Serro 2 Virus Highlights the Fundamental Genomic and Biological Features of a Natural Vaccinia Virus Infecting Humans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laboratory Assays
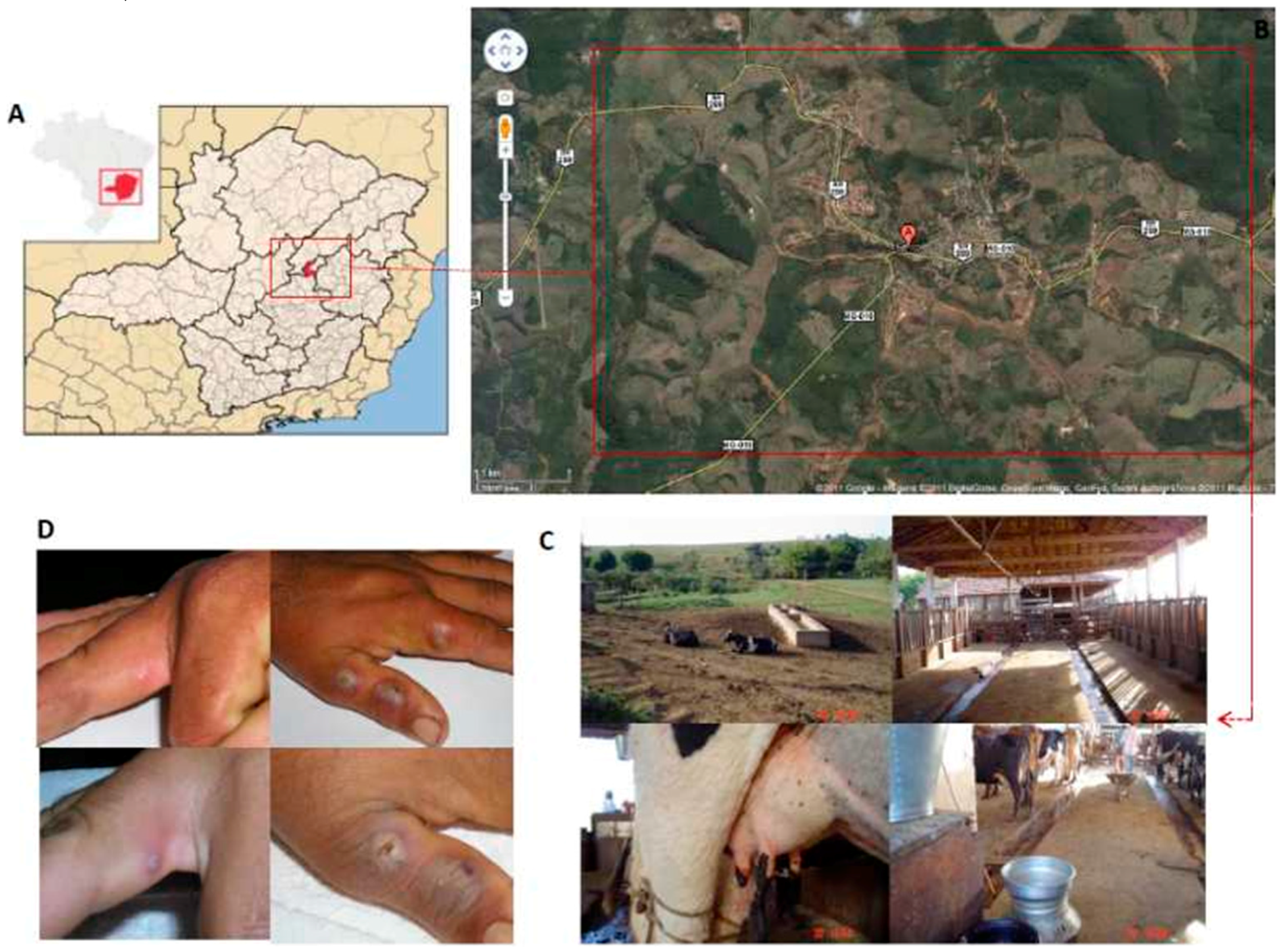
2.1.1 Virus Isolation and Clonal Expansion
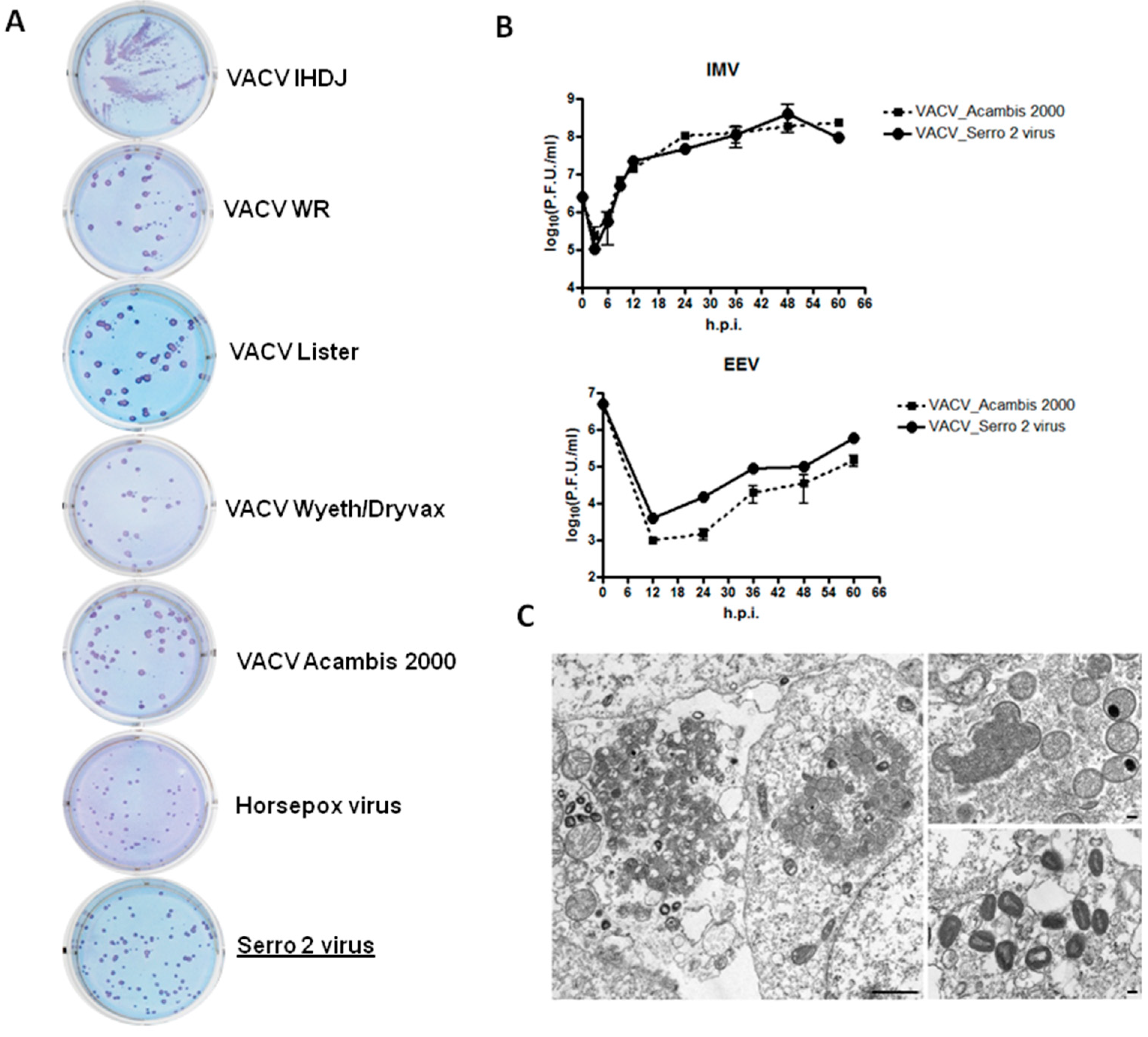
2.1.2. Plaque Phenotype Assay
2.1.3. One-Step Growth Curve
2.1.4. Electron Microscopy
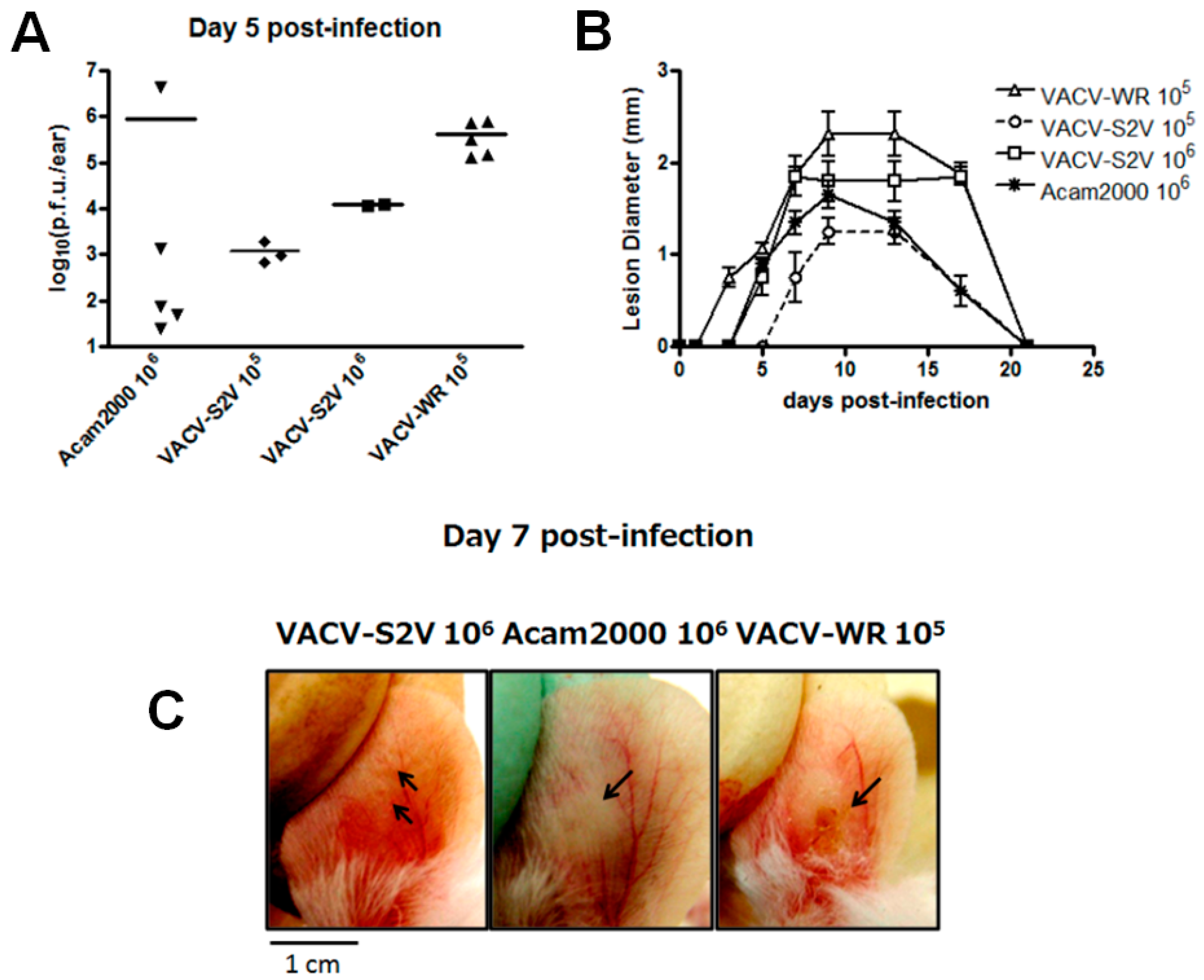
2.1.5. In Vivo Analysis
2.1.6. Viral Titration
2.2. Molecular Characterization
2.2.1. DNA Extraction and Sequencing Protocols
2.2.2. Sequence Analysis and Alignment
3. Results
3.1. Virus Isolation and Phenotypic Characterization
3.2. In Vivo Analysis
3.3. The S2V Genome
3.4. Comparison with Other VACV Strains
3.5. Genes Associated with Humoral Immune Response
3.6. Immunomodulatory Genes and Virulence Factors
3.7. Targets of Antiviral Drug Therapies
3.8. Genetic Diversity between the Brazilian VACV
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fenner, F.; Henderson, D.A.; Arita, I.; Jezek, A.; Ladnyi, I.D. Smallpox and Its Eradication; World Health Organization Press: Geneva, Switzerland, 1988; p. 49. [Google Scholar]
- Smith, G.L.; Mackett, M.; Moss, B. Recombinant vaccinia viruses as new live vaccines. Biotechnol. Genet. Eng. Rev. 1984, 2, 383–407. [Google Scholar] [CrossRef] [PubMed]
- Kirn, D.H.; Thorne, S.H. Targeted and armed oncolytic poxviruses: A novel multi-mechanistic therapeutic class for cancer. Nat. Rev. Cancer 2009, 9, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Damon, I. Poxviruses. In Fields Virology; Raven Press: New York, NY, USA, 2007; pp. 2049–2075. [Google Scholar]
- International Committee on Taxonomy of Viruses (ICTV). 2009. Available online: http://www.ncbi.nlm.nih.gov/ICTVdb/ (accessed on 1 November 2011).
- Moss, B. Poxvirus entry and membrane fusion. Virology 2006, 344, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Moss, B. Poxviridae and their replication. In Fields Virology; Raven Press: New York, NY, USA, 2007; pp. 2906–2945. [Google Scholar]
- Damaso, C.R.; Esposito, J.J.; Condit, R.C.; Moussatche, N. An emergent poxvirus from humans and cattle in Rio de Janeiro State: Cantagalo virus may derive from Brazilian smallpox vaccine. Virology 2000, 277, 439–449. [Google Scholar] [CrossRef] [PubMed]
- De Souza Trindade, G.; da Fonseca, F.G.; Marques, J.T.; Nogueira, M.L.; Mendes, L.C.N.; Borges, A.S.; Peiró, J.R.; Pituco, E.M.; Bonjardim, C.A.; Ferreira, P.C.P.; et al. Aracatuba virus: A vaccinia-like virus associated with infection in humans and cattle. Emerg. Infect. Dis. 2003, 9, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Trindade, G.S.; Lobato, Z.I.; Drumond, B.P.; Leite, J.A.; Trigueiro, R.C.; Guedes, M.I.; da Fonseca, F.G.; dos Santos, J.R.; Bonjardim, C.A.; Ferreira, P.C.; et al. Short report: Isolation of two vaccinia virus strains from a single bovine vaccinia outbreak in rural area from Brazil: Implications on the emergence of zoonotic orthopoxviruses. Am. J. Trop. Med. Hyg. 2006, 75, 486–490. [Google Scholar] [PubMed]
- Leite, J.A.; Drumond, B.P.; Trindade, G.S.; Lobato, Z.I.; da Fonseca, F.G.; Madureira, M.C.; Guedes, M.I.; Ferreira, J.M.; Bonjardim, C.A.; Ferreira, P.C.; et al. Passatempo virus, a vaccinia virus strain, Brazil. Emerg. Infect. Dis. 2005, 11, 1935–1938. [Google Scholar] [CrossRef] [PubMed]
- Abrahão, J.S.; Guedes, M.I.; Trindade, G.S.; Fonseca, F.G.; Campos, R.K.; Mota, B.F.; Lobato, Z.I.; Silva-Fernandes, A.T.; Rodrigues, G.O.; Lima, L.S.; et al. One More Piece in the VACV Ecological Puzzle: Could Peridomestic Rodents Be the Link between Wildlife and Bovine Vaccinia Outbreaks in Brazil? PLoS ONE 2009, 4, e7428. [Google Scholar]
- Megid, J.; Appolinário, C.M.; Langoni, H.; Pituco, E.M.; Okuda, L.H. Vaccinia virus in humans and cattle in southwest region of Sao Paulo state, Brazil. Am. J. Trop. Med. Hyg. 2008, 5, 647–651. [Google Scholar]
- Quixabeira-Santos, J.C.; Medaglia, M.L.; Pescador, C.A.; Damaso, C.R. Animal movement and establishment of vaccinia virus Cantagalo strain in Amazon biome, Brazil. Emerg. Infect. Dis. 2011, 4, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Medaglia, M.L.; Pessoa, L.C.; Sales, E.R.; Freitas, T.R.; Damaso, C.R. Spread of cantagalo virus to northern Brazil. Emerg. Infect. Dis. 2009, 7, 1142–1143. [Google Scholar] [CrossRef] [PubMed]
- Lopes de, S.; Lacerda, J.P.; Fonseca, I.E.; Castro, D.P.; Forattini, O.P.; Rabello, E.X. Cotia Virus: A new agent isolated from sentinel mice in São Paulo, Brazil. Am. J. Trop. Med. Hyg. 1965, 14, 156–157. [Google Scholar]
- Da Fonseca, F.G.; Trindade, G.S.; Silva, R.L.; Bonjardim, C.A.; Ferreira, P.C.; Kroon, E.G. Characterization of a vaccinia-like virus isolated in a Brazilian forest. J. Gen. Virol. 2002, 83, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Moussatché, N.; Damaso, C.R.; McFadden, G. When good vaccines go wild: Feral Orthopoxvirus in developing countries and beyond. J. Infect. Dev. Ctries. 2008, 3, 156–173. [Google Scholar] [CrossRef]
- Trindade, G.S.; Emerson, G.L.; Carroll, D.S.; Kroon, E.G.; Damon, I.K. Brazilian vaccinia viruses and their origins. Emerg. Infect. Dis. 2007, 13, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Drumond, B.P.; Leite, J.A.; da Fonseca, F.G.; Bonjardim, C.A.; Ferreira, P.C.; Kroon, E.G. Brazilian Vaccinia virus strains are genetically divergent and differ from the Lister vaccine strain. Microbes Infect. 2008, 10, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Medaglia, M.L.; Moussatché, N.; Nitsche, A.; Dabrowski, P.W.; Li, Y.; Damon, I.K.; Lucas, C.G.; Arruda, L.B.; Damaso, C.R. Genomic Analysis, Phenotype,and Virulence of the Historical Brazilian Smallpox Vaccine Strain IOC: Implications for the Origins and Evolutionary Relationships of Vaccinia virus. J. Virol. 2015, 89, 11909–11925. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.M.; Drumond, B.P.; Guedes, M.I.; Pascoal-Xavier, M.A.; Almeida-Leite, C.M.; Arantes, R.M.; Mota, B.E.; Alves, P.A.; Oliveira, F.M.; Ferreira, P.C.; et al. Virulence in murine model shows the existence of two distinct populations of Brazilian Vaccinia virus strains. PLoS ONE 2008, 3, e3043. [Google Scholar] [CrossRef] [PubMed]
- Tscharke, D.C.; Reading, P.C.; Smith, G.L. Dermal infection with vaccinia virus reveals roles for virus proteins not seen using other inoculation routes. J. Gen. Virol. 2002, 8, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.L.; Viana, F.C.; Ribeiro, S.C.A.; Coelho, H.E.; Lúcio, W.F.; Oliveira, P.R. Surto de varíola bovina no município do Prata-MG. Arq. Bras. Med. Vet. Zootec. 1986, 3, 323–330. [Google Scholar]
- Fernandes, T. The smallpox vaccine: Its first century in Brazil (from the Jennerian to the animal vaccine. Hist. Cienc. Saude Manguinhos 1999, 6, 29–51. [Google Scholar] [PubMed]
- Lum, G.S.; Soriano, F.; Trejos, A.; Llerena, J. Vaccinia epidemic and epizootic in El Salvador. Am. J. Trop. Med. Hyg. 1967, 3, 332–338. [Google Scholar]
- Bhattacharjee, A.B. Simultaneous infection with foot and mouth disease and vaccinia virus. Indian Med. J. 1966, 60, 209–210. [Google Scholar] [PubMed]
- Trindade, G.S.; Guedes, M.I.; Drumond, B.P.; Mota, B.E.; Abrahão, J.S.; Lobato, Z.I.; Gomes, J.A.; Corrêa-Oliveira, R.; Nogueira, M.L.; Kroon, E.G.; et al. Zoonotic vaccinia virus: Clinical and immunological characteristics in a naturally infected patient. Clin. Infect. Dis. 2009, 3, 37–40. [Google Scholar] [CrossRef] [PubMed]
- De Souza Trindade, G.; Drumond, B.P.; Guedes, M.I.; Leite, J.A.; Mota, B.E. Zoonotic vaccinia virus infection in Brazil: Clinical description and implications for health professionals. J. Clin. Microbiol. 2007, 4, 1370–1372. [Google Scholar] [CrossRef] [PubMed]
- Joklik, W.K. The purification of four strains of poxvirus. Virology 1962, 18, 9–18. [Google Scholar] [CrossRef]
- Damon, I.K.; Davidson, W.B.; Hughes, C.M.; Olson, V.A.; Smith, S.K.; Holman, R.C.; Frey, S.A.; Newman, F.; Belshe, R.B.; Yan, L.; et al. Evaluation of smallpox vaccines using variola neutralization. J. Gen. Virol. 2009, 90, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, A.; Osborne, J.; Slack, S.; Roper, R.L.; Upton, C. Poxvirus Orthologous Clusters (POCs). Bioinformatics 2002, 18, 1544–1545. [Google Scholar] [CrossRef] [PubMed]
- Besemer, J.; Lomsadze, A.; Borodovsky, M. GeneMarkS: A self-training method for prediction of gene starts in microbial genomes. Implications for finding sequence motifs in regulatory regions. Nucleic Acids Res. 2001, 29, 2607–2618. [Google Scholar] [CrossRef] [PubMed]
- Salzberg, S.L.; Delcher, A.L.; Kasif, S.; White, O. Microbial gene identification using interpolated Markov models. Nucleic Acids Res. 1998, 26, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Eddy, S.R. Multile alignment using hidden Markov models. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1995, 3, 114–120. [Google Scholar] [PubMed]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [PubMed]
- Weltzin, R.; Liu, J.; Pugachev, K.V.; Myers, G.A.; Coughlin, B. Clonal vaccinia virus grown in cell culture as a new smallpox vaccine. Nat. Med. 2003, 9, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Henderson, D.A. Bioterrorism as a public health threat. Emerg. Infect. Dis. 1998, 4, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Shchelkunov, S.N. An Increasing Danger of Zoonotic Orthopoxvirus Infections. PLoS Pathog. 2013, 9, e1003756. [Google Scholar] [CrossRef] [PubMed]
- McCausland, M.M.; Benhnia, M.R.; Crickard, L.; Laudenslager, J.; Granger, S.W.; Tahara, T.; Kubo, R.; Koriazova, L.; Kato, S.; Crotty, S. Combination therapy of vaccinia virus infection with human anti-H3 and anti-B5 monoclonal antibodies in a small animal model. Antivir. Ther. 2010, 4, 661–675. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.H.; McCausland, M.M.; Valdez, C.; Huynh, D.; Hernandez, J.E.; Mu, Y.; Hirst, S.; Villareal, L.; Felgner, P.L.; Crotty, S. Vaccinia virus H3L envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice. J. Virol. 2005, 18, 11724–11733. [Google Scholar] [CrossRef] [PubMed]
- Rudraraju, R.; Ramsay, A.J. Single-shot immunization with recombinant adenovirus encoding vaccinia virus glycoprotein A27L is protective against a virulent respiratory poxvirus infection. Vaccine 2010, 31, 4997–5004. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Hollinshead, R.; Smith, G.L. Antibody-sensitive and antibody-resistant cell-to-cell spread by vaccinia virus: Role of the A33R protein in antibody-resistant spread. J. Gen. Virol. 2002, 83, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Earl, P.; Americo, J.; Damon, I.; Smith, S.K.; Yu, F.; Sebrell, A.; Emerson, S.; Cohen, G.; Eisenberg, R.J.; et al. Characterization of chimpanzee/human monoclonal antibodies to vaccinia virus A33 glycoprotein and its variola virus homolog in vitro and in a vaccinia virus mouse protection model. J. Virol. 2007, 17, 8989–8995. [Google Scholar] [CrossRef] [PubMed]
- Berhanu, A.; Wilson, R.L.; Kirkwood-Watts, D.L.; King, D.S.; Warren, T.K. Vaccination of BALB/c mice with Escherichia coli-expressed vaccinia virus proteins A27L, B5R, and D8L protects mice from lethal vaccinia virus challenge. J. Virol. 2008, 82, 3517–3529. [Google Scholar] [CrossRef] [PubMed]
- Benhnia, M.R.; McCausland, M.M.; Moyron, J.; Laudenslager, J.; Granger, S. Vaccinia virus extracellular enveloped virion neutralization in vitro and protection in vivo depend on complement. J. Virol. 2009, 83, 1201–1215. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.; Shamim, M.; Whitbeck, J.C.; Sfyroera, G.; Lambris, J.D.; Isaacs, S.N. Antibodies against the extracellular enveloped virus B5R protein are mainly responsible for the EEV neutralizing capacity of vaccinia immune globulin. Virology 2004, 325, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Heraud, J.M.; Edghill-Smith, Y.; Ayala, V.; Kalisz, I.; Parrino, J.; Kalyanaraman, V.S.; Manischewitz, J.; King, L.R.; Hryniewicz, A.; Trindade, C.J.; et al. Subunit recombinant vaccine protects against monkeypox. J. Immunol. 2006, 177, 2552–2564. [Google Scholar] [CrossRef] [PubMed]
- Leite, J.A.; da Fonseca, F.G.; de Souza Trindade, G.; Abrahão, J.S.; Arantes, R.M.; de Almeida-Leite, C.M.; dos Santos, J.R.; Guedes, M.I.; Ribeiro, B.M.; Bonjardim, C.A.; et al. A-type inclusion bodies: A factor influencing cowpox virus lesion pathogenesis. Arch. Virol. 2011, 156, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Leite, J.A.; Drumond, B.P.; de Souza Trindade, G.; Bonjardim, C.A.; Ferreira, P.C.; Kroon, E.G. Brazilian Vaccinia virus strains show genetic polymorphism at the ati gene. Virus Genes 2007, 35, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Shchelkunov, S.N.; Blinov, V.M.; Sandakhchiev, L.S. Ankyrin-like proteins of variola and vaccinia viruses. FEBS Lett. 1993, 319, 163–165. [Google Scholar] [CrossRef]
- Jackson, S.S.; Ilyinskii, P.; Philippon, V.; Gritz, L.; Yafal, A.G.; Zinnack, K.; Beaudry, K.R.; Manson, K.H.; Lifton, M.A.; Kuroda, M.J.; et al. Role of genes that modulate host immune responses in the immunogenicity and pathogenicity of vaccinia virus. J. Virol. 2005, 79, 6554–6559. [Google Scholar] [CrossRef] [PubMed]
- Puehler, F.; Weining, K.C.; Symons, J.A.; Smith, G.L.; Staeheli, P. Vaccinia virus-encoded cytokine receptor binds and neutralizes chicken interferon-gamma. Virology 1998, 248, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Myskiw, C.; Arsenio, J.; van Bruggen, R.; Deschambault, Y.; Cao, J. Vaccinia virus E3 suppresses expression of diverse cytokines through inhibition of the PKR, NF-κappaB, and IRF3 pathways. J. Virol. 2009, 83, 6757–6768. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.P.; Alcami, A. Inhibition of interferons by ectromelia virus. J. Virol. 2002, 76, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Oguiura, N.; Spehner, D.; Drillien, R. Detection of a protein encoded by the vaccinia virus C7L open reading frame and study of its effect on virus multiplication in different cell lines. J. Gen. Virol. 1993, 74, 1409–1413. [Google Scholar] [CrossRef] [PubMed]
- Symons, J.A.; Adams, E.; Tscharke, D.C.; Reading, P.C.; Waldmann, H.; Smith, G.L. The vaccinia virus C12L protein inhibits mouse IL-18 and promotes virus virulence in the murine intranasal model. J. Gen. Virol. 2002, 83, 2833–2844. [Google Scholar] [CrossRef] [PubMed]
- Blasco, R.; Moss, B. Extracellular vaccinia virus formation and cell-to-cell virus transmission are prevented by deletion of the gene encoding the 37,000-Dalton outer envelope protein. J. Virol. 1991, 11, 5910–5920. [Google Scholar]
- Grosenbach, D.W.; Berhanu, A.; King, D.S.; Mosier, S.; Jones, K.F.; Jordan, R.A.; Bolken, T.C.; Hruby, D.E. Efficacy of ST-246 versus lethal poxvirus challenge in immunodeficient mice. Proc. Natl. Acad. Sci. USA 2009, 2, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Pevear, D.C.; Davies, M.H.; Collett, M.S.; Bailey, T.; Rippen, S.; Barone, L.; Burns, C.; Rhodes, G.; Tohan, S.; et al. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus challenge. J. Virol. 2005, 20, 13139–13149. [Google Scholar] [CrossRef] [PubMed]
- Andrei, G.; Snoeck, R. Cidofovir Activity against Poxvirus Infections. Viruses 2010, 2, 2803–2830. [Google Scholar] [CrossRef] [PubMed]
- Gammon, D.B.; Snoeck, R.; Fiten, P.; Krecmerová, M.; Holý, A.; De Clercq, E.; Opdenakker, G.; Evans, D.H.; Andrei, G. Mechanism of antiviral drug resistance of vaccinia virus: Identification of residues in the viral DNA polymerase conferring differential resistance to antipoxvirus drugs. J. Virol. 2008, 82, 12520–12534. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.N.; Obraztsova, M.; Kern, E.R.; Quenelle, D.C.; Keith, K.A.; Prichard, M.N.; Luo, M.; Moyer, R.W. Isolation and characterization of cidofovir resistant vaccinia viruses. Virol. J. 2008, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Kornbluth, R.S.; Smee, D.F.; Sidwell, R.W.; Snarsky, V.; Evans, D.H.; Hostetler, K.Y. Mutations in the E9L polymerase gene of cidofovir-resistant vaccinia virus strain WR are associated with the drug resistance phenotype. Antimicrob. Agents Chemother. 2006, 50, 4038–4043. [Google Scholar] [CrossRef] [PubMed]
- Smee, D.F.; Wandersee, M.K.; Bailey, K.W.; Hostetler, K.Y.; Holy, A.; Sidwell, R.W. Characterization and treatment of cidofovir-resistant vaccinia (WR strain) virus infections in cell culture and in mice. Antivir. Chem. Chemother. 2005, 16, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Huemer, H.P.; Lassnig, C.; Nowotny, N. Cowpox virus isolate virulent in humans shows attenuated phenotype in mice. Res. Vet. Sci. 2012, 92, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Kinnunen, P.M.; Henttonen, H.; Hoffmann, B.; Kallio, E.R.; Korthase, C. Cow pox virus infections in Eurasian wild rodents. Vector Borne Zoonotic Dis. 2011, 8, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Domi, A.; Weisberg, A.S.; Moss, B. Vaccinia virus E2L null mutants exhibit a major reduction in extracellular virion formation and virus spread. J. Virol. 2008, 82, 4215–4226. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.; Ward, B.M.; Weisberg, A.S.; Moss, B. Mutations in the vaccinia virus A33R and B5R envelope proteins that enhance release of extracellular virions and eliminate formation of actin-containing microvilli without preventing tyrosine phosphorylation of the A36R protein. J. Virol. 2003, 77, 12266–12275. [Google Scholar] [CrossRef] [PubMed]
- Aldaz-Carroll, L.; Whitbeck, J.C.; Ponce de Leon, M.; Lou, H.; Hirao, L.; Isaacs, S.N.; Moss, B.; Eisenberg, R.J.; Cohen, G.H. Epitope-mapping studies define two major neutralization sites on the vaccinia virus extracellular enveloped virus glycoprotein B5R. J. Virol. 2005, 79, 6260–6271. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, S.; Sakiyama, T.; Hasegawa, H.; Saijo, M.; Maeda, A.; Kurane, I.; Maeno, G.; Kimura, J.; Hirama, C.; Yoshida, T.; et al. An attenuated LC16m8 smallpox vaccine: Analysis of full-genome sequence and induction of immune protection. J. Virol. 2005, 79, 11873–11891. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.; Huang, X.; Yang, Y. Direct TLR2 signaling is critical for NK cell activation and function in response to vaccinia viral infection. PLoS Pathog. 2010, 6, e1000811. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.K.; Regner, M.; Bonefeld, C.M.; Boding, L.; Kongsbak, M.; Ødum, N.; Müllbacher, A.; Geisler, C.; von Essen, M.R. TCR down-regulation boosts T-cell-mediated cytotoxicity and protection against poxvirus infections. Eur. J. Immunol. 2011, 41, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Tewari, M.; Dixit, V.M. Fas- and tumor necrosis factor-induced apoptosis is inhibited by the poxvirus crmA gene product. J. Biol. Chem. 1995, 270, 3255–3260. [Google Scholar] [PubMed]
- Isaacs, S.N.; Kotwal, G.J.; Moss, B. Vaccinia virus complement-control protein prevents antibody-dependent complement-enhanced neutralization of infectivity and contributes to virulence. Proc. Natl. Acad. Sci. USA 1992, 89, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Bugert, J.J.; Lohmüller, C.; Damon, I.; Moss, B.; Darai, G. Chemokine Homolog of Molluscum Contagiosum Virus: Sequence Conservation and Expression. Virology 1998, 242, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Smith, T.D.; Smolak, P.J.; Friend, D.; Hagen, H.; Gerhart, M.; Park, L.; Pickup, D.J.; Torrance, D.; Mohler, K.; et al. Poxvirus genomes encode a secreted, soluble protein that preferentially inhibits beta chemokine activity yet lacks sequence homology to known chemokine receptors. Virology 1997, 236, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.L.; Howard, S.T.; Chan, Y.S. Vaccinia virus encodes a family of genes with homology to serine proteinase inhibitors. J. Gen. Virol. 1989, 70, 2333–2343. [Google Scholar] [CrossRef] [PubMed]
- Kotwal, G.J. Virokines: Mediators of virus-host interaction and future immunomodulators in medicine. Arch. Immunol. Ther. Exp. 1999, 47, 135–138. [Google Scholar]
- Medaglia, M.L.; Moussatché, N.; Nitsche, A.; Dabrowski, P.W.; Li, Y.; Damon, I.K.; Lucas, C.G.; Arruda, L.B.; Damaso, C.R. Genomic Analysis, Phenotype, and Virulence of the Historical Brazilian Smallpox Vaccine Strain IOC: Implications for the Origins and Evolutionary Relationships of Vaccinia Virus. J. Virol. 2015, 89, 11909–11925. [Google Scholar] [CrossRef] [PubMed]
- Assis, F.L.; Borges, I.A.; Ferreira, P.C.P.; Bonjardim, C.A.; Trindade, G.S.; Lobato, Z.I.P.; Guedes, M.I.M.; Vaz, M.; Kroon, E.G.; Abrahão, J.S. Group 2 vaccinia virus, Brazil. Emerg. Infect. Dis. 2012, 18, 2035–2038. [Google Scholar] [CrossRef] [PubMed]

| Virus Species | Strain (Abbreviation) | GenBank Accession Number(s) |
|---|---|---|
| Cowpox virus | GRI-90 (CPXV-GRI) | X94355 |
| Monkeypox virus | Zaire-96-I-16 (MPXV Zaire96) | AF380138 |
| Vaccinia virus | 3737 (VACV-3737) | DQ377945 |
| Acambis-2000 (VACV Acam2000) | AY313847 | |
| Acambis-3000 (VACV-Acam3000) | AY603355 | |
| Acambis clone 3 (VACV-Acam3) | AY313848 | |
| Araçatuba | EF051269, EF051277, EF051285, EF175987, EF175965, DQ194389, AY523994, EF175973, DQ194382 | |
| BeAn-58058 | EF051270, EF051278, EF051286, EF175990, EF175968, DQ194388, DQ206442, EF175976, AF261890 | |
| Belo Horizonte | EF051276, EF051284, EF051292, EF175993, EF175971, DQ194390, DQ206435, EF175979, DQ194383 | |
| Cantagalo # | KT013210 | |
| Chorioallantois Vaccinia Ankara (VACV-CVA) | AM501482 | |
| Copenhagen (VACV-COP) | M35027 | |
| Duke (VACV-DUKE) | DQ439815 | |
| Guarani P1 | EF051271, EF051279, EF051287, EF175991, EF175969, DQ194385, DQ206436, EF175977, DQ194380 | |
| Guarani P2 | EF051272, EF051280, EF051288, EF175988, EF175966, DQ194386, DQ206437, EF175974, DQ194381 | |
| Horsepox virus (HSPV-MNR76) | DQ792504 | |
| IOC Brazil # (VACV-IOC-B388) | KT184691 | |
| Lister 107 France | DQ121394 | |
| Lister Butantã * Brazil | EF175981, EF175982, EF175983, EF175994, EF175972, EF175984, EF175985, EF175980 | |
| Lister Japan (VACV-Lister) | AY678276 | |
| Lister LC16m0 (VACV-LC16m0) | AY678277 | |
| Lister LC16m8 (VACV-LC16m8) | AY678276 | |
| Western Reserve (VACV-WR) | AY243312 | |
| Modified Vaccinia Ankara (VACV-MVA) | U94848 | |
| Modified Vaccinia Ankara 1721 (VACV-MVA-1721) | DQ983236 | |
| Passatempo | EF051274, EF051282, EF051290, EF175989, EF175967, DQ530240, DQ070848, EF175975, DQ530239 | |
| Rabbitpox virus Utrecht (RPXV-Utr) | AY484669 | |
| Serro2 (S2V) | KF179385 | |
| SpAn232 | EF051283, EF051291, EF175992, EF175970, DQ194387, DQ222922, EF175978, DQ194384, EF051275 |
| Gene | Viral Product | Characteristic/Function | Coding Region in S2V |
|---|---|---|---|
| A46R | Blocks TLR-mediated signaling | Antagonizes TLR signaling. Inhibits NF-κB activation. Blocks IFN response. | Present |
| A52R | Blocks TLR-mediated signaling | Antagonizes TLR signaling. Blocks NF-κB activation by multiple TLRs and associates with IRAK2 and TRAF6. Blocks IFN response. | Present |
| A53R | Binds to TNF-α | CrmC. Viroceptor. Secreted TNF inhibitor. TNF receptor homolog. | Present |
| B19R | IFNα/β receptor homolog | Viroceptor. Mimics IFNα/β receptor. Binds and inhibits the activity of type I IFN. B18R in VACV-WR. | Present |
| B8R | IFNγ receptor | Viroceptor. Mimics IFNγ receptor. Binds and inhibits the activity of type II IFN. | Present |
| B13R | Serpin-1, -2, -3 gene family (SPI-2/CrmA) | Inhibits IL-1 converting enzyme (caspase). | Present; truncated as in VACV-COP |
| B16R | IL-1β receptor homolog | Viroceptor. Blocks febrile response in a poxvirus infection. B15R inVACV-WR. | Early stop codon |
| C3L | Complement control protein | Virokine. Inhibits the classical and alternative complement activation pathways. | Present |
| C7L | Antiapoptotic protein | Apoptosis inhibitor; host range virulence factor. | Present |
| C12L | IL-18 binding protein | Virokine. Natural antagonist of IL-18. Inhibits IL-18 induced IFN-γ production. | Present |
| C11R | Vaccinia growth factor | Virokine. Stimulates cell growth. Virulence factor. | Present |
| E3L | dsRNA binding protein | IFN inhibitor. Antiapoptotic protein. Sequesters dsRNA and prevents activation of PKR and OAS. | Present |
| K3L | eIF-2α mimic | Antiapoptotic protein. Mimics eIF-2α and prevents activation of PKR. | Present |
| F1L | Mitochondrial-localized protein | Virokine. Protects cells from apoptotic death and inhibits cytochrome c release. Antiapoptotic protein. | Present |
| K1L | Host range protein | Virokine. Blocks signaling pathway for NF-κB activation. Inhibits proinflammatory genes expression. | Present |
| M2L | NF-κB inhibitor | Antiapoptotic factor. | Present |
| N1L | Antiapoptotic protein | Virokine. Blocks signaling pathway for NF-κB activation by TNF. | Present |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trindade, G.D.S.; Emerson, G.L.; Sammons, S.; Frace, M.; Govil, D.; Fernandes Mota, B.E.; Abrahão, J.S.; De Assis, F.L.; Olsen-Rasmussen, M.; Goldsmith, C.S.; et al. Serro 2 Virus Highlights the Fundamental Genomic and Biological Features of a Natural Vaccinia Virus Infecting Humans. Viruses 2016, 8, 328. https://doi.org/10.3390/v8120328
Trindade GDS, Emerson GL, Sammons S, Frace M, Govil D, Fernandes Mota BE, Abrahão JS, De Assis FL, Olsen-Rasmussen M, Goldsmith CS, et al. Serro 2 Virus Highlights the Fundamental Genomic and Biological Features of a Natural Vaccinia Virus Infecting Humans. Viruses. 2016; 8(12):328. https://doi.org/10.3390/v8120328
Chicago/Turabian StyleTrindade, Giliane De Souza, Ginny L. Emerson, Scott Sammons, Michael Frace, Dhwani Govil, Bruno Eduardo Fernandes Mota, Jônatas Santos Abrahão, Felipe Lopes De Assis, Melissa Olsen-Rasmussen, Cynthia S. Goldsmith, and et al. 2016. "Serro 2 Virus Highlights the Fundamental Genomic and Biological Features of a Natural Vaccinia Virus Infecting Humans" Viruses 8, no. 12: 328. https://doi.org/10.3390/v8120328
APA StyleTrindade, G. D. S., Emerson, G. L., Sammons, S., Frace, M., Govil, D., Fernandes Mota, B. E., Abrahão, J. S., De Assis, F. L., Olsen-Rasmussen, M., Goldsmith, C. S., Li, Y., Carroll, D., Guimarães da Fonseca, F., Kroon, E., & Damon, I. K. (2016). Serro 2 Virus Highlights the Fundamental Genomic and Biological Features of a Natural Vaccinia Virus Infecting Humans. Viruses, 8(12), 328. https://doi.org/10.3390/v8120328








