Development of Neutralization Assay Using an eGFP Chikungunya Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines, Viruses, Antibodies
2.2. Plasmid Construction
2.3. RNA Transcription and Transfection
2.4. Plaque Assay
2.5. Immunofluorescence Assay (IFA)
2.6. Antiviral Assay of eGFP-CHIKV by Ribavirin
2.7. Neutralization Assay Based on eGFP-CHIKV Reporter Virus
2.8. Plaque Reduction Neutralization Tests (PRNTs)
2.9. Real-Time RT-PCR Analysis
2.10. Statistical Analysis
3. Results
3.1. Isolation and Characterization of CHIKV
3.2. Construction and Characterization of of the Full-Length CHIKV cDNA Clone
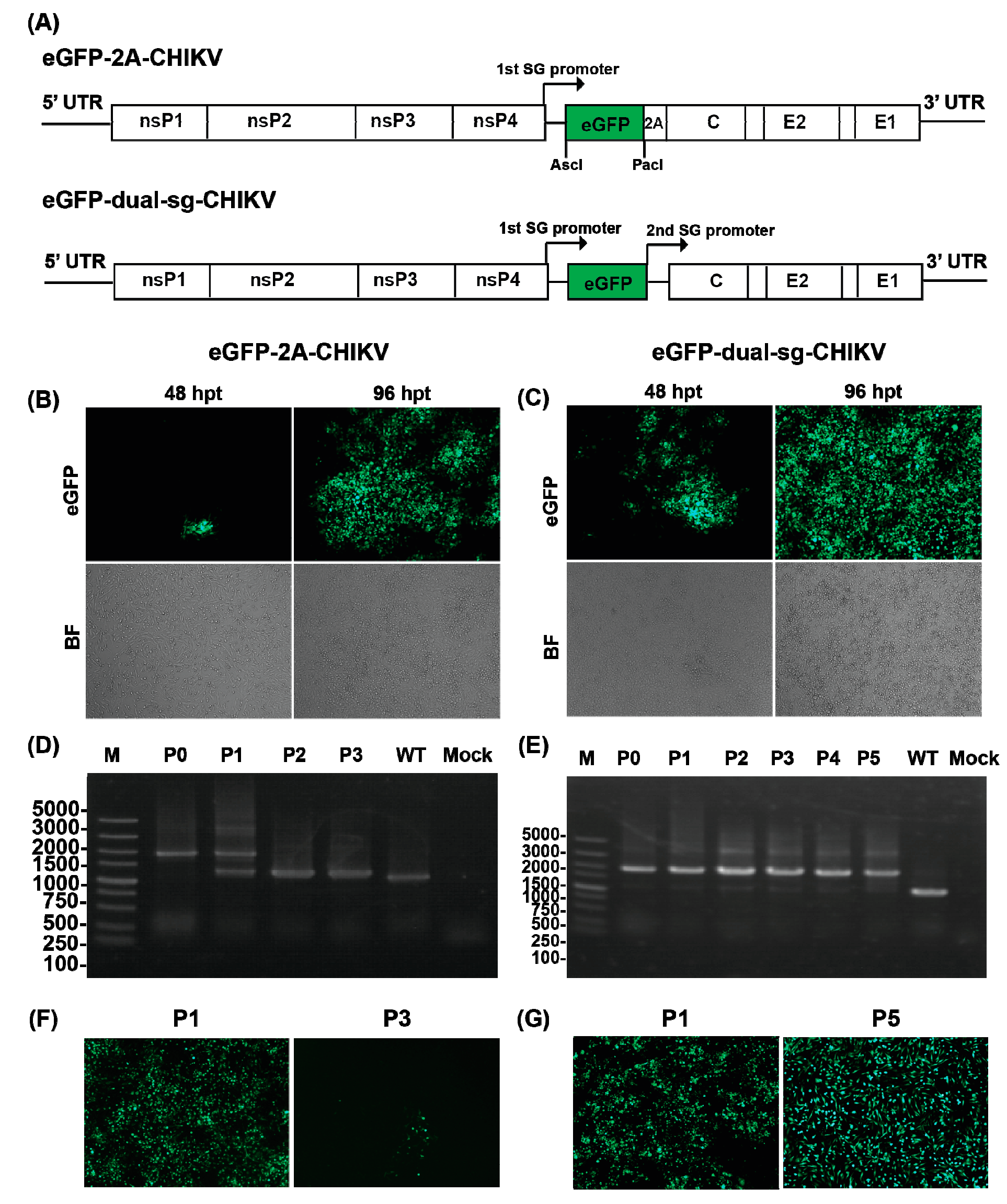
3.3. Construction of the CHIKV Reporter Virus with eGFP
3.4. Characterization of the CHIKV Reporter Virus
3.5. Inhibitory Effects of Ribavirin on eGFP-CHIKV Reporter Virus
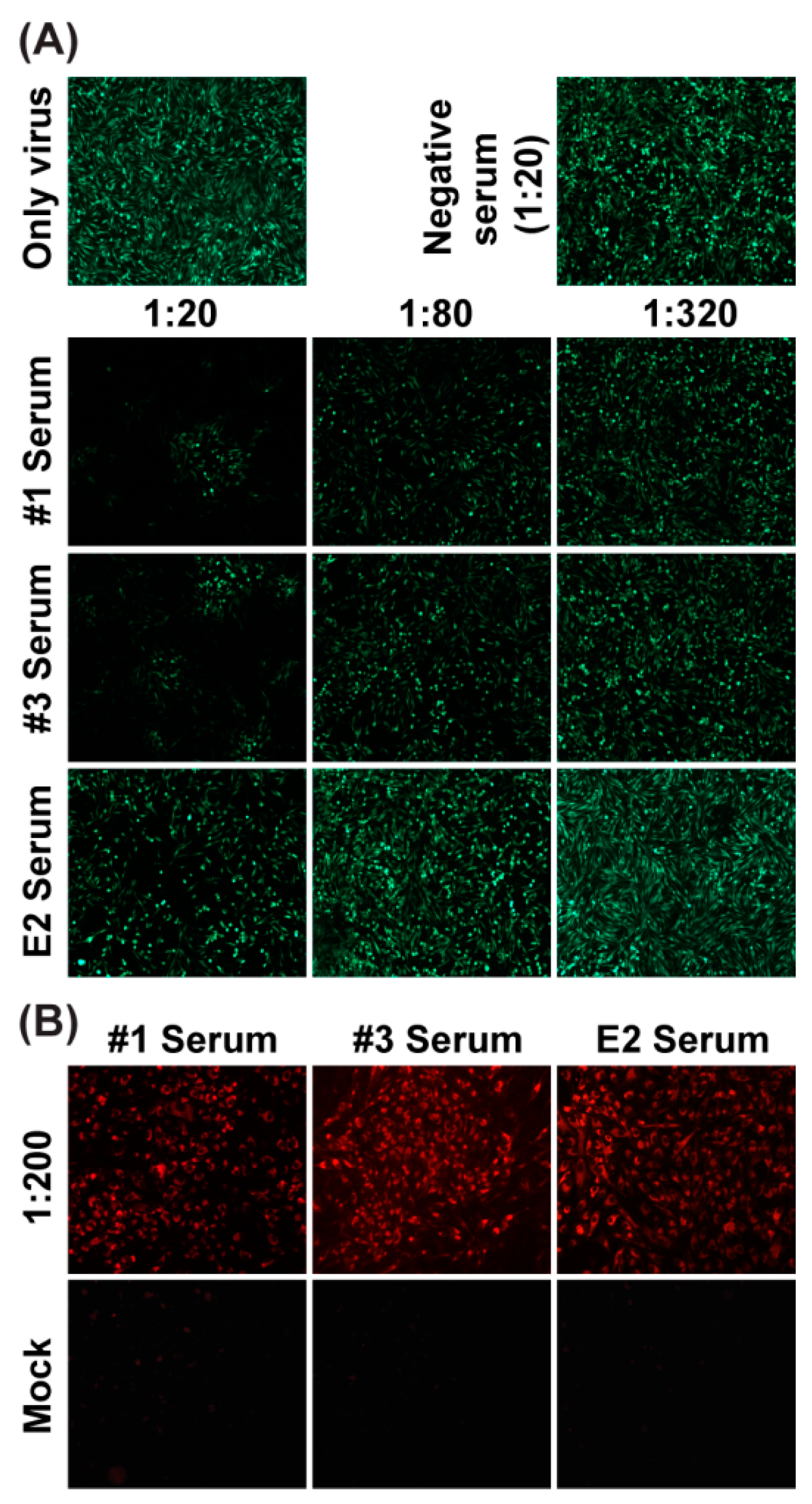
3.6. Confirmation of Neutralization Assay Using eGFP-CHIKV with CHIKV Patients’ Sera
3.7. Neutralization Assay with Sera from Mice Using eGFP-CHIKV
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CHIKV | Chikungunya virus |
| mAbs | monoclonal antibodies |
| NT | neutralization |
| MOI | multiplicity of infection |
| eGFP | enhanced green fluorescent protein |
| Gluc | Gaussia luciferase |
References
- Burt, F.J.; Rolph, M.S.; Rulli, N.E.; Mahalingam, S.; Heise, M.T. Chikungunya: A re-emerging virus. Lancet 2012, 379, 662–671. [Google Scholar] [CrossRef]
- Morrison, T.E. Reemergence of Chikungunya Virus. J. Virol. 2014, 88, 11644–11647. [Google Scholar] [CrossRef] [PubMed]
- Cruz, D.J.M.; Bonotto, R.M.; Gomes, R.G.B.; da Silva, C.T.; Taniguchi, J.B.; No, J.H.; Lombardot, B.; Schwartz, O.; Hansen, M.A.E.; Freitas-Junior, L.H. Identification of novel compounds inhibiting Chikungunya virus-induced cell death by high throughput screening of a kinase inhibitor library. PLoS Neglect. Trop. Dis. 2013, 7, e2471. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, S.A.; Nuckols, J.; McGee, C.E.; Huang, Y.-J.S.; Vanlandingham, D.L.; Tesh, R.B.; Higgs, S. In vivo imaging of Chikungunya virus in mice and Aedes mosquitoes using a Renilla luciferase clone. Vector Borne Zoonotic Dis. 2011, 11, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Reisen, W.K. Present and future arboviral threats. Antivir. Res. 2010, 85, 328–345. [Google Scholar] [CrossRef] [PubMed]
- Josseran, L.; Paquet, C.; Zehgnoun, A.; Caillere, N.; le Tertre, A.; Solet, J.-L.; Ledrans, M. Chikungunya disease outbreak, Reunion island. Emerg. Infect. Dis. 2006, 12, 1994–1995. [Google Scholar] [CrossRef] [PubMed]
- Tsetsarkin, K.A.; Vanlandingham, D.L.; McGee, C.E.; Higgs, S. A single mutation in Chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007, 3, e201. [Google Scholar] [CrossRef] [PubMed]
- Dash, A.; Bhatia, R.; Sunyoto, T.; Mourya, D. Emerging and re-emerging arboviral diseases in Southeast Asia. J. Vector Borne Dis. 2013, 50, 77–84. [Google Scholar] [PubMed]
- Zheng, K.; Li, J.; Zhang, Q.; Liang, M.; Li, C.; Lin, M.; Huang, J.; Li, H.; Xiang, D.; Wang, N.; et al. Genetic analysis of chikungunya viruses imported to mainland China in 2008. Virol. J. 2010, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhang, Y.; Zhouhui, Q.; Kou, J.; Liang, W.; Zhang, H.; Monagin, C.; Zhang, Q.; Li, W.; Zhong, H.; et al. Chikungunya virus with E1-A226V mutation causing two outbreaks in 2010, Guangdong, China. Virol. J. 2013, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; He, J.; Wu, D.; Wang, Z.; Zhong, X.; Zhong, H.; Ding, F.; Liu, Z.; Wang, S.; Huang, Z.; et al. Maiden outbreak of Chikungunya in Dongguan City, Guangdong Province, China: Epidemiological characteristics. PLoS ONE 2012, 7, e42830. [Google Scholar]
- García-Arriaza, J.; Cepeda, V.; Hallengärd, D.; Sorzano, C.Ó.S.; Kümmerer, B.M.; Liljeström, P.; Esteban, M. A novel poxvirus-based vaccine, MVA-CHIKV, is highly immunogenic and protects mice against Chikungunya infection. J. Virol. 2014, 88, 3527–3547. [Google Scholar] [CrossRef] [PubMed]
- Hallengärd, D.; Kakoulidou, M.; Lulla, A.; Kümmerer, B.M.; Johansson, D.X.; Mutso, M.; Lulla, V.; Fazakerley, J.K.; Roques, P.; le Grand, R.; et al. Novel attenuated Chikungunya vaccine candidates elicit protective immunity in C57BL/6 mice. J. Virol. 2014, 88, 2858–2866. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.J.; Adams, A.P.; Wang, E.; Plante, K.; Gorchakov, R.; Seymour, R.L.; Vinet-Oliphant, H.; Weaver, S.C. Chikungunya vaccine candidate is highly attenuated and protects nonhuman primates against telemetrically monitored disease following a single dose. J. Infect. Dis. 2014, 209, 1891–1899. [Google Scholar] [CrossRef] [PubMed]
- Tretyakova, I.; Hearn, J.; Wang, E.; Weaver, S.; Pushko, P. DNA vaccine initiates replication of live attenuated Chikungunya virus in vitro and elicits protective immune response in mice. J. Infect. Dis. 2014, 209, 1882–1890. [Google Scholar] [CrossRef] [PubMed]
- Piper, A.; Ribeiro, M.; Smith, K.M.; Briggs, C.M.; Huitt, E.; Nanda, K.; Spears, C.J.; Quiles, M.; Cullen, J.; Thomas, M.E.; et al. Chikungunya virus host range E2 transmembrane deletion mutants induce protective immunity against challenge in C57BL/6J mice. J. Virol. 2013, 87, 6748–6757. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Kim, D.Y.; Weaver, S.C.; Frolov, I. Chimeric Chikungunya viruses are nonpathogenic in highly sensitive mouse models but efficiently induce a protective immune response. J. Virol. 2011, 85, 9249–9252. [Google Scholar] [CrossRef] [PubMed]
- Selvarajah, S.; Sexton, N.R.; Kahle, K.M.; Fong, R.H.; Mattia, K.-A.; Gardner, J.; Lu, K.; Liss, N.M.; Salvador, B.; Tucker, D.F.; et al. A neutralizing monoclonal antibody targeting the acid-sensitive region in Chikungunya virus E2 protects from disease. PLoS Neglect. Trop. Dis. 2013, 7, e2423. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Silva, L.A.; Fox, J.M.; Flyak, A.I.; Kose, N.; Sapparapu, G.; Khomandiak, S.; Ashbrook, A.W.; Kahle, K.M.; Fong, R.H.; et al. Isolation and characterization of broad and ultrapotent human monoclonal antibodies with therapeutic activity against Chikungunya virus. Cell Host Microbe 2015, 18, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Sudeep, A.B.; Arankalle, V.A. Evaluation of recombinant E2 protein-based and whole-virus inactivated candidate vaccines against Chikungunya virus. Vaccine 2012, 30, 6142–6149. [Google Scholar] [CrossRef] [PubMed]
- Gläsker, S.; Lulla, A.; Lulla, V.; Couderc, T.; Drexler, J.F.; Liljeström, P.; Lecuit, M.; Drosten, C.; Merits, A.; Kümmerer, B.M. Virus replicon particle based Chikungunya virus neutralization assay using Gaussia luciferase as readout. Virol. J. 2013, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kishishita, N.; Takeda, N.; Anuegoonpipat, A.; Anantapreecha, S. Development of a pseudotyped-lentiviral-vector-based neutralization assay for Chikungunya virus infection. J. Clin. Microbiol. 2013, 51, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-H.; Ma, W.-Q.; Yang, Y.-L.; Wang, H.-M.; Dong, F.-T.; Huang, Z.-X. Median effective effect-site concentration of sufentanil for wake-up test in adolescents undergoing surgery: A randomized trial. BMC Anesthesiol. 2015, 15, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Sim, A.C.N.; Lin, W.; Tan, G.K.X.; Sim, M.S.T.; Chow, V.T.K.; Alonso, S. Induction of neutralizing antibodies against dengue virus type 2 upon mucosal administration of a recombinant Lactococcus lactis strain expressing envelope domain III antigen. Vaccine 2008, 26, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Ding, G.-Y.; Zhou, H.-Q.; Xie, X.-M.; Li, X.-B.; Shi, Y.-X.; Su, J.-K.; Huang, J.-C. Rapid detection of Dengue virus and Chikungunya virus by multiplexreal-time RT-PCR assay with an internal control. Chin. J. Zoonoses 2013, 29, 242–247. (In Chinese) [Google Scholar]
- Briolant, S.; Garin, D.; Scaramozzino, N.; Jouan, A.; Crance, J.M. In vitro inhibition of Chikungunya and Semliki Forest viruses replication by antiviral compounds: Synergistic effect of interferon-α and ribavirin combination. Antivir. Res. 2004, 61, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Scholte, F.E.M.; Tas, A.; Martina, B.E.E.; Cordioli, P.; Narayanan, K.; Makino, S.; Snijder, E.J.; van Hemert, M.J. Characterization of synthetic Chikungunya viruses based on the consensus sequence of recent E1–226V isolates. PLoS ONE 2013, 8, e71047. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, O.; Albert, M.L. Biology and pathogenesis of Chikungunya virus. Nat. Rev. Microbiol. 2010, 8, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Osorio, J.E.; Livengood, J.A.; Chen, R.; Stinchcomb, D.T. Chikungunya virus and prospects for a vaccine. Expert Rev. Vaccines 2012, 11, 1087–1101. [Google Scholar] [CrossRef] [PubMed]
- Frolova, E.; Gorchakov, R.; Garmashova, N.; Atasheva, S.; Vergara, L.A.; Frolov, I. Formation of nsP3-specific protein complexes during Sindbis virus replication. J. Virol. 2006, 80, 4122–4134. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, B.M.; Grywna, K.; Gläsker, S.; Wieseler, J.; Drosten, C. Construction of an infectious Chikungunya virus cDNA clone and stable insertion of mCherry reporter genes at two different sites. J. Gen. Virol. 2012, 93, 1991–1995. [Google Scholar] [CrossRef] [PubMed]
- Patterson, M.; Poussard, A.; Taylor, K.; Seregin, A.; Smith, J.; Peng, B.-H.; Walker, A.; Linde, J.; Smith, J.; Salazar, M.; et al. Rapid, non-invasive imaging of alphaviral brain infection: Reducing animal numbers and morbidity to identify efficacy of potential vaccines and antivirals. Vaccine 2011, 29, 9345–9351. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.T.; Stauft, C.B.; Aboellail, T.A.; Toth, A.M.; Jarvis, D.L.; Powers, A.M.; Olson, K.E. Bioluminescent imaging and histopathologic characterization of WEEV neuroinvasion in outbred CD-1 mice. PLoS ONE 2013, 8, e53462. [Google Scholar] [CrossRef] [PubMed]
- Poussard, A.; Patterson, M.; Taylor, K.; Seregin, A.; Smith, J.; Smith, J.; Salazar, M.; Paessler, S. In vivo imaging systems (IVIS) detection of a neuro-invasive encephalitic virus. J. Vis. Exp. 2012. [Google Scholar] [CrossRef] [PubMed]
- Tamberg, N.; Lulla, V.; Fragkoudis, R.; Lulla, A.; Fazakerley, J.K.; Merits, A. Insertion of EGFP into the replicase gene of Semliki Forest virus results in a novel, genetically stable marker virus. J. Gen. Virol. 2007, 88, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Delogu, I.; Pastorino, B.; Baronti, C.; Nougairède, A.; Bonnet, E.; de Lamballerie, X. In vitro antiviral activity of arbidol against Chikungunya virus and characteristics of a selected resistant mutant. Antivir. Res. 2011, 90, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Tsetsarkin, K.; Higgs, S.; McGee, C.E.; Lamballerie, X.D.; Charrel, R.N.; Vanlandingham, D.L. Infectious clones of Chikungunya virus (La Reunion isolate) for vector competence studies. Vector Borne Zoonotic Dis. 2006, 6, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Vanlandingham, D.L.; Tsetsarkin, K.; Hong, C.; Klingler, K.; McElroy, K.L.; Lehane, M.J.; Higgs, S. Development and characterization of a double subgenomic Chikungunya virus infectious clone to express heterologous genes in Aedes aegypti mosqutioes. Insect Biochem. Mol. Biol. 2005, 35, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Gardner, C.L.; Watson, A.M.; Ryman, K.D.; Klimstra, W.B. Stable, high-level expression of reporter proteins from improved alphavirus expression vectors to track replication and dissemination during encephalitic and arthritogenic disease. J. Virol. 2014, 88, 2035–2046. [Google Scholar] [CrossRef] [PubMed]
- Tsetsarkin, K.A.; Chen, R.; Leal, G.; Forrester, N.; Higgs, S.; Huang, J.; Weaver, S.C. Chikungunya virus emergence is constrained in Asia by lineage-specific adaptive landscapes. Proc. Natl. Acad. Sci. USA 2011, 108, 7872–7877. [Google Scholar] [CrossRef] [PubMed]


| Primer | Sequence |
|---|---|
| CHIKV-5′UTR′-PsiI-F | cgcTTATAATAATACGACTCACTATAGatggctgcgtgagacacacg |
| CHIKV-nsP2-BamHI-R(2490) | cgcGGATCCctcaccaaggcgatcaaggc |
| CHIKV-nsP2-PsiI-F(1877) | cgcTTATAACGTACGATGGCCGAGTCCTAGTG |
| CHIKV-nsP3-BamHI-R(4087) | cgcGGATCCgatatccatgcgttttacccggtac |
| CHIKV-nsP3-PsiI-F(4103) | cgcTTATAAgatatcGCGAAGAACGATGAAGAG |
| CHIKV-nsP4-BamHI-R(6802) | cgcGGATCCgcattaaggcggtaagcgca |
| CHIKV-nsP4-PsiI-F(6604) | cgcTTATAACTTGGCAACAGCGTACCTATG |
| CHIKV-E2-BamHI-R(9308) | cgcGGATCCtgcatgtcacatttgccagag |
| CHIKV-nsP2-PsiI-F(8713) | cgcTTATAACATGATTGGACCAAGCTGCG |
| CHIKV-E1-BamHI-R(10727) | cgcGGATCCggtgctgtgtgctgcagcgacg |
| CHIKV-E1-PsiI-F(10196) | cgcTTATAAGCCTACCTGATTACAGC |
| CHIKV-3′UTR-BamHI-R | cgcGGATCCACTAGTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTgaaatatt |
| CHIKV-AscI-PacI-2A-F | TAAATACCAATCAGCCATAcggcgcgccaagACATACgccttaattaatCAGCTGTTGAATTTTGACCTTCTCAA |
| CHIKV-AscI-PacI-2A-R | GGTTGGGATAAACTCCATTGGCCCAGGGTTGGACTCGACGTCTCCCGCCAGCTTGAGAAGGTCAAAATTCAACA |
| CHIKV-PacI-2SG promoter-F | gccttaattaatGTCATAACCTTGTACGG |
| CHIKV-2SG promoter-C-F | AATCAGCCATAATGGAGTTTATCCCAACC |
| CHIKV-2SG promoter-C-R | ATAAACTCCATTATGGCTGATTGGTATTT |
| eGFP-AscI-F | ttggcgcgccatggtgagcaagggcgaggag |
| PacI-eGFP-stop-R | TccttaattaaCTActtgtacagctcgtccatgcc |
| CHIKV-7376-F | gaagtgcagggtatatcag |
| CHIKV-8498-R | gatgcttgtagcagctgat |
| CHIKV-9923-F | TGAGCGTCGGTGCCCAC |
| CHIKV-10003-R | GAGTCTTATACCGTACTCCCACCGT |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, C.-L.; Liu, S.-Q.; Zhou, D.-G.; Xu, L.-L.; Li, X.-D.; Zhang, P.-T.; Li, P.-H.; Ye, H.-Q.; Wei, H.-P.; Yuan, Z.-M.; et al. Development of Neutralization Assay Using an eGFP Chikungunya Virus. Viruses 2016, 8, 181. https://doi.org/10.3390/v8070181
Deng C-L, Liu S-Q, Zhou D-G, Xu L-L, Li X-D, Zhang P-T, Li P-H, Ye H-Q, Wei H-P, Yuan Z-M, et al. Development of Neutralization Assay Using an eGFP Chikungunya Virus. Viruses. 2016; 8(7):181. https://doi.org/10.3390/v8070181
Chicago/Turabian StyleDeng, Cheng-Lin, Si-Qing Liu, Dong-Gen Zhou, Lin-Lin Xu, Xiao-Dan Li, Pan-Tao Zhang, Peng-Hui Li, Han-Qing Ye, Hong-Ping Wei, Zhi-Ming Yuan, and et al. 2016. "Development of Neutralization Assay Using an eGFP Chikungunya Virus" Viruses 8, no. 7: 181. https://doi.org/10.3390/v8070181
APA StyleDeng, C.-L., Liu, S.-Q., Zhou, D.-G., Xu, L.-L., Li, X.-D., Zhang, P.-T., Li, P.-H., Ye, H.-Q., Wei, H.-P., Yuan, Z.-M., Qin, C.-F., & Zhang, B. (2016). Development of Neutralization Assay Using an eGFP Chikungunya Virus. Viruses, 8(7), 181. https://doi.org/10.3390/v8070181






