Measles to the Rescue: A Review of Oncolytic Measles Virus
Abstract
:1. Introduction
2. MV as an Oncolytic Virus
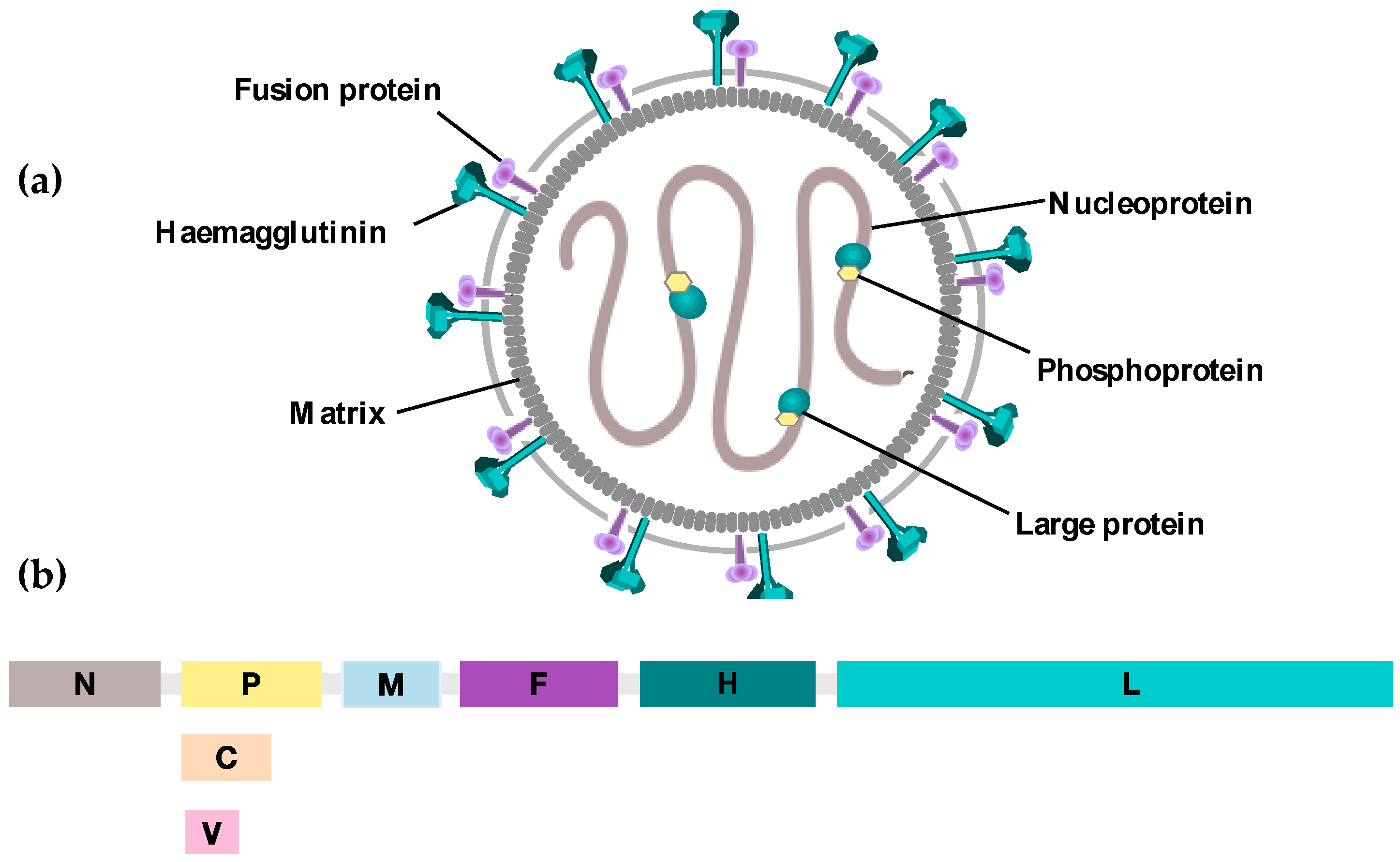
3. Oncolytic Measles Virus: Biological Characteristics and Cytopathic Effects
4. MV Receptors
5. Mechanisms of Specificity
5.1. Relationship of Receptor Expression to Oncolytic Activity
5.2. Defects in Interferon Response
6. Role of the Immune System in MV-Mediated Oncolysis
7. Engineering Oncolytic Measles Virus
7.1. Targeting MV Entry
7.2. Monitoring and Tracking Viral Replication
7.3. Enhancing Oncolytic MV Activity
7.3.1. “Radiovirotherapy”
7.3.2. “Chemovirotherapy”
7.3.3. “Immunovirotherapy”
8. Overcoming Anti-Viral Host Immune Responses
8.1. Concomitant Immunosuppressive Therapy
8.2. Shielding from Immune Attack
9. Clinical Trials
10. Conclusions
Acknowledgments
Conflicts of Interest
References
- Dock, G. The influence of complicating diseases upon leukaemia. Am. J. Med. Sci. 1904, 127, 563–592. [Google Scholar] [CrossRef]
- Pasquinucci, G. Possible effect of measles on leukaemia. Lancet 1971, 1, 136. [Google Scholar] [CrossRef]
- Ziegler, J.L. Spontaneous remission in burkitt’s lymphoma. Natl. Cancer Inst. Monogr. 1976, 44, 61–65. [Google Scholar] [PubMed]
- Zygiert, Z. Hodgkin’s disease: Remissions after measles. Lancet 1971, 1, 593. [Google Scholar] [CrossRef]
- Enders, J.F.; Peebles, T.C. Propagation in tissue cultures of cytopathogenic agents from patients with measles. Proc. Soc. Exp. Biol. Med. 1954, 86, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Enders, J.F.; Katz, S.L.; Milovanovic, M.V.; Holloway, A. Studies on an attenuated measles-virus vaccine. I. Development and preparations of the vaccine: Technics for assay of effects of vaccination. N. Engl. J. Med. 1960, 263, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Bellini, W.J.; Rota, J.S.; Rota, P.A. Virology of measles virus. J. Infect. Dis. 1994, 170, S15–S23. [Google Scholar] [CrossRef] [PubMed]
- Angel, J.B.; Walpita, P.; Lerch, R.A.; Sidhu, M.S.; Masurekar, M.; DeLellis, R.A.; Noble, J.T.; Snydman, D.R.; Udem, S.A. Vaccine-associated measles pneumonitis in an adult with aids. Ann. Intern. Med. 1998, 129, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Vaccine Safety Committe, Institute of Medicine. Adverse Events Associated with Childhood Vaccines: Evidence Bearing on Causality; Stratton, K.R., Howe, C.J., Johnston, R.B., Jr., Eds.; National Academies Press: Washington, DC, USA, 1994. [Google Scholar]
- Hashiguchi, T.; Kajikawa, M.; Maita, N.; Takeda, M.; Kuroki, K.; Sasaki, K.; Kohda, D.; Yanagi, Y.; Maenaka, K. Crystal structure of measles virus hemagglutinin provides insight into effective vaccines. Proc. Natl. Acad. Sci. USA 2007, 104, 19535–19540. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.J.; Federspiel, M.J.; Peng, K.W.; Tong, C.; Dingli, D.; Morice, W.G.; Lowe, V.; O’Connor, M.K.; Kyle, R.A.; Leung, N.; et al. Remission of disseminated cancer after systemic oncolytic virotherapy. Mayo Clin. Proc. 2014, 89, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.E.; Oldstone, M.B. Measles. History and basic biology. Introduction. Curr. Top. Microbiol. Immunol. 2009, 329, 1. [Google Scholar] [PubMed]
- Moss, W.J.; Griffin, D.E. Global measles elimination. Nat. Rev. Microbiol. 2006, 4, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Navaratnarajah, C.K.; Oezguen, N.; Rupp, L.; Kay, L.; Leonard, V.H.; Braun, W.; Cattaneo, R. The heads of the measles virus attachment protein move to transmit the fusion-triggering signal. Nat. Struct. Mol. Biol. 2011, 18, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Long, M.A.; Rossi, F.M. Targeted cell fusion facilitates stable heterokaryon generation in vitro and in vivo. PLoS ONE 2011, 6, e26381. [Google Scholar] [CrossRef] [PubMed]
- Galanis, E.; Bateman, A.; Johnson, K.; Diaz, R.M.; James, C.D.; Vile, R.; Russell, S.J. Use of viral fusogenic membrane glycoproteins as novel therapeutic transgenes in gliomas. Hum. Gene Ther. 2001, 12, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Herschke, F.; Plumet, S.; Duhen, T.; Azocar, O.; Druelle, J.; Laine, D.; Wild, T.F.; Rabourdin-Combe, C.; Gerlier, D.; Valentin, H. Cell-cell fusion induced by measles virus amplifies the type i interferon response. J. Virol. 2007, 81, 12859–12871. [Google Scholar] [CrossRef] [PubMed]
- Tatsuo, H.; Ono, N.; Tanaka, K.; Yanagi, Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature 2000, 406, 893–897. [Google Scholar] [PubMed]
- Cocks, B.G.; Chang, C.C.; Carballido, J.M.; Yssel, H.; de Vries, J.E.; Aversa, G. A novel receptor involved in T-cell activation. Nature 1995, 376, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Sidorenko, S.P.; Clark, E.A. Characterization of a cell surface glycoprotein IPO-3, expressed on activated human B and T lymphocytes. J. Immunol. 1993, 151, 4614–4624. [Google Scholar] [PubMed]
- Kruse, M.; Meinl, E.; Henning, G.; Kuhnt, C.; Berchtold, S.; Berger, T.; Schuler, G.; Steinkasserer, A. Signaling lymphocytic activation molecule is expressed on mature CD83+ dendritic cells and is up-regulated by IL-1β. J. Immunol. 2001, 167, 1989–1995. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, H.; Tanaka, K.; Ono, N.; Tatsuo, H.; Yanagi, Y. Induction of the measles virus receptor slam (CD150) on monocytes. J. Gen. Virol. 2001, 82, 2913–2917. [Google Scholar] [CrossRef] [PubMed]
- Hsu, E.C.; Sarangi, F.; Iorio, C.; Sidhu, M.S.; Udem, S.A.; Dillehay, D.L.; Xu, W.; Rota, P.A.; Bellini, W.J.; Richardson, C.D. A single amino acid change in the hemagglutinin protein of measles virus determines its ability to bind CD46 and reveals another receptor on marmoset B cells. J. Virol. 1998, 72, 2905–2916. [Google Scholar] [PubMed]
- Naniche, D.; Varior-Krishnan, G.; Cervoni, F.; Wild, T.F.; Rossi, B.; Rabourdin-Combe, C.; Gerlier, D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 1993, 67, 6025–6032. [Google Scholar] [PubMed]
- Dorig, R.E.; Marcil, A.; Chopra, A.; Richardson, C.D. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 1993, 75, 295–305. [Google Scholar] [CrossRef]
- Muhlebach, M.D.; Mateo, M.; Sinn, P.L.; Prufer, S.; Uhlig, K.M.; Leonard, V.H.; Navaratnarajah, C.K.; Frenzke, M.; Wong, X.X.; Sawatsky, B.; et al. Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature 2011, 480, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Noyce, R.S.; Bondre, D.G.; Ha, M.N.; Lin, L.T.; Sisson, G.; Tsao, M.S.; Richardson, C.D. Tumor cell marker pvrl4 (nectin 4) is an epithelial cell receptor for measles virus. PLoS Pathog. 2011, 7, e1002240. [Google Scholar] [CrossRef] [PubMed]
- Leonard, V.H.; Sinn, P.L.; Hodge, G.; Miest, T.; Devaux, P.; Oezguen, N.; Braun, W.; McCray, P.B., Jr.; McChesney, M.B.; Cattaneo, R. Measles virus blind to its epithelial cell receptor remains virulent in rhesus monkeys but cannot cross the airway epithelium and is not shed. J. Clin. Invest. 2008, 118, 2448–2458. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.D.; Nakamura, T.; Russell, S.J.; Peng, K.W. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 2004, 64, 4919–4926. [Google Scholar] [CrossRef] [PubMed]
- Miest, T.S.; Frenzke, M.; Cattaneo, R. Measles virus entry through the signaling lymphocyte activation molecule governs efficacy of mantle cell lymphoma radiovirotherapy. Mol. Ther. 2013, 21, 2019–2031. [Google Scholar] [CrossRef] [PubMed]
- Derycke, M.S.; Pambuccian, S.E.; Gilks, C.B.; Kalloger, S.E.; Ghidouche, A.; Lopez, M.; Bliss, R.L.; Geller, M.A.; Argenta, P.A.; Harrington, K.M.; et al. Nectin 4 overexpression in ovarian cancer tissues and serum: Potential role as a serum biomarker. Am. J. Clin. Pathol. 2010, 134, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Fabre-Lafay, S.; Garrido-Urbani, S.; Reymond, N.; Goncalves, A.; Dubreuil, P.; Lopez, M. Nectin-4, a new serological breast cancer marker, is a substrate for tumor necrosis factor-alpha-converting enzyme (TACE)/ADAM-17. J. Biol. Chem. 2005, 280, 19543–19550. [Google Scholar] [CrossRef] [PubMed]
- Takano, A.; Ishikawa, N.; Nishino, R.; Masuda, K.; Yasui, W.; Inai, K.; Nishimura, H.; Ito, H.; Nakayama, H.; Miyagi, Y.; et al. Identification of nectin-4 oncoprotein as a diagnostic and therapeutic target for lung cancer. Cancer Res. 2009, 69, 6694–6703. [Google Scholar] [CrossRef] [PubMed]
- Fujiyuki, T.; Yoneda, M.; Amagai, Y.; Obayashi, K.; Ikeda, F.; Shoji, K.; Murakami, Y.; Sato, H.; Kai, C. A measles virus selectively blind to signaling lymphocytic activation molecule shows anti-tumor activity against lung cancer cells. Oncotarget 2015, 6, 24895–24903. [Google Scholar] [CrossRef] [PubMed]
- Awano, M.; Fujiyuki, T.; Shoji, K.; Amagai, Y.; Murakami, Y.; Furukawa, Y.; Sato, H.; Yoneda, M.; Kai, C. Measles virus selectively blind to SLAM has oncolytic efficacy against nectin-4-expressing pancreatic cancer cells. Cancer Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.; Russell, S.J. Engineering oncolytic viruses to exploit tumor specific defects in innate immune signaling pathways. Expert Opin. Biol. Ther. 2009, 9, 1163–1176. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, A.; Tamura, T.; Taniguchi, T. Interferon regulatory factor family of transcription factors and regulation of oncogenesis. Cancer Sci. 2008, 99, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Berchtold, S.; Lampe, J.; Weiland, T.; Smirnow, I.; Schleicher, S.; Handgretinger, R.; Kopp, H.G.; Reiser, J.; Stubenrauch, F.; Mayer, N.; et al. Innate immune defense defines susceptibility of sarcoma cells to measles vaccine virus-based oncolysis. J. Virol. 2013, 87, 3484–3501. [Google Scholar] [CrossRef] [PubMed]
- Grote, D.; Cattaneo, R.; Fielding, A.K. Neutrophils contribute to the measles virus-induced antitumor effect: Enhancement by granulocyte macrophage colony-stimulating factor expression. Cancer Res. 2003, 63, 6463–6468. [Google Scholar] [PubMed]
- Dey, A.; Zhang, Y.; Castleton, A.Z.; Bailey, K.; Beaton, B.; Patel, B.; Fielding, A.K. The role of neutrophils in measles virus-mediated oncolysis differs between B-cell malignancies and is not always enhanced by GCSF. Mol. Ther. 2016, 24, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Guillerme, J.B.; Boisgerault, N.; Roulois, D.; Menager, J.; Combredet, C.; Tangy, F.; Fonteneau, J.F.; Gregoire, M. Measles virus vaccine-infected tumor cells induce tumor antigen cross-presentation by human plasmacytoid dendritic cells. Clin. Cancer Res. 2013, 19, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, O.G.; Errington-Mais, F.; Steele, L.; Hadac, E.; Jennings, V.; Scott, K.; Peach, H.; Phillips, R.M.; Bond, J.; Pandha, H.; et al. Measles virus causes immunogenic cell death in human melanoma. Gene Ther. 2013, 20, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Bucheit, A.D.; Kumar, S.; Grote, D.M.; Lin, Y.; von Messling, V.; Cattaneo, R.B.; Fielding, A.K. An oncolytic measles virus engineered to enter cells through the CD20 antigen. Mol. Ther. 2003, 7, 62–72. [Google Scholar] [CrossRef]
- Hammond, A.L.; Plemper, R.K.; Zhang, J.; Schneider, U.; Russell, S.J.; Cattaneo, R. Single-chain antibody displayed on a recombinant measles virus confers entry through the tumor-associated carcinoembryonic antigen. J. Virol. 2001, 75, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.W.; Donovan, K.A.; Schneider, U.; Cattaneo, R.; Lust, J.A.; Russell, S.J. Oncolytic measles viruses displaying a single-chain antibody against CD38, a myeloma cell marker. Blood 2003, 101, 2557–2562. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.T.; Trejo, T.R.; Pham, L.D.; Oberg, A.L.; Russell, S.J.; Peng, K.W. Intravascularly administered RGD-displaying measles viruses bind to and infect neovessel endothelial cells in vivo. Mol. Ther. 2009, 17, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Hallak, L.K.; Merchan, J.R.; Storgard, C.M.; Loftus, J.C.; Russell, S.J. Targeted measles virus vector displaying echistatin infects endothelial cells via αvβ3 and leads to tumor regression. Cancer Res. 2005, 65, 5292–5300. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.W.; Holler, P.D.; Orr, B.A.; Kranz, D.M.; Russell, S.J. Targeting virus entry and membrane fusion through specific peptide/MHC complexes using a high-affinity T-cell receptor. Gene Ther. 2004, 11, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Hadac, E.M.; Peng, K.W.; Nakamura, T.; Russell, S.J. Reengineering paramyxovirus tropism. Virology 2004, 329, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Peng, K.W.; Harvey, M.; Greiner, S.; Lorimer, I.A.; James, C.D.; Russell, S.J. Rescue and propagation of fully retargeted oncolytic measles viruses. Nat. Biotechnol. 2005, 23, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Hirano, A.; Stenglein, S.; Nelson, J.; Thomas, G.; Wong, T.C. Engineered serine protease inhibitor prevents furin-catalyzed activation of the fusion glycoprotein and production of infectious measles virus. J. Virol. 1995, 69, 3206–3210. [Google Scholar] [PubMed]
- Richardson, C.; Hull, D.; Greer, P.; Hasel, K.; Berkovich, A.; Englund, G.; Bellini, W.; Rima, B.; Lazzarini, R. The nucleotide sequence of the mRNA encoding the fusion protein of measles virus (Edmonston strain): A comparison of fusion proteins from several different paramyxoviruses. Virology 1986, 155, 508–523. [Google Scholar] [CrossRef]
- Maisner, A.; Mrkic, B.; Herrler, G.; Moll, M.; Billeter, M.A.; Cattaneo, R.; Klenk, H.D. Recombinant measles virus requiring an exogenous protease for activation of infectivity. J. Gen. Virol. 2000, 81, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Springfeld, C.; von Messling, V.; Frenzke, M.; Ungerechts, G.; Buchholz, C.J.; Cattaneo, R. Oncolytic efficacy and enhanced safety of measles virus activated by tumor-secreted matrix metalloproteinases. Cancer Res. 2006, 66, 7694–7700. [Google Scholar] [CrossRef] [PubMed]
- Muhlebach, M.D.; Schaser, T.; Zimmermann, M.; Armeanu, S.; Hanschmann, K.M.; Cattaneo, R.; Bitzer, M.; Lauer, U.M.; Cichutek, K.; Buchholz, C.J. Liver cancer protease activity profiles support therapeutic options with matrix metalloproteinase-activatable oncolytic measles virus. Cancer Res. 2010, 70, 7620–7629. [Google Scholar] [CrossRef] [PubMed]
- Leber, M.F.; Bossow, S.; Leonard, V.H.; Zaoui, K.; Grossardt, C.; Frenzke, M.; Miest, T.; Sawall, S.; Cattaneo, R.; von Kalle, C.; et al. MicroRNA-sensitive oncolytic measles viruses for cancer-specific vector tropism. Mol. Ther. 2011, 19, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Baertsch, M.A.; Leber, M.F.; Bossow, S.; Singh, M.; Engeland, C.E.; Albert, J.; Grossardt, C.; Jager, D.; von Kalle, C.; Ungerechts, G. Microrna-mediated multi-tissue detargeting of oncolytic measles virus. Cancer Gene Ther. 2014, 21, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.W.; Facteau, S.; Wegman, T.; O’Kane, D.; Russell, S.J. Non-invasive in vivo monitoring of trackable viruses expressing soluble marker peptides. Nat. Med. 2002, 8, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Duprex, W.P.; McQuaid, S.; Hangartner, L.; Billeter, M.A.; Rima, B.K. Observation of measles virus cell-to-cell spread in astrocytoma cells by using a green fluorescent protein-expressing recombinant virus. J. Virol. 1999, 73, 9568–9575. [Google Scholar] [PubMed]
- Duprex, W.P.; McQuaid, S.; Roscic-Mrkic, B.; Cattaneo, R.; McCallister, C.; Rima, B.K. In vitro and in vivo infection of neural cells by a recombinant measles virus expressing enhanced green fluorescent protein. J. Virol. 2000, 74, 7972–7979. [Google Scholar] [CrossRef] [PubMed]
- Go, V.L. Carcinoembryonic antigen: Clinical application. Cancer 1976, 37, 562–566. [Google Scholar] [CrossRef]
- Dingli, D.; Peng, K.W.; Harvey, M.E.; Greipp, P.R.; O’Connor, M.K.; Cattaneo, R.; Morris, J.C.; Russell, S.J. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood 2004, 103, 1641–1646. [Google Scholar] [CrossRef] [PubMed]
- Jhiang, S.M.; Cho, J.Y.; Ryu, K.Y.; DeYoung, B.R.; Smanik, P.A.; McGaughy, V.R.; Fischer, A.H.; Mazzaferri, E.L. An immunohistochemical study of Na+/I− symporter in human thyroid tissues and salivary gland tissues. Endocrinology 1998, 139, 4416–4419. [Google Scholar] [PubMed]
- Spitzweg, C.; Morris, J.C. The sodium iodide symporter: Its pathophysiological and therapeutic implications. Clin. Endocrinol. 2002, 57, 559–574. [Google Scholar] [CrossRef]
- Dingli, D.; Russell, S.J.; Morris, J.C., 3rd. In vivo imaging and tumor therapy with the sodium iodide symporter. J. Cell. Biochem. 2003, 90, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.; Vongpunsawad, S.; Nakamura, T.; James, C.D.; Schroeder, M.; Cattaneo, R.; Giannini, C.; Krempski, J.; Peng, K.W.; Goble, J.M.; et al. Retargeted oncolytic measles strains entering via the EGFRvIII receptor maintain significant antitumor activity against gliomas with increased tumor specificity. Cancer Res. 2006, 66, 11840–11850. [Google Scholar] [CrossRef] [PubMed]
- Msaouel, P.; Iankov, I.D.; Allen, C.; Aderca, I.; Federspiel, M.J.; Tindall, D.J.; Morris, J.C.; Koutsilieris, M.; Russell, S.J.; Galanis, E. Noninvasive imaging and radiovirotherapy of prostate cancer using an oncolytic measles virus expressing the sodium iodide symporter. Mol. Ther. 2009, 17, 2041–2048. [Google Scholar] [CrossRef] [PubMed]
- Hutzen, B.; Pierson, C.R.; Russell, S.J.; Galanis, E.; Raffel, C.; Studebaker, A.W. Treatment of medulloblastoma using an oncolytic measles virus encoding the thyroidal sodium iodide symporter shows enhanced efficacy with radioiodine. BMC Cancer 2012. [Google Scholar] [CrossRef] [PubMed]
- Ungerechts, G.; Springfeld, C.; Frenzke, M.E.; Lampe, J.; Johnston, P.B.; Parker, W.B.; Sorscher, E.J.; Cattaneo, R. Lymphoma chemovirotherapy: CD20-targeted and convertase-armed measles virus can synergize with fludarabine. Cancer Res. 2007, 67, 10939–10947. [Google Scholar] [CrossRef] [PubMed]
- Ungerechts, G.; Springfeld, C.; Frenzke, M.E.; Lampe, J.; Parker, W.B.; Sorscher, E.J.; Cattaneo, R. An immunocompetent murine model for oncolysis with an armed and targeted measles virus. Mol. Ther. 2007, 15, 1991–1997. [Google Scholar] [CrossRef] [PubMed]
- Hartkopf, A.D.; Bossow, S.; Lampe, J.; Zimmermann, M.; Taran, F.A.; Wallwiener, D.; Fehm, T.; Bitzer, M.; Lauer, U.M. Enhanced killing of ovarian carcinoma using oncolytic measles vaccine virus armed with a yeast cytosine deaminase and uracil phosphoribosyltransferase. Gynecol. Oncol. 2013, 130, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, J.K.; Bossow, S.; Grossardt, C.; Sawall, S.; Kupsch, J.; Erbs, P.; Hassel, J.C.; von Kalle, C.; Enk, A.H.; Nettelbeck, D.M.; et al. Chemovirotherapy of malignant melanoma with a targeted and armed oncolytic measles virus. J. Invest. Dermatol. 2013, 133, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.; Lampe, J.; Bossow, S.; Zimmermann, M.; Neubert, W.; Bitzer, M.; Lauer, U.M. A novel armed oncolytic measles vaccine virus for the treatment of cholangiocarcinoma. Hum. Gene Ther. 2013, 24, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Lampe, J.; Bossow, S.; Weiland, T.; Smirnow, I.; Lehmann, R.; Neubert, W.; Bitzer, M.; Lauer, U.M. An armed oncolytic measles vaccine virus eliminates human hepatoma cells independently of apoptosis. Gene Ther. 2013, 20, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Grossardt, C.; Engeland, C.E.; Bossow, S.; Halama, N.; Zaoui, K.; Leber, M.F.; Springfeld, C.; Jaeger, D.; von Kalle, C.; Ungerechts, G. Granulocyte-macrophage colony-stimulating factor-armed oncolytic measles virus is an effective therapeutic cancer vaccine. Hum. Gene Ther. 2013, 24, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Peng, K.W.; Dingli, D.; Kratzke, R.A.; Russell, S.J. Oncolytic measles viruses encoding interferon β and the thyroidal sodium iodide symporter gene for mesothelioma virotherapy. Cancer Gene Ther. 2010, 17, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Iankov, I.D.; Allen, C.; Federspiel, M.J.; Myers, R.M.; Peng, K.W.; Ingle, J.N.; Russell, S.J.; Galanis, E. Expression of immunomodulatory neutrophil-activating protein of helicobacter pylori enhances the antitumor activity of oncolytic measles virus. Mol. Ther. 2012, 20, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.; Paraskevakou, G.; Iankov, I.; Giannini, C.; Schroeder, M.; Sarkaria, J.; Schroeder, M.; Puri, R.K.; Russell, S.J.; Galanis, E. Interleukin-13 displaying retargeted oncolytic measles virus strains have significant activity against gliomas with improved specificity. Mol. Ther. 2008, 16, 1556–1564. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Erlichman, C.; McDonald, C.J.; Ingle, J.N.; Zollman, P.; Iankov, I.; Russell, S.J.; Galanis, E. Heat shock protein inhibitors increase the efficacy of measles virotherapy. Gene Ther. 2008, 15, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Quezada, S.A.; Peggs, K.S. Exploiting CTLA-4, PD-1 and PD-L1 to reactivate the host immune response against cancer. Br. J. Cancer 2013, 108, 1560–1565. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Hodi, F.S.; Robert, C. CTLA-4 and PD-1/PD-L1 blockade: New immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin. Cancer Res. 2013, 19, 5300–5309. [Google Scholar] [CrossRef] [PubMed]
- Engeland, C.E.; Grossardt, C.; Veinalde, R.; Bossow, S.; Lutz, D.; Kaufmann, J.K.; Shevchenko, I.; Umansky, V.; Nettelbeck, D.M.; Weichert, W.; et al. CTLA-4 and PD-L1 checkpoint blockade enhances oncolytic measles virus therapy. Mol. Ther. 2014, 22, 1949–1959. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; Dey, A.; Ghorani, E.; Kumar, S.; Malam, Y.; Rai, L.; Steele, A.J.; Thomson, J.; Wickremasinghe, R.G.; Zhang, Y.; et al. Differential cytopathology and kinetics of measles oncolysis in two primary B-cell malignancies provides mechanistic insights. Mol. Ther. 2011, 19, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Heinzerling, L.; Kunzi, V.; Oberholzer, P.A.; Kundig, T.; Naim, H.; Dummer, R. Oncolytic measles virus in cutaneous T-cell lymphomas mounts antitumor immune responses in vivo and targets interferon-resistant tumor cells. Blood 2005, 106, 2287–2294. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.W.; Ahmann, G.J.; Pham, L.; Greipp, P.R.; Cattaneo, R.; Russell, S.J. Systemic therapy of myeloma xenografts by an attenuated measles virus. Blood 2001, 98, 2002–2007. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.; Greiner, S.; Harvey, M.; Soeffker, D.; Frenzke, M.; Abraham, K.; Shaw, A.; Rozenblatt, S.; Federspiel, M.J.; Russell, S.J.; et al. Oncolytic activities of approved mumps and measles vaccines for therapy of ovarian cancer. Cancer Gene Ther. 2005, 12, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Hu, C.; Nakamura, T.; Marks, J.D.; Russell, S.J.; Peng, K.W. Affinity thresholds for membrane fusion triggering by viral glycoproteins. J. Virol. 2007, 81, 13149–13157. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hasegawa, K.; Russell, S.J.; Sadelain, M.; Peng, K.W. Prostate-specific membrane antigen retargeted measles virotherapy for the treatment of prostate cancer. Prostate 2009, 69, 1128–1141. [Google Scholar] [CrossRef] [PubMed]
- Bach, P.; Abel, T.; Hoffmann, C.; Gal, Z.; Braun, G.; Voelker, I.; Ball, C.R.; Johnston, I.C.; Lauer, U.M.; Herold-Mende, C.; et al. Specific elimination of CD133+ tumor cells with targeted oncolytic measles virus. Cancer Res. 2013, 73, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, K.; Hanauer, J.R.; Prufer, S.; Munch, R.C.; Volker, I.; Filippis, C.; Jost, C.; Hanschmann, K.M.; Cattaneo, R.; Peng, K.W.; et al. Darpin-targeting of measles virus: Unique bispecificity, effective oncolysis, and enhanced safety. Mol. Ther. 2013, 21, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Pham, L.; O’Connor, M.K.; Federspiel, M.J.; Russell, S.J.; Peng, K.W. Dual therapy of ovarian cancer using measles viruses expressing carcinoembryonic antigen and sodium iodide symporter. Clin. Cancer Res. 2006, 12, 1868–1875. [Google Scholar] [CrossRef] [PubMed]
- Galanis, E.; Hartmann, L.C.; Cliby, W.A.; Long, H.J.; Peethambaram, P.P.; Barrette, B.A.; Kaur, J.S.; Haluska, P.J., Jr.; Aderca, I.; Zollman, P.J.; et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 2010, 70, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Phuong, L.K.; Allen, C.; Peng, K.W.; Giannini, C.; Greiner, S.; TenEyck, C.J.; Mishra, P.K.; Macura, S.I.; Russell, S.J.; Galanis, E.C. Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme. Cancer Res. 2003, 63, 2462–2469. [Google Scholar] [PubMed]
- National Institutes of Health. Nct00390299: Viral Therapy in Treating Patients with Recurrent Glioblastoma Multuforme. Available online: http://www.clinicaltrials.gov/ct2/show/NCT00390299 (accessed on 27 July 2016).
- McDonald, C.J.; Erlichman, C.; Ingle, J.N.; Rosales, G.A.; Allen, C.; Greiner, S.M.; Harvey, M.E.; Zollman, P.J.; Russell, S.J.; Galanis, E. A measles virus vaccine strain derivative as a novel oncolytic agent against breast cancer. Breast Cancer Res. Treat. 2006, 99, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Blechacz, B.; Splinter, P.L.; Greiner, S.; Myers, R.; Peng, K.W.; Federspiel, M.J.; Russell, S.J.; LaRusso, N.F. Engineered measles virus as a novel oncolytic viral therapy system for hepatocellular carcinoma. Hepatology 2006, 44, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Msaouel, P.; Iankov, I.D.; Allen, C.; Morris, J.C.; von Messling, V.; Cattaneo, R.; Koutsilieris, M.; Russell, S.J.; Galanis, E. Engineered measles virus as a novel oncolytic therapy against prostate cancer. Prostate 2009, 69, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Russell, S.J.; Peng, K.W. Systemic therapy of disseminated myeloma in passively immunized mice using measles virus-infected cell carriers. Mol. Ther. 2010, 18, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.; Opyrchal, M.; Aderca, I.; Schroeder, M.A.; Sarkaria, J.N.; Domingo, E.; Federspiel, M.J.; Galanis, E. Oncolytic measles virus strains have significant antitumor activity against glioma stem cells. Gene Ther. 2013, 20, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Penheiter, A.R.; Wegman, T.R.; Classic, K.L.; Dingli, D.; Bender, C.E.; Russell, S.J.; Carlson, S.K. Sodium iodide symporter (NIS)-mediated radiovirotherapy for pancreatic cancer. Am. J. Roentgenol. 2010, 195, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Peng, K.W.; Russell, S.J. Oncolytic measles virus encoding thyroidal sodium iodide symporter for squamous cell cancer of the head and neck radiovirotherapy. Hum. Gene Ther. 2012, 23, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Touchefeu, Y.; Khan, A.A.; Borst, G.; Zaidi, S.H.; McLaughlin, M.; Roulstone, V.; Mansfield, D.; Kyula, J.; Pencavel, T.; Karapanagiotou, E.M.; et al. Optimising measles virus-guided radiovirotherapy with external beam radiotherapy and specific checkpoint kinase 1 inhibition. Radiother. Oncol. 2013, 108, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Steele, M.B.; Suksanpaisan, L.; Federspiel, M.J.; Russell, S.J.; Peng, K.W.; Bakkum-Gamez, J.N. Oncolytic measles and vesicular stomatitis virotherapy for endometrial cancer. Gynecol. Oncol. 2014, 132, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Kunzi, V.; Oberholzer, P.A.; Heinzerling, L.; Dummer, R.; Naim, H.Y. Recombinant measles virus induces cytolysis of cutaneous T-cell lymphoma in vitro and in vivo. J. Invest. Dermatol. 2006, 126, 2525–2532. [Google Scholar] [CrossRef] [PubMed]
- Grote, D.; Russell, S.J.; Cornu, T.I.; Cattaneo, R.; Vile, R.; Poland, G.A.; Fielding, A.K. Live attenuated measles virus induces regression of human lymphoma xenografts in immunodeficient mice. Blood 2001, 97, 3746–3754. [Google Scholar] [CrossRef] [PubMed]
- Iankov, I.D.; Hillestad, M.L.; Dietz, A.B.; Russell, S.J.; Galanis, E. Converting tumor-specific markers into reporters of oncolytic virus infection. Mol. Ther. 2009, 17, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Ungerechts, G.; Frenzke, M.E.; Yaiw, K.C.; Miest, T.; Johnston, P.B.; Cattaneo, R. Mantle cell lymphoma salvage regimen: Synergy between a reprogrammed oncolytic virus and two chemotherapeutics. Gene Ther. 2010, 17, 1506–1516. [Google Scholar] [CrossRef] [PubMed]
- Bossow, S.; Grossardt, C.; Temme, A.; Leber, M.F.; Sawall, S.; Rieber, E.P.; Cattaneo, R.; von Kalle, C.; Ungerechts, G. Armed and targeted measles virus for chemovirotherapy of pancreatic cancer. Cancer Gene Ther. 2011, 18, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Yurttas, C.; Berchtold, S.; Malek, N.; Bitzer, M.; Lauer, U.M. “PULSED” versus “CONTINUOUS” application of the prodrug 5-FC for enhancing oncolytic effectiveness of a measles vaccine virus armed with a suicide gene. Hum. Gene Ther. Clin. Dev. 2014, 25, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Tong, C.; Dispenzieri, A.; Federspiel, M.J.; Russell, S.J.; Peng, K.W. Polyinosinic acid decreases sequestration and improves systemic therapy of measles virus. Cancer Gene Ther. 2012, 19, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Sistigu, A.; Viaud, S.; Chaput, N.; Bracci, L.; Proietti, E.; Zitvogel, L. Immunomodulatory effects of cyclophosphamide and implementations for vaccine design. Semin. Immunopathol. 2011, 33, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.W.; Myers, R.; Greenslade, A.; Mader, E.; Greiner, S.; Federspiel, M.J.; Dispenzieri, A.; Russell, S.J. Using clinically approved cyclophosphamide regimens to control the humoral immune response to oncolytic viruses. Gene Ther. 2013, 20, 255–261. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02192775 (accessed on 4 March 2016).
- Najar, M.; Raicevic, G.; Crompot, E.; Fayyad-Kazan, H.; Bron, D.; Toungouz, M.; Lagneaux, L. The immunomodulatory potential of mesenchymal stromal cells: A story of a regulatory network. J. Immunother. 2016, 39, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Castleton, A.; Dey, A.; Beaton, B.; Patel, B.; Aucher, A.; Davis, D.M.; Fielding, A.K. Human mesenchymal stromal cells deliver systemic oncolytic measles virus to treat acute lymphoblastic leukemia in the presence of humoral immunity. Blood 2014, 123, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.T.; Federspiel, M.J.; Guo, C.M.; Ooi, L.L.; Russell, S.J.; Peng, K.W.; Hui, K.M. Systemically delivered measles virus-infected mesenchymal stem cells can evade host immunity to inhibit liver cancer growth. J. Hepatol. 2013, 59, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Mader, E.K.; Maeyama, Y.; Lin, Y.; Butler, G.W.; Russell, H.M.; Galanis, E.; Russell, S.J.; Dietz, A.B.; Peng, K.W. Mesenchymal stem cell carriers protect oncolytic measles viruses from antibody neutralization in an orthotopic ovarian cancer therapy model. Clin. Cancer Res. 2009, 15, 7246–7255. [Google Scholar] [CrossRef] [PubMed]
- Almstatter, I.; Mykhaylyk, O.; Settles, M.; Altomonte, J.; Aichler, M.; Walch, A.; Rummeny, E.J.; Ebert, O.; Plank, C.; Braren, R. Characterization of magnetic viral complexes for targeted delivery in oncology. Theranostics 2015, 5, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Miest, T.S.; Yaiw, K.C.; Frenzke, M.; Lampe, J.; Hudacek, A.W.; Springfeld, C.; von Messling, V.; Ungerechts, G.; Cattaneo, R. Envelope-chimeric entry-targeted measles virus escapes neutralization and achieves oncolysis. Mol. Ther. 2011, 19, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Galanis, E.; Atherton, P.J.; Maurer, M.J.; Knutson, K.L.; Dowdy, S.C.; Cliby, W.A.; Haluska, P., Jr.; Long, H.J.; Oberg, A.; Aderca, I.; et al. Oncolytic measles virus expressing the sodium iodide symporter to treat drug-resistant ovarian cancer. Cancer Res. 2015, 75, 22–30. [Google Scholar] [CrossRef] [PubMed]
| Virus Strain | Genetic Modification | Tumour Type | |
|---|---|---|---|
| Unmodified attenuated vaccine strain | MV-Edm | Unmodified | Leukemias/lymphomas [69,83,84], multiple myeloma [85], ovarian cancers [86], breast cancer, melanoma, renal cell carcinoma, fibrosarcoma |
| Retargeted virus | MV-CD20 | Retargeted to CD20 | Leukemias, fibrosarcoma [43] |
| MV-CD38 | Retargeted to CD38 | MM, Erythroleukemia, Burkitt’s lymphoma, ovarian cancer, GBM [50] | |
| MV-HER2/neu | Retargeted to her2/neu | Ovarian cancer, medulloblastoma [87] | |
| MV0EGFRvIII | Retargeted to EGFR | Erythroleukemia, Burkitt’s lymphoma, ovarian cancer, GBM [66] | |
| MV-PSMA | Retargeted to PSMA | Prostate cancer [88] | |
| MV-CD133 | Retargeted to CD133 | Hepatocellular carcinoma, colon, glioma [89] | |
| MV-aVb3-integrin targeted | Retargeted to aVb3-integrin | Multiple myeloma [47] | |
| MV-MMP | MMP-activated virus | Hepatocellular carcinoma, fibrosarcoma, cholangio carcinoma [54,55] | |
| MV-miRNA-sensitive | Retargeted to miRNA sequences | GBM [56] | |
| MV-DARPins | Retargeted to EGFR, Her2/neu and EpCAM | Adenocarcinoma, breast ductal carcinoma, colon adenocarcinoma, fibrosarcoma, ovarian carcinoma, glioblastoma [90] | |
| Reporter genes | MV-CEA | CEA reporter gene | Ovarian cancer [91,92], GBM [93,94], breast cancer [95], hepatocellular carcinoma [96], prostate cancer [97], rhabdomyosarcoma [79] |
| MV-NIS | NIS reporter gene | Ovarian cancer [91], MM [98], GBM [99], hepatocellular carcinoma [96], pancreatic cancer [100], prostate [97], head oral squamous cell carcinoma, anaplastic thyroid carcinoma, hypopharyngeal carcinoma [101], colorectal cancer [102], endometrial cancer [103], T cell lymphoma [104] | |
| MV-LacZ | Beta-galactosidase reporter gene | Lymphoma [105] | |
| MV-lambda | Human light immunoglobulin chain reporter gene | MM [106] | |
| Suicide genes | MV-PNP | Prodrug convertase | Leukemia/lymphoma [107], colorectal cancer [70], pancreatic cancer [108] |
| MV-SCD/FCU1 | Prodrug convertase | Ovarian cancer [71], melanoma [72], cholangiocarcinoma [73], hepatocellular carcinoma [74], colorectal carcinoma [109] | |
| Immuno-stimulating genes | MV-GMCSF | GM-CSF gene | ALL cells, Burkitt’s lymphoma [39,75] |
| MV-NAP | Neutrophil activating protein gene | Breast cancer [77] | |
| MV-alphaCTLA4 | Retargeted to CTLA-4 antibody | Malignant melanoma [82] | |
| MV-alphaPDL-1 | Retargeted to PDL-1 antibody | Malignant melanoma [82] | |
| MV-Hblind-IL13 | Retargeted to IL13 | GBM [78] | |
| MV-IFN | IFN immunomodulatory gene | Mesothelioma [76] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aref, S.; Bailey, K.; Fielding, A. Measles to the Rescue: A Review of Oncolytic Measles Virus. Viruses 2016, 8, 294. https://doi.org/10.3390/v8100294
Aref S, Bailey K, Fielding A. Measles to the Rescue: A Review of Oncolytic Measles Virus. Viruses. 2016; 8(10):294. https://doi.org/10.3390/v8100294
Chicago/Turabian StyleAref, Sarah, Katharine Bailey, and Adele Fielding. 2016. "Measles to the Rescue: A Review of Oncolytic Measles Virus" Viruses 8, no. 10: 294. https://doi.org/10.3390/v8100294
APA StyleAref, S., Bailey, K., & Fielding, A. (2016). Measles to the Rescue: A Review of Oncolytic Measles Virus. Viruses, 8(10), 294. https://doi.org/10.3390/v8100294





