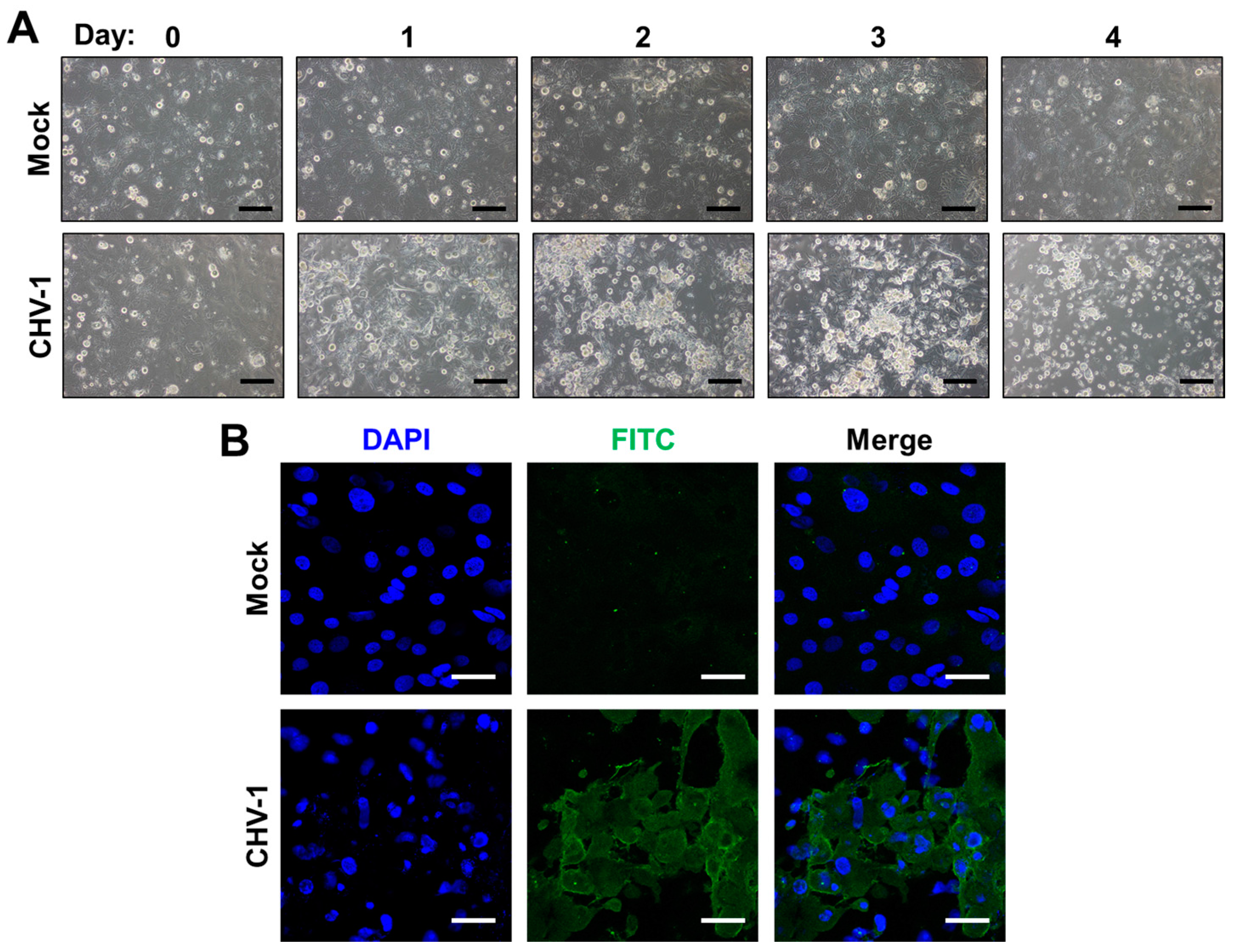
3.2. Explants
3.3. Limitations of 3D Cell Culture/Explant Systems
4. In Vivo Systems
4.1. Mice
4.2. Rabbits
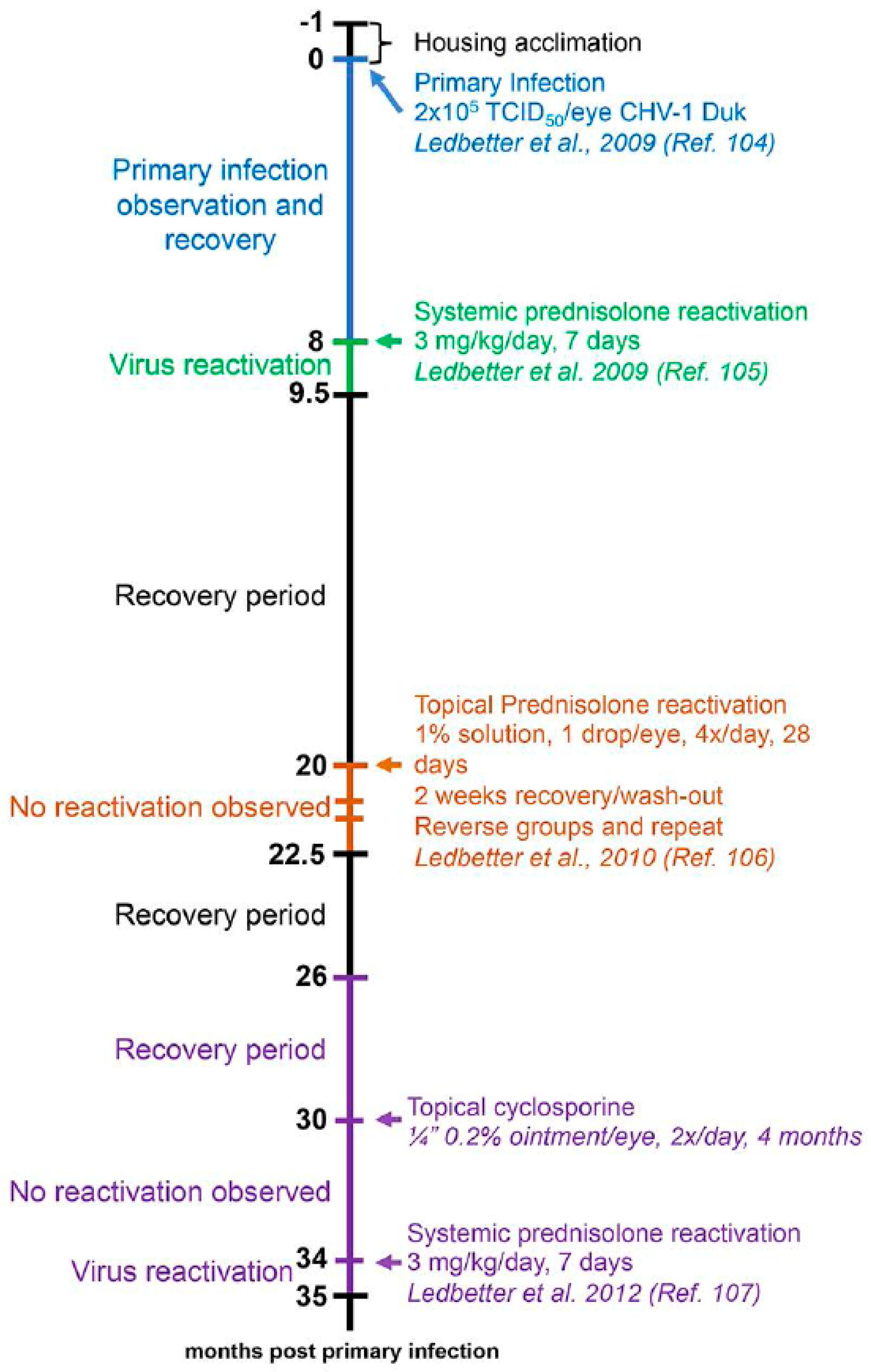
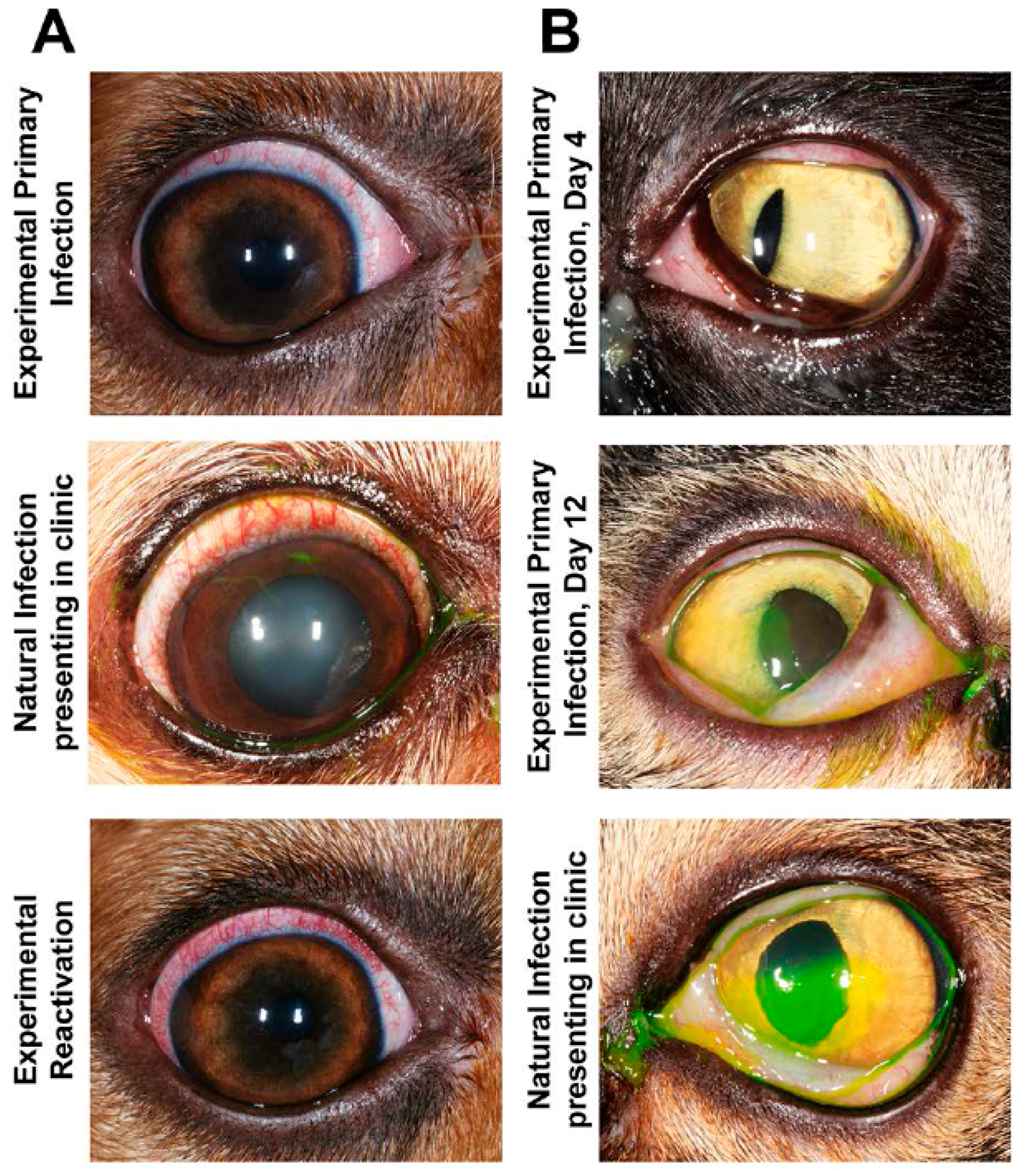
4.3. Dogs
4.4. Cats
4.5. Limitations of Non-Traditional In Vivo Models
5. Conclusions and Future Prospects
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gould, D. Feline herpesvirus-1: Ocular manifestations, diagnosis and treatment options. J. Feline Med. Surg. 2011, 13, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, E.C. Canine herpesvirus-1 ocular diseases of mature dogs. N. Z. Vet. J. 2013, 61, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Rowe, A.M.; St Leger, A.J.; Jeon, S.; Dhaliwal, D.K.; Knickelbein, J.E.; Hendricks, R.L. Herpes keratitis. Prog. Retin. Eye Res. 2013, 32, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Gaskell, R.; Dawson, S.; Radford, A.; Thiry, E. Feline herpesvirus. Vet. Res. 2007, 38, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Maes, R. Felid herpesvirus type 1 infection in cats: A natural host model for alphaherpesvirus pathogenesis. ISRN Vet. Sci. 2012, 2012, 495830. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhu, H. Ocular herpes: The pathophysiology, management and treatment of herpetic eye diseases. Virol. Sin. 2014, 29, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Ogawa, H.; Maeda, K.; Imai, A.; Ohashi, E.; Matsunaga, S.; Tohya, Y.; Ohshima, T.; Mochizuki, M. Nosocomial outbreak of serious canine infectious tracheobronchitis (kennel cough) caused by canine herpesvirus infection. J. Clin. Microbiol. 2010, 48, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Harman, R.M.; Bussche, L.; Ledbetter, E.C.; van de Walle, G.R. Establishment and characterization of an air-liquid canine corneal organ culture model to study acute herpes keratitis. J. Virol. 2014, 88, 13669–13677. [Google Scholar] [CrossRef] [PubMed]
- Pennington, M.R.; van de Walle, G.R. Electric cell-substrate impedance sensing to monitor viral growth and study cellular responses to infection with alphaherpesviruses in real time. mSphere 2017, 2, e00039-17. [Google Scholar] [CrossRef] [PubMed]
- Labetoulle, M.; Auquier, P.; Conrad, H.; Crochard, A.; Daniloski, M.; Bouée, S.; El Hasnaoui, A.; Colin, J. Incidence of herpes simplex virus keratitis in France. Ophthalmology 2005, 112, 888.e1–895.e1. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Sternberg, M.R.; Kottiri, B.J.; McQuillan, G.M.; Lee, F.K.; Nahmias, A.J.; Berman, S.M.; Markowitz, L.E. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 2006, 296, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Young, R.C.; Hodge, D.O.; Liesegang, T.J.; Baratz, K.H. Incidence, recurrence and outcomes of herpes simplex virus eye disease in Olmsted County, Minnesota, 1976–2007: The effect of oral antiviral prophylaxis. Arch. Ophthalmol. 2010, 128, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Burcea, M.; Gheorghe, A.; Pop, M. Incidence of herpes simplex virus keratitis in HIV/AIDS patients compared with the general population. J. Med. Life 2015, 8, 62–63. [Google Scholar] [PubMed]
- Looker, K.J.; Magaret, A.S.; May, M.T.; Turner, K.M.; Vickerman, P.; Gottlieb, S.L. Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS ONE 2015, 10, e0140765. [Google Scholar] [CrossRef] [PubMed]
- Nöthling, J.O.; Hüssy, D.; Steckler, D.; Ackermann, M. Seroprevalence of canine herpesvirus in breeding kennels in the Gauteng Province of South Africa. Theriogenology 2008, 69, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Babaei, H.; Akhtardanesh, B.; Ghanbarpour, R.; Namjoo, A. Serological evidence of canine herpesvirus-1 in dogs of Kerman city, south-east of Iran. Transbound. Emerg. Dis. 2010, 57, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, E.C.; Kim, S.G.; Dubovi, E.J. Outbreak of ocular disease associated with naturally-acquired canine herpesvirus-1 infection in a closed domestic dog colony. Vet. Ophthalmol. 2009, 12, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Krogenæs, A.; Rootwelt, V.; Larsen, S.; Sjøberg, E.K.; Akselsen, B.; Skår, T.M.; Myhre, S.S.; Renström, L.H.M.; Klingeborn, B.; Lund, A. A serologic study of canine herpesvirus-1 infection in the Norwegian adult dog population. Theriogenology 2012, 78, 153–158. [Google Scholar]
- Krogenæs, A.; Rootwelt, V.; Larsen, S.; Renström, L.; Farstad, W.; Lund, A. A serological study of canine herpesvirus-1 infection in a population of breeding bitches in Norway. Acta Vet. Scand. 2014, 56, 19. [Google Scholar] [CrossRef] [PubMed]
- Larsen, R.W.; Kiupel, M.; Balzer, H.J.; Agerholm, J.S. Prevalence of canid herpesvirus-1 infection in stillborn and dead neonatal puppies in Denmark. Acta Vet. Scand. 2015, 57, 1. [Google Scholar] [CrossRef] [PubMed]
- Maggs, D.J.; Lappin, M.R.; Nasisse, M.P. Detection of feline herpesvirus-specific antibodies and DNA in aqueous humor from cats with or without uveitis. Am. J. Vet. Res. 1999, 60, 932–936. [Google Scholar] [PubMed]
- Kang, B.T.; Park, H.M. Prevalence of feline herpesvirus 1, feline calicivirus and Chlamydophila felis in clinically normal cats at a Korean animal shelter. J. Vet. Sci. 2008, 9, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Henzel, A.; Brum, M.C.S.; Lautert, C.; Martins, M.; Lovato, L.T.; Weiblen, R. Isolation and identification of feline calicivirus and feline herpesvirus in Southern Brazil. Braz. J. Microbiol. 2012, 43, 560–568. [Google Scholar] [CrossRef] [PubMed]
- McManus, C.M.; Levy, J.K.; Andersen, L.A.; McGorray, S.P.; Leutenegger, C.M.; Gray, L.K.; Hilligas, J.; Tucker, S.J. Prevalence of upper respiratory pathogens in four management models for unowned cats in the Southeast United States. Vet. J. 2014, 201, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Rypuła, K.; Płoneczka-Janeczko, K.; Bierowiec, K.; Kumala, A.; Sapikowski, G. Prevalence of viral infections in cats in southwestern Poland in the years 2006 to 2010. Berl Munch Tierarztl Wochenschr. 2014, 127, 163–165. [Google Scholar] [PubMed]
- Shaikh, S.; Ta, C.N. Evaluation and management of herpes zoster ophthalmicus. Am. Fam. Physician 2002, 66, 1723–1730. [Google Scholar] [PubMed]
- Borkar, D.S.; Tham, V.M.; Esterberg, E.; Ray, K.J.; Vinoya, A.C.; Parker, J.V.; Uchida, A.; Acharya, N.R. Incidence of herpes zoster ophthalmicus: Results from the pacific ocular inflammation study. Ophthalmology 2013, 120, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.W.; Alvarez-Pasquin, M.J.; Bijl, M.; Franco, E.; Gaillat, J.; Clara, J.G.; Labetoulle, M.; Michel, J.P.; Naldi, L.; Sanmarti, L.S.; et al. Herpes zoster epidemiology, management and disease and economic burden in Europe: A multidisciplinary perspective. Ther. Adv. Vaccines 2015, 3, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.P.; Bolin, D.C.; Bryant, U.; Carter, C.N.; Giles, R.C.; Harrison, L.R.; Hong, C.B.; Jackson, C.B.; Poonacha, K.; Wharton, R.; et al. Prevalence of latent, neuropathogenic equine herpesvirus-1 in the thoroughbred broodmare population of central Kentucky. Equine Vet. J. 2008, 40, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Lunn, D.P.; Davis-Poynter, N.; Flaminio, M.J.B.F.; Horohov, D.W.; Osterrieder, K.; Pusterla, N.; Townsend, H.G.G. Equine herpesvirus-1 consensus statement. J. Vet. Intern. Med. 2009, 23, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Hussey, G.S.; Goehring, L.S.; Lunn, D.P.; Hussey, S.B.; Huang, T.; Osterrieder, N.; Powell, C.; Hand, J.; Holz, C.; Slater, J. Experimental infection with equine herpesvirus type 1 (EHV-1) induces chorioretinal lesions. Vet. Res. 2013, 44, 118. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, Y.; Yilmaz, V.; Kirmizigul, A.H. Equine herpesvirus type 1 (EHV-1) and 4 (EHV-4) infections in horses and donkeys in northeastern Turkey. Iran. J. Vet. Res. 2015, 16, 341–344. [Google Scholar] [PubMed]
- Collinson, P.N.; O’Rielly, J.L.; Ficorilli, N.; Studdert, M.J. Isolation of equine herpesvirus type 2 (equine gammaherpesvirus 2) from foals with keratoconjunctivitis. J. Am. Vet. Med. Assoc. 1994, 205, 329–331. [Google Scholar] [PubMed]
- Borchers, K.; Wolfinger, U.; Goltz, M.; Broll, H.; Ludwig, H. Distribution and relevance of equine herpesvirus type 2(EHV-2) infections. Arch. Virol. 1997, 142, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Borchers, K.; Frölich, K.; Ludwig, H. Detection of equine herpesvirus types 2 and 5 (EHV-2 and EHV-5) in Przewalski’s wild horses. Arch. Virol. 1999, 144, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, O.; von Oppen, T.; Glitz, F.; Deegen, E.; Ludwig, H.; Borchers, K. Detection of equine herpesvirus type 2 (EHV-2) in horses with keratoconjunctivitis. Virus Res. 2001, 80, 93–99. [Google Scholar] [CrossRef]
- Craig, M.I.; Barrandeguy, M.E.; Fernández, F.M. Equine herpesvirus 2 (EHV-2) infection in thoroughbred horses in Argentina. BMC Vet. Res. 2005, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Borchers, K.; Ebert, M.; Fetsch, A.; Hammond, T.; Sterner-Kock, A. Prevalence of equine herpesvirus type 2 (EHV-2) DNA in ocular swabs and its cell tropism in equine conjunctiva. Vet Microbiol. 2006, 118, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Webber, J.J.; Selby, L.A. Risk factors related to the prevalence of infectious bovine keratoconjunctivitis. J. Am. Vet. Med. Assoc. 1981, 179, 823–826. [Google Scholar] [PubMed]
- Nandi, S.; Kumar, M.; Manohar, M.; Chauhan, R.S. Bovine herpesvirus infections in cattle. Anim. Health Res. Rev. 2009, 10, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Raaperi, K.; Orro, T.; Viltrop, A. Epidemiology and control of bovine herpesvirus 1 infection in Europe. Vet. J. 2014, 201, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Bartha, A.; Juhász, M.; Liebermann, H. Isolation of a bovine herpesvirus from calves with respiratory disease and keratoconjunctivitis. A preliminary report. Acta Vet. Acad. Sci. Hung. 1966, 16, 357–358. [Google Scholar] [PubMed]
- Graham, D.A.; McNeill, G.J.; Calvert, V.; Mawhinney, K.; Curran, W.; Ball, N.W.; Todd, D. Virological and serological evidence of bovine herpesvirus type 4 in cattle in Northern Ireland. Vet. Rec. 2005, 157, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Aslan, M.E.; Azkur, A.K.; Gazyagci, S. Epidemiology and genetic characterization of BVDV, BHV-1, BHV-4, BHV-5 and Brucella spp. infections in cattle in Turkey. J. Vet. Med. Sci. 2015, 77, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Cvetojević, Đ.; Savić, B.; Milićević, V.; Kureljušić, B.; Jezdimirović, N.; Jakić-Dimić, D.; Pavlović, M.; Spalević, L. Prevalence of Bovine herpesvirus type 4 in aborting dairy cows. Pol. J. Vet. Sci. 2016, 19, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Seal, B.S.; Heuschele, W.P.; Klieforth, R.B. Prevalence of antibodies to alcelaphine herpesvirus-1 and nucleic acid hybridization analysis of viruses isolated from captive exotic ruminants. Am. J. Vet. Res. 1989, 50, 1447–1453. [Google Scholar] [PubMed]
- Russell, G.C.; Stewart, J.P.; Haig, D.M. Malignant catarrhal fever: A review. Vet. J. 2009, 179, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Bremer, C.W. The prevalence of ovine herpesvirus-2 in 4 sheep breeds from different regions in South Africa. J. S. Afr. Vet. Assoc. 2010, 81, 93–96. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, D.; Li, H. The pathology of malignant catarrhal fever, with an emphasis on ovine herpesvirus 2. Vet. Pathol. 2014, 51, 437–452. [Google Scholar] [CrossRef] [PubMed]
- Thiry, J.; Keuser, V.; Muylkens, B.; Meurens, F.; Gogev, S.; Vanderplasschen, A.; Thiry, E. Ruminant alphaherpesviruses related to bovine herpesvirus 1. Vet. Res. 2006, 37, 169–190. [Google Scholar] [CrossRef] [PubMed]
- Tryland, M.; Das Neves, C.G.; Sunde, M.; Mork, T. Cervid herpesvirus 2, the primary agent in an outbreak of infectious keratoconjunctivitis in semidomesticated reindeer. J. Clin. Microbiol. 2009, 47, 3707–3713. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.L.; das Neves, C.G.; Finstad, G.F.; Beckmen, K.B.; Skjerve, E.; Nymo, I.H.; Tryland, M. Evidence of alphaherpesvirus infections in Alaskan caribou and reindeer. BMC Vet. Res. 2012, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Squires, R.; Wilson, P.; Whelan, N.; Johnstone, A.; Ayanegui-Alcérreca, M.; Castillo-Alcala, F.; Knight, D. Alpha and gamma herpesvirus detection in two herds of farmed red deer (Cervus elaphus) in New Zealand. N. Z. Vet. J. 2012, 60, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Rola, J.; Larska, M.; Socha, W.; Rola, J.G.; Materniak, M.; Urban-Chmiel, R.; Thiry, E.; Żmudziński, J.F. Seroprevalence of bovine herpesvirus 1 related alphaherpesvirus infections in free-living and captive cervids in Poland. Vet. Microbiol. 2017, 204, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Tryland, M.; Romano, J.S.; Marcin, N.; Nymo, I.H.; Josefsen, T.D.; Sørensen, K.K.; Mørk, T. Cervid herpesvirus 2 and not Moraxella bovoculi caused keratoconjunctivitis in experimentally inoculated semi-domesticated Eurasian tundra reindeer. Acta Vet. Scand. 2017, 59, 23. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.P.; Waugh, L.F.; Goldstein, T.; Freeman, K.S.; Kelly, T.R.; Wheeler, E.A.; Smith, B.R.; Gulland, F.M.; Goldstein, T. Evaluation of viruses and their association with ocular lesions in pinnipeds in rehabilitation. Vet. Ophthalmol. 2015, 18, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Webre, J.M.; Hill, J.M.; Nolan, N.M.; Clement, C.; McFerrin, H.E.; Bhattacharjee, P.S.; Hsia, V.; Neumann, D.M.; Foster, T.P.; Lukiw, W.J.; et al. Rabbit and mouse models of HSV-1 latency, reactivation and recurrent eye diseases. J. Biomed. Biotechnol. 2012, 2012, 612316. [Google Scholar] [CrossRef] [PubMed]
- Bean, A.G.D.; Baker, M.L.; Stewart, C.R.; Cowled, C.; Deffrasnes, C.; Wang, L.F.; Lowenthal, J.W. Studying immunity to zoonotic diseases in the natural host—keeping it real. Nat. Rev. Immunol. 2013, 13, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Menne, S.; Cote, P.J. The woodchuck as an animal model for pathogenesis and therapy of chronic hepatitis B virus infection. World J. Gastroenterol. 2007, 13, 104–124. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, K.; Jacobson, I.M.; Tennant, B.C. The role of the woodchuck model in the treatment of hepatitis B virus infection. Clin. Liver Dis. 2007, 11, 707–725. [Google Scholar] [CrossRef] [PubMed]
- Domingo, E.; Parrish, C.R.; Holland, J.J. Origin and Evolution of Viruses; Elsevier Academic Press: New York, NY, USA, 2008; ISBN 9780080564968. [Google Scholar]
- White, D.W.; Suzanne Beard, R.; Barton, E.S. Immune modulation during latent herpesvirus infection. Immunol. Rev. 2012, 245, 189–208. [Google Scholar] [CrossRef] [PubMed]
- Wertheim, J.O.; Smith, M.D.; Smith, D.M.; Scheffler, K.; Kosakovsky Pond, S.L. Evolutionary origins of human herpes simplex viruses 1 and 2. Mol. Biol. Evol. 2014, 31, 2356–2364. [Google Scholar] [CrossRef] [PubMed]
- Field, H.J.; Huang, M.L.; Lay, E.M.; Mickleburgh, I.; Zimmermann, H.; Birkmann, A. Baseline sensitivity of HSV-1 and HSV-2 clinical isolates and defined acyclovir-resistant strains to the helicase—primase inhibitor pritelivir. Antivir. Res. 2013, 100, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Piret, J.; Boivin, G. Antiviral drug resistance in herpesviruses other than cytomegalovirus. Rev. Med. Virol. 2014, 24, 186–218. [Google Scholar] [CrossRef] [PubMed]
- Kongyingyoes, B.; Priengprom, T.; Pientong, C.; Aromdee, C.; Suebsasana, S.; Ekalaksananan, T. 3,19-isopropylideneandrographolide suppresses early gene expression of drug-resistant and wild type herpes simplex viruses. Antivir. Res. 2016, 132, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Novoa, B.; Romero, A.; Álvarez, Á.L.; Moreira, R.; Pereiro, P.; Costa, M.M.; Dios, S.; Estepa, A.; Parra, F.; Figueras, A. Antiviral activity of myticin C peptide from mussel: An ancient defense against herpesviruses. J. Virol. 2016, 90, 7692–7702. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Yang, K.; Wills, E.; Tang, L.; Baines, J.D. A mutation in the DNA polymerase accessory factor of herpes simplex virus 1 restores viral DNA replication in the presence of raltegravir. J. Virol. 2014, 88, 11121–11129. [Google Scholar] [CrossRef] [PubMed]
- Maggs, D.J.; Clarke, H.E. In vitro efficacy of ganciclovir, cidofovir, penciclovir, foscarnet, idoxuridine and acyclovir against feline herpesvirus type-1. Am. J. Vet. Res. 2004, 65, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Van der Meulen, K.; Garré, B.; Croubels, S.; Nauwynck, H. In vitro comparison of antiviral drugs against feline herpesvirus 1. BMC Vet. Res. 2006, 2, 13. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, E.C.; Spertus, C.B.; Pennington, M.R.; van de Walle, G.R.; Judd, B.E.; Mohammed, H.O. In vitro and in vivo evaluation of cidofovir as a topical ophthalmic antiviral for ocular canine herpesvirus-1 infections in dogs. J. Ocul. Pharmacol. Ther. 2015, 31, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Pennington, M.R.; Fort, M.W.; Ledbetter, E.C.; van de Walle, G.R. A novel corneal explant model system to evaluate antiviral drugs against feline herpesvirus type 1 (FHV-1). J. Gen. Virol. 2016, 97, 1414–1425. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, E.C.; Nicklin, A.M.; Spertus, C.B.; Pennington, M.R.; van de Walle, G.R.; Mohammed, H.O. Evaluation of topical ophthalmic ganciclovir gel in the treatment of experimentally induced ocular canine herpesvirus-1 infection. Am. J. Vet. Res. 2017, in press. [Google Scholar]
- Kim, H.S.; Jun Song, X.; de Paiva, C.S.; Chen, Z.; Pflugfelder, S.C.; Li, D.Q. Phenotypic characterization of human corneal epithelial cells expanded ex vivo from limbal explant and single cell cultures. Exp. Eye Res. 2004, 79, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Sandmeyer, L.S.; Keller, C.B.; Bienzle, D. Culture of feline corneal epithelial cells and infection with feline herpesvirus-1 as an investigative tool. Am. J. Vet. Res. 2005, 66, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Werner, A.; Braun, M.; Kietzmann, M. Isolation and cultivation of canine corneal cells for in vitro studies on the anti-inflammatory effects of dexamethasone. Vet. Ophthalmol. 2008, 11, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Farooq, A.V.; Tiwari, V.; Kim, M.J.; Shukla, D. HSV-1 infection of human corneal epithelial cells: Receptor-mediated entry and trends of re-infection. Mol. Vis. 2010, 16, 2476–2486. [Google Scholar] [PubMed]
- García-Posadas, L.; Arranz-Valsero, I.; López-García, A.; Soriano-Romaní, L.; Diebold, Y. A new human primary epithelial cell culture model to study conjunctival inflammation. Investig. Ophthalmol. Vis. Sci. 2013, 54, 7143–7152. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.M.; Li, L.; Fisher, S.; Pearce, V.P.; Shay, J.W.; Wright, W.E.; Cavanagh, H.D.; Jester, J.V. Characterization of growth and differentiation in a telomerase-immortalized human corneal epithelial cell line. Investig. Opthalmol. Vis. Sci. 2005, 46, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Araki-Sasaki, K.; Ohashi, Y.; Sasabe, T.; Hayashi, K.; Watanabe, H.; Tano, Y.; Handa, H. An SV40-immortalized human corneal epithelial cell line and its characterization. Investig. Ophthalmol. Vis. Sci. 1995, 36, 614–621. [Google Scholar]
- Rolinski, J.; Hus, I. Immunological aspects of acute and recurrent herpes simplex keratitis. J. Immunol. Res. 2014, 2014, 513560. [Google Scholar] [CrossRef] [PubMed]
- Giménez, F.; Suryawanshi, A.; Rouse, B.T. Pathogenesis of herpes stromal keratitis—A focus on corneal neovascularization. Prog. Retin. Eye Res. 2013, 33, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Astashkina, A.; Mann, B.; Grainger, D.W. A critical evaluation of in vitro cell culture models for high-throughput drug screening and toxicity. Pharmacol. Ther. 2012, 134, 82–106. [Google Scholar] [CrossRef] [PubMed]
- Nasisse, M.P.; Guy, J.S.; Davidson, M.G.; Sussman, W.; de Clercq, E. In vitro susceptibility of feline herpesvirus-1 to vidarabine, idoxuridine, trifluridine, acyclovir, or bromovinyldeoxyuridine. Am. J. Vet. Res. 1989, 50, 158–160. [Google Scholar] [PubMed]
- Stiles, J. Treatment of cats with ocular disease attributable to herpesvirus infection: 17 cases (1983–1993). J. Am. Vet. Med. Assoc. 1995, 207, 599–603. [Google Scholar] [PubMed]
- Nasisse, M.P.; Dorman, D.C.; Jamison, K.C.; Weigler, B.J.; Hawkins, E.C.; Stevens, J.B. Effects of valacyclovir in cats infected with feline herpesvirus 1. Am. J. Vet. Res. 1997, 58, 1141–1144. [Google Scholar] [PubMed]
- Williams, D.L.; Robinson, J.C.; Lay, E.; Field, H. Efficacy of topical aciclovir for the treatment of feline herpetic keratitis: Results of a prospective clinical trial and data from in vitro investigations. Vet. Rec. 2005, 157, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Sandmeyer, L.S.; Keller, C.B.; Bienzle, D. Effects of cidofovir on cell death and replication of feline herpesvirus-1 in cultured feline corneal epithelial cells. Am. J. Vet. Res. 2005, 66, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Fontenelle, J.P.; Powell, C.C.; Veir, J.K.; Radecki, S.V.; Lappin, M.R. Effect of topical ophthalmic application of cidofovir on experimentally induced primary ocular feline herpesvirus-1 infection in cats. Am. J. Vet. Res. 2008, 69, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Thiry, E.; Addie, D.; Belák, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Hosie, M.J.; Lloret, A.; et al. Feline herpesvirus infection ABCD guidelines on prevention and management. J. Feline Med. Surg. 2009, 11, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Rajaiya, J.; Zhou, X.; Barequet, I.; Gilmore, M.S.; Chodosh, J. Novel model of innate immunity in corneal infection. In Vitro Cell. Dev. Biol. Anim. 2015, 51, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, C.E.; Rnjak-Kovacina, J.; Kaplan, D.L. Corneal tissue engineering: Recent advances and future perspectives. Tissue Eng. Part B. Rev. 2015, 21, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.Y.; Li, Y.T.; Cho, C.H.; Yu, T.C. Nanoscale modification of porous gelatin scaffolds with chondroitin sulfate for corneal stromal tissue engineering. Int. J. Nanomedicine 2012, 7, 1101–1114. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, M.A.; Gemeinhart, R.A.; Yue, B.Y.J.T.; Djalilian, A.R. Decellularized human cornea for reconstructing the corneal epithelium and anterior stroma. Tissue Eng. Part C Methods 2012, 18, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Funamoto, S.; Sasaki, S.; Honda, T.; Hattori, S.; Nam, K.; Kimura, T.; Mochizuki, M.; Fujisato, T.; Kobayashi, H.; et al. Preparation and characterization of decellularized cornea using high-hydrostatic pressurization for corneal tissue engineering. Biomaterials 2010, 31, 3941–3948. [Google Scholar] [CrossRef] [PubMed]
- Bayyoud, T.; Thaler, S.; Hofmann, J.; Maurus, C.; Spitzer, M.S.; Bartz-Schmidt, K.U.; Szurman, P.; Yoeruek, E. Decellularized bovine corneal posterior lamellae as carrier matrix for cultivated human corneal endothelial cells. Curr. Eye Res. 2012, 37, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.; Sun, W.; Chen, G.; Tang, S.; Li, M.; Shao, Z.; Mi, S. Tissue-engineered cornea constructed with compressed collagen and laser-perforated electrospun mat. Sci. Rep. 2017, 7, 970. [Google Scholar] [CrossRef] [PubMed]
- Resau, J.H.; Sakamoto, K.; Cottrell, J.R.; Hudson, E.A.; Meltzer, S.J. Explant organ culture: A review. Cytotechnology 1991, 7, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959. [Google Scholar]
- Armitage, W.J. Preservation of human cornea. Transfus. Med. Hemother. 2011, 38, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Richard, N.R.; Anderson, J.A.; Weiss, J.L.; Binder, P.S. Air/liquid corneal organ culture: A light microscopic study. Curr. Eye Res. 1991, 10, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Collin, H.B.; Anderson, J.A.; Richard, N.R.; Binder, P.S. In vitro model for corneal wound healing; organ-cultured human corneas. Curr. Eye Res. 1995, 14, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Alekseev, O.; Tran, A.H.; Azizkhan-Clifford, J. Ex vivo organotypic corneal model of acute epithelial herpes simplex virus type I infection. J. Vis. Exp. 2012, e3631. [Google Scholar] [CrossRef] [PubMed]
- Alekseev, O.; Limonnik, V.; Donovan, K.; Azizkhan-Clifford, J. Activation of checkpoint kinase 2 is critical for herpes simplex virus type 1 replication in corneal epithelium. Ophthalmic Res. 2015, 53, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Drevets, P.; Chucair-Elliott, A.; Shrestha, P.; Jinkins, J.; Karamichos, D.; Carr, D.J.J. The use of human cornea organotypic cultures to study herpes simplex virus type 1 (HSV-1)-induced inflammation. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, N.; Jaishankar, D.; Agelidis, A.; Yadavalli, T.; Mangano, K.; Patel, S.; Tekin, S.Z.; Shukla, D. Cultured corneas show dendritic spread and restrict herpes simplex virus infection that is not observed with cultured corneal cells. Sci. Rep. 2017, 7, 42559. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; van Cleemput, J.; Qiu, Y.; Reddy, V.R.; Mateusen, B.; Nauwynck, H.J. Ex vivo modeling of feline herpesvirus replication in ocular and respiratory mucosae, the primary targets of infection. Virus Res. 2015, 210, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.B.; Jensen, S.B.; Nielsen, C.; Quartin, E.; Kato, H.; Chen, Z.J.; Silverman, R.H.; Akira, S.; Paludan, S.R. Herpes simplex virus infection is sensed by both toll-like receptors and retinoic acid-inducible gene-like receptors, which synergize to induce type I interferon production. J. Gen. Virol. 2009, 90, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.R.; Kaminski, J.J.; Kurt-Jones, E.A.; Fitzgerald, K.A. Pattern recognition receptors and the innate immune response to viral infection. Viruses 2011, 3, 920–940. [Google Scholar] [CrossRef] [PubMed]
- Hamrah, P.; Huq, S.O.; Liu, Y.; Zhang, Q.; Dana, M.R. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J. Leukoc. Biol. 2003, 74, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.R.R.; Naranjo, C.; Leiva, M.; Fondevila, D.; Iborra, A.; Martinez, P.; Peña, T. Canine normal corneal epithelium bears a large population of CD45-positive cells. Vet. J. 2009, 179, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.V.; Abrams, K.L.; Kern, T.J. Feline eosinophilic keratitis: A retrospective study of 54 cases: (1989–1994). Vet. Comp. Ophthalmol. 1996, 6, 131–134. [Google Scholar]
- Novak, N.; Peng, W.M. Dancing with the enemy: The interplay of herpes simplex virus with dendritic cells. Clin. Exp. Immunol. 2005, 142, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Kaye, S.; Choudhary, A. Herpes simplex keratitis. Prog. Retin. Eye Res. 2006, 25, 355–380. [Google Scholar] [CrossRef] [PubMed]
- Röck, T.; Hofmann, J.; Thaler, S.; Bramkamp, M.; Bartz-Schmidt, K.U.; Yoeruek, E.; Röck, D. Factors that influence the suitability of human organ-cultured corneas. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Hagenah, M.; Böhnke, M.; Engelmann, K.; Winter, R. Incidence of bacterial and fungal contamination of donor corneas preserved by organ culture. Cornea 1995, 14, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Armitage, W.J.; Easty, D.L. Factors influencing the suitability of organ-cultured corneas for transplantation. Investig. Ophthalmol. Vis. Sci. 1997, 38, 16–24. [Google Scholar]
- Gain, P.; Thuret, G.; Chiquet, C.; Vautrin, A.C.; Carricajo, A.; Acquart, S.; Maugery, J.; Aubert, G. Use of a pair of blood culture bottles for sterility testing of corneal organ culture media. Br. J. Ophthalmol. 2001, 85, 1158–1162. [Google Scholar] [CrossRef] [PubMed]
- Pels, L. Organ culture: The method of choice for preservation of human donor corneas. Br. J. Ophthalmol. 1997, 81, 523–525. [Google Scholar] [CrossRef] [PubMed]
- Spelsberg, H.; Reinhard, T.; Sengler, U.; Daeubener, W.; Sundmacher, R. Organ-cultured corneal grafts from septic donors: A retrospective study. Eye 2002, 16, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Borderie, V.M.; Laroche, L. Microbiologic study of organ-cultured donor corneas. Transplantation 1998, 66, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Röck, D.; Wude, J.; Bartz-Schmidt, K.U.; Yoeruek, E.; Thaler, S.; Röck, T. Factors influencing the contamination rate of human organ-cultured corneas. Acta Ophthalmol. 2017, in press. [Google Scholar]
- Gruenert, A.K.; Rosenbaum, K.; Geerling, G.; Fuchsluger, T.A. The influence of donor factors on corneal organ culture contamination. Acta Ophthalmol. 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Seiler, T.G.; Tschopp, M.; Zimmerli, S.; Tappeiner, C.; Wittwer, V.V.; Frueh, B.E. Time course of antibiotic and antifungal concentrations in corneal organ culture. Cornea 2016, 35, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.M.; Clement, C. Herpes simplex virus type 1 DNA in human corneas: What are the virological and clinical implications? J. Infect. Dis. 2009, 200, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.E.; Azcuy, A.M.; Varnell, E.D.; Sloop, G.D.; Thompson, H.W.; Hill, J.M. HSV-1 DNA in tears and saliva of normal adults. Investig. Ophthalmol. Vis. Sci. 2005, 46, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Farooq, A.V.; Shukla, D. Corneal latency and transmission of herpes simplex virus-1. Future Virol. 2011, 6, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Cleator, G.M.; Klapper, P.E.; Dennett, C.; Sullivan, A.L.; Bonshek, R.E.; Marcyniuk, B.; Tullo, A.B. Corneal donor infection by herpes simplex virus: Herpes simplex virus DNA in donor corneas. Cornea 1994, 13, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Openshaw, H.; McNeill, J.I.; Lin, X.H.; Niland, J.; Cantin, E.M. Herpes simplex virus DNA in normal corneas: Persistence without viral shedding from ganglia. J. Med. Virol. 1995, 46, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Garweg, J.G.; Boehnke, M. Low rate shedding of HSV-1 DNA but not of infectious virus from human donor corneae into culture media. J. Med. Virol. 1997, 52, 320–325. [Google Scholar] [CrossRef]
- Van Gelderen, B.E.; van der Lelij, A.; Treffers, W.F.; van der Gaag, R. Detection of herpes simplex virus type 1, 2 and varicella zoster virus DNA in recipient corneal buttons. Br. J. Ophthalmol. 2000, 84, 1238–1243. [Google Scholar] [CrossRef] [PubMed]
- Broniek, G.; Langwińska-Wośko, E.; Sybilska, M.; Szaflik, J.P.; Przybylski, M.; Wróblewska, M. Occurrence of viral DNA in paired samples of corneal rim and cornea preservation fluid. J. Med. Virol. 2016, 89, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Sengler, U.; Reinhard, T.; Adams, O.; Krempe, C.; Sundmacher, R. Herpes simplex virus infection in the media of donor corneas during organ culture: Frequency and consequences. Eye (Lond.) 2001, 15, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Stiles, J.; Pogranichniy, R. Detection of virulent feline herpesvirus-1 in the corneas of clinically normal cats. J. Feline Med. Surg. 2008, 10, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Sawtell, N.M.; Poon, D.K.; Tansky, C.S.; Thompson, R.L. The latent herpes simplex virus type 1 genome copy number in individual neurons is virus strain specific and correlates with reactivation. J. Virol. 1998, 72, 5343–5350. [Google Scholar] [PubMed]
- Mulik, S.; Xu, J.; Reddy, P.B.J.; Rajasagi, N.K.; Gimenez, F.; Sharma, S.; Lu, P.Y.; Rouse, B.T. Role of miR-132 in angiogenesis after ocular infection with herpes simplex virus. Am. J. Pathol. 2012, 18, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Matundan, H.; Mott, K.R.; Ghiasi, H. Role of CD8+ T cells and lymphoid dendritic cells in protection from ocular herpes simplex virus 1 challenge in immunized mice. J. Virol. 2014, 88, 8016–8027. [Google Scholar] [CrossRef] [PubMed]
- Chucair-Elliott, A.J.; Zheng, M.; Carr, D.J.J. Degeneration and regeneration of corneal nerves in response to HSV-1 infection. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yin, X.; Stuart, P.M.; Leib, D.A. Dendritic cell autophagy contributes to herpes simplex virus-driven stromal keratitis and immunopathology. mBio 2015, 6, e01426-15. [Google Scholar] [CrossRef] [PubMed]
- Royer, D.J.; Zheng, M.; Conrady, C.D.; Carr, D.J.J. Granulocytes in ocular HSV-1 infection: Opposing roles of mast cells and neutrophils. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3763–3775. [Google Scholar] [CrossRef] [PubMed]
- Feldman, L.T.; Ellison, A.R.; Voytek, C.C.; Yang, L.; Krause, P.; Margolis, T.P. Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proc. Natl. Acad. Sci. USA 2002, 99, 978–983. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, B.M.; Halford, W.P. Evidence that spontaneous reactivation of herpesvirus does not occur in mice. Virol. J. 2005, 2, 67. [Google Scholar] [CrossRef] [PubMed]
- Margolis, T.P.; Elfman, F.L.; Leib, D.; Pakpour, N.; Apakupakul, K.; Imai, Y.; Voytek, C. Spontaneous reactivation of herpes simplex virus type 1 in latently infected murine sensory ganglia. J. Virol. 2007, 81, 11069–11074. [Google Scholar] [CrossRef] [PubMed]
- Jester, J.V.; Morishige, N.; BenMohamed, L.; Brown, D.J.; Osorio, N.; Hsiang, C.; Perng, G.C.; Jones, C.; Wechsler, S.L. Confocal microscopic analysis of a rabbit eye model of high-incidence recurrent herpes stromal keratitis. Cornea 2016, 35, 81–88. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Cosby, R.; Hill, J.M.; Bazan, H.E.P. Changes in corneal innervation after HSV-1 latency established with different reactivation phenotypes. Curr. Eye Res. 2016, 42, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Dervillez, X.; Khan, A.A.; Chentoufi, A.A.; Chilukuri, S.; Shukr, N.; Fazli, Y.; Ong, N.N.; Afifi, R.E.; Osorio, N.; et al. The herpes simplex virus latency-associated transcript gene is associated with a broader repertoire of virus-specific exhausted CD8+ T cells retained within the trigeminal ganglia of latently infected HLA transgenic rabbits. J. Virol. 2016, 90, 3913–3928. [Google Scholar] [CrossRef] [PubMed]
- Kollias, C.M.; Huneke, R.B.; Wigdahl, B.; Jennings, S.R. Animal models of herpes simplex virus immunity and pathogenesis. J. Neurovirol. 2015, 21, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Chentoufi, A.A.; Dasgupta, G.; Christensen, N.D.; Hu, J.; Choudhury, Z.S.; Azeem, A.; Jester, J.V.; Nesburn, A.B.; Wechsler, S.L.; BenMohamed, L. A novel HLA (HLA-A*0201) transgenic rabbit model for preclinical evaluation of human CD8+ T cell epitope-based vaccines against ocular herpes. J. Immunol. 2010, 184, 2561–2571. [Google Scholar] [CrossRef] [PubMed]
- Evermann, J.F.; Ledbetter, E.C.; Maes, R.K. Canine reproductive, respiratory and ocular diseases due to canine herpesvirus. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 1097–1120. [Google Scholar] [CrossRef] [PubMed]
- Wright, N.G.; Cornwell, H.J.C. The susceptibility of six-week old puppies to canine jerpes virus. J. Small Anim. Pract. 1969, 10, 669–674. [Google Scholar] [CrossRef]
- Ledbetter, E.C.; Dubovi, E.J.; Kim, S.G.; Maggs, D.J.; Bicalho, R.C. Experimental primary ocular canine herpesvirus-1 infection in adult dogs. Am. J. Vet. Res. 2009, 70, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, E.C.; Kim, S.G.; Dubovi, E.J.; Bicalho, R.C. Experimental reactivation of latent canine herpesvirus-1 and induction of recurrent ocular disease in adult dogs. Vet. Microbiol. 2009, 138, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, E.C.; Kice, N.C.; Matusow, R.B.; Dubovi, E.J.; Kim, S.G. The effect of topical ocular corticosteroid administration in dogs with experimentally induced latent canine herpesvirus-1 infection. Exp. Eye Res. 2010, 90, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, E.C.; da Silva, E.C.; Kim, S.G.; Dubovi, E.J.; Schwark, W.S. Frequency of spontaneous canine herpesvirus-1 reactivation and ocular viral shedding in latently infected dogs and canine herpesvirus-1 reactivation and ocular viral shedding induced by topical administration of cyclosporine and systemic administration of corticosteroids. Am. J. Vet. Res. 2012, 73, 1079–1084. [Google Scholar] [PubMed]
- Mundy, P.; da Silva, E.C.; Ledbetter, E.C. Effects of cyclophosphamide myelosuppression in adult dogs with latent canine herpesvirus-1 infection. Vet. Microbiol. 2012, 159, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Nicklin, A.M.; McEntee, M.C.; Ledbetter, E.C. Effects of ocular surface strontium-90 β radiotherapy in dogs latently infected with canine herpesvirus-1. Vet. Microbiol. 2014, 174, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Spertus, C.B.; Mohammed, H.O.; Ledbetter, E.C. Effects of topical ocular application of 1% trifluridine ophthalmic solution in dogs with experimentally induced recurrent ocular canine herpesvirus-1 infection. Am. J. Vet. Res. 2016, 77, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, E.C.; Kim, K.; Dubovi, E.J.; Mohammed, H.O.; Felippe, M.J.B. Clinical and immunological assessment of therapeutic immunization with a subunit vaccine for recurrent ocular canine herpesvirus-1 infection in dogs. Vet. Microbiol. 2016, 197, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Nasisse, M.P.; Guy, J.S.; Davidson, M.G.; Sussman, W.A.; Fairley, N.M. Experimental ocular herpesvirus infection in the cat. Sites of virus replication, clinical features and effects of corticosteroid administration. Investig. Ophthalmol. Vis. Sci. 1989, 30, 1758–1768. [Google Scholar]
- Haid, C.; Kaps, S.; Gönczi, E.; Hässig, M.; Metzler, A.; Spiess, B.M.; Richter, M. Pretreatment with feline interferon omega and the course of subsequent infection with feline herpesvirus in cats. Vet. Ophthalmol. 2007, 10, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Thomasy, S.M.; Lim, C.C.; Reilly, C.M.; Kass, P.H.; Lappin, M.R.; Maggs, D.J. Evaluation of orally administered famciclovir in cats experimentally infected with feline herpesvirus type-1. Am. J. Vet. Res. 2011, 72, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Hamano, M.; Maeda, K.; Mizukoshi, F.; Une, Y.; Mochizuki, M.; Tohya, Y.; Akashi, H.; Kai, K. Experimental infection of recent field isolates of feline herpesvirus type 1. J. Vet. Med. Sci. 2003, 65, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Vaz, P.K.; Job, N.; Horsington, J.; Ficorilli, N.; Studdert, M.J.; Hartley, C.A.; Gilkerson, J.R.; Browning, G.F.; Devlin, J.M. Low genetic diversity among historical and contemporary clinical isolates of felid herpesvirus 1. BMC Genomics 2016, 17, 704. [Google Scholar] [CrossRef] [PubMed]
- Maggs, D.J.; Nasisse, M.P.; Kass, P.H. Efficacy of oral supplementation with l-lysine in cats latently infected with feline herpesvirus. Am. J. Vet. Res. 2003, 64, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, J.W. Attitudes toward Animals: Species Ratings. Soc. Anim. 1995, 3, 139–150. [Google Scholar] [CrossRef]
- Baumans, V. Use of animals in experimental research: An ethical dilemma? Gene Ther. 2004, 11, S64–S66. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.J.; Lee, H.S.; Yu, X.F.; Choi, E.; Koo, B.C.; Kwon, M.S.; Lee, Y.S.; Cho, S.J.; Jin, G.Z.; Kim, L.H.; et al. Generation of cloned transgenic cats expressing red fluorescence protein. Biol. Reprod. 2008, 78, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.G.; Kim, M.K.; Jang, G.; Oh, H.J.; Park, J.E.; Kang, J.T.; Koo, O.J.; Kim, T.; Kwon, M.S.; Koo, B.C.; et al. Generation of red fluorescent protein transgenic dogs. Genesis 2009, 47, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.E.; Keller, G.L.; Dresser, B.L. In vitro fertilization in domestic and non-domestic cats including sequences of early nuclear events, development in vitro, cryopreservation and successful intra- and interspecies embryo transfer. J. Reprod. Fertil. Suppl. 1993, 47, 189–201. [Google Scholar] [PubMed]
- Nagashima, J.B.; Sylvester, S.R.; Nelson, J.L.; Cheong, S.H.; Mukai, C.; Lambo, C.; Flanders, J.A.; Meyers-Wallen, V.N.; Songsasen, N.; Travis, A.J. Live births from domestic dog (Canis familiaris) embryos produced by in vitro rertilization. PLoS ONE 2015, 10, e0143930. [Google Scholar] [CrossRef] [PubMed]
- Robert-Tissot, C.; Rüegger, V.L.; Cattori, V.; Meli, M.L.; Riond, B.; Gomes-Keller, M.A.; Vögtlin, A.; Wittig, B.; Juhls, C.; Hofmann-Lehmann, R.; et al. The innate antiviral immune system of the cat: Molecular tools for the measurement of its state of activation. Vet. Immunol. Immunopathol. 2011, 143, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Decker, B.; Parker, H.G.; Dhawan, D.; Kwon, E.M.; Karlins, E.; Davis, B.W.; Ramos-Vara, J.A.; Bonney, P.L.; McNiel, E.A.; Knapp, D.W.; et al. Homologous mutation to human BRAF V600E is common in naturally occurring canine bladder cancer—Evidence for a relevant model system and urine-based diagnostic test. Mol. Cancer Res. 2015, 13, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Brachelente, C.; Cappelli, K.; Capomaccio, S.; Porcellato, I.; Silvestri, S.; Bongiovanni, L.; de Maria, R.; Verini Supplizi, A.; Mechelli, L.; Sforna, M. Transcriptome analysis of canine cutaneous melanoma and melanocytoma reveals a modulation of genes regulating extracellular matrix metabolism and cell cycle. Sci. Rep. 2017, 7, 6386. [Google Scholar] [CrossRef] [PubMed]
- Pomari, E.; Stefanon, B.; Colitti, M. Effect of Arctium lappa (burdock) extract on canine dermal fibroblasts. Vet. Immunol. Immunopathol. 2013, 156, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Ertl, R.; Klein, D. Transcriptional profiling of the host cell response to feline immunodeficiency virus infection. Virol. J. 2014, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Kafarnik, C.; Fritsche, J.; Reese, S. In vivo confocal microscopy in the normal corneas of cats, dogs and birds. Vet. Ophthalmol. 2007, 10, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Kafarnik, C.; Fritsche, J.; Reese, S. Corneal innervation in mesocephalic and brachycephalic dogs and cats: Assessment using in vivo confocal microscopy. Vet. Ophthalmol. 2008, 11, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.J.; Kipar, A.; Sample, J.T.; Stewart, J.P. Pathogenesis of a model gammaherpesvirus in a natural host. J. Virol. 2010, 84, 3949–3961. [Google Scholar] [CrossRef] [PubMed]
- McHugh, K.J.; Saint-Geniez, M.; Tao, S.L. Topographical control of ocular cell types for tissue engineering. J. Biomed. Mater. Res. B. Appl. Biomater. 2013, 101, 1571–1584. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.W. Ocular immune privilege and transplantation. Front. Immunol. 2016, 7, 37. [Google Scholar] [CrossRef] [PubMed]
| Virus | Abbreviations | Subfamily | Associated Ocular Diseases | Overall Prevalence | Herpesvirus-Associated Ocular Disease Prevalence | References |
|---|---|---|---|---|---|---|
| Human alphaherpesvirus 1 | HHV-1/HSV-1 | Simplexvirus | Corneal lesions, stromal & epithelial keratitis, conjunctivitis | 67–90% | 12–36/100,000 | [3,10,11,12,13,14] |
| Canid alphaherpesvirus 1 | CHV-1 | Varicellovirus | Corneal lesions, stromal & epithelial keratitis, conjunctivitis | 21–98% | Unknown | [2,15,16,17,18,19,20] |
| Felid alphaherpesvirus 1 | FHV-1 | Varicellovirus | Corneal lesions, stromal & epithelial keratitis, conjunctivitis | 40–97% | Unknown | [21,22,23,24,25] |
| Human alphaherpesvirus 3 | HHV-3/VZV | Varicellovirus | Herpes zoster ophthalmicus | > 95% | 19–31/100,000 | [26,27,28] |
| Equid alphaherpesvirus 1 | EHV-1 | Varicellovirus | Chorioretinitis | 52%-“endemic” | 50–90% of choroidal lesions in experimental infection | [29,30,31,32] |
| Equid gammaherpesvirus 2 | EHV-2 | Percavirus | Keratoconjunctivitis | 51–93% | 8–60% of keratoconjunctivitis cases tested | [33,34,35,36,37,38] |
| Bovine alphaherpesvirus 1 | BoHV-1 | Varicellovirus | Keratoconjunctivitis | 20–97% | 4.95/100 | [39,40,41] |
| Bovine gammaherpesvirus 4 | BoHV-4 | Rhadinovirus | Keratoconjunctivitis & ocular discharge | 21–35% | Unknown | [42,43,44,45] |
| Alcelpahine gammaherpesvirus 1 & Ovine gammaherpesvirus 2 | AlHV-1 OvHV-2 | Macavirus | Ocular discharge | 29–77% | Typical symptom of malignant catarrhal fever | [46,47,48,49] |
| Cervid alphaherpesvirus 1 & 2 | CvHV-1 CvHV-2 | Varicellovirus | Keratoconjunctivitis & keratitis | 18–47% | ~5% in free-ranging, 30% in animals | [50,51,52,53,54,55] |
| Otariid herpesviruses & Phocid herpesviruses (Various species) | OtHV PhHV | Gammaherpes-viruses | Corneal lesions, keratoconjunctivitis | 26–76% | Unknown | [56] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pennington, M.R.; Ledbetter, E.C.; Van de Walle, G.R. New Paradigms for the Study of Ocular Alphaherpesvirus Infections: Insights into the Use of Non-Traditional Host Model Systems. Viruses 2017, 9, 349. https://doi.org/10.3390/v9110349
Pennington MR, Ledbetter EC, Van de Walle GR. New Paradigms for the Study of Ocular Alphaherpesvirus Infections: Insights into the Use of Non-Traditional Host Model Systems. Viruses. 2017; 9(11):349. https://doi.org/10.3390/v9110349
Chicago/Turabian StylePennington, Matthew R., Eric C. Ledbetter, and Gerlinde R. Van de Walle. 2017. "New Paradigms for the Study of Ocular Alphaherpesvirus Infections: Insights into the Use of Non-Traditional Host Model Systems" Viruses 9, no. 11: 349. https://doi.org/10.3390/v9110349
APA StylePennington, M. R., Ledbetter, E. C., & Van de Walle, G. R. (2017). New Paradigms for the Study of Ocular Alphaherpesvirus Infections: Insights into the Use of Non-Traditional Host Model Systems. Viruses, 9(11), 349. https://doi.org/10.3390/v9110349





